Abstract
The segregation of homologous chromosomes during the Meiosis I division requires an obligate crossover per homolog pair (crossover assurance). In Saccharomyces cerevisiae and mammals, Msh4 and Msh5 proteins stabilize Holliday junctions and its progenitors to facilitate crossing over. S. cerevisiae msh4/5 hypomorphs that reduce crossover levels up to twofold at specific loci on chromosomes VII, VIII, and XV without affecting homolog segregation were identified recently. We use the msh4–R676W hypomorph to ask if the obligate crossover is insulated from variation in crossover frequencies, using a S. cerevisiae S288c/YJM789 hybrid to map recombination genome-wide. The msh4–R676W hypomorph made on average 64 crossovers per meiosis compared to 94 made in wild type and 49 in the msh4Δ mutant confirming the defect seen at individual loci on a genome-wide scale. Crossover reductions in msh4–R676W and msh4Δ were significant across chromosomes regardless of size, unlike previous observations made at specific loci. The msh4–R676W hypomorph showed reduced crossover interference. Although crossover reduction in msh4–R676W is modest, 42% of the four viable spore tetrads showed nonexchange chromosomes. These results, along with modeling of crossover distribution, suggest the significant reduction in crossovers across chromosomes and the loss of interference compromises the obligate crossover in the msh4 hypomorph. The high spore viability of the msh4 hypomorph is maintained by efficient segregation of the natural nonexchange chromosomes. Our results suggest that variation in crossover frequencies can compromise the obligate crossover and also support a mechanistic role for interference in obligate crossover formation.
Keywords: crossover assurance, obligate crossover, genome-wide recombination map, whole-genome sequencing, nonexchange chromosomes
SEXUALLY reproducing organisms undergo meiosis to produce haploid gametes from diploid progenitor cells (Roeder 1997; Zickler and Kleckner 1999). This reduction in ploidy is achieved through the segregation of homologous chromosomes at the first meiotic division (MI). Accurate homolog segregation is facilitated by crossovers that establish physical connections between homolog pairs and provide tension necessary for generation of the bipolar spindle (Petronczki et al. 2003). Meiotic crossing over is highly regulated to ensure at least one crossover per homolog pair (crossover assurance) despite limited number of crossovers per meiosis (Berchowitz and Copenhaver 2010; Rosu et al. 2011).
Although crossovers are thought to be essential for accurate meiotic chromosome segregation, population genetic studies in humans suggest that there is considerable variation in crossover frequencies between populations, sexes, and individuals (Cheung et al. 2007; Chowdhury et al. 2009; Fledel-Alon et al. 2009; Kong et al. 2010; Kong et al. 2014). Analysis of meiotic crossovers in single sperm cells using whole-genome sequencing reinforces the fact that within individuals, crossover numbers per meiosis vary widely (Lu et al. 2012). The average number of crossovers per sperm was observed to be 26, but with a large variation from 17 to 35 crossovers per sperm (Lu et al. 2012). Although a lower frequency of crossovers increases the chances of aneuploidy in sperm, studies in Saccharomyces cerevisiae, Drosophila, and humans have shown that nonexchange chromosomes can undergo accurate segregation frequently (Dawson et al. 1986; Mann and Davis 1986; Guacci and Kaback 1991; Dernburg et al. 1996; Karpen et al. 1996; Kemp et al. 2004; Cheslock et al. 2005; Fledel-Alon et al. 2009; Gladstone et al. 2009; Newnham et al. 2010). Identification of genetic variants associated with such variation in crossover frequencies is of considerable interest.
Meiotic crossovers are initiated by the programmed introduction of DNA double-strand breaks (DSBs) (Keeney et al. 1997). Repair of meiotic DSBs results in the formation of crossover as well as noncrossover products through distinct pathways (Allers and Lichten 2001; Hunter and Kleckner 2001). In S. cerevisiae and mammals, a majority of the crossovers are formed through a pathway mediated by the MutS mismatch repair homologs Msh4, Msh5, and MutL mismatch repair homologs Mlh1, Mlh3 (Ross-Macdonald and Roeder 1994; Hollingsworth et al. 1995; Baker et al. 1996; Barlow and Hulten 1998; De Vries et al. 1999; Edelmann et al. 1999; Woods et al. 1999; Kneitz et al. 2000; Novak et al. 2001; Lipkin et al. 2002; Santucci-Darmanin et al. 2002; Argueso et al. 2004; Guillon et al. 2005; Kolas et al. 2005; Lynn et al. 2007; Cole et al. 2012). The Msh4/5 proteins are part of an ensemble of proteins called the ZMM complex that stabilizes single end invasion intermediates generated during invasion of an intact homolog by a resected DSB end (Chua and Roeder 1998; Agarwal and Roeder 2000; Borner et al. 2004; Tsubouchi et al. 2006; Shinohara et al. 2008). The Msh4/5 complex also binds and stabilizes double Holliday junctions and promotes their resolution into crossover products in association with other repair factors that include Mlh1/3, Exo1, and Sgs1 (Borner et al. 2004; Snowden et al. 2004; Nishant et al. 2008; Snowden et al. 2008; Zakharyevich et al. 2010; De Muyt et al. 2012; Zakharyevich et al. 2012).
Recent human studies have implicated polymorphisms in ZMM genes such as RNF212 (putative S. cerevisiae ZIP3 ortholog) and MSH4 with genome-wide crossover frequency variation (Kong et al. 2014). Similar observations have been made in S. cerevisiae, where a series of msh4/5 hypomorphic alleles that showed up to twofold reduction in crossovers at specific loci on chromosomes VII, VIII, and XV with high spore viability were identified (Nishant et al. 2010). The high spore viability observed in S. cerevisiae msh4/5 hypomorphs and in other mutants like mlh3Δ mms4Δ (Brown et al. 2013) provide further evidence that a reduction in crossovers is not directly correlated with nondisjunction. In this study we use the S. cerevisiae msh4–R676W hypomorph as a tool to study how variation in crossover frequencies is buffered by the cell to ensure chromosome segregation. The msh4–R676W hypomorph is predicted to be defective in ATP hydrolysis by the Msh4/5 complex (Kijas et al. 2003; Nishant et al. 2010; Rakshambikai et al. 2013). S. cerevisiae, msh4∆, and msh5∆ mutants have ∼2.5-fold reduction in crossing over and ∼60% reduction in meiotic viability, and nonexchange chromosomes are observed in the viable spores (Ross-Macdonald and Roeder 1994; Hollingsworth et al. 1995; Novak et al. 2001; Argueso et al. 2004; Chen et al. 2008; Oke et al. 2014). Comparison of spore viability and genetic map distances for wild type (97%, 96 cM), msh4–R676W (90%, 56 cM), and msh4Δ (36%, 39 cM) showed that the msh4 hypomorph has high spore viability despite up to a twofold decrease in crossing over on specific genetic intervals on chromosome XV (Argueso et al. 2004; Nishant et al. 2010). Two mutually exclusive possibilities can explain this phenomenon. Either msh4/5 hypomorphs continue to ensure one crossover per homolog pair (crossover assurance) or they segregate nonexchange chromosomes efficiently. To distinguish between these two mechanisms, we sought to examine genome-wide crossover distribution in the msh4–R676W hypomorph.
In whole-genome studies, segregation of single nucleotide polymorphisms (SNPs) in crosses of yeast strains are used to track recombination events (Chen et al. 2008; Mancera et al. 2008; Qi et al. 2009; Anderson et al. 2011; Martini et al. 2011; Oke et al. 2014). We made high-resolution genome-wide recombination maps in the msh4–R676W hypomorph using an S288c/YJM789 hybrid strain. The msh4–R676W hypomorph showed on average ∼30% genome-wide reduction in crossover numbers and reduced interference compared to wild type. Although the reduction in crossovers was modest, 42% of the four viable spore meiotic events were observed to have one, or more than one, nonexchange chromosome in the msh4–R676W hypomorph. Combined loss of crossovers and interference therefore compromise the obligate crossover in msh4–R676W. These results, along with modeling of crossover distribution patterns in wild type and msh4 mutants, support a mechanistic role for interference in crossover assurance. Despite the large number of meioses with nonexchange chromosomes in the msh4–R676W hypomorph, analysis of spore viability patterns suggests efficient segregation of natural nonexchange chromosomes in S. cerevisiae.
Materials and Methods
Media and strains
S. cerevisiae S288c and YJM789 yeast strains were grown on either yeast extract–peptone–dextrose (YPD) or synthetic complete medium at 30° (Mortimer and Johnston 1986; Rose et al. 1990; McCusker et al. 1994). All strains constructed in this study were derived by transformation of S288c and YJM789 S. cerevisiae strains with integration plasmids using standard techniques (Gietz et al. 1995). When required, the drugs geneticin (Invitrogen), nourseothricin (Werner BioAgents, Germany), and hygromycin (Sigma) were added to the media at prescribed concentrations (Goldstein and McCusker 1999). Strains are listed in Supporting Information, Table S1. Sporulation medium was prepared as described in Argueso et al. (2004).
Tetrad analysis
The haploid strains were patched together on synthetic complete medium and incubated for 4 hr. The resulting diploids were sporulated using standard zero-growth-mating protocol (Argueso et al. 2003). After 48 hr in sporulation medium, tetrads were dissected on synthetic complete medium using a Zeiss dissection microscope.
DNA extraction and whole-genome sequencing of meiotic spores
Spore colonies from tetrads were independently cultured overnight at 30° in 4 ml of YPD liquid medium. DNA was extracted from each culture using the PrepEase DNA isolation kit from Affymetrix following the manufacturer’s protocol. Genomic DNA fragmentation and library preparation were performed as described previously (Wilkening et al. 2013). Briefly, 2 μg of genomic DNA was sheared using a Bandelin Sonorex RX 102 sonicating water bath to obtain DNA fragments of 250–500 bp. End repair, dA-tailing, and ligation were done as per the Illumina library preparation protocol with heat inactivation instead of column/magnetic bead- based cleanups. The multiplexed libraries were amplified and size selected for 350–-400 bp using Invitrogen E-Gel (SizeSelect 2%). The size-selected DNA was sequenced (100PE) on Illumina HiSeq 2000 machines at the EMBL Genomics Core Facilities (GeneCore), Heidelberg, Germany. The raw reads obtained were demultiplexed using the FASTX-toolkit (https://github.com/agordon/fastx_toolkit). Demultiplexed reads were processed for quality control (QC) using the NGS QC Toolkit (Patel and Jain 2012). These QC-filtered high-quality reads were used for further analysis. The sequence data are available from the National Centre for Biotechnology Information Sequence Read Archive under accession no. SRP041856.
Read mapping, genotyping, and annotation of recombination events
We used “ReCombine”—a set of programs developed to analyze the meiotic recombination events from whole-genome sequencing and microarray data from yeast tetrads (Anderson et al. 2011). Reads were aligned to both the S288c and YJM789 references using ReadAligner program, which uses native bowtie aligner for alignment (Langmead et al. 2009). Since, our read length was ∼100 bp and bowtie 1 has limitations in aligning longer reads, each read was broken down into two separate reads of 45 bp each to increase the overall alignment rate. Input parameters for the bowtie aligner in ReadAligner program were also modified to support paired-end alignment. From the alignment, 58,655 SNPs were genotyped. Finally various events such as crossovers, noncrossovers (type 0 gene conversions exhibiting 1:3 or 3:1 segregation of SNP markers), gene conversions not associated with crossovers (type 0, 2, 3, and 4 gene conversions), and crossover-associated gene conversions were detected using the Crossover program (Anderson et al. 2011). A 2.5-kb range was set to merge closely placed crossover and noncrossover events. Custom R scripts were written to parse the output files. Plotting was done in R using grammar for graphics 2 (ggplot2) and other base packages. All test statistics were calculated in R (v. 2.15.2, CRAN). The SNP segregation files, data output from the Crossover program, and the custom R scripts are deposited at http://figshare.com (http://dx.doi.org/10.6084/m9.figshare. 1192705).
Detection of copy-number variation
For each alignment of each segregant, read counts were tabulated for consecutive 5000-bp windows using cn.MOPS (Klambauer et al. 2012). With the read counts table, segmentation and smoothing was done using default settings in DNAcopy to infer chromosomal copy number (Olshen et al. 2004). Allele frequency for each SNP in each segregant was calculated as the number of bases called from the YJM789 allele as a proportion of the total number of bases called.
Interference analysis
Previous studies have suggested that ∼250 intercrossover distances (genome-wide crossover data from three tetrads) is sufficient to distinguish between strains with wild-type interference and absence of interference (Chen et al. 2008; Anderson et al. 2011). We calculated intercrossover distance as the physical distance between consecutive crossovers. These values were converted into genetic distance using the formula: Morgan = 12.07 × 106 × 2/mean crossovers. A 1-cM genetic distance was equivalent to 2.56 kb (wild type), 3.75 kb (msh4–R676W), and 4.8 kb (msh4Δ). Genetic distances between crossovers were modeled as a gamma distribution. The model parameters (α and β) were fitted by maximum-likelihood method. Estimated parameters were tested for goodness of fit by Kolmogorov–Smirnov statistic tests (P < 0.05) and Bayesian information criterion (BIC).
Results
High-resolution genome-wide recombination map in the msh4–R676W hypomorph using high-throughput sequencing
We used the S. cerevisiae S288c/YJM789 hybrid to generate a high-resolution genome-wide recombination map in the msh4–R676W hypomorph. The S288c/YJM789 hybrid has a spore viability of 84%, recombination parameters are similar to the SK1 and S288c strains, and crossovers display interference (Winzeler et al. 1998; Chen et al. 2008; Mancera et al. 2008). The msh4–R676W hypomorph was previously identified in the SK1 strain background (Nishant et al. 2010) (Figure S1). The SK1 Msh4 protein sequence is different from S288c at one amino acid and from YJM789 at two amino acid positions. Similarly, the SK1 Msh5 protein sequence shows polymorphisms relative to S288c at four amino acid and YJM789 at 17 amino acid positions. To avoid a possible incompatibility between Msh4 and Msh5 due to these polymorphisms, we analyzed the msh4–R676W hypomorphic mutation in an SK1 context in the S288c/YJM789 hybrid. We introduced the SK1 allele of MSH5 in the S288c strain-bearing deletion of msh4Δ. SK1 MSH4 and msh4–R676W alleles were introduced into the YJM789 strain with msh5Δ mutation. The SK1 MSH4/5 and msh4–R676W alleles were analyzed as heterozygotes over their respective null mutations in the S288c/YJM789 hybrid (Figure S2). The S288c/YJM789 hybrid with SK1 MSH4/5 genes showed high spore viability similar to the wild-type cross (84%) (Table 1). The msh4–R676W hypomorph also showed high spore viability of 76% compared to a msh4Δ mutant, which has 41% viability in the S288c/YJM789 hybrid (Table 1). To generate high-resolution genome-wide recombination maps in msh4 mutants and controls, high-coverage whole-genome sequence data were obtained from a total of 80 of the four viable spore tetrads (Table 1 and Table S2). We selected four viable spore tetrads to analyze how variation in crossover frequencies can be tolerated without affecting chromosome segregation. These include 38 tetrads in the msh4–R676W hypomorph and 18 tetrads in msh4Δ. Among the controls we sequenced 20 tetrads in the S288c/YJM789 hybrid and four tetrads in the S288c/YJM789 hybrid with SK1 MSH4/5 genes. The sequence data are available from the National Centre for Biotechnology Information Sequence Read Archive under accession no. SRP041856. A total of 58,655 markers were genotyped across the mutant and control strains with a median intermarker interval size of 72 bp (Figure S3). Plots showing segregation information of SNPs in all 80 tetrads sequenced are in File S1. We observed on average 94.4 crossovers in the S288c × YJM789 wild-type strain and 56.8 noncrossovers (Table 1). These values are statistically similar to the crossover (90.4, t-test, P = 0.27) and noncrossover counts (46.0, t-test, P = 0.05) observed in the Mancera et al. (2008) study. As discussed previously, these numbers do not account for noncrossovers that occur between two consecutive SNP markers or noncrossovers that had restoration repair in the wild-type and msh4 mutants (Mancera et al. 2008). The S288c × YJM789 hybrid with SK1 MSH4/5 genes had on average 85.7 crossovers and 55.5 noncrossovers (Table 1), which is not significantly different from wild type (t-test, P = 0.22 for crossovers and P = 0.88 for noncrossovers). These results suggest SK1 MSH4/5 genes are functional in the S288c/YJM789 hybrid with a minor reduction in crossover frequency that does not affect spore viability. The minor reduction in crossover frequency may be because of replacement of the S288c and YJM789 MSH4/5 alleles with the SK1 MSH4/5 alleles. Crossover and noncrossover counts for each of the 80 tetrads are shown in Table S3.
Table 1. Spore viability and recombination parameters of the S288c/YJM789 hybrid bearing SK1 MSH4/5 or msh4 mutant alleles.
| Genotype | N | S.V% | Tetrads sequenced | Avg. CO counts ±SD (median) | Avg. NCO counts ±SD (median) |
|---|---|---|---|---|---|
| S288c × YJM789 | 180 | 84 | 20 | 94.4 ± 14.6 (95) | 56.8 ± 22.6 (55) |
| S288c × YJM789 with SK1 MSH4/MSH5 | 209 | 84 | 4 | 85.7 ± 8.5 (83.5) | 55.5 ± 6.9 (50) |
| S288c × YJM789 with SK1 msh4–R676W/MSH5 | 239 | 76 | 38 | 64.2 ± 12.1 (62) | 55.2 ± 13.4 (51.5) |
| S288c × YJM789 msh4Δ | 100 | 41 | 18 | 49.5 ± 16.7 (47) | 69.7 ± 27 (56) |
CO, crossover; NCO, noncrossover; N, number of tetrads analyzed; SD, standard deviation.
The msh4–R676W hypomorph shows a genome-wide decrease in crossing over with high spore viability
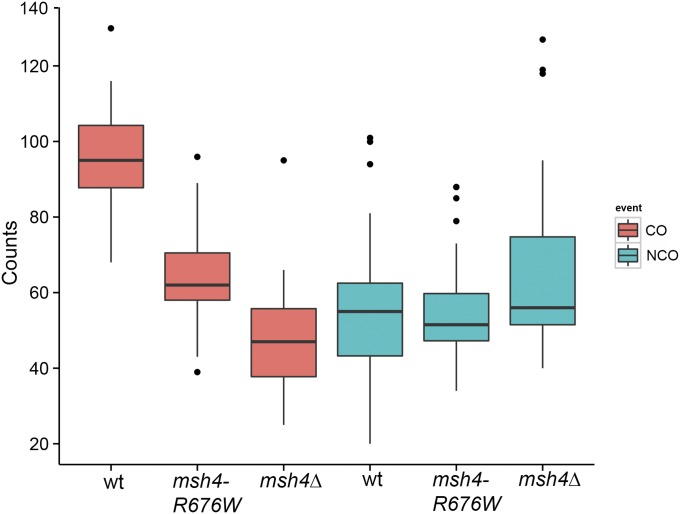
We analyzed crossover and noncrossover events in the msh4–R676W hypomorph. The average number of crossovers was reduced to 64.2 / meiosis while the noncrossovers were 55.2 / meiosis (Table 1 and Figure 1). The reduction in crossovers compared to wild type is statistically significant for the msh4–R676W hypomorph (t-test, P = 4.18 × 10−9). Noncrossovers were not statistically different from wild type (t-test, P = 0.77). These results suggest there is a genome-wide reduction in crossing over in the msh4–R676W hypomorph while noncrossovers are similar to wild type. Representative crossover and noncrossover distributions along chromosome IV for wild type and msh4–R676W hypomorph are shown in Figure 2, A and B. For the msh4Δ mutant, average crossovers were reduced to 49.5 / meiosis (t-test, P = 2.93 × 10−10), while noncrossovers (69.7 / meiosis, 56 median) were similar to wild type (t-test, P = 0.12). The data for msh4Δ are consistent with previous analysis of msh4Δ tetrads in the S288c x YJM789 hybrid where crossovers showed a twofold reduction while noncrossovers were unchanged (Chen et al. 2008; Mancera et al. 2008; Oke et al. 2014). Collectively these results suggest that in the msh4–R676W hypomorph and msh4Δ mutant, crossovers are reduced on a genome-wide scale while noncrossovers are maintained (Figure 1). These results suggest that most of the DSBs that cannot be repaired as crossovers in msh4 mutants are repaired using inter-sister recombination. It is also possible that the restoration/conversion ratio is perturbed in the msh4 mutants in favor of restoration of the SNP markers so that more noncrossovers are not detected.
Figure 1.
Frequencies of crossovers and noncrossovers per meiosis for wild type, msh4–R676W, and msh4Δ. Box plots show minimum, first quantile, median, third quantile, and maximum count.
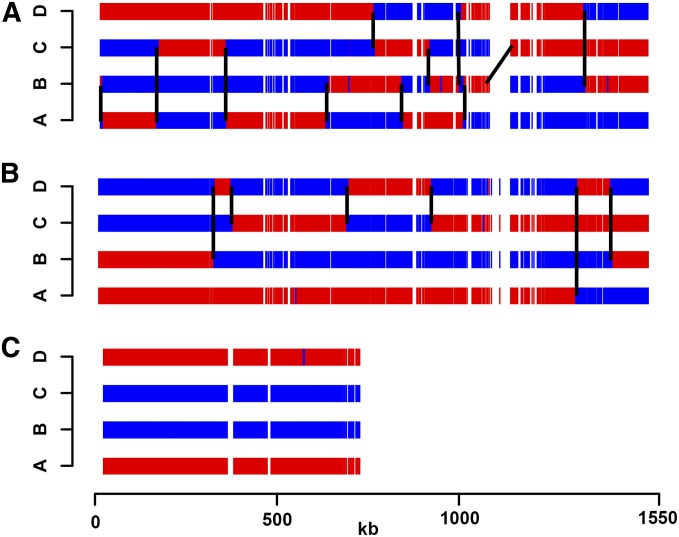
Figure 2.
Representative meiotic crossover map for wild-type and msh4–R676W mutant. A tetrad showing 11 crossovers on chromosome IV in the wild-type (A) compared to 6 in the msh4–R676W mutant (B). (C) msh4–R676W tetrad with nonexchange chromosome X. S288c and YJM789 SNPs are shown in blue and red, respectively.
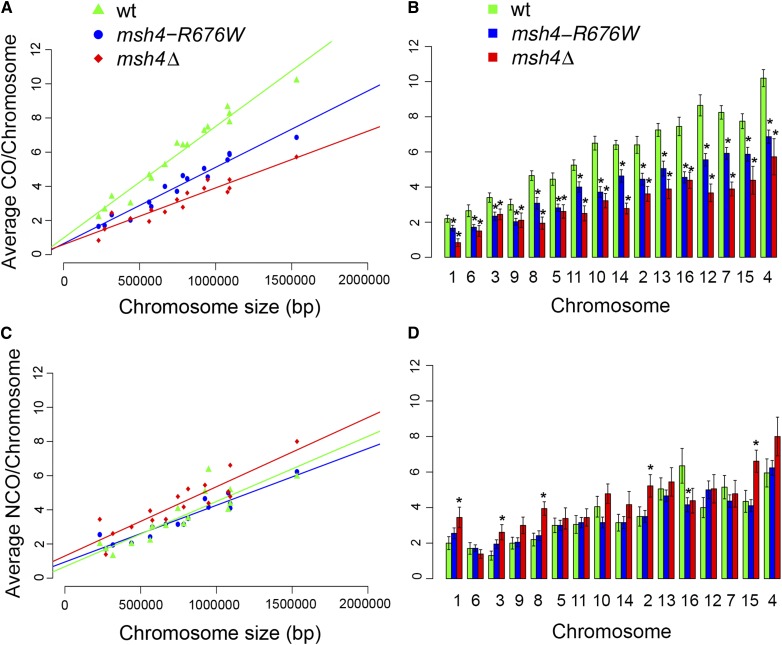
To study the effects of chromosome size on crossover distribution, average crossover counts per chromosome were plotted as a function of chromosome size for the wild type, msh4–R676W and the msh4Δ mutants (Table S4 and Figure 3A). Crossover counts were directly proportional to the chromosome size for wild type with an intercept of 0.98 (95% confidence interval: 0.44, 1.5). For the msh4–R676W and msh4Δ mutants crossover counts correlated with chromosome size but with lower intercepts of 0.64 (95% confidence interval: 0.20, 1.1) and 0.59 (95% confidence interval: 0.09, 1.1). Both the msh4–R676W and msh4Δ mutant showed a proportional loss of crossovers from small and large chromosomes (Figure 3B). These results suggest msh4 mutations result in similar crossover defects on small and large chromosomes, which could increase the probability of nonexchange events for small chromosomes as discussed later. Noncrossover counts for the wild type, msh4–R676W and the msh4Δ mutants are also proportional to chromosome size (Table S4 and Figure 3C). While overall noncrossover levels were the same, the msh4Δ mutant had statistically significant increase in noncrossover levels on some of the chromosomes (Figure 3D). The noncrossover intercepts for wild type (0.67), msh4–R676W (0.94) and msh4Δ (1.3) were greater for msh4 mutants compared to wild type (Figure 3C). The greater noncrossover intercepts in msh4 mutants compared to wild type maybe due to the increase in noncrossovers on some of the chromosomes in the msh4 mutants (Figure 3D). Comparison of crossover distribution along the chromosomes showed similar patterns in wild type, msh4–R676W and msh4Δ mutants (File S2 and Figure S4). Gene conversion information for wild type and msh4 mutants is shown in File S3.
Figure 3.
Average crossover and noncrossover counts per chromosome for wild type, msh4–R676W, and msh4Δ. (A and C) Scatter plot of average crossover and noncrossover counts per chromosome against chromosome size for wild type, msh4–R676W, and msh4Δ. Best fit line is obtained through linear regression analysis. The equations for the best fit lines are: wild type (CO = 0.0000065 × chr. size + 0.98; NCO = 0.0000034 × chr. size + 0.67), msh4–R676W (CO = 0.0000045 × chr. size + 0.64; NCO = 0.0000033 × chr. size + 0.94), msh4Δ (CO = 0.0000033 × chr. size + 0.59, NCO = 0.0000041 × chr. size + 1.29). (B and D) Bar plot of average crossover and noncrossover counts per chromosome for wild type, msh4–R676W, and msh4Δ. Chromosomes (msh4–R676W and msh4Δ) with significant difference (two-tailed t-test for difference in mean; P < 0.05) in crossover/noncrossover counts compared to wild type are shown with an asterisk symbol (*). Chromosomes are ordered according to size from left to right. Error bars are mean ±SE.
Crossover reduction is associated with reduced crossover interference in the msh4–R676W hypomorph
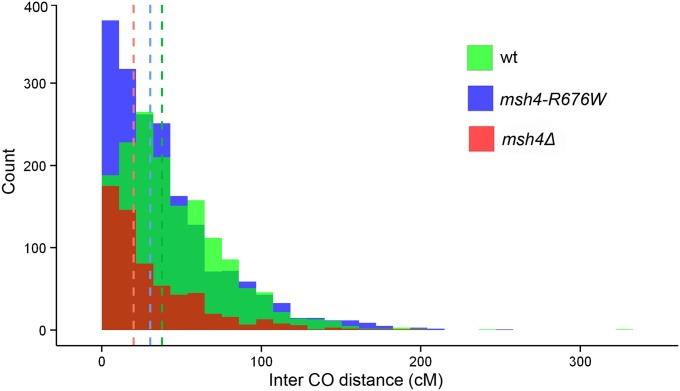
Interference limits crossover number and ensures that crossover events are widely spaced along the chromosome (Muller 1916; Hillers 2004; Kleckner et al. 2004; Stahl et al. 2004). Crossover interference was calculated by modeling the inter-crossover distances as a gamma distribution. The gamma distribution is characterized by the shape parameter (γ) and the scale parameter (β). γ = 1 corresponds to no interference, while γ > 1 indicates positive interference (Anderson et al. 2011). Larger values of γ suggest stronger interference making the method suitable for quantitative analysis of interference instead of just indicating presence or loss of interference. To compare interference between wild type and msh4 mutants that make fewer crossovers, inter-crossover distances in physical units were converted into genetic distances (cM) to account for differences in crossover numbers (Materials and Methods). For the wild-type strain the γ value was 1.77 which suggests presence of interference. This value is also comparable to the γ value of 1.96 obtained from analysis of inter-crossover distances for 46 wild-type tetrads (Mancera et al. 2008; Anderson et al. 2011). γ = 0.95 for msh4Δ suggests loss of genetic interference consistent with previous analysis of crossover data at specific loci and genome-wide (Novak et al. 2001; Argueso et al. 2004; Chen et al. 2008; Mancera et al. 2008). For msh4–R676W, γ = 1.23 that suggests a moderate loss of interference. The median inter-crossover distances in wild type, msh4–R676W and msh4Δ were 37.6 cM, 30.3 cM and 20 cM respectively consistent with reduction in inter-crossover distances caused by loss of interference (Figure 4). These differences in inter-crossover distances between msh4–R676W vs. wild type (P = 3.33 × 10−11); msh4–R676W vs. msh4Δ (P = 7.18 × 10−10) and wild type vs. msh4Δ (P = 2.2 × 10−16) are statistically significant using the Wilcoxon rank-sum test. The reduction in interference with reduced crossovers in the msh4–R676W hypomorph is consistent with the two pathway model for crossover formation (Stahl et al. 2004; Getz et al. 2008). The simultaneous loss of crossovers and interference has mechanistic consequences for the maintenance of crossover assurance in the msh4–R676W hypomorph (see next section).
Figure 4.
Histogram of intercrossover distances in centimorgans for wild type, msh4–R676W, and msh4Δ. The vertical lines indicate the median intercrossover distance for wild type, msh4–R676W, and msh4Δ.
Chromatid interference was measured as the ratio of observed and expected two, three and four strand double crossovers events between two adjacent intervals. Chi-square tests show no chromatid interference (P > 0.05 for wild type, msh4–R676W and msh4Δ mutants suggesting no difference between observed and expected counts) (Table S5). Absence of chromatid interference is also observed for other zmm mutants (zip1Δ, zip2Δ, zip3Δ, zip4Δ, spo16Δ) (Chen et al. 2008).
Nonexchange chromosomes are frequent in the msh4–R676W hypomorph
The msh4–R676W hypomorph shows genome-wide reduction in crossover number compared to wild type (Figure 1, Table 1, and Table S4). However, this mutant has high spore viability compared to msh4Δ (Nishant et al. 2010) (Table 1). One mechanism by which the msh4–R676W hypomorph can maintain such high spore viability is by distributing the remaining crossovers among all 16 chromosomes to ensure one crossover per homolog pair. The presence of an obligatory crossover on every homolog will be sufficient to ensure high spore viability. When we examined the distribution of crossovers on all chromosomes in the msh4–R676W hypomorph, 42% of the tetrads had at least one (39%) or more (3%) nonexchange chromosomes (Table S3). An example of an msh4–R676W tetrad with a single nonexchange chromosome is shown in Figure 2C. In the wild-type strain only two of the 20 tetrads sequenced (10%) had a single nonexchange event consistent with the low number of tetrads with nonexchange chromosomes reported previously for wild-type cells (Chen et al. 2008; Mancera et al. 2008). In the msh4Δ mutant 72% of the four viable spore tetrads examined had one (39%) or more than one (33%) nonexchange event. Nonexchange chromosomes have been previously observed among the four viable spore tetrads of other zmm mutants including the msh4Δ mutant (Chen et al. 2008). The percentage of tetrads with more than one nonexchange event (33%) was significantly higher (P = 0.003, Fisher’s exact test) in msh4Δ mutants compared to the msh4–R676W hypomorph (3%). Sometimes adjacent crossovers that are closely spaced may be annotated as double noncrossovers inflating the number of nonexchange chromosomes. This problem is more likely to occur in meiotic mutants with reduced or no interference. We inspected each nonexchange chromosome in the wild-type and the msh4 mutants for ambiguity in the annotation for double crossovers and double noncrossovers and did not find any significant difference in our estimate of nonexchange chromosomes (Table S6). The nucleotide divergence between the S288c and YJM789 strains might also contribute to a small degree of loss of crossover assurance in the S288c/YJM789 hybrid as fewer E0s are observed in other S. cerevisiae strains (Kaback et al. 1989; Lao et al. 2013).
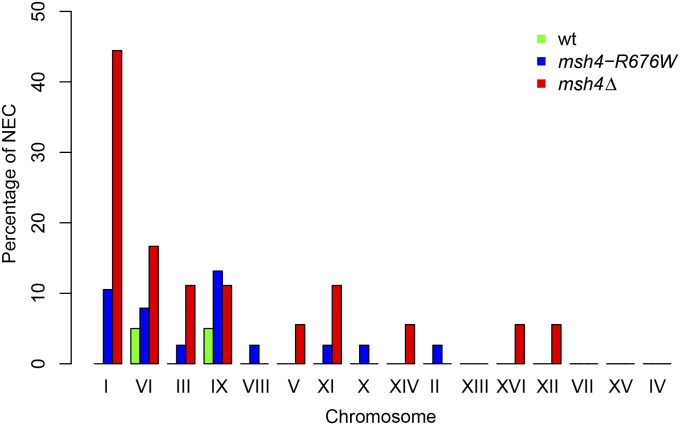
To analyze the influence of chromosome size on occurrence of nonexchange chromosomes in the wild-type, msh4–R676W, and msh4Δ mutant, we determined the percentage of nonexchange events for each chromosome (Figure 5 and Table S7). Small chromosomes I, III, VI, and IX were observed to have nonexchange events in all three genotypes (wild type, msh4–R676W, and msh4Δ). Nonexchange events were observed on medium chromosomes II, V, VIII, X, XI, and XIV only in msh4–R676W and msh4Δ. For large chromosomes IV, VII, XII, XIII, XV, and XVI, nonexchange events occurred only in msh4Δ. These observations suggest that minor variations in crossover frequencies are sufficient to produce nonexchange events on small chromosomes. But for medium and large chromosomes, stronger reductions in crossover frequency as observed with the msh4–R676W and msh4Δ genotypes are required. If all chromosomes are equally likely to receive no crossovers, then a quarter of the E0’s are expected to be small chromosomes. We observed 30 of the 40 E0’s to be small chromosomes and this indicates an overrepresentation of small chromosomes among E0’s (P = 4.6 × 10−11, Binomial test). These results suggest that nonexchange chromosome formation is influenced by chromosome size and small chromosomes are at the greatest risk for loss of crossover assurance when crossover frequencies decrease. Higher frequency of nonexchange events on smaller chromosomes compared to large chromosomes has been observed in other crossover defective mutants and also in human meiosis (Chen et al. 2008; Fledel-Alon et al. 2009). It is also observed that chromosome I shows a disproportionately high percentage of E0’s and the strongest crossover defects in the msh4Δ mutant (Figure 3B, Figure 5, and Table S4). The chromosome I-specific defects observed in msh4Δ may be because it is the smallest chromosome and therefore sensitive to loss of crossovers. Chromosome I was also observed to have the highest number of nonexchange events in zmm mutants in an earlier study by Chen et al. (2008). Analysis of copy-number variation using read-depth information did not detect aneuploidy in any of the 80 sequenced tetrads (Materials and Methods, data not shown).
Figure 5.
Bar plot showing the percentage of nonexchange chromosomes separately for each chromosome. The data are generated from the analysis of 20 wild-type, 38 msh4–R676W, and 18 msh4Δ tetrads in the S288c/YJM789 hybrid background. Chromosomes are arranged in increasing order of size.
To test if the observed number and pattern of nonexchange chromosomes can be predicted from the average crossover counts per chromosome, we modeled the crossover distribution for wild-type and msh4 mutants using a Poisson distribution (Table 2). The expected number of E0’s (fraction of chromosomes with zero crossover) per tetrad were 0.3, 0.8, and 1.4 for wild type, msh4–R676W, and msh4∆, respectively (Table 2). Nonexchange chromosomes are therefore expected in msh4–R676W if the reduction in crossovers is associated with loss of interference (the Poisson distribution assumes that the crossover events are independent). We compared the expected and observed E0 counts for wild type, msh4–R676W, and msh4Δ. The observed E0 count per cell in wild type (0.1) is three times lower due to the presence of interference. The observed E0 count per cell in msh4–R676W (0.45) is two times lower due to partial loss of interference. For msh4Δ there is close concordance between the observed E0 per cell (1.2) and the expected E0 (1.4) as crossover distribution is random. These observations suggest convergence between the expected and observed E0s with diminishing interference (File S4). The presence of interference decreases the proportion of E0’s for the same number of crossovers suggesting a mechanistic role for interference in crossover assurance (File S4). Our calculations suggest that in the complete absence of interference, S. cerevisiae will require a random distribution of up to 200 crossovers to achieve a 0.98 probability of observing no nonexchange chromosomes (File S4). The Poisson model also suggests that the probability of occurrence of nonexchange events in wild type, msh4–R676W, and msh4Δ is influenced by chromosome size (Table 2) supporting the experimental observations (Figure 5). Further, the presence of the obligate crossover will also cause the observed E0 frequency to be less than the E0 frequency expected from a random Poisson distribution. We see this trend for the wild type (Table 2). But for the msh4 mutants the observed E0 frequencies are closer to the expected E0 frequency suggesting loss of the obligate crossover. We did a similar analysis by modeling the noncrossover distribution for wild-type and msh4 mutants using a Poisson distribution (Table S8). Since noncrossovers do not show interference (Mancera et al. 2008), we expect that the observed number of chromosomes without a noncrossover should be close to that predicted from a Poisson distribution. The observed frequency of chromosomes with zero noncrossovers per cell for wild type (1.2), msh4–R676W (1.1), and msh4Δ (0.77) was greater than the expected frequency (1.07, 0.89, and 0.55 for wild type, msh4–R676W, and msh4Δ, respectively) (Table S8). These results suggest that there is no obligate noncrossover in the wild type or msh4 mutants. We looked at the distribution of all gene conversion events not associated with crossovers for the exchange and nonexchange chromosomes in wild type, msh4–R676W, and msh4Δ (Table S9). The nonexchange chromosomes received 75% of the gene conversions compared to chromosomes with a crossover (P = 0.02). This observation suggests that crossover-independent recombination interactions are less favored for nonexchange chromosomes. Reduced gene conversion events have been observed previously on nonexchange chromosome III in the dmc1hed1 mutant by Lao et al. (2013).
Table 2. Expected poisson probabilities of observing a nonexchange chromosome given the average crossovers per chromosome.
| Chromosome | Probability of zero crossover in wild type | Probability of zero crossover in msh4–R676W | Probability of zero crossover in msh4Δ |
|---|---|---|---|
| I | 0.11 | 0.19 | 0.44 |
| II | 0.00 | 0.01 | 0.03 |
| III | 0.03 | 0.10 | 0.09 |
| IV | 0.00 | 0.00 | 0.00 |
| V | 0.01 | 0.06 | 0.07 |
| VI | 0.07 | 0.18 | 0.22 |
| VII | 0.00 | 0.00 | 0.02 |
| VIII | 0.01 | 0.05 | 0.14 |
| IX | 0.05 | 0.13 | 0.12 |
| X | 0.00 | 0.02 | 0.04 |
| XI | 0.01 | 0.02 | 0.08 |
| XII | 0.00 | 0.00 | 0.03 |
| XIII | 0.00 | 0.01 | 0.02 |
| XIV | 0.00 | 0.01 | 0.06 |
| XV | 0.00 | 0.00 | 0.01 |
| XVI | 0.00 | 0.01 | 0.01 |
| Expected frequency of E0 chromosomes per cell | 0.30 | 0.80 | 1.39 |
| Observed frequency of E0 chromosomes per cell | 0.1 | 0.45 | 1.2 |
The probability of nonexchange events are chromosome size dependent. Small chromosomes show higher probability of nonexchange events in wild type while small and medium chromosomes show higher probability of nonexchange events in msh4–R676W and msh4Δ. The expected Poisson probability of observing no crossover on a chromosome was calculated using the mean number of crossovers observed for that particular chromosome (λ) from experimental data and the formula: P(k) = λke-λ/k!. For example, since the mean number of crossovers on chromosome III is 3.4, the expected probability of no crossovers (k = 0), is P(k = 0 | mean crossover = 3.4) = e−3.4 = 0.03.
Nonexchange chromosomes are efficiently segregated in the msh4–R676W hypomorph
Given the large number of E0 chromosomes in the msh4–R676W hypomorph we examined how the high spore viability is maintained. We tested the role of specific crossover-independent mechanisms for nonexchange chromosome segregation such as the spindle checkpoint and the contribution of random segregation. Spindle checkpoint proteins Mad2 and Mad3 are known to be involved in nonexchange chromosome segregation (Shonn et al. 2000; Cheslock et al. 2005; Lacefield and Murray 2007). Mad2 causes a metaphase delay in response to inappropriate microtubule attachment. Mad3 causes a prophase delay in every meiosis to properly align any nonexchange chromosomes to the bipolar spindle (Cheslock et al. 2005). We deleted MAD2 and MAD3 genes in the msh4 mutant background in the S288c/YJM789 hybrid to check whether these proteins contribute to the high spore viability observed in the msh4–R676W hypomorph. mad2Δ and mad3Δ single mutants have viability of 39 and 83%, respectively (Table 3 and Figure S5). Double mutants mad3∆ msh4–R676W and mad3∆ msh4∆ showed viability of 69 and 38%. When mad2Δ mutant was analyzed with msh4 mutants, the double mutants mad2∆ msh4–R676W and mad2Δ msh4Δ showed viability of 27 and 10%, respectively. The observed double mutant viability of the msh4 mutants with mad2Δ or mad3Δ was not significantly different from the expected viability of the double mutants assuming a multiplicative model for the genetic interaction (Table 3; Dixon et al. 2009). These results suggest absence of significant negative genetic interaction between the msh4 mutants and mad2Δ, mad3Δ mutants. Therefore the spindle checkpoint has only a minor role in the high spore viability of the msh4–R676W hypomorph, but other crossover-independent mechanisms might still contribute (see Discussion).
Table 3. Analysis of genetic interaction of mutations in spindle checkpoint genes (mad2Δ, mad3Δ) with msh4 mutants.
| Genotype | N | Expected viability (%) | Observed viability (%) | P-value (χ2 test) |
|---|---|---|---|---|
| Wild type | 180 | 84 | ||
| mad2∆ | 140 | 39 | ||
| msh4∆ | 100 | 41 | ||
| msh4–R676W | 239 | 76 | ||
| mad2∆ msh4∆ | 120 | 16 | 10 | P = 0.09 |
| mad2∆ msh4–R676W | 140 | 30 | 27 | P = 0.49 |
| mad3∆ | 60 | 83 | ||
| mad3∆ msh4∆ | 60 | 34 | 38 | P = 0.76 |
| mad3∆ msh4–R676W | 140 | 63 | 69 | P = 0.17 |
All mutants are analyzed in the S288c/YJM789 background. N is the number of tetrads analyzed. P-values indicate statistical significance of the difference between expected and observed viability of the double-mutant combinations The expected spore viability for the double mutants is calculated as the product of the spore viabilities of the individual single mutants (Dixon et al. 2009).
We tested whether random segregation of the nonexchange chromosomes can explain the spore viability observed in the msh4–R676W hypomorph. We calculated the expected four and two spore viability based on E0 numbers for msh4–R676W and msh4Δ assuming random segregation (Table 4). For msh4Δ there is no difference between the expected (24%) and observed four viable spore class (24%). For msh4–R676W an 11% increase in the observed four viable spore class (54%) compared to the expected (47%) is seen. The difference between the observed four viable spore frequency and expected frequency from random segregation is statistically significant for msh4–R676W (P = 0.018, one-sided binomial test). For the two viable spore class, the difference between expected and observed viability for msh4Δ (41 and 20%, respectively) and msh4–R676W (30 and 15%, respectively) is twofold, which suggests efficient segregation of the nonexchange chromosomes at MI. It is also possible that the two viable spore class is underpopulated because there are more zero viable spores. Overall these results suggest that crossover-independent mechanisms enhance the fidelity of chromosome segregation in the msh4 hypomorph, significantly more than random segregation.
Table 4. Calculation of estimated four viable and two viable spore tetrads based on E0 counts.
| Wild type | msh4–R676W | msh4Δ | ||||
|---|---|---|---|---|---|---|
| No. of E0 chromosomes | No. of tetrads | Expected number of nonviable tetrads | No. of tetrads | Expected number of nonviable tetrads | No. of tetrads | Expected number of nonviable tetrads |
| 0 | 18 | 0 | 22 | 0 | 5 | 0 |
| 1 | 2 | 2 | 15 | 15 | 7 | 7 |
| 2 | 0 | 0 | 1 | 3 | 4 | 12 |
| 3 | 0 | 0 | 0 | 0 | 2 | 14 |
| Total | 20 | 2 | 38 | 18 | 18 | 33 |
| Expected fraction of four viable spore tetrads | 0.91 | 0.68 | 0.35 | |||
| Observed fraction of four viable spore tetrads | 0.63 | |||||
| Fraction of four viable spore tetrads expected after correction | 0.47 | 0.24 | ||||
| Observed four viable spore tetrads | 54% | 24% | ||||
| Fraction of expected two viable spore tetrads | 0.09 | 0.3 | 0.41 | |||
| Observed two viable spore tetrads | 10% | 15% | 20% | |||
We calculated the expected number of four viable spore tetrads based on the proportion of nonviable tetrads that arise due to mis-segregation of chromosomes. Assuming random segregation of nonexchange chromosomes, if we observe one tetrad with one nonexchange chromosome, we expect there is one other nonviable tetrad (i.e., not four viable spore tetrad). Likewise for one tetrad with two nonexchange chromosomes we expect 22 - 1 = 3 other nonviable tetrads and for one tetrad with three nonexchange chromosomes, one expects 23 - 1 = 7 other non-four viable spore tetrads. We can then estimate the expected number of four viable spore tetrads for each genotype by dividing the observed count of four viable spore tetrads over the total (expected number of nonviable tetrads + observed four viable spore tetrads). After obtaining this expected proportion, we scaled it down by the fraction observed in wild type (0.90 / 0.63) to obtain a corrected estimate. A similar logic is used to calculate the expected number of two viable spore tetrads.
Previous estimates of the efficiency of segregation of nonexchange homeologous/nonhomologous or artificial chromosome pairs in S. cerevisiae have ranged from 75 to 90% (Dawson et al. 1986; Mann and Davis 1986; Guacci and Kaback 1991; Ross et al. 1996; Kemp et al. 2004; Cheslock et al. 2005; Gladstone et al. 2009; Newnham et al. 2010). These disjunction estimates are significantly more than the disjunction efficiency observed in the msh4 mutants. The higher disjunction frequency observed for the nonexchange chromosomes in these studies (Dawson et al. 1986; Mann and Davis 1986; Guacci and Kaback 1991; Ross et al. 1996; Kemp et al. 2004; Cheslock et al. 2005; Gladstone et al. 2009; Newnham et al. 2010) may be because of the use of a single homeologous/nonhomologous/artificial nonexchange chromosome, which is better segregated by crossover-independent pathways compared to the heterogeneous pool of natural nonexchange chromosomes in the msh4 mutants (Figure 5). It is also important to note that recombination interactions involving gene conversions will be absent from the nonhomologous or artificial chromosomes used to study nonexchange chromosome segregation.
Discussion
Although the msh4–R676W hypomorph makes sufficient crossovers (64/meiosis) to ensure the obligate crossover, crossover assurance was disrupted due to decreased crossover interference. Nonexchange chromosomes are observed in 42% of the meiotic events analyzed. These observations support a mechanistic role for interference in the formation of obligatory crossovers. Genome-wide analysis of crossovers in other meiotic mutants where obligate crossovers are assumed from spore viability or localized measures of crossing over may be necessary to verify any aspect of crossover assurance.
Why is crossover assurance perturbed in the msh4–R676W hypomorph?
Wild-type S. cerevisiae meiosis is thought to have a mechanism for crossover assurance, which ensures that one crossover per homolog pair will be maintained (Bishop and Zickler 2004; Shinohara et al. 2008). The presence of crossover assurance is supported by (a) the low frequency of nonexchange chromosomes in wild-type S. cerevisiae meiosis (Kaback et al. 1989; Chen et al. 2008; Mancera et al. 2008; Shinohara et al. 2008) and (b) the phenomenon of crossover homeostasis that maintains crossover number when DSB levels are reduced (Martini et al. 2006). This study, as well as previous genome-wide crossover mapping studies, suggests that wild-type strains do maintain crossover assurance although nonexchange chromosomes are occasionally seen (Mancera et al. 2008). However, in msh4–R676W, 42% of the meioses had at least one nonexchange chromosome. Why does crossover assurance not work in the msh4–R676W hypomorph with 64 crossovers and only 16 chromosomes? One reason is that msh4 mutants show significant reduction in crossovers across all chromosomes, making it possible that small chromosomes do not receive an obligate crossover in every meiosis (Figure 3B). Previous analyses of crossing over at specific loci in msh4/5 hypomorphs as well as msh4/5Δ mutants have suggested a chromosome size effect that is different from our results. Crossover defects were observed in these studies to be stronger on the larger chromosomes compared to the small chromosomes (Abdullah et al. 2004; Stahl et al. 2004; Nishant et al. 2010). This may be due to the small number of loci analyzed on a few chromosomes and highlights the need to look at crossover defects globally to discern chromosome-specific patterns.
The other reason that contributes to the loss of crossover assurance in the msh4–R676W hypomorph is the partial loss of interference (Figure 4). In other organisms with a lower number of total crossovers, the interference observed is much higher, such as in Drosophila (γ = ∼4) and mouse (γ = ∼10) (Foss and Stahl 1995; Broman et al. 2002; de Boer et al. 2006), supposedly to ensure the obligate crossover. S. cerevisiae (γ = 1.77) has reduced interference compared to these organisms and as a consequence the distribution of crossovers in S. cerevisiae is closer to that of a Poisson distribution. The reduced interference may explain why up to 90 crossovers are required to ensure an obligate crossover on every homolog pair for a system with only 16 homolog pairs (File S4). The presence of occasional nonexchange chromosomes in wild-type strains (this study, Mancera et al. 2008) may also be due to the reduced stringency of crossover distribution mechanisms in S. cerevisiae compared to other model systems. Any reduction in crossovers through a mutation/ polymorphism or random fluctuation in crossover numbers coupled with the loss of interference therefore results in increased incidence of nonexchange pairs.
The alternate possibility is that Msh4/5 has a role in crossover assurance that is compromised in the msh4–R676W hypomorph. Since the crossover/noncrossover decision is made before the action of ZMM proteins, the proper execution of the crossover decision might require the wild-type activity of the Msh4/5 proteins (Hunter and Kleckner 2001; Bishop and Zickler 2004; Storlazzi et al. 2010). So in the msh4–R676W hypomorph the crossovers are not made at the designated sites and hence crossover assurance is perturbed.
How efficiently are natural nonexchange chromosomes segregated in S. cerevisiae?
Nonexchange chromosomes may be segregated randomly or through mechanisms such as centromere pairing, the spindle checkpoint, or heterochromatin association that facilitate accurate segregation (Li and Murray 1991; Dernburg et al. 1996; Shonn et al. 2000; Kemp et al. 2004). Crossover-independent meiotic chromosome segregation has been analyzed previously in S. cerevisiae, Schizosaccharomyces pombe, and Drosophila (Sturtevant and Beadle 1936; Carpenter 1973; Dawson et al. 1986; Mann and Davis 1986; Davis and Smith 2003). In S. cerevisiae, centromere pairing (mediated by Zip1) is thought to decrease rotational freedom of the homologous kinetochores and ensure that they attach to microtubules from opposite poles (Ostergren 1951; Lacefield and Murray 2007; Gladstone et al. 2009; Newnham et al. 2010; Obeso et al. 2013). The spindle checkpoint is activated in response to improper or unattached kinetochores (Li and Murray 1991; Rieder et al. 1994; Li and Nicklas 1995). Improper spindle-kinetochore attachments are common in case of nonexchange chromosomes. Spindle checkpoint genes MAD1, MAD2, MAD3 have been shown to facilitate segregation of nonexchange pairs in S. cerevisiae or chromosome pairs with crossovers placed far from the centromere (Shonn et al. 2000; Cheslock et al. 2005; Lacefield and Murray 2007). Cell-cycle delays introduced by MAD1 (metaphase I), MAD2 (metaphase I), and MAD3 (prophase I) are thought to provide additional time for chromosome pairs to establish bipolar attachment of spindles to kinetochores (Cheslock et al. 2005). These backup pathways can mask defects in crossover assurance. Analysis of genetic interactions between the msh4–R676W hypomorph and spindle checkpoint genes MAD2, MAD3 suggest that the spindle checkpoint is not critical for proper segregation of the nonexchange homologous chromosomes in the msh4–R676W hypomorphic background, but other mechanisms might still play a role.
For example, the high spore viability of the msh4–R676W hypomorph can also be explained by a role for the Msh4/5 proteins in nonexchange chromosome segregation, which is distinct from its pro-crossover role. The predicted early roles of Msh4/5 proteins in homologous pairing supports such a possibility (Storlazzi et al. 2010). The msh4–R676W hypomorph might be proficient in such chromosome segregation functions but not able to execute crossover formation. A possible role for the Msh4/5 complex in segregation of nonexchange chromosomes can also explain why the mlh3Δ mms4Δ double mutant make very few crossovers but has good viability (Argueso et al. 2004; Brown et al. 2013). These mechanisms need not be mutually exclusive and may all contribute to the efficiency of nonexchange chromosome segregation.
Our study also sheds light on how many nonexchange chromosomes can be efficiently segregated. In the msh4–R676W hypomorph, only 3% of the meiosis had more than one nonexchange chromosome compared to 33% in msh4∆ mutants. But in none of the cases we found more than three nonexchange chromosomes in a given meiosis (Table S3). These results support the idea that there are limits on how many nonexchange chromosomes can be handled. With four E0’s per meiotic cell, the expected four viable spore class from random segregation will be close to 6% (1 of 24 tetrads will contain four viable spores), which may not be observable unless large number of tetrads are analyzed. The presence of multiple nonexchange chromosomes may be the reason crossover-independent pathways cannot improve spore viability in msh4Δ mutants.
Chromosome size and shape have a role in nonexchange chromosome segregation in Drosophila (Grell 1964; Hawley et al. 1992). In S. cerevisiae, analysis of segregation of nonhomologous natural chromosomes and a centromere plasmid showed absence of shape and size bias (Guacci and Kaback 1991). Similar results were observed with the segregation analysis of artificial nonexchange chromosomes that are of comparable or different sizes within a twofold range (Ross et al. 1996). These studies may have failed to detect the effect of chromosome size on segregation of natural nonexchange S. cerevisiae chromosomes. Our analysis using msh4 mutants indicates that chromosome size might play some role in nonexchange segregation (Figure 5 and Table 2). In the msh4–R676W hypomorph, nonexchange events are mainly restricted to small and medium-sized chromosomes as predicted from the Poisson probabilities of observing a nonexchange chromosome (Table 2). Whereas in msh4∆ mutants nonexchange events are found on small, medium, and large chromosomes. We hypothesize that the viability defect found in msh4∆ mutants could be due to the presence of nonexchange events on large chromosomes. Crossover-independent mechanisms might be efficient in segregating small and medium-sized nonexchange chromosomes, whereas the process may be less efficient with large chromosomes.
A major challenge in studying achiasmate chromosome segregation mechanisms is that most of the mutants that generate natural nonexchange chromosomes have very poor spore viability. Some exceptions include the msh4–R676W hypomoprh (this study) or mlh3Δ mms4Δ mutants that show a 6- to 17-fold reduction in crossing over at specific loci (Brown et al. 2013). On the other hand, wild-type cells have high frequencies of crossovers that result in very few achiasmate chromosomes (Kaback et al. 1992). Cytological approaches that do not require viable spores are one alternative. We suggest that the msh4–R676W hypomorph can be used to study the efficiency of crossover-independent segregation mechanisms as it has a large number of meioses with nonexchange chromosomes and still maintains high spore viability.
To conclude, we used the baker’s yeast Saccharomyces cerevisiae as a model to study whether the obligate crossover is insulated from variation in crossover numbers. An S. cerevisiae msh4–R676W hypomorphic allele that has crossover defects but maintains good viability was used to experimentally induce variation in crossover numbers. The msh4–R676W hypomorph showed ∼30% genome-wide reduction in crossovers and reduced crossover interference. Statistically significant reduction in crossover numbers was observed on all chromosomes in the msh4 mutants. Crossover assurance was lost in 42% of the meioses in the msh4–R676W hypomorph, especially on small and medium-sized chromosomes that were most sensitive to even minor fluctuations in crossover number. Since Msh4/5 is part of the ZMM complex, the loss of assurance in msh4–R676W may also be in part due to the direct role of Msh4/5 in crossover assurance. The distribution of nonexchange chromosomes observed in msh4 mutants was consistent with predictions based on modeling the crossovers as a Poisson distribution and provides a mechanistic link between interference and the obligate crossover. The high spore viability of the msh4–R676W hypomorph is maintained by efficient segregation of a limited number of nonexchange chromosomes. Our results suggest that variation in crossover frequencies can disrupt obligate crossover formation without affecting viability. Such nonexchange chromosomes may be more common than previously thought and highlight the need to use genome-wide crossover mapping methods to analyze crossover assurance.
Acknowledgments
We thank Eric Alani, Michael Lichten, and Eugenio Mancera for critical reading of the manuscript and helpful discussions. We also thank Jennifer Fung for suggestions on using the ReCombine suite of programs. This study was technically supported by the European Molecular Biology Laboratory Genomics Core Facility (GeneCore). K.T.N. is supported by a Wellcome Trust-Department of Biotechnology (DBT) India Alliance Intermediate fellowship (IA/I/11/2500268) and Indian Institute of Science Education and Research Thiruvananthapuram (IISER-TVM) intramural funds. The research leading to these results has received funding from the National Institutes of Health and the European Research Council under the European Union’s Seventh Framework Programme (FP7/2007-2013)/ERC Grant agreement no. AdG-294542 to L.M.S. G.N.K. is also supported by a fellowship from the Council for Scientific and Industrial Research, New Delhi. The funders had no role in study design, data collection and interpretation, preparation of the manuscript or decision to publish.
Footnotes
Supporting information is available online at http://www.genetics.org/lookup/suppl/doi:10.1534/genetics.114.172320/-/DC1.
Available freely online through the author-supported open access option.
Sequence data from this article have been deposited with the National Centre for Biotechnology Information Sequence Read Archive under accession no. SRP041856.
Processed data and custom R scripts are available from http://figshare.com (http://dx.doi.org/10.6084/m9.figshare.1192705).
Communicating editor: N. M. Hollingsworth
Literature Cited
- Abdullah M. F., Hoffmann E. R., Cotton V. E., Borts R. H., 2004. A role for the MutL homologue MLH2 in controlling heteroduplex formation and in regulating between two different crossover pathways in budding yeast. Cytogenet. Genome Res. 107: 180–190. [DOI] [PubMed] [Google Scholar]
- Agarwal S., Roeder G. S., 2000. Zip3 provides a link between recombination enzymes and synaptonemal complex proteins. Cell 102: 245–255. [DOI] [PubMed] [Google Scholar]
- Allers T., Lichten M., 2001. Differential timing and control of noncrossover and crossover recombination during meiosis. Cell 106: 47–57. [DOI] [PubMed] [Google Scholar]
- Anderson C. M., Chen S. Y., Dimon M. T., Oke A., DeRisi J. L., et al. , 2011. ReCombine: a suite of programs for detection and analysis of meiotic recombination in whole-genome datasets. PLoS ONE 6: e25509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argueso J. L., Kijas A. W., Sarin S., Heck J., Waase M., et al. , 2003. Systematic mutagenesis of the Saccharomyces cerevisiae MLH1 gene reveals distinct roles for Mlh1p in meiotic crossing over and in vegetative and meiotic mismatch repair. Mol. Cell. Biol. 23: 873–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argueso J. L., Wanat J., Gemici Z., Alani E., 2004. Competing crossover pathways act during meiosis in Saccharomyces cerevisiae. Genetics 168: 1805–1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker S. M., Plug A. W., Prolla T. A., Bronner C. E., Harris A. C., et al. , 1996. Involvement of mouse Mlh1 in DNA mismatch repair and meiotic crossing over. Nat. Genet. 13: 336–342. [DOI] [PubMed] [Google Scholar]
- Barlow A. L., Hulten M. A., 1998. Crossing over analysis at pachytene in man. Eur. J. Hum. Genet. 6: 350–358. [DOI] [PubMed] [Google Scholar]
- Berchowitz L. E., Copenhaver G. P., 2010. Genetic interference: don’t stand so close to me. Curr. Genomics 11: 91–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop D. K., Zickler D., 2004. Early decision; meiotic crossover interference prior to stable strand exchange and synapsis. Cell 117: 9–15. [DOI] [PubMed] [Google Scholar]
- Borner G. V., Kleckner N., Hunter N., 2004. Crossover/noncrossover differentiation, synaptonemal complex formation, and regulatory surveillance at the leptotene/zygotene transition of meiosis. Cell 117: 29–45. [DOI] [PubMed] [Google Scholar]
- Broman K. W., Rowe L. B., Churchill G. A., Paigen K., 2002. Crossover interference in the mouse. Genetics 160: 1123–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M. S., Lim E., Chen C., Nishant K. T., Alani E., 2013. Genetic analysis of mlh3 mutations reveals interactions between crossover promoting factors during meiosis in baker’s yeast. G3 Genes Genomes Genet. 3: 9–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter A. T., 1973. A meiotic mutant defective in distributive disjunction in Drosophila melanogaster. Genetics 73: 393–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S. Y., Tsubouchi T., Rockmill B., Sandler J. S., Richards D. R., et al. , 2008. Global analysis of the meiotic crossover landscape. Dev. Cell 15: 401–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheslock P. S., Kemp B. J., Boumil R. M., Dawson D. S., 2005. The roles of MAD1, MAD2 and MAD3 in meiotic progression and the segregation of nonexchange chromosomes. Nat. Genet. 37: 756–760. [DOI] [PubMed] [Google Scholar]
- Cheung V. G., Burdick J. T., Hirschmann D., Morley M., 2007. Polymorphic variation in human meiotic recombination. Am. J. Hum. Genet. 80: 526–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury R., Bois P. R., Feingold E., Sherman S. L., Cheung V. G., 2009. Genetic analysis of variation in human meiotic recombination. PLoS Genet. 5: e1000648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua P. R., Roeder G. S., 1998. Zip2, a meiosis-specific protein required for the initiation of chromosome synapsis. Cell 93: 349–359. [DOI] [PubMed] [Google Scholar]
- Cole F., Kauppi L., Lange J., Roig I., Wang R., et al. , 2012. Homeostatic control of recombination is implemented progressively in mouse meiosis. Nat. Cell Biol. 14: 424–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis L., Smith G. R., 2003. Nonrandom homolog segregation at meiosis I in Schizosaccharomyces pombe mutants lacking recombination. Genetics 163: 857–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson D. S., Murray A. W., Szostak J. W., 1986. An alternative pathway for meiotic chromosome segregation in yeast. Science 234: 713–717. [DOI] [PubMed] [Google Scholar]
- de Boer E., Stam P., Dietrich A. J., Pastink A., Heyting C., 2006. Two levels of interference in mouse meiotic recombination. Proc. Natl. Acad. Sci. USA 103: 9607–9612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Muyt A., Jessop L., Kolar E., Sourirajan A., Chen J., et al. , 2012. BLM helicase ortholog Sgs1 is a central regulator of meiotic recombination intermediate metabolism. Mol. Cell 46: 43–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries S. S., Baart E. B., Dekker M., Siezen A., de Rooij D. G., et al. , 1999. Mouse MutS-like protein Msh5 is required for proper chromosome synapsis in male and female meiosis. Genes Dev. 13: 523–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dernburg A. F., Sedat J. W., Hawley R. S., 1996. Direct evidence of a role for heterochromatin in meiotic chromosome segregation. Cell 86: 135–146. [DOI] [PubMed] [Google Scholar]
- Dixon S. J., Costanzo M., Baryshnikova A., Andrews B., Boone C., 2009. Systematic mapping of genetic interaction networks. Annu. Rev. Genet. 43: 601–625. [DOI] [PubMed] [Google Scholar]
- Edelmann W., Cohen P. E., Kneitz B., Winand N., Lia M., et al. , 1999. Mammalian MutS homologue 5 is required for chromosome pairing in meiosis. Nat. Genet. 21: 123–127. [DOI] [PubMed] [Google Scholar]
- Fledel-Alon A., Wilson D. J., Broman K., Wen X., Ober C., et al. , 2009. Broad-scale recombination patterns underlying proper disjunction in humans. PLoS Genet. 5: e1000658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foss E. J., Stahl F. W., 1995. A test of a counting model for chiasma interference. Genetics 139: 1201–1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Getz T. J., Banse S. A., Young L. S., Banse A. V., Swanson J., et al. , 2008. Reduced mismatch repair of heteroduplexes reveals “non”-interfering crossing over in wild-type Saccharomyces cerevisiae. Genetics 178: 1251–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gietz R. D., Schiestl R. H., Willems A. R., Woods R. A., 1995. Studies on the transformation of intact yeast cells by the LiAc/SS-DNA/PEG procedure. Yeast 11: 355–360. [DOI] [PubMed] [Google Scholar]
- Gladstone M. N., Obeso D., Chuong H., Dawson D. S., 2009. The synaptonemal complex protein Zip1 promotes bi-orientation of centromeres at meiosis I. PLoS Genet. 5: e1000771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein A. L., McCusker J. H., 1999. Three new dominant drug resistance cassettes for gene disruption in Saccharomyces cerevisiae. Yeast 15: 1541–1553. [DOI] [PubMed] [Google Scholar]
- Grell R. F., 1964. Distributive pairing: the size-dependent mechanism for regular segregation of the fourth chromosomes in Drosophila Melanogaster. Proc. Natl. Acad. Sci. USA 52: 226–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guacci V., Kaback D. B., 1991. Distributive disjunction of authentic chromosomes in Saccharomyces cerevisiae. Genetics 127: 475–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillon H., Baudat F., Grey C., Liskay R. M., de Massy B., 2005. Crossover and noncrossover pathways in mouse meiosis. Mol. Cell 20: 563–573. [DOI] [PubMed] [Google Scholar]
- Hawley R. S., Irick H., Zitron A. E., Haddox D. A., Lohe A., et al. , 1992. There are two mechanisms of achiasmate segregation in Drosophila females, one of which requires heterochromatic homology. Dev. Genet. 13: 440–467. [DOI] [PubMed] [Google Scholar]
- Hillers K. J., 2004. Crossover interference. Curr. Biol. 14: R1036–R1037. [DOI] [PubMed] [Google Scholar]
- Hollingsworth N. M., Ponte L., Halsey C., 1995. MSH5, a novel MutS homolog, facilitates meiotic reciprocal recombination between homologs in Saccharomyces cerevisiae but not mismatch repair. Genes Dev. 9: 1728–1739. [DOI] [PubMed] [Google Scholar]
- Hunter N., Kleckner N., 2001. The single-end invasion: an asymmetric intermediate at the double-strand break to double-holliday junction transition of meiotic recombination. Cell 106: 59–70. [DOI] [PubMed] [Google Scholar]
- Kaback D. B., Steensma H. Y., de Jonge P., 1989. Enhanced meiotic recombination on the smallest chromosome of Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 86: 3694–3698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaback D. B., Guacci V., Barber D., Mahon J. W., 1992. Chromosome size-dependent control of meiotic recombination. Science 256: 228–232. [DOI] [PubMed] [Google Scholar]
- Karpen G. H., Le M. H., Le H., 1996. Centric heterochromatin and the efficiency of achiasmate disjunction in Drosophila female meiosis. Science 273: 118–122. [DOI] [PubMed] [Google Scholar]
- Keeney S., Giroux C. N., Kleckner N., 1997. Meiosis-specific DNA double-strand breaks are catalyzed by Spo11, a member of a widely conserved protein family. Cell 88: 375–384. [DOI] [PubMed] [Google Scholar]
- Kemp B., Boumil R. M., Stewart M. N., Dawson D. S., 2004. A role for centromere pairing in meiotic chromosome segregation. Genes Dev. 18: 1946–1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kijas A. W., Studamire B., Alani E., 2003. Msh2 separation of function mutations confer defects in the initiation steps of mismatch repair. J. Mol. Biol. 331: 123–138. [DOI] [PubMed] [Google Scholar]
- Klambauer G., Schwarzbauer K., Mayr A., Clevert D. A., Mitterecker A., et al. , 2012. cn.MOPS: mixture of Poissons for discovering copy number variations in next-generation sequencing data with a low false discovery rate. Nucleic Acids Res. 40: e69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleckner N., Zickler D., Jones G. H., Dekker J., Padmore R., et al. , 2004. A mechanical basis for chromosome function. Proc. Natl. Acad. Sci. USA 101: 12592–12597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kneitz B., Cohen P. E., Avdievich E., Zhu L., Kane M. F., et al. , 2000. MutS homolog 4 localization to meiotic chromosomes is required for chromosome pairing during meiosis in male and female mice. Genes Dev. 14: 1085–1097. [PMC free article] [PubMed] [Google Scholar]
- Kolas N. K., Svetlanov A., Lenzi M. L., Macaluso F. P., Lipkin S. M., et al. , 2005. Localization of MMR proteins on meiotic chromosomes in mice indicates distinct functions during prophase I. J. Cell Biol. 171: 447–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong A., Thorleifsson G., Gudbjartsson D. F., Masson G., Sigurdsson A., et al. , 2010. Fine-scale recombination rate differences between sexes, populations and individuals. Nature 467: 1099–1103. [DOI] [PubMed] [Google Scholar]
- Kong A., Thorleifsson G., Frigge M. L., Masson G., Gudbjartsson D. F., et al. , 2014. Common and low-frequency variants associated with genome-wide recombination rate. Nat. Genet. 46: 11–16. [DOI] [PubMed] [Google Scholar]
- Lacefield S., Murray A. W., 2007. The spindle checkpoint rescues the meiotic segregation of chromosomes whose crossovers are far from the centromere. Nat. Genet. 39: 1273–1277. [DOI] [PubMed] [Google Scholar]
- Langmead B., Trapnell C., Pop M., Salzberg S. L., 2009. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 10: R25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lao J. P., Cloud V., Huang C. C., Grubb J., Thacker D., et al. , 2013. Meiotic crossover control by concerted action of Rad51-Dmc1 in homolog template bias and robust homeostatic regulation. PLoS Genet. 9: e1003978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R., Murray A. W., 1991. Feedback control of mitosis in budding yeast. Cell 66: 519–531. [DOI] [PubMed] [Google Scholar]
- Li X., Nicklas R. B., 1995. Mitotic forces control a cell-cycle checkpoint. Nature 373: 630–632. [DOI] [PubMed] [Google Scholar]
- Lipkin S. M., Moens P. B., Wang V., Lenzi M., Shanmugarajah D., et al. , 2002. Meiotic arrest and aneuploidy in MLH3-deficient mice. Nat. Genet. 31: 385–390. [DOI] [PubMed] [Google Scholar]
- Lu S., Zong C., Fan W., Yang M., Li J., et al. , 2012. Probing meiotic recombination and aneuploidy of single sperm cells by whole-genome sequencing. Science 338: 1627–1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynn A., Soucek R., Borner G. V., 2007. ZMM proteins during meiosis: crossover artists at work. Chromosome Res. 15: 591–605. [DOI] [PubMed] [Google Scholar]
- Mancera E., Bourgon R., Brozzi A., Huber W., Steinmetz L. M., 2008. High-resolution mapping of meiotic crossovers and non-crossovers in yeast. Nature 454: 479–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann C., Davis R. W., 1986. Meiotic disjunction of circular minichromosomes in yeast does not require DNA homology. Proc. Natl. Acad. Sci. USA 83: 6017–6019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martini E., Diaz R. L., Hunter N., Keeney S., 2006. Crossover homeostasis in yeast meiosis. Cell 126: 285–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martini E., Borde V., Legendre M., Audic S., Regnault B., et al. , 2011. Genome-wide analysis of heteroduplex DNA in mismatch repair-deficient yeast cells reveals novel properties of meiotic recombination pathways. PLoS Genet. 7: e1002305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCusker J. H., Clemons K. V., Stevens D. A., Davis R. W., 1994. Genetic characterization of pathogenic Saccharomyces cerevisiae isolates. Genetics 136: 1261–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortimer R. K., Johnston J. R., 1986. Genealogy of principal strains of the yeast genetic stock center. Genetics 113: 35–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller H. J., 1916. The mechanism of crossing over. Am. Nat. 50: 284–305. [Google Scholar]
- Newnham L., Jordan P., Rockmill B., Roeder G. S., Hoffmann E., 2010. The synaptonemal complex protein, Zip1, promotes the segregation of nonexchange chromosomes at meiosis I. Proc. Natl. Acad. Sci. USA 107: 781–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishant K.T., Plys A.J., Alani E., 2008. A mutation in the putative MLH3 endonuclease domain confers a defect in both mismatch repair and meiosis in Saccharomyces cerevisiae. Genetics 179: 747–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishant K. T., Chen C., Shinohara M., Shinohara A., Alani E., 2010. Genetic analysis of baker’s yeast Msh4–Msh5 reveals a threshold crossover level for meiotic viability. PLoS Genet. 6: e1001083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak J. E., Ross-Macdonald P. B., Roeder G. S., 2001. The budding yeast Msh4 protein functions in chromosome synapsis and the regulation of crossover distribution. Genetics 158: 1013–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obeso D., Pezza R. J., Dawson D., 2013. Couples, pairs, and clusters: mechanisms and implications of centromere associations in meiosis. Chromosoma 123: 43–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oke A., Anderson C. M., Yam P., Fung J. C., 2014. Controlling meiotic recombination repair-specifying the roles of ZMMs, Sgs1 and Mus81/Mms4 in crossover formation. PLoS Genet. 10: e1004690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olshen A. B., Venkatraman E. S., Lucito R., Wigler M., 2004. Circular binary segmentation for the analysis of array-based DNA copy number data. Biostatistics 5: 557–572. [DOI] [PubMed] [Google Scholar]
- Ostergren G., 1951. The mechanism of co-orientation in bivalents and multivalents. Hereditas 37: 156. [Google Scholar]
- Patel R. K., Jain M., 2012. NGS QC Toolkit: a toolkit for quality control of next generation sequencing data. PLoS ONE 7: e30619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petronczki M., Siomos M. F., Nasmyth K., 2003. Un ménage à quatre: the molecular biology of chromosome segregation in meiosis. Cell 112: 423–440. [DOI] [PubMed] [Google Scholar]
- Qi J., Wijeratne A. J., Tomsho L. P., Hu Y., Schuster S. C., et al. , 2009. Characterization of meiotic crossovers and gene conversion by whole-genome sequencing in Saccharomyces cerevisiae. BMC Genomics 10: 475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakshambikai R., Srinivasan N., Nishant K. T., 2013. Structural insights into Saccharomyces cerevisiae Msh4–Msh5 complex function using homology modeling. PLoS ONE 8: e78753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieder C. L., Schultz A., Cole R., Sluder G., 1994. Anaphase onset in vertebrate somatic cells is controlled by a checkpoint that monitors sister kinetochore attachment to the spindle. J. Cell Biol. 127: 1301–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roeder G. S., 1997. Meiotic chromosomes: it takes two to tango. Genes Dev. 11: 2600–2621. [DOI] [PubMed] [Google Scholar]
- Rose M. D., Winston F., Hieter P., 1990. Methods in yeast genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- Ross L. O., Rankin S., Shuster M. F., Dawson D. S., 1996. Effects of homology, size and exchange of the meiotic segregation of model chromosomes in Saccharomyces cerevisiae. Genetics 142: 79–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross-Macdonald P., Roeder G. S., 1994. Mutation of a meiosis-specific MutS homolog decreases crossing over but not mismatch correction. Cell 79: 1069–1080. [DOI] [PubMed] [Google Scholar]
- Rosu S., Libuda D. E., Villeneuve A. M., 2011. Robust crossover assurance and regulated interhomolog access maintain meiotic crossover number. Science 334: 1286–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santucci-Darmanin S., Neyton S., Lespinasse F., Saunieres A., Gaudray P., et al. , 2002. The DNA mismatch-repair MLH3 protein interacts with MSH4 in meiotic cells, supporting a role for this MutL homolog in mammalian meiotic recombination. Hum. Mol. Genet. 11: 1697–1706. [DOI] [PubMed] [Google Scholar]
- Shinohara M., Oh S. D., Hunter N., Shinohara A., 2008. Crossover assurance and crossover interference are distinctly regulated by the ZMM proteins during yeast meiosis. Nat. Genet. 40: 299–309. [DOI] [PubMed] [Google Scholar]
- Shonn M. A., McCarroll R., Murray A. W., 2000. Requirement of the spindle checkpoint for proper chromosome segregation in budding yeast meiosis. Science 289: 300–303. [DOI] [PubMed] [Google Scholar]
- Snowden T., Acharya S., Butz C., Berardini M., Fishel R., 2004. hMSH4-hMSH5 recognizes Holliday Junctions and forms a meiosis-specific sliding clamp that embraces homologous chromosomes. Mol. Cell 15: 437–451. [DOI] [PubMed] [Google Scholar]
- Snowden T., Shim K. S., Schmutte C., Acharya S., Fishel R., 2008. hMSH4–hMSH5 adenosine nucleotide processing and interactions with homologous recombination machinery. J. Biol. Chem. 283: 145–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl F. W., Foss H. M., Young L. S., Borts R. H., Abdullah M. F., et al. , 2004. Does crossover interference count in Saccharomyces cerevisiae? Genetics 168: 35–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storlazzi A., Gargano S., Ruprich-Robert G., Falque M., David M., et al. , 2010. Recombination proteins mediate meiotic spatial chromosome organization and pairing. Cell 141: 94–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturtevant A. H., Beadle G. W., 1936. The relations of inversions in the X chromosome of Drosophila Melanogaster to crossing over and disjunction. Genetics 21: 554–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsubouchi T., Zhao H., Roeder G. S., 2006. The meiosis-specific zip4 protein regulates crossover distribution by promoting synaptonemal complex formation together with zip2. Dev. Cell 10: 809–819. [DOI] [PubMed] [Google Scholar]
- Wilkening S., Tekkedil M. M., Lin G., Fritsch E. S., Wei W., et al. , 2013. Genotyping 1000 yeast strains by next-generation sequencing. BMC Genomics 14: 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winzeler E. A., Richards D. R., Conway A. R., Goldstein A. L., Kalman S., et al. , 1998. Direct allelic variation scanning of the yeast genome. Science 281: 1194–1197. [DOI] [PubMed] [Google Scholar]
- Woods L. M., Hodges C. A., Baart E., Baker S. M., Liskay M., et al. , 1999. Chromosomal influence on meiotic spindle assembly: abnormal meiosis I in female Mlh1 mutant mice. J. Cell Biol. 145: 1395–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakharyevich K., Ma Y., Tang S., Hwang P. Y., Boiteux S., et al. , 2010. Temporally and biochemically distinct activities of Exo1 during meiosis: double-strand break resection and resolution of double Holliday junctions. Mol. Cell 40: 1001–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakharyevich K., Tang S., Ma Y., Hunter N., 2012. Delineation of joint molecule resolution pathways in meiosis identifies a crossover-specific resolvase. Cell 149: 334–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zickler D., Kleckner N., 1999. Meiotic chromosomes: integrating structure and function. Annu. Rev. Genet. 33: 603–754. [DOI] [PubMed] [Google Scholar]