Abstract
A CO-binding heme protein was solubilized and partially purified from the inner membrane fraction of rat liver mitochondria by a modification of a method [Imai, Y. & Sato, R. (1974) Biochem. Biophys. Res. Commun. 60, 8-14] developed to purify cytochrome P-450 from liver microsomes. The partially purified preparation contained protoheme and its spectral properties are characteristic of the heme proteins of the cytochrome P-450 family. The isolated cytochrome P-450 preparation could reconstitute a CO-sensitive, NADPH-dependent 26-hydroxylation activity for 5β-cholestane-3α,7α,-12α-triol when supplemented with NADPH-adrenodoxin reductase and adrenodoxin, both purified from bovine adrenocortical mitochondria. Unlike a cytochrome P-450 purified from liver microsomes of drug-untreated rats, however, the liver mitochondrial cytochrome P-450 could not catalyze NADPH-dependent benzphetamine N-demethylation in the presence of adrenodoxin reductase and adrenodoxin or function with the purified microsomal NADPH-cytochrome c reductase plus Emulgen 913 as an electron-donating system. It is concluded that the rat liver inner mitochondrial membrane houses a species of cytochrome P-450 functional in 5β-cholestane-3α,7α,12α-triol 26-hydroxylation.
Keywords: heme protein, liver mitochondria, bile acid, cholestanetriol 26-hydroxylase
Full text
PDF
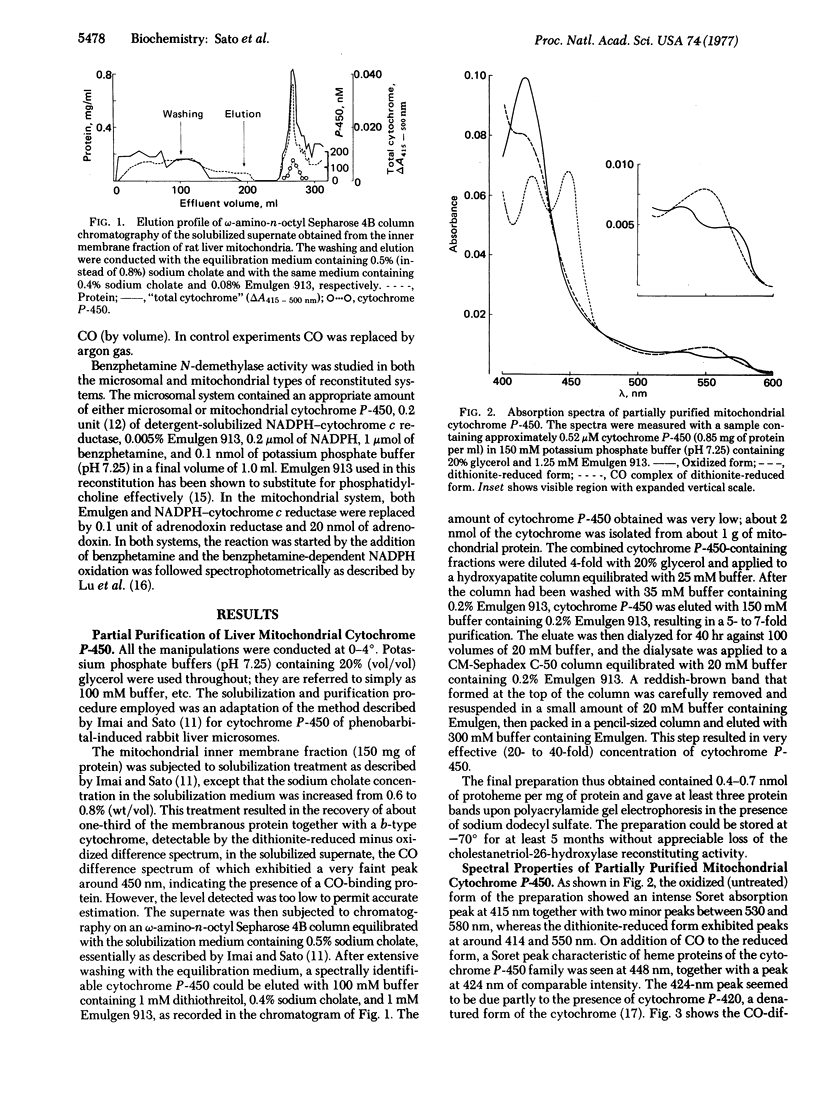
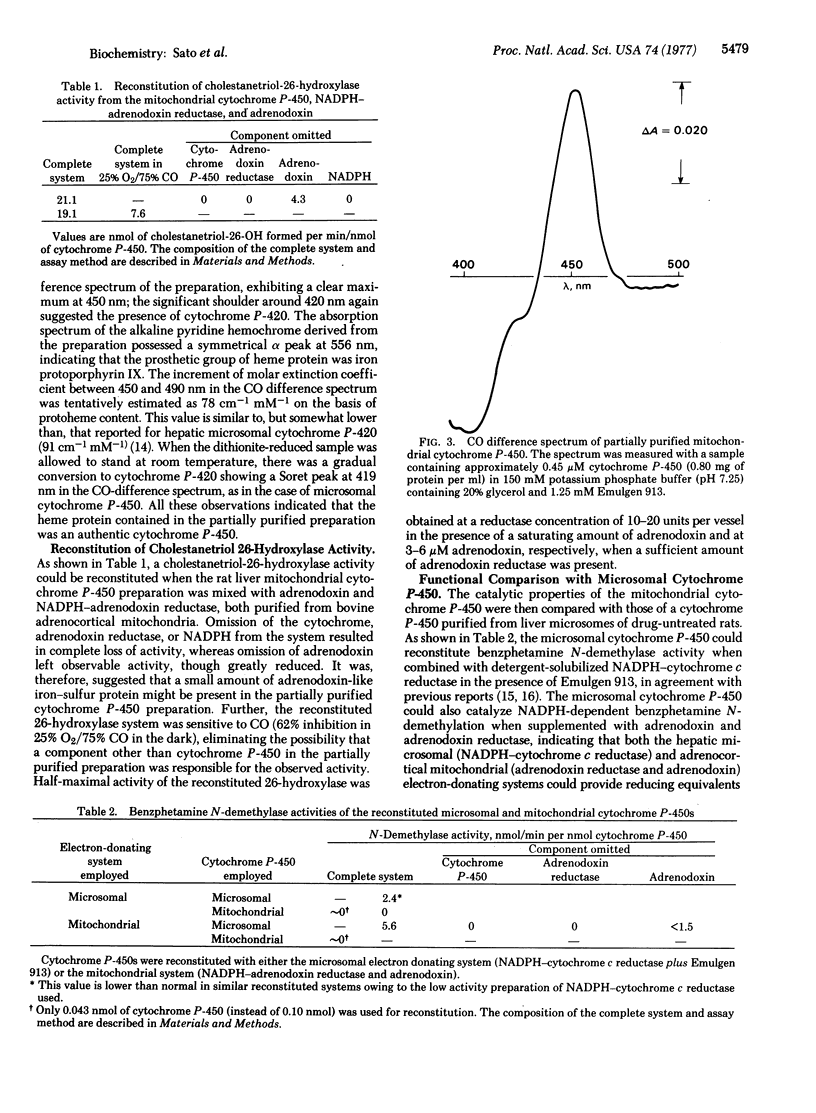


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Björkhem I., Danielsson H., Wikvall K. Side chain hydroxylations in biosynthesis of cholic acid. 25- and 26-Hydroxylation of 5beta-cholestane-3alpha, 7alpha, 12alpha-triol by reconstituted systems from rat liver microsomes. J Biol Chem. 1976 Jun 10;251(11):3495–3495. [PubMed] [Google Scholar]
- Björkhem I., Gustafsson J. Mitochondrial omega-hydroxylation of cholesterol side chain. J Biol Chem. 1974 Apr 25;249(8):2528–2535. [PubMed] [Google Scholar]
- Björkhem I., Gustafsson J. Omega-hydroxylation of steriod side-chain in biosynthesis of bile acids. Eur J Biochem. 1973 Jul 2;36(1):201–212. doi: 10.1111/j.1432-1033.1973.tb02902.x. [DOI] [PubMed] [Google Scholar]
- Cronholm T., Johansson G. Oxidation of 5 beta-cholestane-3alpha, 7alpha, 12alpha-triol by rat liver microsomes. Eur J Biochem. 1970 Oct;16(2):373–381. doi: 10.1111/j.1432-1033.1970.tb01091.x. [DOI] [PubMed] [Google Scholar]
- Ghazarian J. G., Jefcoate C. R., Knutson J. C., Orme-Johnson W. H., DeLuca H. F. Mitochondrial cytochrome p450. A component of chick kidney 25-hydrocholecalciferol-1alpha-hydroxylase. J Biol Chem. 1974 May 25;249(10):3026–3033. [PubMed] [Google Scholar]
- Haugen D. A., Coon M. J. Properties of electrophoretically homogeneous phenobarbital-inducible and beta-naphthoflavone-inducible forms of liver microsomal cytochrome P-450. J Biol Chem. 1976 Dec 25;251(24):7929–7939. [PubMed] [Google Scholar]
- Imai Y., Sato R. A gel-electrophoretically homogeneous preparation of cytochrome P-450 from liver microsomes of phenobarbital-pretreated rabbits. Biochem Biophys Res Commun. 1974 Sep 9;60(1):8–14. doi: 10.1016/0006-291x(74)90164-8. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lu A. Y., Levin W., Kuntzman R. Reconstituted liver microsomal enzyme system that hydroxylates drugs, other foreign compounds and endogenous substrates. VII. Stimulation of benzphetamine N-demethylation by lipid and detergent. Biochem Biophys Res Commun. 1974 Sep 9;60(1):266–272. doi: 10.1016/0006-291x(74)90200-9. [DOI] [PubMed] [Google Scholar]
- Nishibayashi H., Sato R. Preparation of hepatic microsomal particles containing P-450 as the sole heme constituent and absolute spectra of P-450. J Biochem. 1968 Jun;63(6):766–779. doi: 10.1093/oxfordjournals.jbchem.a128842. [DOI] [PubMed] [Google Scholar]
- OMURA T., SATO R. THE CARBON MONOXIDE-BINDING PIGMENT OF LIVER MICROSOMES. I. EVIDENCE FOR ITS HEMOPROTEIN NATURE. J Biol Chem. 1964 Jul;239:2370–2378. [PubMed] [Google Scholar]
- Okuda K., Hoshita N. Oxidation of 5-beta-cholestane-3 alpha, 7 alpha, 12 alpha-triol by rat-liver mitochondria. Biochim Biophys Acta. 1968 Oct 22;164(2):381–388. [PubMed] [Google Scholar]
- Okuda K., Weber P., Ullrich V. Photochemical action spectrum of the co-inhibited 5beta-cholestane- 3alpha, 7alpha, 12alpha-triol 26-hydroxylase system. Biochem Biophys Res Commun. 1977 Feb 7;74(3):1071–1076. doi: 10.1016/0006-291x(77)91627-8. [DOI] [PubMed] [Google Scholar]
- Pedersen J. I., Ghazarian J. G., Orme-Johnson N. R., DeLuca H. F. Isolation of chick renal mitochondrial ferredoxin active in the 25-hydroxyvitamin D3-1alpha-hydroxylase system. J Biol Chem. 1976 Jul 10;251(13):3933–3941. [PubMed] [Google Scholar]
- Suhara K., Ikeda Y., Takemori S., Katagiri M. The purification and properties of NADPH-adrenodoxin reductase from bovine adrenocortical mitochondria. FEBS Lett. 1972 Nov 15;28(1):45–47. doi: 10.1016/0014-5793(72)80673-2. [DOI] [PubMed] [Google Scholar]
- Suhara K., Takemori S., Katagiri M. Improved purification of bovine adrenal iron-sulfur protein. Biochim Biophys Acta. 1972 Apr 15;263(2):272–278. doi: 10.1016/0005-2795(72)90079-7. [DOI] [PubMed] [Google Scholar]
- Taniguchi S., Hoshita N., Okuda K. Enzymatic characteristics of CO-sensitive 26-hydroxylase system for 5beta-cholestane-3 alpha, 7 alpha, 12 alpha-triol in rat-liver mitochondria and its intramitochondrial localization. Eur J Biochem. 1973 Dec 17;40(2):607–617. doi: 10.1111/j.1432-1033.1973.tb03233.x. [DOI] [PubMed] [Google Scholar]


