Abstract
A common feature of many neurodegenerative diseases is the accumulation of toxic proteins that disrupt vital cellular functions. Degradative pathways such as autophagy play an important protective role in breaking down misfolded and long-lived proteins. Neurons are particularly vulnerable to defects in these pathways, but many of the details regarding the link between autophagy and neurodegeneration remain unclear. We previously found that temperature-sensitive paralytic mutants in Drosophila are enriched for those exhibiting age-dependent neurodegeneration. Here we show that one of these mutants, comatose (comt), in addition to locomotor defects, displays shortened lifespan and progressive neurodegeneration, including loss of dopaminerigic (DA) neurons. comt encodes N-ethyl-maleimide sensitive fusion protein (NSF1), which has a well-documented role in synaptic transmission. However, the neurodegenerative phenotypes we observe in comt mutants do not appear to depend on defects in synaptic transmission, but rather from their inability to sustain autophagy under stress, due at least in part to a defect in trafficking of lysosomal proteases such as cathepsin-L. Conversely, overexpression of NSF1 rescues α-synuclein-induced toxicity of DA neurons in a model of Parkinson’s disease. Our results demonstrate a neuroprotective role for NSF1 that involves mediation of fusion events crucial for degradative pathways such as autophagy, providing greater understanding of cellular dysfunctions common to several neurodegenerative diseases.
Keywords: comatose, neurodegeneration, autophagy
RECENT studies have demonstrated the utility of Drosophila models of neurodegeneration in aiding our understanding of a number of neurodegenerative diseases. Specifically, expression of human proteins implicated in diseases including Alzheimer’s disease (Ye and Fortini 1999; Wittmann et al. 2001), Parkinson’s disease (Feany and Bender 2000; Auluck et al. 2002), and polyglutamine expansion disorders (Jackson et al. 1998; Warrick et al. 1998), among others has proven to be a powerful approach for uncovering the mechanisms underlying disease progression.
An alternative strategy to understanding neurodegeneration involves a forward genetics approach, by screening for mutations that result in progressive neurodegeneration to uncover neuroprotective mechanisms that may have further implications for human neurodegenerative diseases. We previously reported a collection of temperature-sensitive (ts) paralytic mutants that is enriched for mutants showing progressive, age-dependent neurodegeneration (Palladino et al. 2002). We have found that other locomotor mutants in our collection also exhibit neurodegeneration. Analysis of these mutants has uncovered several novel neuroprotective functions, ranging from regulation of metabolic processes to regulation of the innate immune response (Palladino et al. 2003; Gnerer et al. 2006; Miller et al. 2012; Cao et al. 2013).
Here we show a neuroprotective role for NSF1 using ts comatose (comt) mutants, which were originally identified in a screen for ts-paralytic mutants (Siddiqi and Benzer 1976). comt encodes N-ethyl-maleimide sensitive fusion protein (NSF1), a AAA-ATPase with a well-characterized role in regulation of membrane fusion events (Whiteheart et al. 1994; Whiteheart et al. 2001), including synaptic transmission (Pallanck et al. 1995; Chen and Scheller 2001). Specifically, NSF1 is involved in the disassembly of SNARE complexes following synaptic vesicle fusion, a process crucial to maintaining the readily releasable pool of synaptic vesicles for subsequent rounds of fusion (Littleton et al. 1998, 2001; Tolar and Pallanck 1998; Sanyal et al. 2001; Hanson and Whiteheart 2005).
Despite its role in synaptic transmission, we find that the neuroprotective function of NSF1 appears to operate via a distinct mechanism. Specifically, we find that NSF1 is required to maintain autophagy during periods of stress. Autophagy is a degradative process whereby misfolded proteins, aggregates, organelles, and other cellular materials are engulfed by autophagosomes, which then fuse with lysosomes where the contents are degraded and recycled (Klionsky 2007).
Although previous studies have revealed a link between perturbations in autophagy and neurodegeneration in both mice and humans (Hara et al. 2006; Komatsu et al. 2006; McCray and Taylor 2008), NSF has not previously been implicated in any neurodegenerative disorder. However, recent evidence demonstrates that SNARE proteins such as neuronal synaptobrevin (nSyb) have important neuroprotective functions in addition to mediating synaptic transmission (Haberman et al. 2012). Nonetheless, the question of whether recycling of SNARE complexes following fusion and its dependence on NSF1 is as important for these neuroprotective functions as for rapid transmitter release in synaptic transmission has not previously been addressed. By investigating the neuroprotective role of NSF1 in Drosophila and determining which particular mechanisms are particularly sensitive to proper NSF1 function, we have further elucidated key steps required for maintenance of neuronal integrity that may ultimately fail in some neurodegenerative diseases.
Materials and Methods
Fly stocks
Canton-S was used as a wild-type control. The temperature-sensitive comatose alleles comtTP7 (provided by K. S. Krishnan, Tata Institute, Bangalore, India), comtST17, and comtST53 (Siddiqi and Benzer 1976) were previously described. UAS-lamp1-GFP (Pulipparacharuvil et al. 2005) was provided by Helmut Kramer (University of Texas Southwestern Medical Center, Dallas) and UAS-mCherry-GFP-Atg8a (Filimonenko et al. 2007) was provided by Thomas Neufeld (University of Minnesota, Minneapolis). snapI65 (Babcock et al. 2004) and UAS-NSF1 (Golby et al. 2001) were provided by Leo Pallanck (University of Washington, Seattle). seiTS2 (Jackson et al. 1985) was previously described. The following fly lines were obtained from the Bloomington Stock Center: elavC155-Gal4, Cg-Gal4, TH-Gal4, UAS-αSyn, UAS-Syx17RNAI, UAS-ubisnapRNAi, UAS-VAMP7RNAi, and UAS-Atg5RNAi. UAS-comtRNAi (no. 105552) was obtained from the Vienna Drosophila RNAi Center.
Histology
Newly eclosed flies were shifted to 29° for up to 48 hr. Heads were severed and placed in fresh Carnoy’s fixative overnight at 4°. Samples were then washed in 70% ethanol and embedded in paraffin using standard histological procedures. The 5-μm frontal sections were cut, stained in hematoxylin and eosin, and imaged under a light microscope. Vacuoles of at least 2 μm in diameter were recorded for each sample. To avoid potential artifacts, vacuoles were only recorded if they appeared in at least two consecutive sections.
Lifespan analysis
Flies were collected shortly after eclosion and cohorts of 10 flies were placed in single vials. A total of 10 vials (100 flies) were used for each genotype. Flies shifted to 29° were transferred to fresh food every day and the percentage of surviving flies was measured. Flies remaining at room temperature (22°) were transferred and measured every 2 days.
Climbing assay
Newly emerged flies were collected and placed into cohorts of 10 flies per vial. A total of 10 cohorts of flies were assessed for each genotype. Climbing ability was assessed by transferring a cohort of flies into an empty climbing cylinder (diameter, 2.5 cm; height, 20 cm). For each trial, flies were tapped to the bottom of the vial, and the percentage of flies passing an 8-cm mark within 20 sec was recorded. This percentage of flies was recorded as the climbing index. Each cohort of flies was assessed across five trials, with a 1-min recovery between trials.
Lysotracker staining
Lysotracker staining was performed as previously described (Wong et al. 2012). Briefly, larval fat bodies were dissected in cold PBS and immediately transferred to a solution of LysoTracker Red DND-99 (Invitrogen) (1:500) for 45 min at room temperature. Samples were washed four times in PBS and fixed in 4% formaldehyde in PBS for 20 min at room temperature. Samples were washed four times in PBS and mounted in Vectashield (Vector Laboratories) along with DAPI (1:1000.) The size of LysoTracker-positive puncta was measured using ImageJ software (National Institutes of Health, NIH).
Immunohistochemistry
Brains and fat body samples were dissected and fixed in 4% formaldehyde in PBS for 20 min at room temperature. Samples were then placed in blocking buffer (PBS with 0.1% Triton X-100 and 0.1% normal goat serum) for 1 hr. Samples were then incubated in primary antibodies overnight at 4°. Next, samples were washed five times in PBS, then incubated in secondary antibodies for 2 hr at room temperature. Finally, samples were washed five times in PBS and mounted in Vectashield. Primary antibodies used were: rabbit anti-tyrosine hydroxylase (1:100) (Millipore), mouse anti-GFP (1:500) (Invitrogen), rabbit anti-DsRed (1:500) (Clontech Laboratories), and mouse anti-bruchpilot (nc82) (1:50) (Developmental Studies Hybridoma Bank, University of Iowa). Secondary antibodies used were: goat anti-rabbit Alexa-568, goat anti-mouse Alexa-488, and goat anti-rabbit Alexa-488 (1:200) (Invitrogen).
Imaging and quantification
Images were obtained on a Leica LSM 500 confocal microscope. Serial 1-μm z-stacks were obtained for each image using a ×20 numerical aperture (N.A.) 10.8 or ×63 N.A. 1.4 oil objective. Brightness and contrast were adjusted using ImageJ software (NIH) and Adobe Photoshop CS5 (Adobe).
Dopaminergic neurons were counted as previously described (Whitworth et al. 2005; Barone and Bohmann 2013). Briefly, dissected brains were mounted between two glass coverslips to aid in visualization of PPL1 neurons. The number of neurons in each cluster was assessed with the scorer blind to the genotype and condition of each sample.
Starvation
Flies were collected shortly after eclosion and placed in groups of 10 in individual vials. Ten vials (100 flies) were used per genotype. Flies were raised on standard fly media for 2 days, and then transferred to empty vials lined with water-soaked filter paper to prevent desiccation. Flies were transferred to fresh vials every 8 hr, and the percentage of flies surviving was recorded. For the larval starvation, early third-instar larvae were collected and placed in a 20% sucrose solution for 3 or 12 hr.
Western blotting
Two adult flies of each genotype were homogenized using 20 μl of homogenization buffer (100 mM Tris-HCl, pH 6.8, 2% SDS). The homogenate was then boiled for 10 min, and centrifuged at 21,000 × g for 10 min to remove debris. A total of 5 μl of each sample was run on a 10% acrylamide gel. Western blots were probed with mouse anti-cathepsin-L at 1:5000 (R&D Biosystems) and goat anti-actin at 1:1000 (Santa Cruz Biotechnology). Protein levels were quantified using an Odyssey Imaging System (Li-Cor) by normalizing band intensities to the level of actin. Experiments were repeated at least three times.
Statistical analysis
Significance for lifespan and starvation survival curves was analyzed using the log-rank test. Climbing behavior and number of dopaminergic neurons were analyzed using the Student’s t-test, with Bonferroni corrections for multiple comparisons when applicable. Statistical analysis was computed using SPSS software (IBM Corporation).
Results
comt mutants exhibit shortened lifespan, locomotor defects, and neurodegeneration
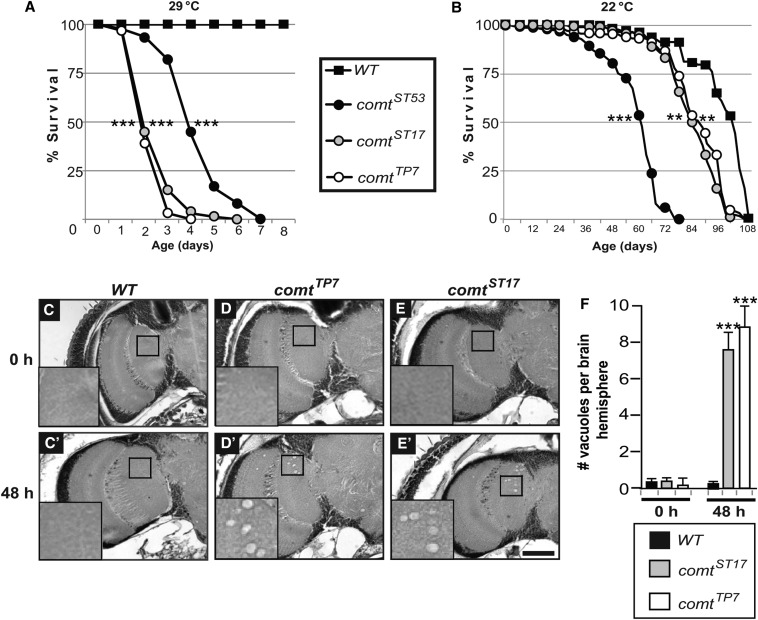
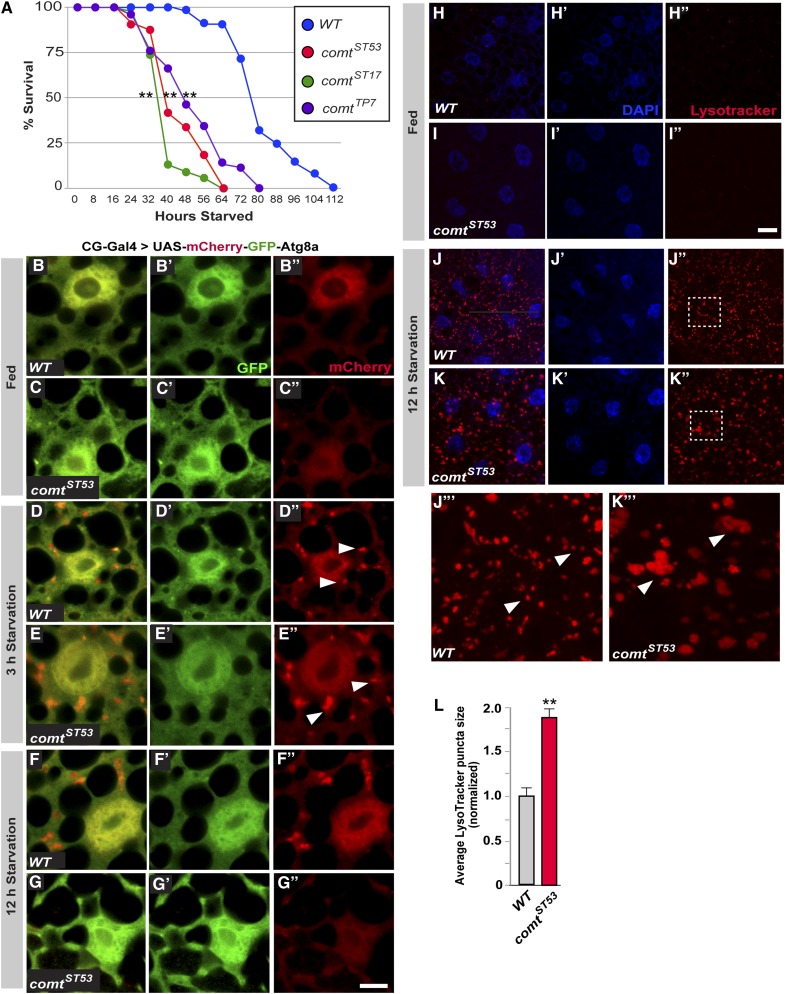
comt is a recessive X-linked ts-paralytic mutant with a well-documented defect in synaptic transmission at temperatures above 38° (Littleton et al. 1998, 2001; Tolar and Pallanck 1998). Although comt flies are not paralyzed below 38°, we found that lifespan was greatly reduced compared with wild-type controls for flies shifted to 29° after eclosion. Three different hypomorphic alleles of comt had severely reduced lifespans, with median lifespans ranging from 2 to 4 days, compared with >30 days for controls (Figure 1A). When raised at room temperature (22°), comt mutants also displayed shortened lifespan compared with controls, but the phenotype was much milder (Figure 1B). These results are consistent with the idea that comt mutants have an underlying unconditional impairment other than a synaptic transmission defect, with the consequences of this impairment becoming more severe under the stress of elevated temperature. In parallel with the shortened lifespan, we also observed progressive neurodegeneration in comt mutant brains. This neurodegeneration was assessed in histological sections as vacuolar pathology in the neuropil after 48-hr exposure to 29° (Figure 1, C–F).
Figure 1.
comatose mutants exhibit shortened lifespan and neurodegeneration. (A and B) Lifespan of comt mutants at 29° (A) and at room temperature (B). (C–E) Histological frontal sections of control (C), and comt (D and E) mutant brains before or after 48-hr exposure to 29°. Neurodegeneration is characterized by vacuolar lesions in the neuropil. Insets in C–E are enlarged images of boxed area. (F) Average number of vacuoles per brain hemisphere for each genotype. Bar in E′, 50 μm. (**P < 0.01 and ***P < 0.001, log-rank test.)
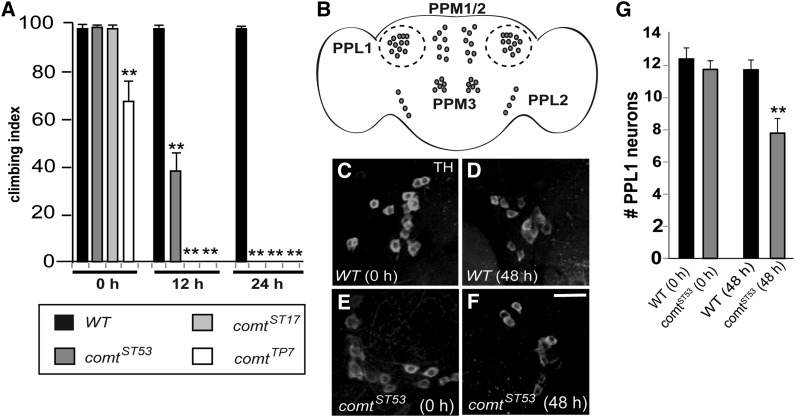
Along with neurodegeneration, comt mutants also displayed a marked decrease in climbing ability (Figure 2A). All comt alleles tested showed significantly reduced climbing as early as 12 hr after shifting to 29°, with a complete lack of climbing ability by 24 hr, even though the restrictive temperature for paralysis is about 38°. The most severe allele, comtTP7, had a moderate climbing defect even before shifting to 29°, again indicating an underlying mutant defect that is independent of temperature.
Figure 2.
Climbing defects and loss of dopaminergic neurons in comatose mutants. (A) Climbing index for controls and comt mutants after shifting to 29°. Climbing index is assessed as the percentage of flies able to climb at least 8 cm within 20 sec. (B) Diagram of the Drosophila adult brain, with major posterior clusters of dopaminergic neurons labeled. Protocerebral posterial lateral 1 (PPL1) clusters are outlined. (C–F) PPL1 clusters of dopaminergic neurons in both control (C and D) and comtST53 (E and F) brains both before or after shifting to 29°. Dopaminergic neurons are stained with anti-tyrosine hydroxylase. Bar in F, 10 μm. (G) Average number of dopaminergic neurons in each PPL1 cluster in control and comt brains. (*P < 0.01 and **P < 0.001, Student’s t-test with Bonferroni correction for multiple comparisons.)
To investigate neurodegeneration in comt mutants in more detail, we asked whether specific populations of neurons were preferentially lost. Because dopaminergic (DA) neuron loss is strongly correlated with locomotor dysfunction, including impaired climbing ability in Drosophila (Feany and Bender 2000), we first tested whether DA neurons are among those lost in comt mutants. DA neurons are found in distinct clusters throughout the central brain of Drosophila. Several of these clusters, particularly on the posterior side of the brain, contain relatively small numbers of neurons, making them amenable to counting (Figure 2B). We found that several DA neurons in the protocerebral posterial lateral 1 (PPL1) cluster were lost in comt mutant brains after 48 hr at 29° (Figure 2, C–G). Thus, we conclude that NSF1 function is required for neuronal viability, particularly under stressful conditions such as elevated temperature.
The neuroprotective role of NSF1 is distinct from its role in synaptic transmission
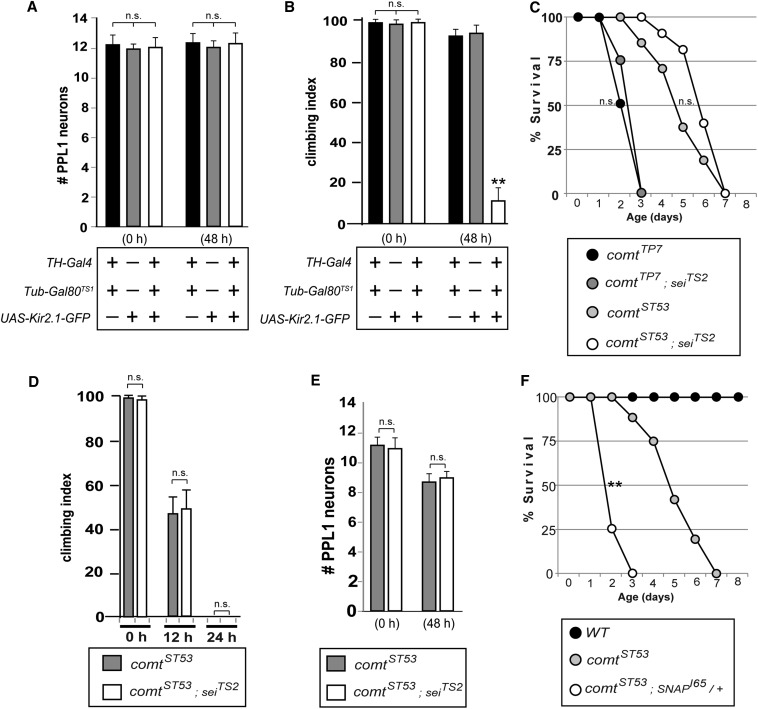
As mentioned previously, NSF1 has a well-characterized role in synaptic transmission. Although synaptic transmission is blocked in photoreceptors at 38° in comt mutants (Littleton et al. 1998, 2001), without direct electrophysiological recordings from the synaptic partners of DA neurons, it is not possible to determine whether synaptic transmission is completely normal in these cells at the 29° incubation temperature used in the experiments above. Thus, to test whether a defect in synaptic transmission can cause loss of DA neurons, we hyperpolarized DA neurons by expressing the inward-rectifying potassium channel, Kir2.1 (Baines et al. 2001) using the TH-Gal4 driver, which should block synaptic transmission in these cells. Because TH-Gal4 also drives expression in non-neuronal cells required for embryonic development (Friggi-Grelin et al. 2003), we repressed Gal4 expression until the adult stage by using tubulin-Gal80ts1 (McGuire et al. 2003) and raising the flies at 18° until eclosion, after which flies were shifted to 29° to inactivate Gal80. We found that expression of UAS-Kir2.1 had no effect on the viability of DA neurons (Figure 3A), although it did disrupt climbing ability (Figure 3B). This result suggests that a defect in synaptic transmission alone in DA neurons is capable of disrupting signaling of these neurons, but is not sufficient to cause their loss.
Figure 3.
Neuroprotective role of NSF1 that is distinct from its role in synaptic transmission. (A and B) Average number of dopaminergic neurons in each PPL1 cluster (A) and climbing ability (B) for flies expressing UAS-Kir2.1-GFP in dopaminergic neurons using the TH-Gal4 driver. (**P < 0.01, Student’s t-test with Bonferroni correction for multiple comparisons.) (C–E) Lifespan (C), climbing ability (D), and loss of dopaminergic neurons (E) in comt mutants alone, compared with comt; seiTS2 double mutants when shifted to 29°. (F) Lifespan of controls, comt mutants, and comt mutants heterozygous for a SNAP mutation when shifted to 29°. (**P < 0.01, log-rank test.)
Additionally, if neurodegeneration and shortened lifespan in comt were due to impaired recycling of SNARE complexes involved in synaptic transmission, we would expect the phenotype to be exacerbated in combination with hyperexcitability mutants, as the pool of fusion-competent vesicles would be depleted more rapidly. However, we found that comt; seits2 double mutants (Jackson et al. 1985; Elkins and Ganetzky 1990; Titus et al. 1997; Wang et al. 1997) were no more severe than comt mutants alone when measuring lifespan, locomotor defects, or loss of DA neurons (Figure 3, C–E).
Although we cannot completely rule out the possibility of some synaptic defect in comt mutants at 29° that contributes to neurodegeneration, our results are consistent with previous data showing that selectively blocking dopamine synthesis in DA neurons, which would effectively eliminate all synaptic transmission in these cells, causes no apparent deficit in their viability (Riemensperger et al. 2011). These results are also consistent with a recent study demonstrating that loss of nSyb in Drosophila photoreceptors causes degeneration that is independent of synaptic transmission defects (Haberman et al. 2012). Together, these data suggest that the neurodegeneration observed in comt mutants is likely to involve a mechanism other than synaptic transmission.
We also tested whether known binding partners of NSF1 modify the shortened lifespan and neurodegeneration phenotypes we observed. One such protein is α-SNAP (Clary and Rothman 1990), which serves as an adaptor between NSF1 and various SNARES. We found that the shortened lifespan of comt mutants is dominantly enhanced by SNAPI65 (Babcock et al. 2004), which significantly lowered the median lifespan (Figure 3F). This observation suggests that the neuroprotective role of NSF1 is still likely to involve SNARE-mediated processes.
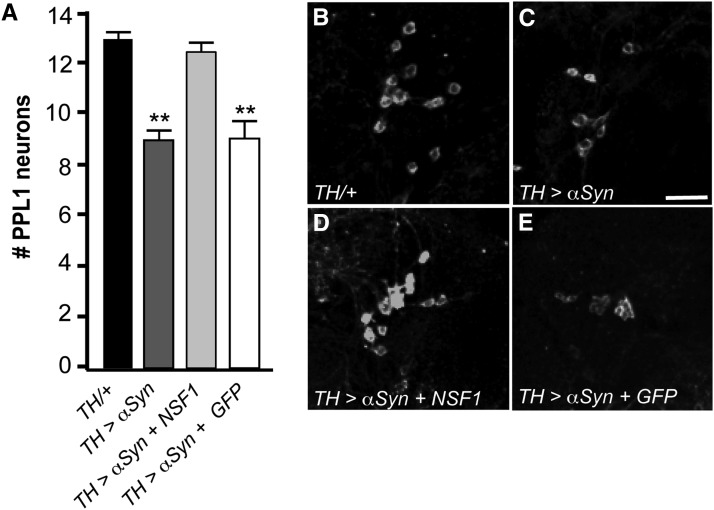
Because comt mutants affect viability of DA neurons, which also undergo degeneration in Parkinson’s disease models, we tested whether the neuroprotective role of NSF1 intersects with one of these models. Expression of human α-synuclein in DA neurons results in progressive neurodegeneration (Auluck et al. 2002). This neurodegeneration is rescued by coexpression of heat-shock protein 70 (HSP70), which belongs to the AAA family of ATPases and is involved in protein quality control mechanisms. Since NSF1 is a member of the same family of ATPases, we tested whether NSF1 could also rescue α-synuclein-mediated loss of DA neurons. Consistent with previous studies, we found that expression of α-synuclein in DA neurons resulted in neurodegeneration by 20 days (Figure 4, A–C). This phenotype was rescued when UAS-NSF1 (Golby et al. 2001) was coexpressed in the same population of neurons (Figure 4D). To rule out the possibility that this suppression is due to the presence of multiple UAS-linked transgenes that titrate Gal4 levels and limit α-synuclein expression, we also coexpressed α-synuclein with UAS-GFP. In this case, the loss of DA neurons was identical to expression of α-synuclein alone (Figure 4E), supporting the hypothesis that it is the expression of UAS-NSF1 that alleviates α-synuclein-induced toxicity.
Figure 4.
NSF1 is protective against α-synuclein-mediated toxicity of dopaminergic neurons. (A) Average number of dopaminergic neurons in each PPL1 cluster for 20-day-old flies raised at 25° expressing α-synuclein in dopaminergic neurons alone, or coexpressed with NSF1 or GFP using TH-Gal4. (B–E) Representative images of PPL1 clusters for each genotype. Dopaminergic neurons are stained with anti-tyrosine hydroxylase. Bar in E, 10 μm. (**P < 0.01, Student’s t-test with Bonferroni correction for multiple comparisons.)
comt mutants cannot sustain autophagy
Although the neuroprotective function of NSF1 seems to be independent of its role in synaptic transmission, there are many other significant cellular functions requiring the recycling of SNARE complexes. For example, previous studies in yeast found that the NSF homolog Sec18p was required for fusion of autophagosomes to the vacuole (Ishihara et al. 2001), a critical step in autophagy. Because autophagy has been associated with neurodegeneration, we investigated the possibility that this process is impaired in comt mutants.
If comt mutants were defective in autophagy, we hypothesized that they would exhibit many of the same phenotypes as mutants known to play key roles in the autophagy pathway. Indeed, shortened lifespan and neurodegeneration have been observed in autophagy-defective (Atg) mutants in Drosophila (Juhasz et al. 2007; Simonsen et al. 2008). Another common phenotype among autophagy mutants is an increased sensitivity to starvation (Scott et al. 2004; Juhasz et al. 2007), due to the inability to break down stored nutrients. To examine if comt mutants are more sensitive to starvation, 3-day-old comt mutant flies were placed in empty vials with only water-soaked filter paper to prevent desiccation. Whereas control flies had a median lifespan of ∼80 hr under starvation conditions, comt mutants died much sooner, with median lifespans ranging from 40 to 48 hr (Figure 5A). These data are consistent with a defect in autophagy in comt mutants.
Figure 5.
Autophagy is defective in comatose mutants. (A) Lifespan analysis of comt mutant flies and controls during prolonged starvation at room temperature (22°). (B–G) Images of fat body in well-fed larvae (B and C) and after starvation for 3 hr (D and E) or 12 hr (F and G). Bar in G, 10 μm. (H–K) LysoTracker (red) and DAPI (blue) staining of fat body in well-fed larvae (H and I) and after 12-hr starvation (J and K). (L) Average size of LysoTracker-positive puncta for each genotype. Bar in I, 20 μm. J′′′ and K′′′ are enlarged images of boxed areas in J and K, respectively. (**P < 0.01, log-rank test.)
To assay autophagy in comt mutants more directly, we examined starvation-induced autophagy in the larval fat body (Scott et al. 2004) using a tandem-tagged Atg8a reporter (UAS-mCherry-GFP-Atg8a) containing a pH-sensitive GFP and a pH-insensitive RFP to measure autophagic flux (Filimonenko et al. 2007). Because Atg8a localizes to the autophagosome, fusion with a lysosome can be seen by the loss of GFP fluorescence, leaving RFP+ puncta. We expressed this reporter in the larval fat body in wild type and comt mutants using the CG-Gal4 driver, and found that fed larvae displayed minimal autophagic flux (Figure 5, B and C). After 3 hr of starvation, the induced autophagy response is near its peak, with several fusion events visible per cell (Figure 5, D and E, arrowheads). It is noteworthy that the initial response in comt mutants is indistinguishable from that of controls, suggesting that comt mutants are capable of normal induction of autophagy. We also monitored autophagic flux after longer periods of starvation. After 12 hr, autophagic flux in wild-type larvae is still strong. However, autophagic flux in comt mutants at this point has greatly diminished (Figure 5, F and G). In fact, we found a complete absence of autophagosomes at this point, suggesting that membrane recycling required for the formation of new autophagic compartments could be defective in comt. The fact that autophagic flux is only impaired in comt mutants following prolonged stress suggests that the defect is not with the induction of autophagy, but rather with sustaining it under periods of stress. This explanation is consistent with the role of NSF1 in synaptic transmission, where neurotransmission is normal until prolonged activity caused a depletion of recycled SNARE complexes for subsequent rounds of fusion (Littleton et al. 2001; Sanyal et al. 2001).
In addition to the tandem-tagged reporter, we also monitored autophagy using LysoTracker, a pH-sensitive dye often used to label lysosomes or autolysosomes (Berry and Baehrecke 2007; Scott et al. 2007). In fed larvae, there was minimal staining with LysoTracker both in comt mutants and in wild-type controls (Figure 5, H and I). After a 12-hr starvation period, however, robust staining could be seen in both comt mutants and control samples but with some important differences between the two genotypes (Figure 5, J and K). Notably, there appeared to be fewer, although much larger lysosomes in comt mutants (Figure 5, J–L, arrowheads). This enlargement of lysosomes is similar to that observed in cathepsin-D mutants (Khurana et al. 2010), as well as in a variety of lysosomal storage diseases (Fukuda et al. 2006). The enlarged lysosomes could result from a lack of lysosomal turnover. A defect in recycling membranes out of lysosomes is consistent with the absence of autophagosomes under similar condtions (Figure 5G). Alternatively, lysosomal enlargement could be a form of compensation that attempts to increase lysosomal capacity in response to the decrease in autophagy. These data provide further support for the idea that comt mutants are defective in the critical events necessary to sustain autophagy.
Loss of DA neurons in comt mutants is due to inability to sustain autophagy
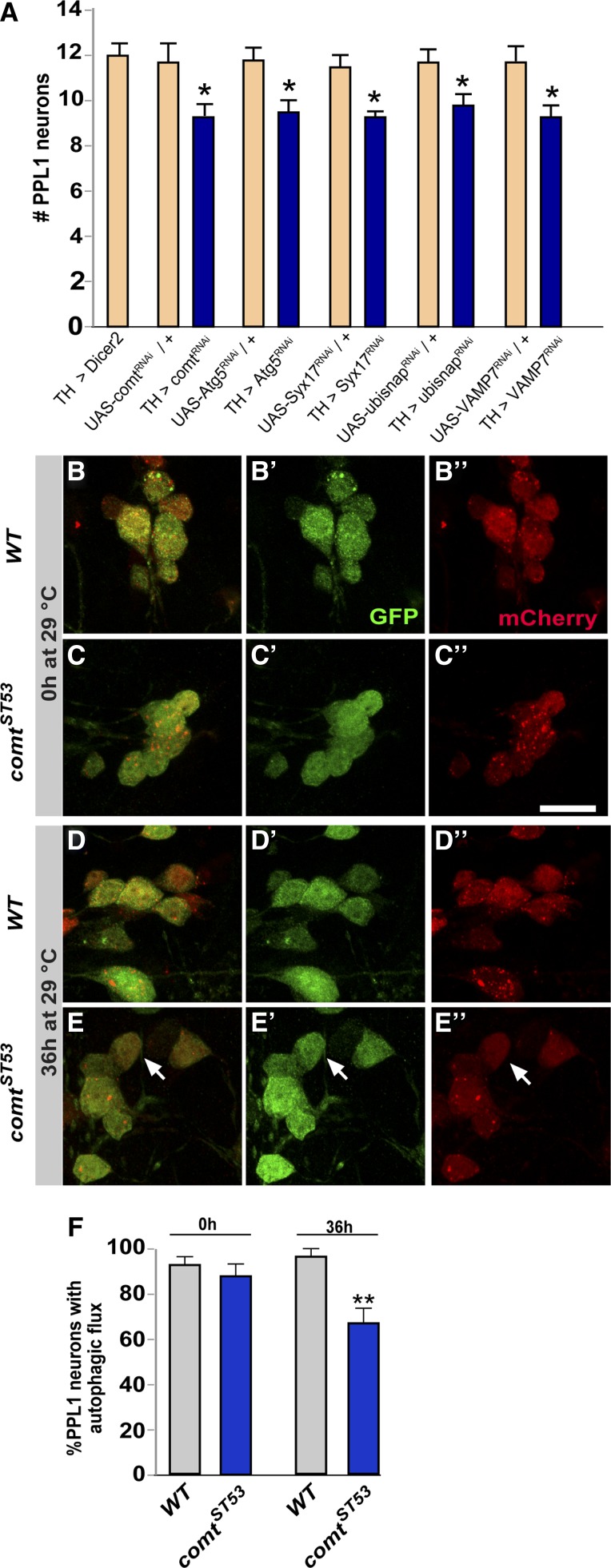
Neurons are often particularly vulnerable to defects in the ability to degrade aberrant proteins and organelles. It was thus of interest to examine the role of NSF1 and autophagy in maintaining the viability of DA neurons. We knocked down expression of NSF1 in DA neurons by expressing UAS-comtRNAi with TH-Gal4, along with a UAS-dicer2 transgene to enhance RNAi knockdown. Knockdown of NSF1 in DA neurons caused a loss of neurons after 48-hr exposure to 29° comparable to that observed in comt mutants (Figure 6A). This observation indicates that the role of NSF1 in the loss of DA neurons is cell autonomous. We found a similar loss of DA neurons when expressing UAS-Atg5RNAi, showing that a reduction in autophagy is sufficient to cause degeneration of DA neurons. We also tested knockdown of three SNARE proteins recently discovered to be required for the fusion of autophagosomes to late endosomes and lysosomes (Takats et al. 2013) for their effects on DA neuron survival. We found that knocking down syntaxin17, ubisnap, or VAMP7 using TH-Gal4 resulted in loss of DA neurons (Figure 6A), demonstrating that activity of these SNARE proteins required for autophagy is necessary to maintain viability of DA neurons.
Figure 6.
Loss of dopaminergic neurons in comatose mutants is due to a defect in autophagy. (A) Average number of dopaminergic neurons in each PPL1 cluster with knockdown of NSF1, ATG5, or SNAREs mediating autophagosome–lysosome fusion after 48 hr at 29°. UAS-Dicer2 was used to enhance RNAi knockdown. Controls were Gal4 and UAS transgenes alone. (B–E) Dopaminergic neurons expressing UAS-mCherry-GFP-Atg8a in either a wild-type or comt mutant background after 0 hr (B and C) or 36 hr (D and E) at 29°. Bar in C, 10 μm. (F) Percentage of dopaminergic neurons with at least one autophagic RFP+ punctum. (*P < 0.05, **P < 0.01, Student’s t-test with Bonferroni correction for multiple comparisons.)
Taken together, the results presented so far support the idea that degeneration of DA neurons in comt mutants results from a defect in some step in autophagy. To test this hypothesis, we monitored autophagic flux directly in DA neurons by expressing the UAS-mCherry-GFP-Atg8a reporter using the TH-Gal4 driver. Because DA loss in comt mutants is evident by 48 hr at 29°, we examined whether there was a decrease in autophagic flux at earlier time points. When flies were maintained at room temperature, we noted autophagic flux in nearly all neurons both in comt mutants and controls (Figure 6, B, C, and F). We saw similar autophagic activity when control flies were placed at 29° for 36 hr (Figure 6D). In comt mutants, however, we found that ∼30% of DA neurons showed little to no autophagic activity (Figure 6E, arrow). Importantly, the percentage of neurons lacking autophagic activity closely matches the percentage of those ultimately lost in comt mutants (Figure 6F and Figure 2G). From these observations, we conclude that a decrease in autophagic flux precedes neurodegeneration of DA neurons in comt mutants.
Lysosomal trafficking is disrupted in comt mutants
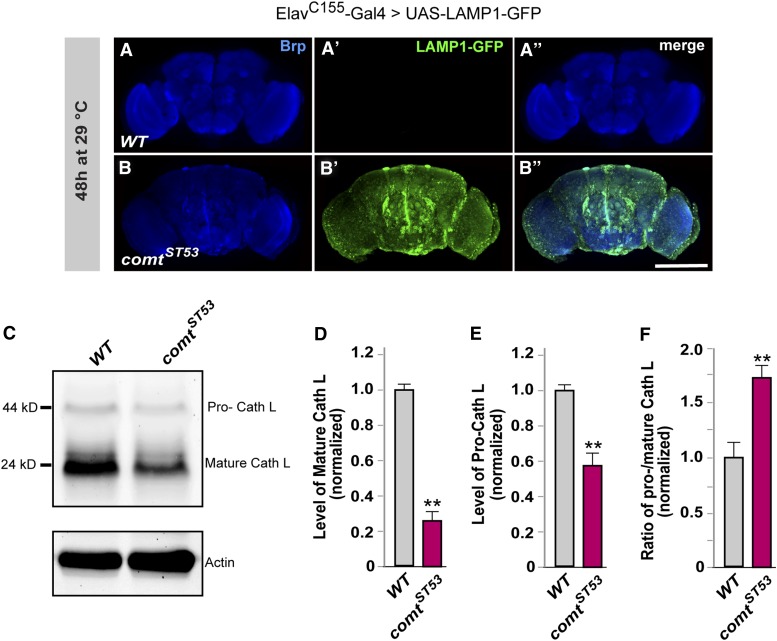
As described above, LysoTracker staining indicated that acidification of lysosomes was normal (Figure 4K), but this technique does not measure the degradative capacity of the lysosomes. Therefore, to determine more precisely the step at which autophagy could be disrupted, we examined lysosomal trafficking in comt mutants using pan-neuronal expression of a LAMP1-GFP fusion protein. LAMP1-GFP is trafficked from the Golgi to late endosomes and lysosomes, where hydrolases degrade the GFP signal (Pulipparacharuvil et al. 2005). In wild-type brains, proper lysosomal trafficking prevents the accumulation of LAMP1-GFP (Figure 7A). In comt brains, however, we found widespread accumulation of LAMP1-GFP throughout the brain (Figure 7B). The failure to efficiently degrade the GFP signal indicates that lysosomal trafficking is impaired in comt mutants.
Figure 7.
Defective lysosomal trafficking in comatose mutants. (A and B) Pan-neuronal expression of a LAMP1-GFP fusion protein (green) in either a wild-type (A) or comt mutant background (B) after 48 hr at 29°. Neuropil (blue) is stained with anti-bruchpilot (Brp). Bar in B, 100 μm. (C) Western blot of cathepsin-L protein in wild-type and comt samples after 48 hr at 29°. (D–F) Quantification of relative protein levels of the mature- (D) and pro- (E) forms of cathepsin-L, along with the ratio of the two forms (F). (**P < 0.01, Student’s t-test.)
The ability of lysosomes to carry out their degradative functions depends on the delivery of cargo, including cathepsins (Futerman and van Meer 2004). Cathepsins are lysosomal proteases that are synthesized in a higher molecular weight pro-form, which then undergo proteolytic cleavage into a mature form in acidic compartments such as autophagosomes or lysosomes (Lee et al. 2010). The pro- and mature-forms of cathepsins, such as cathepsin-L, can be easily distinguished on a Western blot, providing a measurement of trafficking of cathepsins to the lysosome. We found that mature cathepsin-L was reduced by ∼75% in comt mutants (Figure 7, C and D), which is consistent with impaired delivery to the lysosome. If production of mature cathepsin-L is reduced, this might be expected to result in excess accumulation of the uncleaved pro-form of cathepsin-L. We did not observe this increase but instead found a decrease in pro-cathepsin L in comt mutants (Figure 7C). However, compared with wild-type controls, the decrease in pro-cathepsin-L levels in comt was less than that of the mature-form (Figure 7, D and E) and the ratio of the pro-form to the mature-form is significantly higher in comt mutants (Figure 7E). While the reason for lower levels of mature cathepsin-L are unclear, there may be a decrease in synthesis or stability of the pro-form, in addition to the defect in cathepsin-L trafficking in comt mutants. Taken together, these data suggest that trafficking of lysosomal proteases is impaired in comt mutants, which accounts at least in part for the diminished degradative capacity of lysosomes in these mutants.
Discussion
The occurrence of progressive neuronal loss, particularly of DA neurons, in comt mutants has enabled us to uncover and characterize a novel neuroprotective role for NSF1. comt mutants display stress-induced locomotor defects, severely shortened lifespan, and neurodegeneration. This neurodegeneration is apparently independent of the role of NSF1 in synaptic transmission similar to what has been observed in nSyb mutants (Haberman et al. 2012). Autophagy is disrupted in comt mutants, due at least in part to a defect in trafficking of cathepsin-L to lysosomes. In addition to cathepsin-L, it is likely that other lyososmal proteases such as cathepsin-D are also affected. Flies lacking cathepsin-D exhibit several key features of neuronal ceroid lipofuscinoses, a lysosomal storage disorder, including the accumulation of storage material and neurodegeneration (Myllykangas et al. 2005).
Most of our focus on neurodegeneration here has centered on the loss of DA neurons for several reasons: the stereotypical clusters of these neurons in the brain are amenable to quantitative analysis of neurodegeneration; they are a particularly valuable subset of neurons for studying mechanisms of neurodegeneration because of their vulnerability to insults such as oxidative stress; and they are especially relevant to human neurodegenerative disease because DA neurons are preferentially lost in Parkinson’s disease. However, the widespread accumulation of LAMP1-GFP throughout the brain (Figure 7B), reveals that disruption of lysosomal degradation is not limited to DA neurons. Thus, it will be interesting to examine the consequences of decreased NSF1 function in various other subsets of neurons and to determine whether DA neurons are for some reason more susceptible to impairments in autophagy and lysosomal trafficking. If so, we anticipate that association studies for Parkinson’s disease in humans will eventually uncover hits in genes affecting these pathways. In this context, it is also of interest that overexpression of NSF1 rescues α-synuclein-induced toxicity of DA neurons in a fly model of Parkinson’s disease.
Although our results indicate that neurodegeneration in comt mutants is not a secondary consequence of impaired synaptic transmission, there are similarities in the role of NSF1 between the two processes. For example, NSF1 is critical for the recycling of SNARE complexes involved in synaptic transmission, allowing for subsequent rounds of fusion. Similarly, there are a number of SNARE-dependent fusion events required for efficient endolysosomal signaling. These include the trafficking of lysosomal proteins, as well as the fusion between autophagosomes and lysosomes. Our results suggest that recycling of these SNARE complexes requires NSF1 for disassembly and that disassembly is required to allow subsequent rounds of fusion. This idea is consistent with our demonstration that α-SNAP as well as the SNARE proteins syntaxin17, ubisnap, or VAMP7, are also required to maintain viability of DA neurons.
We have demonstrated that trafficking of cathepsin-L, a lysosomal protease, is disrupted in comt mutants. Although this disruption is likely to contribute to the observed defects in autophagic degradation in comt mutants, it should be emphasized that cathepsin-L merely serves as a convenient marker to assess lysosomal trafficking. There is no reason to believe that trafficking of this protein is specifically impaired by comt or that this impairment alone is responsible for the observed defects in neuronal maintenance in comt mutants. Indeed, it is likely that trafficking of many, if not most, proteins required for autophagic degradation is disrupted in comt mutants. As such, it is difficult to determine the primary defect in comt mutants because there are numerous fusion events involved, all of which are likely to require NSF1 for recycling. Thus, comt mutants exhibit deficits at multiple stages of the degradative pathway. The earliest problems to arise would likely be fusion events involving SNAREs with the smallest available pool, or those that require the most frequent recycling.
There is still much to be discovered about which SNARE proteins are involved in which specific fusion events in the cell. Recent studies examining specific fusion events have been very successful, however, including the discovery of particular SNARE complexes required for fusion of autophagosomes with lysosomes (Takats et al. 2013). Matching SNARE proteins to a particular fusion could be complex, however. As illustrated by the recent characterization of retinal degeneration in nSyb mutants in flies (Haberman et al. 2012), there is precedent for the same SNARE complex proteins independently mediating various distinct fusion events.
In summary, our results demonstrate a neuroprotective role for NSF1, which is required to maintain autophagy under stress. Our findings highlight the requirement of recycling SNARE complexes to maintain the integrity of the degradative pathway and advance our understanding of the cellular mechanisms that may underlie a number of neurodegenerative disorders.
Acknowledgments
We thank K. S. Krishnan, Leo Pallanck, Thomas Neufeld, and Helmut Kramer for fly stocks and reagents. We also thank all members of the Ganetzky lab for critical comments and helpful discussion of the manuscript. This work was supported by National Institutes of Health grants F32 NS078958 (D.T.B.) R01 AG033620 and R01 NS15390 (B.G.), and a predoctoral fellowship (0910048G) from the American Heart Association (W.S.).
Footnotes
Communicating editor: H. J. Bellen
Literature Cited
- Auluck P. K., Chan H. Y., Trojanowski J. Q., Lee V. M., Bonini N. M., 2002. Chaperone suppression of alpha-synuclein toxicity in a Drosophila model for Parkinson’s disease. Science 295: 865–868. [DOI] [PubMed] [Google Scholar]
- Babcock M., Macleod G. T., Leither J., Pallanck L., 2004. Genetic analysis of soluble N-ethylmaleimide-sensitive factor attachment protein function in Drosophila reveals positive and negative secretory roles. J. Neurosci. 24: 3964–3973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baines R. A., Uhler J. P., Thompson A., Sweeney S. T., Bate M., 2001. Altered electrical properties in Drosophila neurons developing without synaptic transmission. J. Neurosci. 21: 1523–1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barone M. C., Bohmann D., 2013. Assessing neurodegenerative phenotypes in Drosophila dopaminergic neurons by climbing assays and whole brain immunostaining. J. Vis. Exp. e50339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry D. L., Baehrecke E. H., 2007. Growth arrest and autophagy are required for salivary gland cell degradation in Drosophila. Cell 131: 1137–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y., Chtarbanova S., Petersen A. J., Ganetzky B., 2013. Dnr1 mutations cause neurodegeneration in Drosophila by activating the innate immune response in the brain. Proc. Natl. Acad. Sci. USA 110: E1752–E1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y. A., Scheller R. H., 2001. SNARE-mediated membrane fusion. Nat. Rev. Mol. Cell Biol. 2: 98–106. [DOI] [PubMed] [Google Scholar]
- Clary D. O., Rothman J. E., 1990. Purification of three related peripheral membrane proteins needed for vesicular transport. J. Biol. Chem. 265: 10109–10117. [PubMed] [Google Scholar]
- Elkins T., Ganetzky B., 1990. Conduction in the giant nerve fiber pathway in temperature-sensitive paralytic mutants of Drosophila. J. Neurogenet. 6: 207–219. [DOI] [PubMed] [Google Scholar]
- Feany M. B., Bender W. W., 2000. A Drosophila model of Parkinson’s disease. Nature 404: 394–398. [DOI] [PubMed] [Google Scholar]
- Filimonenko M., Stuffers S., Raiborg C., Yamamoto A., Malerod L., et al. , 2007. Functional multivesicular bodies are required for autophagic clearance of protein aggregates associated with neurodegenerative disease. J. Cell Biol. 179: 485–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friggi-Grelin F., Iche M., Birman S., 2003. Tissue-specific developmental requirements of Drosophila tyrosine hydroxylase isoforms. Genesis 35: 175–184. [DOI] [PubMed] [Google Scholar]
- Fukuda T., Ewan L., Bauer M., Mattaliano R. J., Zaal K., et al. , 2006. Dysfunction of endocytic and autophagic pathways in a lysosomal storage disease. Ann. Neurol. 59: 700–708. [DOI] [PubMed] [Google Scholar]
- Futerman A. H., van Meer G., 2004. The cell biology of lysosomal storage disorders. Nat. Rev. Mol. Cell Biol. 5: 554–565. [DOI] [PubMed] [Google Scholar]
- Gnerer J. P., Kreber R. A., Ganetzky B., 2006. wasted away, a Drosophila mutation in triosephosphate isomerase, causes paralysis, neurodegeneration, and early death. Proc. Natl. Acad. Sci. USA 103: 14987–14993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golby J. A., Tolar L. A., Pallanck L., 2001. Partitioning of N-ethylmaleimide-sensitive fusion (NSF) protein function in Drosophila melanogaster: dNSF1 is required in the nervous system, and dNSF2 is required in mesoderm. Genetics 158: 265–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haberman A., Williamson W. R., Epstein D., Wang D., Rina S., et al. , 2012. The synaptic vesicle SNARE neuronal Synaptobrevin promotes endolysosomal degradation and prevents neurodegeneration. J. Cell Biol. 196: 261–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson P. I., Whiteheart S. W., 2005. AAA+ proteins: have engine, will work. Nat. Rev. Mol. Cell Biol. 6: 519–529. [DOI] [PubMed] [Google Scholar]
- Hara T., Nakamura K., Matsui M., Yamamoto A., Nakahara Y., et al. , 2006. Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature 441: 885–889. [DOI] [PubMed] [Google Scholar]
- Ishihara N., Hamasaki M., Yokota S., Suzuki K., Kamada Y., et al. , 2001. Autophagosome requires specific early Sec proteins for its formation and NSF/SNARE for vacuolar fusion. Mol. Biol. Cell 12: 3690–3702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson F. R., Gitschier J., Strichartz G. R., Hall L. M., 1985. Genetic modifications of voltage-sensitive sodium channels in Drosophila: gene dosage studies of the seizure locus. J. Neurosci. 5: 1144–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson G. R., Salecker I., Dong X., Yao X., Arnheim N., et al. , 1998. Polyglutamine-expanded human huntingtin transgenes induce degeneration of Drosophila photoreceptor neurons. Neuron 21: 633–642. [DOI] [PubMed] [Google Scholar]
- Juhasz G., Erdi B., Sass M., Neufeld T. P., 2007. Atg7-dependent autophagy promotes neuronal health, stress tolerance, and longevity but is dispensable for metamorphosis in Drosophila. Genes Dev. 21: 3061–3066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khurana V., Elson-Schwab I., Fulga T. A., Sharp K. A., Loewen C. A., et al. , 2010. Lysosomal dysfunction promotes cleavage and neurotoxicity of tau in vivo. PLoS Genet. 6: e1001026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klionsky D. J., 2007. Autophagy: from phenomenology to molecular understanding in less than a decade. Nat. Rev. Mol. Cell Biol. 8: 931–937. [DOI] [PubMed] [Google Scholar]
- Komatsu M., Waguri S., Chiba T., Murata S., Iwata J., et al. , 2006. Loss of autophagy in the central nervous system causes neurodegeneration in mice. Nature 441: 880–884. [DOI] [PubMed] [Google Scholar]
- Lee J. H., Yu W. H., Kumar A., Lee S., Mohan P. S., et al. , 2010. Lysosomal proteolysis and autophagy require presenilin 1 and are disrupted by Alzheimer-related PS1 mutations. Cell 141: 1146–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littleton J. T., Chapman E. R., Kreber R., Garment M. B., Carlson S. D., et al. , 1998. Temperature-sensitive paralytic mutations demonstrate that synaptic exocytosis requires SNARE complex assembly and disassembly. Neuron 21: 401–413. [DOI] [PubMed] [Google Scholar]
- Littleton J. T., Barnard R. J., Titus S. A., Slind J., Chapman E. R., et al. , 2001. SNARE-complex disassembly by NSF follows synaptic-vesicle fusion. Proc. Natl. Acad. Sci. USA 98: 12233–12238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCray B. A., Taylor J. P., 2008. The role of autophagy in age-related neurodegeneration. Neurosignals 16: 75–84. [DOI] [PubMed] [Google Scholar]
- McGuire S. E., Le P. T., Osborn A. J., Matsumoto K., Davis R. L., 2003. Spatiotemporal rescue of memory dysfunction in Drosophila. Science 302: 1765–1768. [DOI] [PubMed] [Google Scholar]
- Miller D., Hannon C., Ganetzky B., 2012. A mutation in Drosophila Aldolase causes temperature-sensitive paralysis, shortened lifespan, and neurodegeneration. J. Neurogenet. 26: 317–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myllykangas L., Tyynela J., Page-McCaw A., Rubin G. M., Haltia M. J., et al. , 2005. Cathepsin D-deficient Drosophila recapitulate the key features of neuronal ceroid lipofuscinoses. Neurobiol. Dis. 19: 194–199. [DOI] [PubMed] [Google Scholar]
- Palladino M. J., Hadley T. J., Ganetzky B., 2002. Temperature-sensitive paralytic mutants are enriched for those causing neurodegeneration in Drosophila. Genetics 161: 1197–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palladino M. J., Bower J. E., Kreber R., Ganetzky B., 2003. Neural dysfunction and neurodegeneration in Drosophila Na+/K+ ATPase alpha subunit mutants. J. Neurosci. 23: 1276–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallanck L., Ordway R. W., Ganetzky B., 1995. A Drosophila NSF mutant. Nature 376: 25. [DOI] [PubMed] [Google Scholar]
- Pulipparacharuvil S., Akbar M. A., Ray S., Sevrioukov E. A., Haberman A. S., et al. , 2005. Drosophila Vps16A is required for trafficking to lysosomes and biogenesis of pigment granules. J. Cell Sci. 118: 3663–3673. [DOI] [PubMed] [Google Scholar]
- Riemensperger T., Isabel G., Coulom H., Neuser K., Seugnet L., et al. , 2011. Behavioral consequences of dopamine deficiency in the Drosophila central nervous system. Proc. Natl. Acad. Sci. USA 108: 834–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanyal S., Tolar L. A., Pallanck L., Krishnan K. S., 2001. Genetic interaction between shibire and comatose mutations in Drosophila suggest a role for snap-receptor complex assembly and disassembly for maintenance of synaptic vesicle cycling. Neurosci. Lett. 311: 21–24. [DOI] [PubMed] [Google Scholar]
- Scott R. C., Schuldiner O., Neufeld T. P., 2004. Role and regulation of starvation-induced autophagy in the Drosophila fat body. Dev. Cell 7: 167–178. [DOI] [PubMed] [Google Scholar]
- Scott R. C., Juhasz G., Neufeld T. P., 2007. Direct induction of autophagy by Atg1 inhibits cell growth and induces apoptotic cell death. Curr. Biol. 17: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqi O., Benzer S., 1976. Neurophysiological defects in temperature-sensitive paralytic mutants of Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 73: 3253–3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonsen A., Cumming R. C., Brech A., Isakson P., Schubert D. R., et al. , 2008. Promoting basal levels of autophagy in the nervous system enhances longevity and oxidant resistance in adult Drosophila. Autophagy 4: 176–184. [DOI] [PubMed] [Google Scholar]
- Takats S., Nagy P., Varga A., Pircs K., Karpati M., et al. , 2013. Autophagosomal Syntaxin17-dependent lysosomal degradation maintains neuronal function in Drosophila. J. Cell Biol. 201: 531–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titus S. A., Warmke J. W., Ganetzky B., 1997. The Drosophila erg K+ channel polypeptide is encoded by the seizure locus. J. Neurosci. 17: 875–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolar L. A., Pallanck L., 1998. NSF function in neurotransmitter release involves rearrangement of the SNARE complex downstream of synaptic vesicle docking. J. Neurosci. 18: 10250–10256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X. J., Reynolds E. R., Deak P., Hall L. M., 1997. The seizure locus encodes the Drosophila homolog of the HERG potassium channel. J. Neurosci. 17: 882–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warrick J. M., Paulson H. L., Gray-Board G. L., Bui Q. T., Fischbeck K. H., et al. , 1998. Expanded polyglutamine protein forms nuclear inclusions and causes neural degeneration in Drosophila. Cell 93: 939–949. [DOI] [PubMed] [Google Scholar]
- Whiteheart S. W., Rossnagel K., Buhrow S. A., Brunner M., Jaenicke R., et al. , 1994. N-ethylmaleimide-sensitive fusion protein: a trimeric ATPase whose hydrolysis of ATP is required for membrane fusion. J. Cell Biol. 126: 945–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteheart S. W., Schraw T., Matveeva E. A., 2001. N-ethylmaleimide sensitive factor (NSF) structure and function. Int. Rev. Cytol. 207: 71–112. [DOI] [PubMed] [Google Scholar]
- Whitworth A. J., Theodore D. A., Greene J. C., Benes H., Wes P. D., et al. , 2005. Increased glutathione S-transferase activity rescues dopaminergic neuron loss in a Drosophila model of Parkinson’s disease. Proc. Natl. Acad. Sci. USA 102: 8024–8029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittmann C. W., Wszolek M. F., Shulman J. M., Salvaterra P. M., Lewis J., et al. , 2001. Tauopathy in Drosophila: neurodegeneration without neurofibrillary tangles. Science 293: 711–714. [DOI] [PubMed] [Google Scholar]
- Wong C. O., Li R., Montell C., Venkatachalam K., 2012. Drosophila TRPML is required for TORC1 activation. Curr. Biol. 22: 1616–1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Y., Fortini M. E., 1999. Apoptotic activities of wild-type and Alzheimer’s disease-related mutant presenilins in Drosophila melanogaster. J. Cell Biol. 146: 1351–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]