Dear Editor,
From 423 cases on 22 August to 42 358 on 31 October 2014, the blinding speed at which dengue fever increased in Guangdong province made it a household name in China. In fact, China has experienced the worst dengue outbreak in the past two decades.1 Figure 1 illustrates the cumulative reported cases of dengue fever in association with the average rainfall and temperature from 2004 to 2013 in China (left panel) and the logarithmic increase in reported dengue fever cases in 2014 in Guangdong province alone, with approximately 1000 more cases discovered each week since September of 20141,2 (right panel, colored area). The exact cause of this large outbreak of dengue fever in China is not yet clear. It could be due to the frequent exchange of travelers between China and countries in Southeast Asia or to the large migrant worker population from Africa where mosquito-borne infectious diseases are rampant. It could also be a simple case of an increased mosquito density due to the elevated rainfall between May to July that preceded the dengue fever outbreaks in August. The international community has long recognized the danger that dengue fever poses on global health,3 and thus, China must take action against this reemerging infection with concerted efforts.
Figure 1.
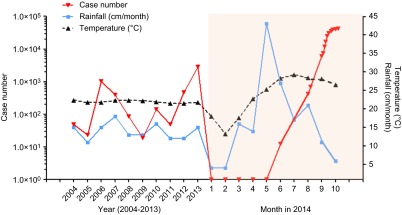
Cumulative dengue case numbers in the past 10 years reported by Chinese CDC (left) and reported cases in 2014 according to the Guangdong CDC (right, colored area). The association between the case number and temperature and rainfall amount is also illustrated.
Dengue fever is caused by dengue virus (DENV) infection, which is transmitted by Aedes aegypti or Aedes albopictus mosquitoes that carry one of the four serologically distinct but genetically related DENV serotypes (DENV-1, 2, 3 and 4). There are an estimated 390 million new infections per year in tropical and subtropical regions.4 The detection of a fifth DENV serotype has further increased its complexity.5 In China, although sporadic cases have been reported in various regions, dengue outbreaks have been traditionally confined to southern coastal regions, including Hainan, Fujian, Guangdong and Guangxi provinces, where hot and humid weather provides an ideal environment for mosquito breeding and the transmission of DENV.
Clinically, fever, rash, lethargy and joint pain are the most common presentations of DENV infection. In severe cases, which are referred to collectively as dengue hemorrhagic fever and dengue shock syndrome, patients may also have pleural effusion, ascites, gallbladder wall thickening, bleeding and shock. Laboratory testing can reveal a range of abnormalities such as leucopenia, thrombocytopenia, hypoalbuminemia, elevated aminotransferase and creatinine levels, and proteinuria. After it is clinically suspected, laboratory confirmation of dengue infection is usually performed using an enzyme-linked immunosorbent assay to detect DENV envelop protein (E) or non-structural protein 1-specific IgM and IgG antibody levels, or reverse transcriptase polymerase chain reaction to measure dengue-specific nucleic acids. A polymerase chain reaction-based assay is also used to differentiate the DENV serotypes.6 Because dengue viremia only lasts for 4–5 days in the majority of cases and sequential infection with distinct dengue serotypes is thought to affect the prognosis,7 improved techniques for serotype differentiation must be developed.
Certain risk factors are thought to contribute to the clinical manifestation of dengue infection. Epidemiological studies have suggested that dengue hemorrhagic fever and dengue shock syndrome occur more often in individuals with secondary heterotypic DENV infections and in primary infection of infants born to dengue-immune mothers. It has been hypothesized that during heterotypic secondary DENV infections, pre-existing cross-reactive antibodies enhance viral entry into host cells by forming virus–antibody complexes and binding to Fcγ receptor-bearing cells, resulting in an increase in viremia and more severe disease.7 Immunological studies have shown that in addition to antibody responses, increased T-cell activation and the production of pro-inflammatory and vasoactive factors may have led to severe disease by increasing vascular permeability, resulting in plasma leakage. Furthermore, complement activation, autoimmunity and host genetics have also been associated with increased disease severity and case fatalities.8 Alternatively, DENV serotypes and genotypes may also affect clinical outcomes;8 however, large virological studies conducted on a population basis are lacking.
Current treatment strategies are mostly geared towards the alleviation of symptoms and the prevention of shock by fluid resuscitation with colloid or crystalloid solutions.9 Further investigations of the specific mechanisms that lead to the development of plasma leakage and shock may provide insight into novel clinical management strategies. Antiviral drugs that target different steps of the DENV viral life cycle are under development, but none of them are yet available in the clinic.8
Mosquitoes carrying dengue viruses mainly breed in water containers in the house and on the patio. Control of this dengue vector can be accomplished by source reduction, such as covering, emptying and cleaning the water storage containers regularly and eliminating containers or solid waste that may accumulate water for mosquito breeding. Alternatively, killing the mosquito vector directly is another effective strategy to control the spread of DENV. Traditional methods of spraying with the insecticide dichlorodiphenyltrichloroethane have been very effective for killing the mosquito vectors of DENV and malaria. However, due to the potential impact of dichlorodiphenyltrichloroethane on human health and the environment, this chemical was banned by the Stockholm Convention on Persistent Organic Pollutants in 2004.10 In the absence of a more potent chemical mosquito killer, preventative measures such as the use of mosquito repellents and mosquito nets can reduce human infection to some extent. The application of knowledge regarding the flavivirus life cycle in mosquito vectors can provide more environmentally friendly methods, such as the infection of mosquitoes with Wolbachia bacteria or the genetic modification of mosquitoes carrying a dominant lethal gene, which have been implemented as novel strategies to block DENV transmission.11,12 Whether such methods can be deployed widely in the field remains to be demonstrated.
Similar to dealing with other infectious diseases at the population level, a preventative dengue vaccine is considered to be the most cost-effective measure to control DENV infection and the subsequent development of dengue fever. Multiple vaccine strategies have been attempted, including an inactivated whole virion vaccine, a live attenuated vaccine (traditional or chimeric), a recombinant subunit vaccine and a DNA vaccine. The major dengue vaccine research efforts during the prior 20 years have been focused on the development of live attenuated vaccines, among which the most notable is the YF17D/DENV chimeric vaccine (CYD) developed by Sanofi Pasteur. Recent phase II and III clinical trials investigating the CYD vaccine have demonstrated that despite eliciting tetravalent neutralizing antibodies, the vaccine did not provide adequate protection against the most endemic DENV-2.13,14 Thus, even if the CYD vaccine could be licensed for use in some countries, a more efficacious next-generation dengue vaccine is still needed. Furthermore, these large clinical vaccine trials have also revealed our ignorance regarding the correlates of protection against DENV infection in humans. The limitations of a live attenuated vaccine in achieving balanced immune responses and effective protection against the four DENV serotypes have provided opportunities for the development of other vaccine modalities such as a recombinant subunit vaccine and a DNA vaccine that encodes DENV prM and E proteins. Both types of vaccines have demonstrated promising results in preclinical studies.8 Recombinant subunit vaccines have notable advantages over traditional vaccines in terms of the associated safety and ease of making dose adjustment to elicit more balanced immune responses. Vector vaccines and viral-like particle vaccine platforms are also worthy of further exploration.
Dengue fever is known to have afflicted humans for over a century. Much is known about the life cycle of the DENV virus and the host immunity elicited during natural dengue viral infection. A promising dengue vaccine, although it is not perfect, is now in advanced stages of clinical testing. However, additional concerted efforts are still needed, including more basic research on the pathogenesis of dengue hemorrhagic fever and a better understanding of the immune correlates of protection. Additional translational research investigating improved methods for discriminating DENV serotypes, the development of efficacious dengue vaccines and prospective clinical studies of novel therapeutic strategies are needed to improve the clinical outcome of DENV infection. It is hoped that through the use of well-designed targeted research programs accompanied by an improvement in environmental hygiene, human suffering from dengue viral infection can be reduced to a minimum.
Acknowledgments
This work was supported in part by a program project grant from the Pasteur Foundation of Shanghai (Y359P41505) to Xia Jin.
References
- Chinese Center for Disease Control and Prevention Reportable infectious disease statistics Beijing; CCDC; 2014. Available athttp://www.chinacdc.cn/tjsj/fdcrbbg/ (accessed 25 November 2014). [Google Scholar]
- Guangdong Hygienic Birth Control Committee Dengue fever cases update Guangzhou; GCDC; 2014. Available at http://www.gdwst.gov.cn/a/yiqingxx/ (accessed 25 November 2014). [Google Scholar]
- Marston HD, Folkers GK, Morens DM, Fauci AS. Emerging viral diseases: confronting threats with new technologies. Sci Transl Med. 2014;6:253ps10. doi: 10.1126/scitranslmed.3009872. [DOI] [PubMed] [Google Scholar]
- Bhatt S, Gething PW, Brady OJ, et al. The global distribution and burden of dengue. Nature. 2013;496:504–507. doi: 10.1038/nature12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Normile D. Tropical medicine. Surprising new dengue virus throws a spanner in disease control efforts. Science. 2013;342:415. doi: 10.1126/science.342.6157.415. [DOI] [PubMed] [Google Scholar]
- World Health Organization Dengue: guidelines for diagnosis, treatment, prevention and control: new edition Geneva; WHO; 2009. Available at whqlibdoc.who.int/publications/2009/9789241547871_eng.pdf/ (accessed 3 October 2014). [PubMed] [Google Scholar]
- Halstead SB. Pathogenesis of dengue: challenges to molecular biology. Science. 1988;239:476–481. doi: 10.1126/science.3277268. [DOI] [PubMed] [Google Scholar]
- Guzman MG, Harris E.Dengue. Lancet 2014. 19 September 2014. DOI: 10.1016/S0140-6736(14)60572-9.
- Wills BA, Nguyen MD, Ha TL, et al. Comparison of three fluid solutions for resuscitation in dengue shock syndrome. N Engl J Med. 2005;353:877–889. doi: 10.1056/NEJMoa044057. [DOI] [PubMed] [Google Scholar]
- Commission of the European Communities. Regulation (EC) No 850/2004 of the European Parliament and of the Council on persistent organic pollutants and amending Directive 79/117/EEC Brussels; EU; 2014. Available at http://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:52006PC0242&from=EN (accessed 3 October 2014). [Google Scholar]
- Hoffmann AA, Montgomery BL, Popovici J, et al. Successful establishment of Wolbachia in Aedes populations to suppress dengue transmission. Nature. 2011;476:454–457. doi: 10.1038/nature10356. [DOI] [PubMed] [Google Scholar]
- Walker T, Johnson PH, Moreira LA, et al. The wMel Wolbachia strain blocks dengue and invades caged Aedes aegypti populations. Nature. 2011;476:450–453. doi: 10.1038/nature10355. [DOI] [PubMed] [Google Scholar]
- Sabchareon A, Wallace D, Sirivichayakul C, et al. Protective efficacy of the recombinant, live-attenuated, CYD tetravalent dengue vaccine in Thai schoolchildren: a randomised, controlled phase 2b trial. Lancet. 2012;380:1559–1567. doi: 10.1016/S0140-6736(12)61428-7. [DOI] [PubMed] [Google Scholar]
- Capeding MR, Tran NH, Hadinegoro SR, et al. Clinical efficacy and safety of a novel tetravalent dengue vaccine in healthy children in Asia: a phase 3, randomised, observer-masked, placebo-controlled trial. Lancet. 2014;384:1358–1365. doi: 10.1016/S0140-6736(14)61060-6. [DOI] [PubMed] [Google Scholar]


