Abstract
The L1CAM family of cell adhesion molecules is a conserved set of single-pass transmembrane proteins that play diverse roles required for proper nervous system development and function. Mutations in L1CAMs can cause the neurological L1 syndrome and are associated with autism and neuropsychiatric disorders. L1CAM expression in the mature nervous system suggests additional functions besides the well-characterized developmental roles. In this study, we demonstrate that the gene encoding the Caenorhabditis elegans L1CAM, sax-7, genetically interacts with gtl-2, as well as with unc-13 and rab-3, genes that function in neurotransmission. These sax-7 genetic interactions result in synthetic phenotypes that are consistent with abnormal synaptic function. Using an inducible sax-7 expression system and pharmacological reagents that interfere with cholinergic transmission, we uncovered a previously uncharacterized nondevelopmental role for sax-7 that impinges on synaptic function.
Keywords: C. elegans, sax-7 L1CAM, GTL-2 TRPM channel, modifier gene, synaptic function
L1CAMS are cell adhesion molecules conserved in Caenorhabditis elegans, Drosophila melanogaster, and vertebrates that are important for nervous system development and function. L1CAMs share a basic body plan of six immunoglobulin-like domains and five fibronectin type III repeats in the extracellular domain, a single transmembrane domain, and a highly conserved cytoplasmic tail. These domains promote both homophilic and heterophilic interactions with diverse molecules to promote cell adhesion and regulation of signal transduction in processes that include axon guidance, myelination, fasciculation, and maintenance of neural architecture, as well as synapse formation and maintenance (Chen and Zhou 2010; Sakurai 2012).
The importance of L1CAM function in nervous system development and function is evident by the diverse neurological symptoms resulting from mutations in the human L1CAM gene family that consists of L1, NrCAM, neurofascin, and CHL1. For example, mutations in NrCAM and CHL1 are associated with autism, addiction, and schizophrenia. In addition, mutations in the X-linked L1 gene result in the L1 syndrome, the symptoms of which include corpus callosum hypoplasia, mental retardation, adducted thumbs, spastic paraplegia, and hydrocephalus (CRASH) (Chen and Zhou 2010; Sakurai 2012). While the severity of these symptoms is dependent on the particular lesion in L1, it is interesting that expressivity of CRASH symptoms can vary among intrafamilial patients (Jouet et al. 1995; Schrander-Stumpel et al. 1995; Fransen et al. 1998). For example, a male L1 patient may exhibit severe hydrocephalus while his male sibling carrying the same allele may not present with hydrocephalus. This variable phenotypic heterogeneity suggests L1 may genetically interact with other genes for disease modification. Supporting this hypothesis is the variable association of Hirschsprung’s disease (HSCR) in L1 patients with hydrocephalus (Okamoto et al. 1997; Parisi et al. 2002; Jackson et al. 2009; Fernandez et al. 2012; Takenouchi et al. 2012). HSCR is a complex, multigenic intestinal aganglionosis disorder where ganglion cell precursors fail to migrate to the distal gut, resulting in congenital constipation. Indeed, murine studies revealed L1 as a modifier gene in intestinal aganglionosis that is consistent with HSCR (Wallace et al. 2010, 2011). Additional studies on L1 as a modifier uncovered a novel role for L1 in promoting the migration and differentiation of ganglion cell precursors in the distal gut (Anderson et al. 2006; Turner et al. 2009), thus illustrating the power of modifier gene studies in identifying gene functions and cellular mechanisms.
In addition to embryonic expression, L1CAMs are also expressed in adults (Liljelund et al. 1994; Wang et al. 1998; Backer et al. 2002; Nikonenko et al. 2006), suggestive of potential L1CAM functions in mature animals that may be masked by their developmental requirement. The impairment of these potential roles may also account for some of the CRASH symptoms in the L1 disorder and possibly the autism and neuropsychiatric disorders that are strongly associated with L1CAMs. This study takes advantage of the ease in performing genetic modifier analyses in C. elegans to dissect the functions of the C. elegans L1CAM, SAX-7, which in addition to controlling dendritic branching morphogenesis, also maintains the position of neurons and their axons to preserve nervous system architecture (Zallen et al. 1999; Sasakura et al. 2005; Wang et al. 2005; Dong et al. 2013; Salzberg et al. 2013). Here, we provide evidence demonstrating that sax-7 acts as a modifier of synaptic regulation, uncovering a novel nondevelopmental neuronal role for sax-7.
Materials and Methods
Strains
C. elegans strains, provided by the Caenorhaditis Genetics Center, were grown on nematode growth medium (NGM) plates at 21°; N2 Bristol served as the wild-type strain (Brenner 1974) and CB4856 served as the Hawaiian strain used for snip single-nucleotide polymorphism (SNP) mapping. The alleles used in this study are listed by linkage groups as follows:
LGI: unc-13(n2813), unc-13(e312), and unc-13(e1091) (Brenner 1974; Kohn et al. 2000; Yook et al. 2001).
LGII: rab-3(js49) (Nonet et al. 1997).
LGIV: unc-24(e138), dpy-20(e1282), and unc-29(e193) (Brenner 1974); sax-7(eq1) (Wang et al. 2005; Zhou et al. 2008); sax-7(nj48) (Sasakura et al. 2005); juIs1 (Hallam and Jin 1998); gtl-2(tm1463) (The Japanese National Bioresource Project); and gtl-2(eq3), which was outcrossed from the LH1 strain (Wang et al. 2005) (see Three-factor cross analysis in Materials and Methods).
LGX: oxIs12 (McIntire et al. 1997).
The integrated oxIs12 (Punc-47::gfp) (McIntire et al. 1997), juIs1 (Punc-25::snb-1::gfp) (Hallam and Jin 1998), evIs111 (rgef-1::gfp), and wdIs20(Punc-4::snb-1::gfp) (Lickteig et al. 2001) transgenes were crossed into respective strains to visualize the architecture of the whole nervous system as well as examine GABA and cholinergic neuron and synapse morphology. The strains generated in this study are as follows:
Three-factor cross analysis
A three-factor cross, which was performed to test for a mutation to the right of sax-7, utilized the unc-24 gene, which maps to the left of sax-7 (positions 3.51 and 3.59, respectively), and the dpy-20 gene, which maps ∼1.6 map units to the right of sax-7. Crossing into the LH1 strain, we generated a strain with the genotype unc-24dpy-20/ sax-7(eq1) and two classes of genetic recombinants were isolated: Unc non-Dpy and Dpy non-Unc. For each recombinant of each class, it was determined whether thin, lethargic worms were among the progeny. If these traits were conferred by eq1, then they would show close linkage to unc-24 so that almost all of the Dpy non-Unc recombinants should segregate thin, lethargic worms.
Of the 30 recombinants analyzed, only 4 of 13 Dpy non-Unc recombinants segregated thin, lethargic progeny while 11 of 17 Unc non-Dpy recombinants segregated thin, lethargic progeny. These data demonstrate that LH1 has a second mutation we designated as eq3 that maps between unc-24 and dpy-20 and is somewhat closer to the latter marker (Figure 1).
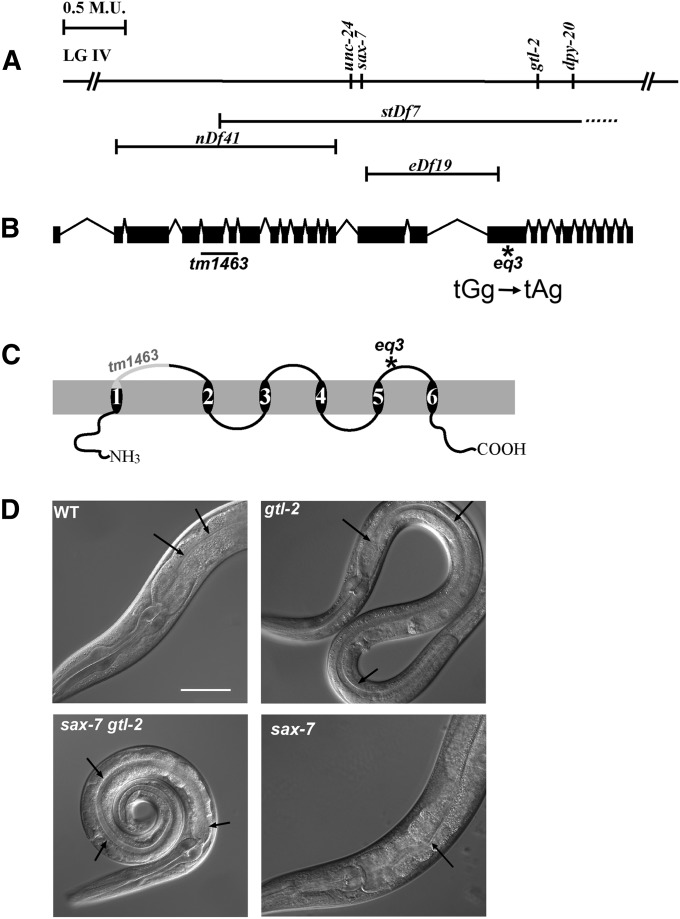
Figure 1.
sax-7 and the closely linked gtl-2 genetically interact to produce synthetic coiling of the body. (A) The positions of sax-7 and gtl-2 on linkage group IV. The chromosomal deficiency stDf7 uncovers sax-7, gtl-2, and dpy-20. The right breakpoint of stDf7 is not known (dashed line). Neither sax-7 nor gtl-2 is uncovered by two other deficiencies in the region, nDf41 and eDf19. (B) The 26 exons of gtl-2 are represented by solid boxes. The positions of the eq3 and tm1463 mutations are indicated. (C) GTL-2 is predicted to have six transmembrane domains that form a pore for cations with the N and C termini depicted as being cytosolic. Also indicated are the regions of the protein that are predicted to be affected by the eq3 or tm1463 mutations. (D) Differential interference contrast micrographs of wild-type (WT), sax-7gtl-2, gtl-2, and sax-7 adult animals. Note that the scrawniness of the gtl-2 and sax-7gtl-2 animals is quite apparent as they are thinner and smaller than wild-type and sax-7 animals. In addition to being scrawny, sax-7gtl-2 animals also tend to coil their bodies, unlike sax-7 or gtl-2 animals. Arrows point to the intestinal lumen, which is enlarged in gtl-2 and sax-7gtl-2 animals, indicating constipation, compared to wild-type and sax-7 animals. Bar, 50 µm.
Molecular characterization of gtl-2 mutants
eq3 or tm1463 homozygotes were lysed to provide template DNA for the generation of PCR products specific for the gtl-2 gene. Primers were chosen such that the DNA sequence could be determined for all of the exons and for short regions of the introns adjoining the exons. The DNA sequence of both strands of PCR products was determined en masse at the Advanced Genetic Analysis Center (University of Minnesota). DNA sequences were compared with the wild-type sequence at wormbase.org. The eq3 mutation was confirmed by sequencing wild-type DNA in the region.
DNA constructs and generation of transgenic animals
Transgenic animals were generated according to standard procedures (Mello et al. 1991). Based on Zhou et al. (2008), we injected sax-7gtl-2 animals with co-injection marker Pstr-1::gfp (Troemel et al. 1995) along with different sax-7 constructs that include 70 ng/μl pLC262 (Psax-7::sax-7) or 10 ng/μl pLC237 (Pmyo-3::sax-7L) or 20 ng/μl pLC227 (Punc-119::sax-7L) or 2.5 ng/μl pLC238 (Pdpy-7::sax-7L). To generate the inducible sax-7 construct (pLC672), we inserted sax-7L into pPD49.78, which contains the HSP16-2 promoter (courtesy of A. Fire, Stanford University School of Medicine, Stanford, CA) between the BamHI and KpnI sites. pLC672 was injected at 50 ng/μl along with 1.5 ng/μl of Pmyo-2::tdtomato as a co-injection marker:
The cosmid F54D1 was injected at 5 ng/μl together with 100 ng/μl pTG96 (Yochem et al. 1998) into gtl-2 animals to generate eqEx72, which was used for mosaic analysis (see Materials and Methods).
Pgtl-2::gtl-2 (pLC645): A 17.5-kb XmaI–KpnI fragment from cosmid F54D1 was subcloned into pBS II SK−. This fragment consists of 6.4 kb upstream of the putative ATG start codon and 347 bp downstream from the poly(A) addition site of the gene (Figure 1). A total of 50 ng/μl pLC pLC645 was injected along with 70 ng/μl Pstr-1::gfp into sax-7gtl-2 animals.
Pgtl-2::gtl-2::gfp (pLC646): GFP DNA PCR’d from pPD95.75 was inserted in frame into pLC645 into a NotI site that was engineered upstream of the stop codon of gtl-2 DNA. gtl-2 animals injected with 30 ng/μl pLC646 along with 70 ng/μl of co-injection marker pRF4 (Mello et al. 1991) were rescued as were sax-7gtl-2 transgenic animals injected with 20 ng/μl pLC646 along with 2 ng/μl of co-injection marker Pmyo-2::tdtomato.
To generate tissue-specific-expressing gtl-2 constructs, tissue-specific promoters were PCR’d and put in frame of (1) pLC606, a derivative of pLC645 that lacks the gtl-2 promoter, or (2) pLC607, a derivative of pLC646 that lacks the gtl-2 promoter. For expression in the excretory cell, we generated Pexc-5::gtl-2 (pLC611) and Pexc-5::gtl-2::gfp (pLC623); both constructs contain 2.6 kb of sequence upstream of the putative exc-5 start codon (Suzuki et al. 2001). A total of 50 ng/μl or 20 ng/μl of the gtl-2 constructs was injected along with 2 ng/μl of co-injection marker Pmyo-2::tdtomato into sax-7gtl-2 or gtl-2 animals. For expression in hyp7, we generated Pdpy-7::gtl-2 (pLC612) and Pdpy-7::gtl-2::gfp (pLC631); both constructs contain 400 bp of sequence upstream of the putative dpy-7 start codon (Gilleard et al. 1997). A total of 2.5 ng/μl of the gtl-2 constructs was injected along with 70 ng/μl of co-injection marker Pstr-1::gfp into sax-7gtl-2 animals. For expression in body-wall muscles, we generated Pmyo-3::gtl-2 (pLC613) and Pmyo-3::gtl-2::gfp (pLC637); both constructs contain 2.3 kb myo-3 promoter sequence (Okkema et al. 1993). A total of 20–50 ng/μl of each construct was injected along 70 ng/μl of co-injection marker Pstr-1::gfp into sax-7gtl-2 or gtl-2 animals. For expression in the nervous system, we generated Prgef-1::gtl-2 (pLC626) and Prgef-1::gtl-2::gfp (pLC639); both constructs contain 3.5 kb of rgef-1 promoter sequence (Altun-Gultekin et al. 2001). A total of 20–40 ng/μl of the gtl-2 constructs was injected along with 70 ng/μl or 2 ng/μl of co-injection markers Pmyo-2::tdtomato into sax-7gtl-2 animals:
Genetic mosaic analysis
Genetic mosaics can be analyzed in C. elegans on the basis of the occasional failure of an extrachromosomal array to disjoin during embryonic cell divisions Yochem et al. 1998. The source of the mosaics was LH104 [gtl-2(eq3); eqEx72[gtl-2(+); sur-5::gfp]], a strain having an extrachromosomal array that contains copies of cosmid clone F54D1, which efficiently rescues mutant, chromosomal copies of gtl-2, and copies of the plasmid pTG96, for nuclear expression of SUR-5::GFP in many cell types (Yochem et al. 1998). Thus, gtl-2(+) and nuclear green fluorescence cosegregate in these strains. Progeny of the strains were examined with a dissecting microscope equipped for epifluorescence for those lacking SUR-5::GFP in some but not all cells, indicating nondisjunction of the array during development. The embryonic cell division at which the nondisjunction occurred was deduced on the basis of the anatomy and nearly invariant cell lineage (Sulston et al. 1983) following an examination of the mosaics with a compound microscope.
Whole-mount immunofluorescence
Young adult animals of wild-type and sax-7(eq1) gtl-2 (eq3) backgrounds were fixed in methanol and stained for indirect immunofluorescence, using the freeze-crack methanol fixation method. Fixed animals were washed, incubated with primary antibodies anti-UNC-17 (1:2000) and anti-UNC-29 (1:100) at 4°, washed again, and incubated in secondary antibody (Alexa 488 and 568; Molecular Probes, Eugene, OR) for 2 hr at room temperature. After washing, the samples were mounted and examined using an Axioplan 2 microscope (Carl Zeiss, Thornwood, NY). Images were acquired using the AxioCam MRM and AxioVision 4.5 software (Carl Zeiss).
Live-animal microscopy
Nervous system architecture was examined in animals containing the evIs111 and oxIs12 GFP markers while synapse morphology in GABA and cholinergic neurons was examined in animals containing the juIs1 and wdIs20 GFP markers, respectively. Synchronized L4 or young adult animals were mounted on 2% agarose pads and anesthetized using 1% (v/v) 1-phenoxy-2-propanol in M9 buffer and scored for defects, using an Axioplan 2 microscope (Carl Zeiss). Images of nervous system architecture in animals containing evIs111 were collected as stacks along the z-axis, using an Olympus Fluoview FV1000 upright confocal microscope and Fluoview software. Images of the other animals were acquired using an AxioCam MRM and AxioVision 4.5 software (Carl Zeiss). Displaced neurons were scored as previously described (Zhou and Chen 2011). To quantitate defects in synapse formation, images of the dorsal nerve cord were acquired and synaptic puncta were analyzed manually over 50 μm. n = 25 animals for each strain.
Aldicarb and Levamisole assays
The assays are based on previously established protocols (Miller et al. 1996; Saifee et al. 1998). Young adults were placed onto NGM plates containing either 0.25 mM Levamisole (≥99% tetramisole hydrochloride; Sigma-Aldrich) or 0.75, 1, 1.25, or 1.5 mM of the acetylcholinesterase inhibitor Aldicarb (Aldicarb Pestanal; Sigma-Aldrich). At 30-min intervals for 2.5–3 hr, we counted the number of paralyzed animals vs. animals that exhibited locomotion after being prodded with a platinum wire. For each strain, we performed 10–12 assays, in which n = 25 animals in each assay.
Growth on varying amounts of magnesium supplementation
gtl-2 and sax-7gtl-2 L4-staged animals were placed in NGM plates containing 0, 1, 2, or 4 mM magnesium supplementation. Their progeny were examined for differences in growth, movement, and Aldicarb sensitivity. Aldicarb assays were performed as described, except the plates used were NGM containing Mg2+ supplementation that corresponded to that of their growth medium.
Thrashing assays
The thrashing assays were performed as previously described (Miller et al. 1996). Young adult hermaphrodites were transferred into a well of a depression slide containing 200 μl M9 buffer. After 2 min of recovery, the number of times an animal thrashed (bending at midbody) was counted for 1 min. n = 50 animals per strain.
Heat-shock induction of sax-7 expression
All strains were cultured at 15° to reduce potential leakiness of the hsp16.2 promoter. L4-staged or young adult transgenic and nontransgenic control animals were transferred and incubated at 34° for 2 hr before they were allowed to recover at 15° for either 3 or 15 hr. After recovery, animals were subjected to the Aldicarb assay (post-heat-shock recovery time of 3 hr), the thrashing assay (post-heat-shock recovery time of 15 hr), or examination of the displaced neuron phenotype (post-heat-shock recovery time of 3 and 15 hr).
Results
eq3 is a mutation in the gtl-2 gene
The LH1 strain, which is homozygous for the sax-7 deletion allele, eq1, was previously described as having a phenotype that is more severe than reported for other sax-7 alleles. In addition to the neuronal position maintenance defects observed in other sax-7 mutant backgrounds, LH1 animals are also scrawny, slow growing (Gro), egg-laying defective (Egl), constipated (Con), and lethargic with a tendency to coil their bodies, behaviors that are not exhibited by other sax-7 alleles (Wang et al. 2005). One possible explanation for this difference is that eq1, unlike the other sax-7 alleles, is a null mutation, as revealed by molecular and genetic analyses and the lack of detectable SAX-7 protein in sax-7(eq1) animals immunostained with an anti-SAX-7 antibody; in contrast, the other sax-7 alleles still show residual SAX-7 protein (Zhou et al. 2008). An alternative explanation is the presence of a second mutation that remained linked to sax-7 after the original isolation and outcrossing of the eq1 mutation. Indeed, examination by three-factor crosses (see Materials and Methods) revealed a second mutation, designated as eq3, to the right of sax-7 in the LH1 strain (Figure 1A). Free of eq3, the sax-7(eq1) phenotype is generally similar to that described for other sax-7 alleles, including defective neuronal position maintenance (Sasakura et al. 2005; Zhou et al. 2008). The scrawny, Gro, Egl, Con, and lethargic phenotype of LH1 is attributed to the eq3 mutation (Figure 1D).
Using SNPs present in a Hawaiian strain of C. elegans, we mapped the eq3 mutation relative to the physical map (Davis et al. 2005). Micro-injection of the F54D1cosmid clone within the mapped interval conferred full rescue of eq3. Further subcloning and PCR analysis of F54D1, together with DNA sequencing of mutant homozygotes, demonstrated that eq3 is a mutation in gtl-2, which encodes a member of the TRPM family of channels that regulate the passage of divalent cations, including Mg2+ and Ca2+ (Venkatachalam and Montell 2007; Teramoto et al. 2010; Nilius and Owsianik 2011).
eq3 is a G-to-A transition that changes amino acid 1064 from a tryptophan to an amber stop, which should result in a truncation of the product in the third extracellular loop (Figure 1, B and C). gtl-2(eq3) homozygotes are indistinguishable from animals homozygous for gtl-2(tm1463), a previously described loss-of-function deletion allele (Stawicki et al. 2011). In addition to the Gro, Egl, and lethargic phenotype, animals homozygous for eq3 or tm1463 are also Con and scrawny, phenotypes that are apparent in Figure 1D. These phenotypes are similar to those of eq3/tm1463 trans-heterozygotes as well as eq3/stDf7 or tm1463/stDf7 hemizygotes, where stDf7 is a chromosomal deficiency that removes multiple genes, including gtl-2 (Figure 1A).
gtl-2 genetically interacts with the cell-adhesion gene sax-7
Unlike the other described phenotypes, the tendency to coil by LH1 animals is not displayed by either sax-7(eq1) or gtl-2(eq3) single-mutant animals (Figure 1D). This synthetic coiling phenotype is also exhibited by additional sax-7gtl-2 strains we generated with gtl-2(tm1463) and another putative null sax-7 allele, nj48. Thus the synthetic coiling in LH1 is not allele specific, indicating sax-7 and gtl-2 genetically interact.
To determine the significance of this genetic interaction, i.e., the cause of the coiling phenotype, we first examined sax-7gtl-2 nervous system morphology. Other than the sax-7-related neuronal displacement, we detected no obvious abnormalities in nervous system architecture, as visualized by a previously established neuronally-expressed GFP marker, evIs111. In addition, we did not detect any significant difference in synapse morphology and spacing, as visualized by synaptobrevin/SNB-1::GFP in both cholinergic and GABAergic neurons (Supporting Information, Figure S1). These results suggest the coiling phenotype is not a result of defects in nervous system architecture or development; rather, this coiling phenotype is more likely due to impaired nervous system function. Indeed, animals with impaired neuromuscular function can exhibit coiling phenotypes (Rand and Russell 1984; Alfonso et al. 1994; Harris et al. 2000). Moreover, gtl-2 was recently shown to have modulatory effects on excitation–inhibition synaptic imbalance caused by increased activity in the neuronal ACR-2R acetylcholine receptor (Stawicki et al. 2011). We thus hypothesized that sax-7gtl-2 animals might have abnormal neuromuscular function.
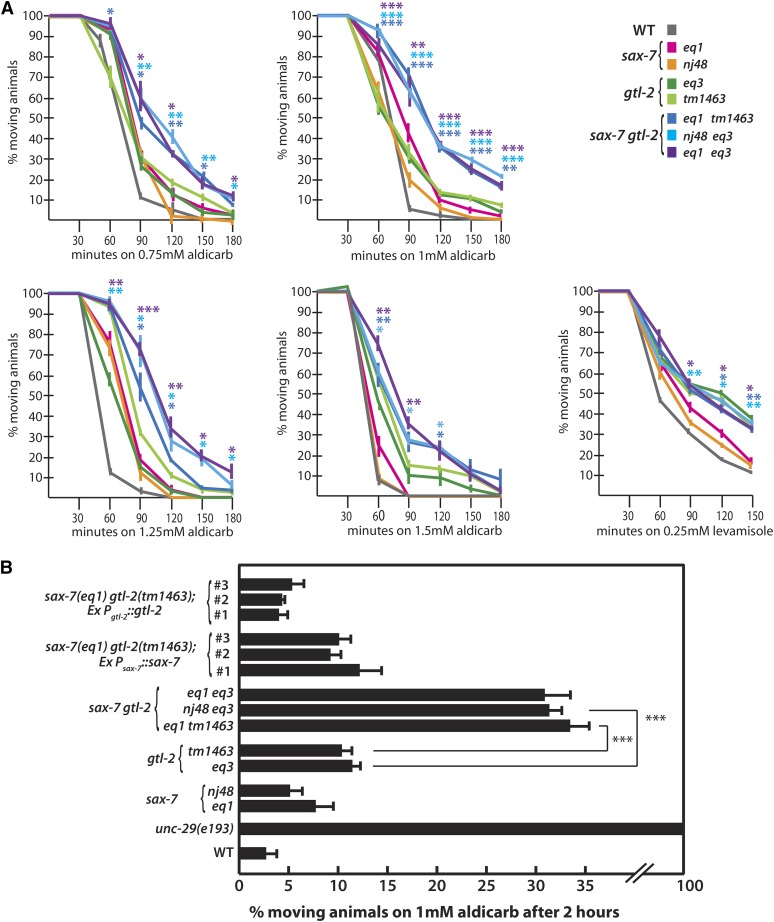
To test this hypothesis, we assessed whether sax-7gtl-2 animals might show resistance to Aldicarb, a cholinesterase inhibitor that has been used to successfully identify synaptic transmission mutants (Nguyen et al. 1995; Miller et al. 1996; Sieburth et al. 2005). Exposure to cholinesterase inhibitors causes acute paralysis in wild-type animals as a result of acetylcholine accumulation at neuromuscular junctions. However, mutants with impaired acetylcholine secretion or acetylcholine receptor function generally exhibit the resistance to inhibitors of cholinesterase (Ric) phenotype. We performed a time-course response to four different Aldicarb concentrations. While the different strains exhibited consistent responses in all conditions tested, the 2-hr time point on 1 mM Aldicarb provided the highest sensitivity to changes in the animals’ response (Figure 2A). A total of 5–7% of sax-7 animals and 10% of gtl-2 animals still exhibited movement by 2 hr of exposure to 1 mM Aldicarb; by comparison, >30% of sax-7gtl-2 animals were still able to move (Figure 2B), thus displaying a level of Aldicarb resistance that is synergistic rather than additive. As expected, virtually no wild-type animals were moving, while 100% of unc-29 animals, which are deficient in nicotinic acetylcholine receptor function (Fleming et al. 1997), were still active and exhibiting a strong level of Aldicarb resistance (data not shown). The sax-7gtl-2 Aldicarb resistance can be reduced with transgenic expression of gtl-2 or sax-7 (Figure 2B). The synthetic coiling and synergistic Ric phenotypes by sax-7gtl-2 animals relative to each single-mutant background reveal a genetic interaction between sax-7 and gtl-2 and suggest that the loss of function of both genes results in abnormal neuromuscular function.
Figure 2.
The response of sax-7gtl-2 as well as sax-7 and gtl-2 single-mutant strains to the cholinesterase inhibitor, Aldicarb, and the acetylcholine receptor agonist, Levamisole. (A) A time-course response of wild-type and mutant strains on NGM media containing 0.75, 1, 1.25, or 1.5 mM Aldicarb and 0.25 mM Levamisole. Controls include wild type (WT), which is sensitive to Aldicarb and Levamisole, and unc-29(e193), which is resistant to Aldicarb and Levamisole (data not shown) (Miller et al. 1996; Fleming et al. 1997). The color-coded *’s in the Aldicarb assays represent the P-values calculated by Student’s t-test comparing sax-7gtl-2 Aldicarb resistance to the corresponding gtl-2 allele. On the other hand, the color-coded *’s in the Levamisole assays represent the P-values when comparing sax-7gtl-2 Levamisole resistance to the corresponding sax-7 allele. *P < 0.05, **P < 0.005, ***P < 0.0001. (B) The percentage of animals moving after a 2-hr exposure to 1 mM Aldicarb. Transgenes containing wild-type gtl-2 can consistently suppress the Aldicarb resistance exhibited by sax-7gtl-2 double mutants. Similarly, transgenes containing wild-type sax-7 restore the level of resistance of the double mutant to that of the gtl-2 single mutant. Three independent transgenic lines were analyzed for each construct assessed. Error bars show the standard error of the mean of at least 12 sample sets, where n = 25 animals for each set.
sax-7 gtl-2 Aldicarb resistance and coiling behavior is suppressed by nonneuronal gtl-2 expression or growth on culture medium with reduced Mg2+ levels
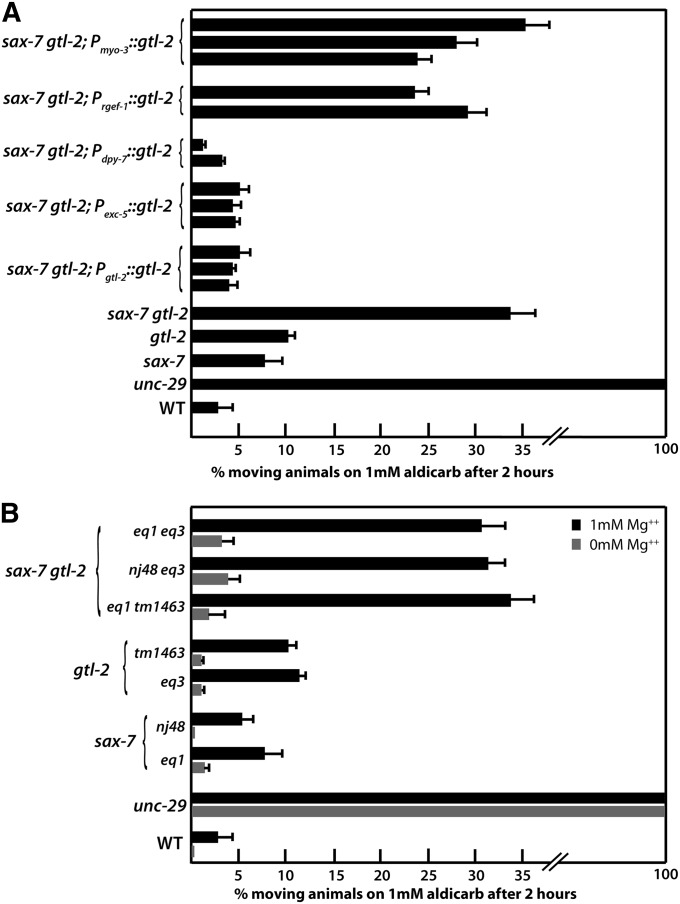
To determine how loss of gtl-2 contributes to sax-7gtl-2 synthetic phenotypes, we examined the site of action for GTL-2, using tissue-specific gtl-2 expression and mosaic analysis. The GTL-2 TRPM channel was previously shown to be expressed in the excretory cell and hyp7 (Teramoto et al. 2010; Stawicki et al. 2011). In agreement with our mosaic analysis (Figure S2), expression of gtl-2::gfp in either of these tissues suppressed the sax-7gtl-2 coiling tendency and gtl-2-related behavioral phenotypes such that transgenic sax-7gtl-2 double-mutant animals are healthy and robust, exhibiting relatively normal movements. Importantly, Aldicarb resistance in these transgenic sax-7gtl-2 animals is suppressed (Figure 3A). In contrast, gtl-2::gfp expressed in body-wall muscle or neurons did not suppress sax-7gtl-2 coiling and Ric phenotypes. The ability for nonneuronal gtl-2 expression to suppress the apparent neuromuscular defects is consistent with a recent study that showed similar gtl-2 expression in either of these nonneuronal tissues can also modulate ACR-2-related synaptic activity (Stawicki et al. 2011).
Figure 3.
The sax-7gtl-2 Ric phenotype can be suppressed with nonneuronal gtl-2 expression and culturing of mutant animals on media with reduced Mg2+ levels. (A) Expression of wild-type gtl-2 driven in hyp7 or in the excretory cell by the dpy-7 and exc-5 promoters, respectively, can suppress the sax-7gtl-2 Aldicarb resistance just as well as the gtl-2 gene. In contrast, gtl-2 expressed in neurons or muscle by the unc-119 and myo-3 promoters, respectively, cannot suppress the sax-7gtl-2 Ric phenotype. Two to three independent transgenic lines were analyzed for each construct assessed. The rescue abilities of these constructs were similar in all sax-7gtl-2 backgrounds but only data for the sax-7(eq1) gtl-2(tm1463) background are shown. (B) The Ric phenotype exhibited by sax-7gtl-2 animals cultured on standard NGM, which is supplemented with 1 mM Mg2+, is dramatically suppressed when animals are cultured on NGM lacking Mg2+ supplementation. Error bars show the standard error of the mean of at least 12 sample sets, where n = 25 for each set.
GTL-2 plays a significant role in maintaining systemic Mg2+ levels; indeed, gtl-2 mutant animals are hypermagnesemic (Teramoto et al. 2010). Because hypermagesemia can affect synaptic transmission to cause neurologic symptoms (Topf and Murray 2003), we hypothesized that the increased systemic Mg2+ level caused by loss of gtl-2 function contributes to the coiling and Ric phenotype in sax-7gtl-2 animals. To test this hypothesis, we assessed the effects of culturing sax-7gtl-2 animals on C. elegans growth medium (NGM) supplemented with different amounts of Mg2+ on movement and response to aldicarb. sax-7gtl-2 animals cultured on standard NGM, which is supplemented with 1 mM Mg2+, display the described phenotypes and Aldicarb resistance (Figure 1 and Figure 2). But when cultured on nonsupplemented NGM [nonsupplemented NGM still contains some residual Mg2+ due to its presence in the bacteria fed to C. elegans as well as the agar used in NGM (Teramoto et al. 2010)], sax-7gtl-2 animals are robust and healthy, exhibiting minimal coiling behavior and importantly, no significant resistance to Aldicarb (Figure 3B). Aldicarb responses were not assessed under conditions of higher levels of Mg2+ (2 and 4 mM) because the already slow growth of the sax-7gtl-2 and gtl-2 mutant animals on standard NGM was further impaired at these higher concentrations (data not shown) (Teramoto et al. 2010), making it difficult to obtain sufficient numbers of adult animals to perform the Aldicarb response assay. Thus reducing Mg2+ levels in the culture medium effectively suppressed the sax-7gtl-2 coiling and Ric phenotypes, consistent with the idea that these phenotypes are due, in part, to a systemic increase in Mg2+.
Tissue-specific suppression of Aldicarb resistance reveals a requirement for sax-7 in neurons
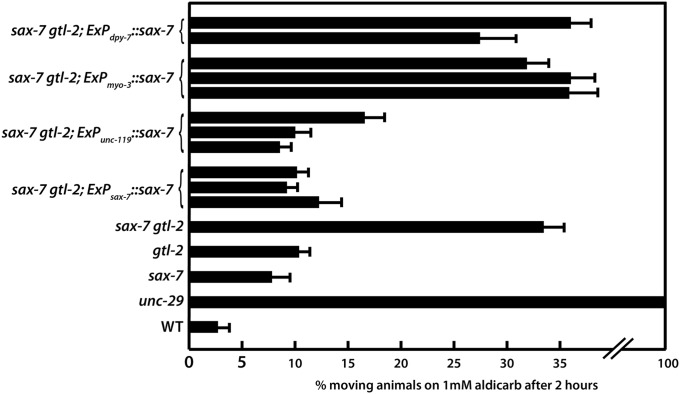
Resistance to Aldicarb is observed in animals with impaired presynaptic or postsynaptic function (Nguyen et al. 1995; Miller et al. 1996; Sieburth et al. 2005). Because sax-7 is widely expressed (Chen et al. 2001; Wang et al. 2005), the sax-7gtl-2 Ric phenotype may result from a loss of sax-7 function in neurons and/or muscles or other tissues. Indeed, sax-7 was previously shown to be required predominantly in neurons but also in the adjacent body-wall muscles and hypodermis for optimal maintenance of motor neuron positions (Wang et al. 2005). We thus assessed the level of Aldicarb resistance in sax-7gtl-2 animals expressing sax-7 in each of the three tissues, using the unc-119, myo-3, or dpy-7 promoters (Okkema et al. 1993; Gilleard et al. 1997; Altun-Gultekin et al. 2001). sax-7 expression in neurons suppressed the sax-7gtl-2 aldicarb resistance equally as well as full-length genomic sax-7 (Figure 4). In contrast, sax-7 expression in body-wall muscles or the hypodermis failed to suppress the resistance.
Figure 4.
Rescue of the sax-7gtl-2 Ric phenotype with tissue-specific sax-7 expression reveals a requirement for sax-7 in neurons. Neuronal expression of sax-7 by the unc-119 promoter suppresses sax-7gtl-2 resistance to Aldicarb to the same level as that of the full-length sax-7 gene, unlike sax-7 expression in body-wall muscle or hyp7. Three independent transgenic lines were analyzed for each construct assessed. The rescue abilities of these constructs are similar in all sax-7gtl-2 strains. For simplicity, only data for the sax-7(eq1) gtl-2(tm1463) background are shown. Error bars show the standard error of the mean of at least 12 sample sets, where n = 25 for each set.
The ability for neuronal but not body-wall muscle expression of sax-7 to suppress the sax-7gtl-2 Ric phenotype is consistent with a neuronal role for sax-7. To test this hypothesis, we assayed responses of the mutant strains to the nicotinic acetylcholine receptor agonist, Levamisole. Wild-type animals exposed to Levamisole become paralyzed due to continued stimulation of body-wall muscles, which are enriched with acetylcholine receptors; in contrast, animals with defective postsynaptic acetylcholine receptor functions show resistance to Levamisole (Lewis et al. 1980; Miller et al. 1996; Fleming et al. 1997). After 2.5 hr of exposure to 0.25 mM Levamisole, sax-7 animals responded similarly to wild-type animals, with most animals paralyzed and only 12–15% animals still moving. By comparison, gtl-2 and sax-7gtl-2 animals showed similar responses, with 32–38% of animals still mobile (Figure 2A). As expected, unc-29 mutant animals, which have impaired acetylcholine receptor function, are resistant to Levamisole (data not shown) (Fleming et al. 1997). The comparable Levamisole resistance levels of gtl-2 and sax-7gtl-2 animals indicate that loss of sax-7 function does not contribute to the sax-7gtl-2 Levamisole resistance. Taken together, these results support a neuronal role for sax-7 that can impinge on synaptic function.
Transient, late-onset sax-7 expression rescues sax-7 gtl-2 Aldicarb resistance but not neuronal displacement, revealing a novel nondevelopmental role for sax-7
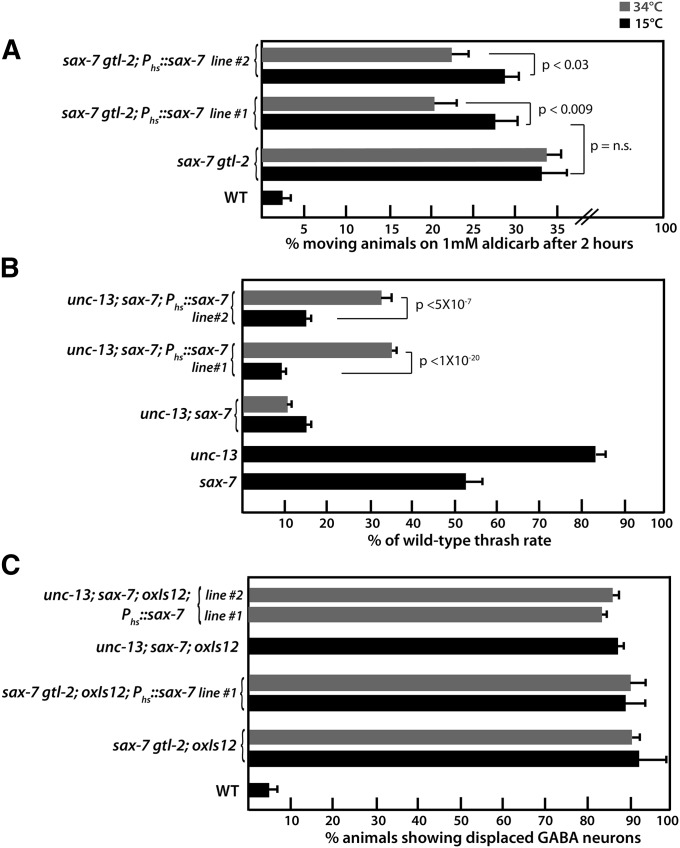
sax-7 mutant animals display progressive displacement of different neurons, including cholinergic and GABA motor neurons (Wang et al. 2005; Zhou et al. 2008). Yet sax-7 single-mutant animals display generally wild-type locomotion and sensitivity to Aldicarb. How does loss of sax-7 contribute to the synthetic sax-7gtl-2 phenotypes? One possible explanation is the sax-7-related neuronal displacement may modestly impair nervous system function to an undetectable level, but when combined with minor gtl-2-related abnormalities in synaptic function, becomes significantly compromised. Alternatively, sax-7 may possess an as-yet-uncharacterized, direct role in synaptic regulation that can be detected only in sensitized backgrounds. To test this possibility, we expressed sax-7, using an inducible heat-shock promoter in sax-7gtl-2 L4-staged larvae or young adults after the peak of neuronal displacement (Wang et al. 2005). A 2-hr induction of the transgene at an elevated temperature of 34°, followed by a 3-hr recovery period at 15°, significantly reduced the level of sax-7gtl-2 Aldicarb resistance (Figure 6A). However, the same sax-7 induction did not rescue the neuronal displacement phenotype (Figure 6C). We conclude that neuronal displacement is not the underlying cause of the sax-7gtl-2 Ric phenotype. Moreover, because of the ability for a late-onset and transient sax-7 expression to rescue the Ric phenotype, we infer that the sax-7gtl-2 Ric phenotype is caused by functional, not developmental defects. Taken together, these results reveal a novel nondevelopmental role for sax-7 in neurons that can modulate synaptic function.
Figure 6.
The synthetic phenotypes displayed by sax-7gtl-2 and unc-13; sax-7 animals are suppressed by late-onset, transient sax-7 expression. (A) Induced sax-7 expression with a heat-shock promoter in young adults suppresses the Aldicarb resistance in sax-7(eq1) gtl-2(tm1463) animals. Two independent transgenic lines were assessed. Error bars show the standard error of the proportion of at least 12 sample sets, where n = 25 for each set. (B) Quantitation of locomotion defects in unc-13(n2813); sax-7 and corresponding single-mutant backgrounds via the thrashing assay, where the thrash rate (number of thrashes per minute) is represented as a percentage of the wild-type thrashing rate, which is 152.44 ± 6.9 thrashes per minute. Error bars show the standard error of the mean. n = 50 animals. (C) Quantitation of young adult animals exhibiting displaced GABA neurons reveals that late-onset transient sax-7 expression cannot rescue the sax-7-related displaced neurons in the different mutant backgrounds.
sax-7 genetically interacts with unc-13 and rab-3, genes that function in neurotransmission
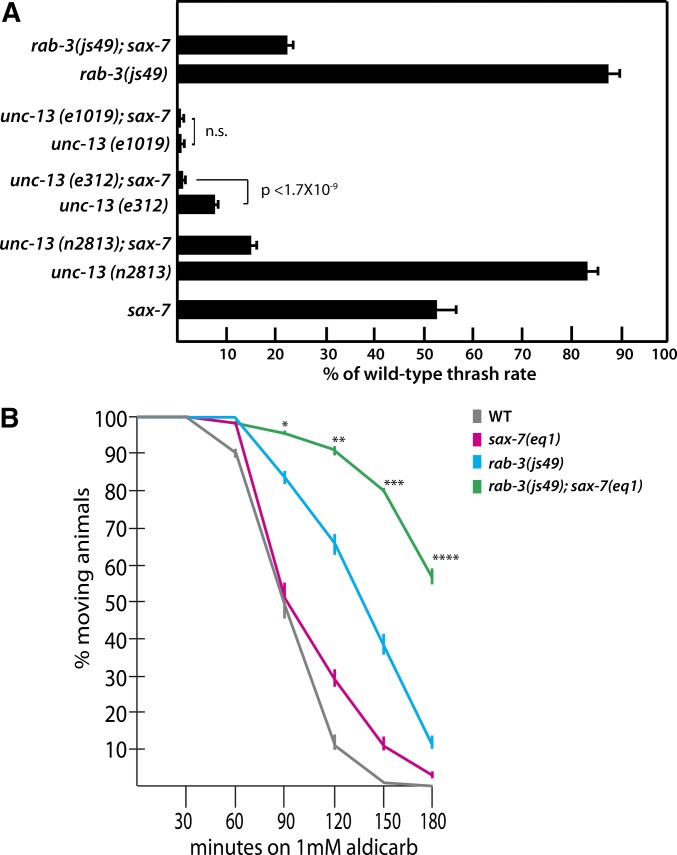
To further investigate the hypothesis that sax-7 modulates synaptic function, we probed whether sax-7 genetically interacts with the core pathway of genes essential for neurotransmitter release. Loss-of-function alleles in these genes generally result in severe locomotion defects, severely abnormal aldicarb responses, and sometimes lethality (Saifee et al. 1998; Kohn et al. 2000), making it difficult to detect potential genetic interactions with sax-7. To bypass this difficulty, we took advantage of a fortuitous missense mutation (n2813) in unc-13, which was previously used as a sensitized background to successfully identify and study genes involved in synaptic transmission (Yook et al. 2001). While unc-13 is essential for synaptic vesicle exocytosis and thus neurotransmitter release, unc-13(n2813) homozygous animals, like sax-7 null animals, move relatively well and are difficult to distinguish from wild-type animals (Kohn et al. 2000; Yook et al. 2001). In contrast, unc-13(n2813); sax-7 double-mutant animals barely move, showing uncoordinated and poor locomotion only when prodded, consistent with a proposed synaptic role for sax-7. We quantitated unc-13; sax-7 movements relative to those of single-mutant animals via a body thrashing assay, which examines the thrashing locomotion behavior of animals placed in liquid (Miller et al. 1996) (also see Materials and Methods). Compared to wild-type animals, which exhibit a thrash rate of 152.44 ± 6.9 thrashes per minute, unc-13 and sax-7 single-mutant animals show reduced thrash rates that are 81.7% and 52.4% of wild-type rates, respectively (Figure 5A). By comparison, the thrash rate of unc-13(n2813); sax-7 double-mutant animals is only 10.4% of the wild-type rate. The sax-7 genetic interaction with unc-13 is not specific to the n2813 allele, since loss of sax-7 also decreases the thrash rate of animals homozygous for a more severe hypomorphic unc-13 allele, e312 (Kohn et al. 2000), from 7.7 to 1.2% of the wild-type rate (Figure 5A). Loss of sax-7 does not reduce the already diminished thrash rate of the severe loss-of-function unc-13(e1091) allele (Figure 5A).
Figure 5.
sax-7 genetically interacts with the neurotransmission genes, unc-13 and rab-3. (A) Quantitation of locomotion defects in unc-13; sax-7, rab-3; sax-7, and the corresponding single-mutant backgrounds via the thrashing assay, where the thrash rate (number of thrashes per minute) is represented as a percentage of the wild-type thrashing rate, which is 152.44 ± 6.9 thrashes per minute. Error bars show the standard error of the mean. n = 50 animals. Error bars show the standard error of the proportion of at least 12 sample sets, where n = 25 for each set. n.s., not significant. (B) A time-course response of wild-type and mutant strains on NGM media containing 1 mM Aldicarb. Controls include wild type (WT), which is sensitive to Aldicarb, and unc-29(e193), which is resistant to Aldicarb (data not shown) (Miller et al. 1996). Error bars show the standard error of the mean of 10 sample sets, where n = 25 animals for each set. *’s represent the P-values calculated by Student’s t-test, comparing rab-3; sax-7 Aldicarb resistance to that of rab-3. *P < 0.005, **P < 0.0005, ***P < 5 × 10−5, ****P < 5 × 10−7.
The striking synergy observed in unc-13(n2813); sax-7 animals afforded us a sensitive system to assess whether transient sax-7 expression could rescue the low thrash rates in unc-13; sax-7 animals. Using the aforementioned inducible heat-shock sax-7 transgenic system, we determined that late-onset transient sax-7 expression could significantly recover the thrash rate in unc-13(n2813); sax-7 L4-staged animals (Figure 6B), even as the neuronal displacement phenotype was not rescued (Figure 6C). This result indicates that the altered nervous system architecture does not contribute to the unc-13(n2813); sax-7 locomotion phenotype and points to a nondevelopmental role for sax-7 that impinges on synaptic function.
The RAB-3 GTPase functions in targeting the synaptic vesicle to the presynaptic density but is not a core factor that is essential for neurotransmission, unlike UNC-13 (Nonet et al. 1997; Gracheva et al. 2008). Indeed, in addition to mild behavioral defects, rab-3(js49) null animals also exhibit modest resistance to Aldicarb (Nonet et al. 1997), thus providing a sensitive platform to further evaluate a role for sax-7 in modulating synaptic signaling. We first assessed whether loss of sax-7 caused a synthetic movement phenotype in rab-3(js49) animals. While rab-3 or sax-7 single-mutant animals move relatively well, rab-3; sax-7 animals exhibit uncoordinated, kinked, and sluggish movements. This synthetic movement defect is reflected in the low thrash rate in rab-3; sax-7 animals of 33.8 ± 2.7 thrashes per minute, which is 22.2% of the wild-type thrash rate as compared to rab-3 animals, which display 133 ± 3.8 thrashes per minute or 87.3% of the wild-type thrash rate (Figure 5A).
How does loss of both rab-3 and sax-7 result in the synthetic movement defects? Unlike the unc-13 alleles we examined, which all exhibit severely Ric phenotypes (Kohn et al. 2000; Yook et al. 2001), loss of rab-3 results only in a modest Ric phenotype that allows us to use the Aldicarb sensitivity assay as a way to assess whether the synthetic rab-3; sax-7 movement defects are a result of abnormal neurotransmission. We observed that loss of sax-7 function reduced rab-3 Aldicarb sensitivity so that 55.6% of rab-3; sax-7 animals were still moving after 3 hr of exposure to 1 mM Aldicarb, in contrast to the 11.2% of rab-3 and 3.6% of sax-7 animals that still showed movement (Figure 5B). Taken together, these results point to abnormal synaptic function as a contributing factor for the synthetic movement defects and enhanced Ric phenotype in rab-3; sax-7 animals, adding support to the hypothesis that sax-7 has a previously uncharacterized function that can modulate synaptic activity.
Discussion
This study reports the identification of the eq3 mutation in gtl-2 in the LH1 strain that accounts for the behavioral phenotypes that are not exhibited by other sax-7 alleles. The vicinity to and genetic interaction with sax-7 initially obscured the presence of the eq3 mutation in LH1. The genetic interaction between sax-7 and gtl-2 is manifested as a synthetic coiling behavior and Aldicarb resistance, phenotypes that are suggestive of abnormal synaptic activity. Consistent with a role in regulating synaptic function, sax-7 also genetically interacts with unc-13 and rab-3, resulting in synthetic movement defects and also enhanced Ric phenotype in rab-3; sax-7 animals. Because the synthetic phenotypes of sax-7gtl-2 and unc-13(n2813); sax-7 animals can be suppressed by late-onset transient expression of sax-7, we conclude that sax-7 has a novel nondevelopmental role that can modulate synaptic function.
Effects of systemic magnesium homeostasis on neuromuscular activity
How does the loss of gtl-2 function contribute to sax-7gtl-2 synthetic phenotypes? The gtl-2 Gro phenotype was previously shown to be a result of the increased systemic Mg2+ levels in gtl-2 animals (Teramoto et al. 2010). Consistent with this finding, the sax-7gtl-2 growth rate was recovered by culturing sax-7gtl-2 animals on media with reduced Mg2+ levels. Reduced Mg2+ levels also suppressed the sax-7gtl-2 Con, Egl, and lethargic phenotypes as well as the synthetic coiling and Ric phenotypes, supporting the idea that increased systemic Mg2+ levels contribute, in large part, to these apparent neuromuscular phenotypes.
Although it was previously shown that systemic ion homeostasis contributes to neuronal network excitation/inhibition balance in C. elegans (Stawicki et al. 2011), it is not clear how the increased systemic levels of Mg2+ may affect neuromuscular function. Similar to gtl-2 animals, patients suffering from hypermagnesemia also present clinically with neuromuscular symptoms that include lethargy, paralysis-inducing deep tendon reflex loss, and impaired cardiac functions. These symptoms are thought to arise in part from the inhibitory actions of Mg2+ on neurotransmission, voltage-gated calcium channels, and the ryanodine receptor (Mordes and Wacker 1977; Lansman et al. 1986; Sonna et al. 1996; Topf and Murray 2003; Wang et al. 2004; Laver 2007; Wang and Berlin 2007; Alexander et al. 2008). It is possible that these processes in C. elegans are similarly affected by the increased systemic Mg2+ levels in gtl-2 backgrounds. Alternatively and/or additionally, the abnormal Mg2+ levels may simply have a negative impact on general animal health that indirectly affects neuromuscular function.
In addition to Mg2+, other divalent ions regulated by gtl-2 may also contribute to the sax-7gtl-2 synthetic phenotypes. TRPM channels are permeable to Ca2+ and trace metal ions, including Zn2+ and Ni2+ (Monteilh-Zoller et al. 2003; Georgiev et al. 2010; Nilius and Owsianik 2011). Indeed, besides being hypermagnesemic, gtl-2 mutant animals also have reduced systemic Ca2+ levels, which are likely to also influence neuromuscular activity (Teramoto et al. 2010). Furthermore, metal ions were implicated in the gtl-2-related suppression of convulsions that resulted from acr-2 hyperactivity (Stawicki et al. 2011).
The role of sax-7 in the sax-7 gtl-2 synthetic phenotypes
It is interesting that no overt abnormalities in nervous system function are observed in sax-7 animals, despite progressive displacement of neurons and their processes. It is only in sensitized gtl-2, unc-13(n2813), and rab-3 backgrounds where synaptic activity is modestly altered that loss of sax-7 function can cause synthetic phenotypes that implicate neurotransmission defects. The genetic interaction between sax-7 and rab-3 null alleles suggests that sax-7 functions in a process distinct from that of rab-3. In contrast, we are unable to discern whether sax-7 functions together with unc-13 or in an unc-13 parallel pathway. Because UNC-13 is a core component of the neurotransmission machinery, loss of unc-13 function results in a severe phenotype that cannot be further enhanced. As a result, the genetic interaction with sax-7 can be observed only with weaker hypomorphic unc-13 alleles. Importantly, the use of tissue-specific and inducible sax-7 expression systems to successfully rescue the synthetic phenotypes but not neuronal displacement reveals a novel function for sax-7 in neurons that is distinct from its well-characterized role in maintaining neural architecture. When taken together, these findings point to this novel sax-7 role as having apparent modulatory activity on synaptic function.
Previous studies have implicated L1CAMs in synapse formation and maturation. For example, L1 has been shown to act transcellularly to organize nicotinic cholinergic synapses (Triana-Baltzer et al. 2006, 2008). Moreover, a previous study revealed a role for the Drosophila L1CAM neuroglian in central synapse formation (Godenschwege et al. 2006). While it is possible that sax-7 participates in synapse formation, we think this is unlikely to contribute to the synthetic phenotypes we observed. First, examination of sax-7gtl-2 cholinergic and GABAergic synapses revealed no overt morphological abnormalities. Second, suppression of the synthetic sax-7gtl-2 Ric and unc-13; sax-7 movement phenotypes by late-onset transient sax-7 expression in L4-staged animals, a time when mature synapses have long been formed, strongly argues against a role for sax-7 in a developmental process such as synaptogenesis. Instead, the temporal nature of this sax-7 rescuing activity is more in line with sax-7 having an acute role in nervous system function, such as modulating synaptic activity. It is not clear how sax-7 may influence synaptic function, but one possibility stems from a previous study demonstrating a role for CHL1 in regulating recycling of synaptic vesicles (Leshchyns’ka et al. 2006). A reduced rate of synaptic vesicle recycling could account for the observed synthetic phenotypes in sax-7 mutant backgrounds.
Supporting this possibility is the significantly reduced thrash rate observed in sax-7 animals when placed in liquid, despite generally normal locomotion on agar, a solid substrate. In contrast to locomotion on solid agar surfaces, the demand on synaptic function is far greater for animals in liquid to execute the quick and continuous thrashing movements. A reduced synaptic vesicle recycling rate, even at modest levels, is likely to be magnified in such an assay.
Modifier genes in uncovering novel L1CAM functions
Modifier gene studies have been essential in revealing mechanisms underlying cellular processes and signal transduction pathways (St. Johnston 2002). For example, much of our understanding of the MAP kinase signaling pathway as well as C. elegans vulval development stemmed from genetic modifier screens that identified suppressors or enhancers of vulval phenotypes (Fay and Yochem 2007; Sundaram 2013). Such modifier gene studies not only reveal mechanisms of cellular processes, but often also uncover novel roles for genes that may be masked by genetic redundancies or other essential functions. Indeed, a previous study uncovered a nonneuronal role for SAX-7 in gastrulation that was made evident only in the absence of HMR-1/cadherin because of functional redundancy by both genes in this process (Grana et al. 2010). Mammalian L1CAMs have also been shown to act as modifier genes. For example, L1 acts as a modifier for intestinal aganglionosis, genetically interacting with the endothelin signaling pathway during enteric nervous system development (Wallace et al. 2010, 2011). In addition, Nrcam acts as a modifier gene in peripheral neuropathy, genetically interacting with Lpin1 to synergistically cause progressive hindlimb paralysis. This genetic interaction demonstrates that in addition to the well-characterized neuronal developmental roles, NrCAM is also important for adult nervous system function (Douglas et al. 2009). In our study, we demonstrate that sax-7 has a previously uncharacterized neuronal role that was uncovered only in sensitized backgrounds with abnormal synaptic function. Similar genetic interactions by L1CAMs with synaptic genes in humans may account for some of the heterogeneity of the CRASH symptoms in the L1 disorder as well as complex neuropsychiatric disorders, addiction, and autism associated with L1CAMs.
Acknowledgments
We thank the C. elegans community at the University of Minnesota, in particular Jocelyn Shaw, David Greenstein, and Brock Grill for intellectual discussion, as well as the Caenorhaditis Genetics Center and The Japanese National Bioresource Project for providing strains used in this study. This study was made possible by grant NS045873 from the National Institute of Neurological Disorders and Stroke.
Footnotes
Supporting information is available online at http://www.genetics.org/lookup/suppl/doi:10.1534/genetics.114.169581/-/DC1.
Communicating editor: M. V. Sundaram
Literature Cited
- Alexander R. T., Hoenderop J. G., Bindels R. J., 2008. Molecular determinants of magnesium homeostasis: insights from human disease. J. Am. Soc. Nephrol. 19: 1451–1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfonso A., Grundahl K., McManus J. R., Rand J. B., 1994. Cloning and characterization of the choline acetyltransferase structural gene (cha-1) from C. elegans. J. Neurosci. 14: 2290–2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altun-Gultekin Z., Andachi Y., Tsalik E. L., Pilgrim D., Kohara Y., et al. , 2001. A regulatory cascade of three homeobox genes, ceh-10, ttx-3 and ceh-23, controls cell fate specification of a defined interneuron class in C. elegans. Development 128: 1951–1969. [DOI] [PubMed] [Google Scholar]
- Anderson R. B., Turner K. N., Nikonenko A. G., Hemperly J., Schachner M., et al. , 2006. The cell adhesion molecule l1 is required for chain migration of neural crest cells in the developing mouse gut. Gastroenterology 130: 1221–1232. [DOI] [PubMed] [Google Scholar]
- Backer S., Sakurai T., Grumet M., Sotelo C., Bloch-Gallego E., 2002. Nr-CAM and TAG-1 are expressed in distinct populations of developing precerebellar and cerebellar neurons. Neuroscience 113: 743–748. [DOI] [PubMed] [Google Scholar]
- Brenner S., 1974. The genetics of Caenorhabditis elegans. Genetics 77: 71–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L., Zhou S., 2010. “CRASH”ing with the worm: insights into L1CAM functions and mechanisms. Dev. Dyn. 239: 1490–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L., Ong B., Bennett V., 2001. LAD-1, the Caenorhabditis elegans L1CAM homologue, participates in embryonic and gonadal morphogenesis and is a substrate for fibroblast growth factor receptor pathway-dependent phosphotyrosine-based signaling. J. Cell Biol. 154: 841–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M. W., Hammarlund M., Harrach T., Hullett P., Olsen S., et al. , 2005. Rapid single nucleotide polymorphism mapping in C. elegans. BMC Genomics 6: 118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong X., Liu O. W., Howell A. S., Shen K., 2013. An extracellular adhesion molecule complex patterns dendritic branching and morphogenesis. Cell 155: 296–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas D. S., Moran J. L., Bermingham J. R., Jr, Chen X. J., Brindley D. N., et al. , 2009. Concurrent Lpin1 and Nrcam mouse mutations result in severe peripheral neuropathy with transitory hindlimb paralysis. J. Neurosci. 29: 12089–12100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fay D. S., Yochem J., 2007. The SynMuv genes of Caenorhabditis elegans in vulval development and beyond. Dev. Biol. 306: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez R. M., Nunez-Torres R., Garcia-Diaz L., de Agustin J. C., Antinolo G., et al. , 2012. Association of X-linked hydrocephalus and Hirschsprung disease: report of a new patient with a mutation in the L1CAM gene. Am. J. Med. Genet. A 158A: 816–820. [DOI] [PubMed] [Google Scholar]
- Fleming J. T., Squire M. D., Barnes T. M., Tornoe C., Matsuda K., et al. , 1997. Caenorhabditis elegans levamisole resistance genes lev-1, unc-29, and unc-38 encode functional nicotinic acetylcholine receptor subunits. J. Neurosci. 17: 5843–5857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransen E., Van Camp G., D’Hooge R., Vits L., Willems P. J., 1998. Genotype-phenotype correlation in L1 associated diseases. J. Med. Genet. 35: 399–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgiev P., Okkenhaug H., Drews A., Wright D., Lambert S., et al. , 2010. TRPM channels mediate zinc homeostasis and cellular growth during Drosophila larval development. Cell Metab. 12: 386–397. [DOI] [PubMed] [Google Scholar]
- Gilleard J. S., Barry J. D., Johnstone I. L., 1997. cis regulatory requirements for hypodermal cell-specific expression of the Caenorhabditis elegans cuticle collagen gene dpy-7. Mol. Cell. Biol. 17: 2301–2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godenschwege T. A., Kristiansen L. V., Uthaman S. B., Hortsch M., Murphey R. K., 2006. A conserved role for Drosophila Neuroglian and human L1-CAM in central-synapse formation. Curr. Biol. 16: 12–23. [DOI] [PubMed] [Google Scholar]
- Gracheva E. O., Hadwiger G., Nonet M. L., Richmond J. E., 2008. Direct interactions between C. elegans RAB-3 and Rim provide a mechanism to target vesicles to the presynaptic density. Neurosci. Lett. 444: 137–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grana T. M., Cox E. A., Lynch A. M., Hardin J., 2010. SAX-7/L1CAM and HMR-1/cadherin function redundantly in blastomere compaction and non-muscle myosin accumulation during Caenorhabditis elegans gastrulation. Dev. Biol. 344: 731–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallam S. J., Jin Y., 1998. lin-14 regulates the timing of synaptic remodelling in Caenorhabditis elegans. Nature 395: 78–82. [DOI] [PubMed] [Google Scholar]
- Harris T. W., Hartwieg E., Horvitz H. R., Jorgensen E. M., 2000. Mutations in synaptojanin disrupt synaptic vesicle recycling. J. Cell Biol. 150: 589–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson S. R., Guner Y. S., Woo R., Randolph L. M., Ford H., et al. , 2009. L1CAM mutation in association with X-linked hydrocephalus and Hirschsprung’s disease. Pediatr. Surg. Int. 25: 823–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jouet M., Moncla A., Paterson J., McKeown C., Fryer A., et al. , 1995. New domains of neural cell-adhesion molecule L1 implicated in X-linked hydrocephalus and MASA syndrome. Am. J. Hum. Genet. 56: 1304–1314. [PMC free article] [PubMed] [Google Scholar]
- Kohn R. E., Duerr J. S., McManus J. R., Duke A., Rakow T. L., et al. , 2000. Expression of multiple UNC-13 proteins in the Caenorhabditis elegans nervous system. Mol. Biol. Cell 11: 3441–3452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lansman J. B., Hess P., Tsien R. W., 1986. Blockade of current through single calcium channels by Cd2+, Mg2+, and Ca2+. Voltage and concentration dependence of calcium entry into the pore. J. Gen. Physiol. 88: 321–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laver D. R., 2007. Ca2+ stores regulate ryanodine receptor Ca2+ release channels via luminal and cytosolic Ca2+ sites. Clin. Exp. Pharmacol. Physiol. 34: 889–896. [DOI] [PubMed] [Google Scholar]
- Leshchyns’ka I., Sytnyk V., Richter M., Andreyeva A., Puchkov D., et al. , 2006. The adhesion molecule CHL1 regulates uncoating of clathrin-coated synaptic vesicles. Neuron 52: 1011–1025. [DOI] [PubMed] [Google Scholar]
- Lewis J. A., Wu C. H., Levine J. H., Berg H., 1980. Levamisole-resistant mutants of the nematode Caenorhabditis elegans appear to lack pharmacological acetylcholine receptors. Neuroscience 5: 967–989. [DOI] [PubMed] [Google Scholar]
- Lickteig K. M., Duerr J. S., Frisby D. L., Hall D. H., Rand J. B., et al. , 2001. Regulation of neurotransmitter vesicles by the homeodomain protein UNC-4 and its transcriptional corepressor UNC-37/groucho in Caenorhabditis elegans cholinergic motor neurons. J. Neurosci. 21: 2001–2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liljelund P., Ghosh P., van den Pol A. N., 1994. Expression of the neural axon adhesion molecule L1 in the developing and adult rat brain. J. Biol. Chem. 269: 32886–32895. [PubMed] [Google Scholar]
- McIntire S. L., Reimer R. J., Schuske K., Edwards R. H., Jorgensen E. M., 1997. Identification and characterization of the vesicular GABA transporter. Nature 389: 870–876. [DOI] [PubMed] [Google Scholar]
- Mello C. C., Kramer J. M., Stinchcomb D., Ambros V., 1991. Efficient gene transfer in C. elegans: extrachromosomal maintenance and integration of transforming sequences. EMBO J. 10: 3959–3970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller K. G., Alfonso A., Nguyen M., Crowell J. A., Johnson C. D., et al. , 1996. A genetic selection for Caenorhabditis elegans synaptic transmission mutants. Proc. Natl. Acad. Sci. USA 93: 12593–12598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteilh-Zoller M. K., Hermosura M. C., Nadler M. J., Scharenberg A. M., Penner R., et al. , 2003. TRPM7 provides an ion channel mechanism for cellular entry of trace metal ions. J. Gen. Physiol. 121: 49–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mordes J. P., Wacker W. E., 1977. Excess magnesium. Pharmacol. Rev. 29: 273–300. [PubMed] [Google Scholar]
- Nguyen M., Alfonso A., Johnson C. D., Rand J. B., 1995. Caenorhabditis elegans mutants resistant to inhibitors of acetylcholinesterase. Genetics 140: 527–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikonenko A. G., Sun M., Lepsveridze E., Apostolova I., Petrova I., et al. , 2006. Enhanced perisomatic inhibition and impaired long-term potentiation in the CA1 region of juvenile CHL1-deficient mice. Eur. J. Neurosci. 23: 1839–1852. [DOI] [PubMed] [Google Scholar]
- Nilius B., Owsianik G., 2011. The transient receptor potential family of ion channels. Genome Biol. 12: 218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonet M. L., Staunton J. E., Kilgard M. P., Fergestad T., Hartwieg E., et al. , 1997. Caenorhabditis elegans rab-3 mutant synapses exhibit impaired function and are partially depleted of vesicles. J. Neurosci. 17: 8061–8073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto N., Wada Y., Goto M., 1997. Hydrocephalus and Hirschsprung’s disease in a patient with a mutation of L1CAM. J. Med. Genet. 34: 670–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okkema P. G., Harrison S. W., Plunger V., Aryana A., Fire A., 1993. Sequence requirements for myosin gene expression and regulation in Caenorhabditis elegans. Genetics 135: 385–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parisi M. A., Kapur R. P., Neilson I., Hofstra R. M., Holloway L. W., et al. , 2002. Hydrocephalus and intestinal aganglionosis: Is L1CAM a modifier gene in Hirschsprung disease? Am. J. Med. Genet. 108: 51–56. [DOI] [PubMed] [Google Scholar]
- Rand J. B., Russell R. L., 1984. Choline acetyltransferase-deficient mutants of the nematode Caenorhabditis elegans. Genetics 106: 227–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saifee O., Wei L., Nonet M. L., 1998. The Caenorhabditis elegans unc-64 locus encodes a syntaxin that interacts genetically with synaptobrevin. Mol. Biol. Cell 9: 1235–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai T., 2012. The role of NrCAM in neural development and disorders–beyond a simple glue in the brain. Mol. Cell. Neurosci. 49: 351–363. [DOI] [PubMed] [Google Scholar]
- Salzberg Y., Diaz-Balzac C. A., Ramirez-Suarez N. J., Attreed M., Tecle E., et al. , 2013. Skin-derived cues control arborization of sensory dendrites in Caenorhabditis elegans. Cell 155: 308–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasakura H., Inada H., Kuhara A., Fusaoka E., Takemoto D., et al. , 2005. Maintenance of neuronal positions in organized ganglia by SAX-7, a Caenorhabditis elegans homologue of L1. EMBO J. 24: 1477–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrander-Stumpel C., Howeler C., Jones M., Sommer A., Stevens C., et al. , 1995. Spectrum of X-linked hydrocephalus (HSAS), MASA syndrome, and complicated spastic paraplegia (SPG1): clinical review with six additional families. Am. J. Med. Genet. 57: 107–116. [DOI] [PubMed] [Google Scholar]
- Sieburth D., Ch’ng Q., Dybbs M., Tavazoie M., Kennedy S., et al. , 2005. Systematic analysis of genes required for synapse structure and function. Nature 436: 510–517. [DOI] [PubMed] [Google Scholar]
- Sonna L. A., Hirshman C. A., Croxton T. L., 1996. Role of calcium channel blockade in relaxation of tracheal smooth muscle by extracellular Mg2+. Am. J. Physiol. 271: L251–L257. [DOI] [PubMed] [Google Scholar]
- St. Johnston D., 2002. The art and design of genetic screens: Drosophila melanogaster. Nat. Rev. Genet. 3: 176–188. [DOI] [PubMed] [Google Scholar]
- Stawicki T. M., Zhou K., Yochem J., Chen L., Jin Y., 2011. TRPM channels modulate epileptic-like convulsions via systemic ion homeostasis. Curr. Biol. 21: 883–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulston J. E., Schierenberg E., White J. G., Thomson J. N., 1983. The embryonic cell lineage of the nematode Caenorhabditis elegans. Dev. Biol. 100: 64–119. [DOI] [PubMed] [Google Scholar]
- Sundaram M. V., 2013. Canonical RTK-Ras-ERK signaling and related alternative pathways (July 1, 2013), WormBook, ed. The C. elegans Research Community, WormBook, /10.1895/wormbook.1.80.2, http://www.wormbook.org. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki N., Buechner M., Nishiwaki K., Hall D. H., Nakanishi H., et al. , 2001. A putative GDP-GTP exchange factor is required for development of the excretory cell in Caenorhabditis elegans. EMBO Rep. 2: 530–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takenouchi T., Nakazawa M., Kanemura Y., Shimozato S., Yamasaki M., et al. , 2012. Hydrocephalus with Hirschsprung disease: severe end of X-linked hydrocephalus spectrum. Am. J. Med. Genet. A 158A: 812–815. [DOI] [PubMed] [Google Scholar]
- Teramoto T., Sternick L. A., Kage-Nakadai E., Sajjadi S., Siembida J., et al. , 2010. Magnesium excretion in C. elegans requires the activity of the GTL-2 TRPM channel. PLoS ONE 5: e9589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topf J. M., Murray P. T., 2003. Hypomagnesemia and hypermagnesemia. Rev. Endocr. Metab. Disord. 4: 195–206. [DOI] [PubMed] [Google Scholar]
- Triana-Baltzer G. B., Liu Z., Berg D. K., 2006. Pre- and postsynaptic actions of L1-CAM in nicotinic pathways. Mol. Cell. Neurosci. 33: 214–226. [DOI] [PubMed] [Google Scholar]
- Triana-Baltzer G. B., Liu Z., Gounko N. V., Berg D. K., 2008. Multiple cell adhesion molecules shaping a complex nicotinic synapse on neurons. Mol. Cell. Neurosci. 39: 74–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troemel E. R., Chou J. H., Dwyer N. D., Colbert H. A., Bargmann C. I., 1995. Divergent seven transmembrane receptors are candidate chemosensory receptors in C. elegans. Cell 83: 207–218. [DOI] [PubMed] [Google Scholar]
- Turner K. N., Schachner M., Anderson R. B., 2009. Cell adhesion molecule L1 affects the rate of differentiation of enteric neurons in the developing gut. Dev. Dyn. 238: 708–715. [DOI] [PubMed] [Google Scholar]
- Venkatachalam K., Montell C., 2007. TRP channels. Annu. Rev. Biochem. 76: 387–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace A. S., Schmidt C., Schachner M., Wegner M., Anderson R. B., 2010. L1cam acts as a modifier gene during enteric nervous system development. Neurobiol. Dis. 40: 622–633. [DOI] [PubMed] [Google Scholar]
- Wallace A. S., Tan M. X., Schachner M., Anderson R. B., 2011. L1cam acts as a modifier gene for members of the endothelin signalling pathway during enteric nervous system development. Neurogastroenterol. Motil. 23: e510–e522. [DOI] [PubMed] [Google Scholar]
- Wang B., Williams H., Du J. S., Terrett J., Kenwrick S., 1998. Alternative splicing of human NrCAM in neural and nonneural tissues. Mol. Cell. Neurosci. 10: 287–295. [DOI] [PubMed] [Google Scholar]
- Wang M., Berlin J. R., 2007. Voltage-dependent modulation of L-type calcium currents by intracellular magnesium in rat ventricular myocytes. Arch. Biochem. Biophys. 458: 65–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M., Tashiro M., Berlin J. R., 2004. Regulation of L-type calcium current by intracellular magnesium in rat cardiac myocytes. J. Physiol. 555: 383–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Kweon J., Larson S., Chen L., 2005. A role for the C. elegans L1CAM homologue lad-1/sax-7 in maintaining tissue attachment. Dev. Biol. 284: 273–291. [DOI] [PubMed] [Google Scholar]
- Yochem J., Gu T., Han M., 1998. A new marker for mosaic analysis in Caenorhabditis elegans indicates a fusion between hyp6 and hyp7, two major components of the hypodermis. Genetics 149: 1323–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yook K. J., Proulx S. R., Jorgensen E. M., 2001. Rules of nonallelic noncomplementation at the synapse in Caenorhabditis elegans. Genetics 158: 209–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zallen J. A., Kirch S. A., Bargmann C. I., 1999. Genes required for axon pathfinding and extension in the C. elegans nerve ring. Development 126: 3679–3692. [DOI] [PubMed] [Google Scholar]
- Zhou S., Chen L., 2011. Neural integrity is maintained by dystrophin in C. elegans. J. Cell Biol. 192: 349–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou S., Opperman K., Wang X., Chen L., 2008. unc-44 Ankyrin and stn-2 gamma-syntrophin regulate sax-7 L1CAM function in maintaining neuronal positioning in Caenorhabditis elegans. Genetics 180: 1429–1443. [DOI] [PMC free article] [PubMed] [Google Scholar]