Abstract
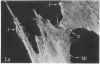
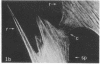
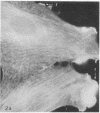
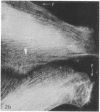
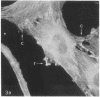
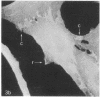
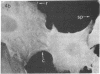
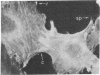
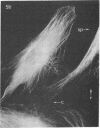
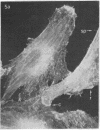
We have used methods that have allowed simultaneous fluorescent staining of intracellular actin together with either myosin, filamin, or tubulin in normal rat kidney fibroblasts in monolayer culture. In the main portions of the cell body, the actin, myosin, and filamin are all present in two structures: in one, the three proteins are present in the same fiber bundles (stress fibers); in the other, there is a diffuse distribution of the three proteins. On portions of the cell periphery however—in the basal regions of microspikes, in ruffles, and in regions of cell-cell contact—actin and filamin are present, but myosin is severely depleted or absent. Microtubules are present in the cell body in a distribution independent of the stress fibers and are mostly absent from the cell periphery. Microspikes and ruffles are highly dynamic structures on the cell surface, and regions of cell-cell contact generally result from the association of ruffles on the two contacting cells. Therefore, the presence of filamin and actin but not myosin in these specialized regions on the cell surface, together with the recent demonstration [Wang, K. & Singer, S. J. (1977) Proc. Natl. Acad. Sci. USA 74, 2021-2025)] that pure filamin interacts with individual F-actin filaments in solution to form fiber bundles and sheet-like structures, suggest that in vivo filamin-actin interactions play an important role in the control of actin filament structure, in cell motility, and in the stabilization of cell-cell contacts.
Keywords: microfilament organization, cell-cell contact, cell motility, filamin
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABERCROMBIE M. The bases of the locomotory behaviour of fibroblasts. Exp Cell Res. 1961;Suppl 8:188–198. doi: 10.1016/0014-4827(61)90348-2. [DOI] [PubMed] [Google Scholar]
- Brandtzaeg P. Conjugates of immunoglobulin G with different fluorochromes. I. Characterization by anionic-exchange chromatography. Scand J Immunol. 1973;2(3):273–290. doi: 10.1111/j.1365-3083.1973.tb02037.x. [DOI] [PubMed] [Google Scholar]
- Brinkley B. R., Fuller E. M., Highfield D. P. Cytoplasmic microtubules in normal and transformed cells in culture: analysis by tubulin antibody immunofluorescence. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4981–4985. doi: 10.1073/pnas.72.12.4981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaysman J. E., Pegrum S. M. Early contacts between fibroblasts. An ultrastructural study. Exp Cell Res. 1973 Mar 30;78(1):71–78. doi: 10.1016/0014-4827(73)90039-6. [DOI] [PubMed] [Google Scholar]
- Heaysman J. E., Pegrum S. M. Early contacts between normal fibroblasts and mouse sarcoma cells. An ultrastructural study. Exp Cell Res. 1973 Apr;78(2):479–481. doi: 10.1016/0014-4827(73)90098-0. [DOI] [PubMed] [Google Scholar]
- Heggeness M. H., Ash J. F. Use of the avidin-biotin complex for the localization of actin and myosin with fluorescence microscopy. J Cell Biol. 1977 Jun;73(3):783–788. doi: 10.1083/jcb.73.3.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huu Duc-Nguyen, Rosenblum E. N., Zeigel R. F. Persistent infection of a rat kidney cell line with Rauscher murine leukemia virus. J Bacteriol. 1966 Oct;92(4):1133–1140. doi: 10.1128/jb.92.4.1133-1140.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarides E. Two general classes of cytoplasmic actin filaments in tissue culture cells: the role of tropomyosin. J Supramol Struct. 1976;5(4):531(383)–563(415). doi: 10.1002/jss.400050410. [DOI] [PubMed] [Google Scholar]
- Lazarides E., Weber K. Actin antibody: the specific visualization of actin filaments in non-muscle cells. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2268–2272. doi: 10.1073/pnas.71.6.2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard T. D., Weihing R. R. Actin and myosin and cell movement. CRC Crit Rev Biochem. 1974 Jan;2(1):1–65. doi: 10.3109/10409237409105443. [DOI] [PubMed] [Google Scholar]
- Sanger J. W. Changing patterns of actin localization during cell division. Proc Natl Acad Sci U S A. 1975 May;72(5):1913–1916. doi: 10.1073/pnas.72.5.1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheetz M. P., Painter R. G., Singer S. J. Relationships of the spectrin complex of human erythrocyte membranes to the actomyosins of muscle cells. Biochemistry. 1976 Oct 5;15(20):4486–4492. doi: 10.1021/bi00665a024. [DOI] [PubMed] [Google Scholar]
- Shelanski M. L., Gaskin F., Cantor C. R. Microtubule assembly in the absence of added nucleotides. Proc Natl Acad Sci U S A. 1973 Mar;70(3):765–768. doi: 10.1073/pnas.70.3.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K., Ash J. F., Singer S. J. Filamin, a new high-molecular-weight protein found in smooth muscle and non-muscle cells. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4483–4486. doi: 10.1073/pnas.72.11.4483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K. Filamin, a new high-molecular-weight protein found in smooth muscle and nonmuscle cells. Purification and properties of chicken gizzard filamin. Biochemistry. 1977 May 3;16(9):1857–1865. doi: 10.1021/bi00628a015. [DOI] [PubMed] [Google Scholar]
- Wang K., Singer S. J. Interaction of filamin with f-actin in solution. Proc Natl Acad Sci U S A. 1977 May;74(5):2021–2025. doi: 10.1073/pnas.74.5.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]