Abstract
Prenatal testosterone exposure may be protective against disordered eating. However, prior studies have produced mixed results. Developmental differences in prenatal testosterone's protective effects on disordered eating may explain these discrepancies. Indeed, studies have differed in the age of participants assessed, with data supporting prenatal testosterone effects on disordered eating in early adolescent and young adult samples but not in late adolescence. The present series of studies are the first to investigate age differences in prenatal testosterone's protective effects on disordered eating. Two indirect markers of higher prenatal testosterone were examined: 1) lower finger-length ratios [index (2D)/ring (4D) finger] (Study 1), and 2) lower disordered eating in females from opposite-sex twin pairs (who are thought to be exposed to higher prenatal testosterone from their male co-twin) relative to female controls (Study 2). Participants were twins from the Michigan State University Twin Registry (Study 1: n = 409; Study 2: n = 1,538) in early adolescence, late adolescence, or young adulthood. Disordered eating was assessed with well-validated questionnaires. Finger-length ratios were measured from hand scans, using electronic computer calipers. Findings were consistent across both studies. Higher prenatal testosterone (lower 2D:4D; females from opposite-sex twin pairs vs. controls) predicted lower disordered eating in early adolescence and young adulthood only. Prenatal testosterone-disordered eating associations were not observed during late adolescence. Results point to the possibility of developmental windows of expression for prenatal testosterone's protective effects on disordered eating and suggest that prior discrepant results may reflect age differences across samples.
Keywords: disordered eating, eating disorder, sex difference, testosterone, hormones, 2D:4D
Sex differences in eating disorder prevalence (female-to-male ratio ranging 3:1 to 10:1) have been largely attributed to sociocultural influences that preferentially increase risk for eating disorders in females, such as pressures for thinness (Striegel-Moore & Bulik, 2007). Although the influence of sociocultural factors is supported by many studies (Keel & Forney, 2013; Striegel-Moore & Bulik, 2007), emerging evidence suggests that biological factors (e.g., gonadal hormones) likely contribute as well (Baker, Girdler, & Bulik, 2012; Hildebrandt, Alfano, Tricamo, & Pfaff, 2010). Exposure to testosterone during prenatal development has been hypothesized, by our group (e.g., Klump et al., 2006; Culbert, Breedlove, Sisk, Burt, & Klump, 2013) and others (e.g., Oinonen & Bird, 2012; Smith, Hawkeswood, & Joiner, 2010), as one biological mechanism that may be particularly important for understanding sex differences in eating pathology.
Prenatal exposure to testosterone, the primary androgen, is critical for the development of sexually-dimorphic characteristics (Arnold & Breedlove, 1985; Berenbaum & Beltz, 2011; Breedlove, 1994) as it drives organizational (i.e., permanent, persisting beyond initial hormone exposure) changes to brain structure and function that result in masculinized (i.e., male-like) behavior later in life. The absence of testosterone exposure early in life averts masculinization, resulting in the expression of female-typical characteristics (Breedlove, 1994; Berenbaum & Beltz, 2011). For example, female rodents that are exposed to higher testosterone during perinatal development, either via intrauterine position effects (i.e., exposure via developing adjacent to males in utero) or exogenous administration of testosterone, subsequently display several masculinized characteristics (e.g., greater food intake, aggression) as compared to control females (Donohoe & Stevens, 1983; Madrid, Lopez-Bote, & Martin, 1993; Ryan & Vandenbergh, 2002). Thus, exposure to testosterone early in life results in male-typical patterns of behavior, including food intake (a key behavior disrupted in eating disorders) in animals.
Since early hormone exposure cannot be experimentally manipulated in humans and direct measures are difficult to obtain, two proxy measures have been used to investigate the organizational effects of prenatal testosterone exposure on eating pathology: 1) finger-length ratios [index finger (2D)/ring finger (4D)] and 2) the study of opposite-sex twin pairs. Finger-length ratios are hypothesized to be a biomarker of prenatal testosterone exposure (for review, see Breedlove, 2010), or more specifically, the balance between prenatal testosterone and estrogen exposure (Manning, Kilduff, Cook, Crewther, & Fink, 2014; Zheng & Cohn, 2011). Direct and indirect evidence from animal and human data suggest that low 2D:4D is indicative of high prenatal testosterone and low prenatal estrogen exposure (Manning et al., 2014). Consistent with this assertion, 2D:4D ratios are sexually dimorphic (i.e., lower 2D:4D in males than in females; Manning, Scutt, Wilson, & Lewis-Jones, 1998) across a range of species (e.g., rodents, primates) and have been shown to be directly influenced by prenatal testosterone and estrogen signaling early in development (Zheng & Cohn, 2011). Further, human data indicate that 2D:4D ratios are sexually-dimorphic as early as the 9th week of gestation (Malas, Dogan, Evcil, & Desdicioglu, 2006), correlate with levels of testosterone (r ∼ -.32) and the relative ratio between testosterone and estradiol (r ∼ -.52) obtained prenatally by amniocentesis (Lutchmaya, Baron-Cohen, Raggatt, Knickmeyer, & Manning, 2004), are masculinized (i.e., ‘male-typical’, lower 2D:4D) in females exposed to high levels of androgens early in life (e.g., females with congenital adrenal hyperplasia; Brown, Hines, Fane, & Breedlove, 2002; Okten, Kalyoncu, & Yari, 2002), and are feminized (i.e. ‘female-typical’, higher 2D:4D) in women with no effective prenatal testosterone exposure (i.e., XY androgen insensitivity syndrome; Berenbaum, Bryk, Nowak, Quigley, & Moffat, 2009).
Opposite-sex twin pairs have also been hypothesized to be a useful paradigm for exploring natural variation in prenatal testosterone exposure because similar to intrauterine position effects observed in rodents, females from opposite-sex twin pairs (herein referred to as OS-F twins) are thought to be exposed to higher levels of testosterone prenatally due to developing in utero with a male co-twin (Miller, 1994). Testosterone transfer from a male co-twin to a female co-twin has been postulated to occur either via maternal circulation or diffusion across fetal membranes (for review, see Tapp, Maybery, & Whitehouse, 2011). Consistent with this notion, OS-F twins are “masculinized” (i.e., OS-F twins do not differ from males or they fall intermediate to males and other females) on several phenotypes (e.g., aggression, sensation seeking; Cohen-Bendahan, Buitelaar, van Goozen, Orlebeke, & Cohen-Kettenis, 2005; Slutske, Bascom, Meier, Medland, & Martin, 2011), including characteristics that are not socially influenced (e.g., auditory systems, cerebral lateralization; Cohen-Bendahan, Buitelaar, van Goozen, & Cohen-Kettenis, 2004; McFadden, 1993). Notably, although the strength of the 2D:4D finger-length ratio and opposite-sex twin methods have been questioned (e.g., Berenbaum et al., 2009; Tapp et al., 2011; Wallen, 2009) and these methods are not completely synonymous with prenatal hormone exposure (Wallen, 2009), they are considered the best and/or most accessible indirect markers of prenatal testosterone exposure (e.g., Breedlove, 2010; Tapp et al., 2011).
Several studies using 2D:4D ratios and opposite-sex twin pairs have provided support for the hypothesis that prenatal testosterone exposure has masculinizing or protective effects (i.e., decreases risk) on eating pathology (Culbert, Breedlove, Burt, & Klump, 2008; Culbert, Breedlove, Sisk, Burt, & Klump, 2013; Klump et al., 2006; Oinonen & Bird, 2012; Quinton, Smith, & Joiner, 2011; Raevuori et al., 2008; Smith et al., 2010). For example, lower 2D:4D digit ratios, which is indicative of higher prenatal testosterone exposure, has been associated with lower levels of disordered eating symptoms such as drive for thinness/leanness, bulimic symptoms, and composite measures of disordered eating attitudes and behaviors (r's = .15-.23; Klump et al., 2006; Oinonen & Bird, 2012; Smith et al., 2010). Similarly, OS-F twins have reported more male-like patterns of eating pathology, i.e., lower levels of disordered eating symptoms (e.g., intentional weight loss, weight preoccupation, body dissatisfaction, and composite measures of disordered eating attitudes and behaviors; Culbert et al., 2008; Culbert et al., 2013; Raevuori et al., 20081) and rates of broadly defined anorexia nervosa (Raevuori et al., 2008) than female controls, such as non-twin females reared with brothers and/or females from same-sex twin pairs (herein referred to as SS-F twins).
However, not all prior research using 2D:4D or opposite-sex twin methodology has provided evidence in support of prenatal testosterone's protective effects on disordered eating. Some studies have found no evidence for prenatal testosterone's protective effects on disordered eating symptoms (e.g., body dissatisfaction, bulimic symptoms; Baker et al., 2009), or effects were observed for some, but not all, eating disorder symptoms (e.g., intentional weight loss and body dissatisfaction, but not bulimic symptoms; Raevuori et al., 2008) or lifetime diagnoses (e.g., for anorexia nervosa but not bulimia nervosa; Quinton et al., 2011; Lydecker et al., 2012; Raevuori et al., 2008). These inconsistent findings could be due to differences in the type of disordered eating symptoms assessed (e.g., attitudinal/cognitive versus behavioral symptoms) or, as suggested by others (e.g., Baker et al., 2009; Raevuori et al., 2008), the use of different questionnaires that may tap slightly different constructs of eating pathology. However, one prior study (i.e., Culbert et al., 2013) found similar results for overall levels of disordered eating and attitudinal/cognitive symptoms (e.g., body weight and shape concerns) assessed by two different questionnaires (i.e., Minnesota Eating Behaviors Survey and Eating Disorder Examination Questionnaire), providing some evidence that the use of different questionnaires is unlikely to completely account for discrepant results.
Another possibility is that discrepant results reflect developmental differences in the expression of prenatal testosterone's protective effects on disordered eating symptoms. Indeed, variation in the age of participants is the most notable difference between studies that have aimed to explore prenatal testosterone-disordered eating effects, and age moderation might help explain the pattern of positive versus negative findings to date. For example, in early adolescence (e.g., after the onset of mid-puberty; Culbert et al., 2013) and in young adulthood (Culbert et al., 2008; Raevuori et al., 2008), OS-F twins showed lower rates of eating pathology than other females (i.e., non-twin females and/or SS-F twins), findings that are suggestive of a protective effect of prenatal testosterone exposure on disordered eating. By contrast, in late adolescence (Baker et al., 2008) and adulthood/midlife (Lydecker et al., 2012), OS-F and SS-F twins showed similar levels of disordered eating symptoms (Baker et al., 2008) or similar lifetime eating disorder prevalence rates (Lydecker et al., 2012), respectively.
Age effects have yet to be observed in studies using 2D:4D finger-length ratios, but this may be because prior research has examined samples that largely span young adulthood (Klump et al., 2006; Oinonen & Bird, 2012; Smith et al., 2010). Results from these studies parallel those of OS-F twins in young adulthood, such that lower 2D:4D finger-length ratios are associated with lower levels of disordered eating symptoms (Klump et al., 2006; Oinonen & Bird, 2012; Smith et al., 2010).
Together, data suggest that prenatal testosterone's protective effects (measured indirectly via 2D:4D or OS-F twin paradigm) on disordered eating may be stronger or weaker depending upon developmental stage (e.g., stronger effects in young adulthood than late adolescence). Thus, while the organizational effects of prenatal testosterone would be expected to exert permanent effects at the biological level (e.g., masculinization of the central nervous system; Breedlove, 1994), there may be developmental differences in prenatal testosterone's influence on the phenotypic expression of disordered eating – especially since disordered eating symptoms and eating disorder onset show developmental variations in risk (i.e., highest risk in late adolescence; see Abebe, Lien, Torgersen, & von Soest, 2012a; Abebe, Lien, & von Soest, 2012b; Jones, Bennett, Olmsted, Lawson, & Rodin, 2001; Steinhausen, Gavez, & Metzke, 2005; Stice, Killen, Hayward, & Taylor, 1998; Stice, Marti, Shaw, & Jaconis, 2009) and are influenced by several etiologic factors that could interact with prenatal testosterone effects across time. In the case of late adolescence, the protective effects of prenatal testosterone may be attenuated by the many other risk factors (e.g., increases in perceived pressures for thinness, autonomy difficulties, initiation of dating; Field et al., 2001; Presnell, Bearman, & Stice, 2004) that contribute to eating disorder risk and increased eating disorder prevalence.
The current series of studies aims to take an initial step at understanding these processes by being the first to examine whether there are age differences in prenatal testosterone's protective effects on disordered eating symptoms. We hypothesized that the protective effects of prenatal testosterone on disordered eating would be evident during early adolescence (i.e., ages 9-14) and young adulthood (i.e., Study 1: ages 20-23; Study 2: ages 20-30), but attenuated during the peak period of eating disorder risk (i.e., late adolescence, ages 15-19). Hypotheses were tested using two indirect markers of prenatal testosterone exposure (i.e., 2D:4D finger-length ratios (Study 1) and OS-F twin design (Study 2)) to ensure that effects replicate across methodologies. Two well-validated self-report measures were used to assess several disordered eating constructs (including body weight and shape concerns, bulimic symptoms, dietary restraint) and to confirm that prenatal testosterone's effects on disordered eating replicate across measures. We focused on these continuous measures of eating pathology in a community/population-based sample to maximize statistical power to detect prenatal testosterone-disordered eating effects. This was an important consideration given that clinical eating disorders (e.g., anorexia nervosa, bulimia nervosa) are relatively rare conditions (prevalence ∼0.3-3.5%; Hudson, Kiripi, Pope, & Kessler, 2007; Swanson, Crow, Le Grange, Swendsen, & Merikangas, 2011), whereas disordered eating symptoms are more prevalent and allow for greater power to detect etiologic effects. Fortunately, these symptoms are also strong prospective predictors of the eventual development of clinical eating disorders (Jacobi, Hayward, de Zwaan, Kraemer, & Stewart, 2004) and thus, results from this study are relevant to knowledge of prenatal testosterone effects across the spectrum of eating pathology.
Methods
Study 1: 2D:4D Finger-length Ratios
This study was approved by the Michigan State University Institutional Review Board and was conducted in compliance with national legislation. Written informed consent/assent was obtained from all participants.
Participants
Study 1 included a population-based sample of 409 SS-F twins ages 9-23 (see Table 1) from the Michigan State University Twin Registry (Klump & Burt, 2006; Burt & Klump, 2013) who were past participants in the Twin Study of Hormones and Behavior across the Menstrual Cycle (Klump et al., 2013) or the Twin Study of Hormones and Disordered Eating across Puberty (see Burt & Klump, 2013). Monozygotic (n = 229) and dizygotic (n = 180) SS-F twins were combined in analyses to maximize the sample size, as mean levels on most disordered eating variables did not significantly differ by zygosity (d's = .00-.22, mean d = .04). Associations between finger-length ratios and disordered eating variables were also similar between monozygotic and dizygotic twins, further suggesting that combining across zygosity would not unduly alter our results (data not shown).
Table 1. Descriptive Statistics (Study 1).
| Early Adolescence | Late Adolescence | Young Adulthood | |
|---|---|---|---|
| Sample Size (n) | 154 | 209 | 46 |
| Age: Mean (SD) | 11.44 (1.69) | 17.23 (1.04) | 21.22 (0.77) |
| 2D:4D | |||
| Range | 0.88-1.08 | 0.87-1.09 | 0.91-1.05 |
| Mean (SD) | 0.97 (0.03) | 0.97 (0.04) | 0.99 (0.03) |
| Minnesota Eating Behavior Survey (MEBS) | |||
| Total Score | |||
| Range (Max = 30) | 0-18 | 0-21 | 0-24 |
| Mean (SD) | 4.57 (4.53) | 5.02 (4.85) | 7.17 (6.42) |
| % > clinical mean | 4.54 | 4.83 | 13.0 |
| Body Dissatisfaction | |||
| Range (Max = 6) | 0-6 | 0-6 | 0-6 |
| Mean (SD) | 1.01 (1.47) | 1.39 (1.82) | 2.22 (2.19) |
| Bulimic Symptoms | |||
| Range (Max = 13) | 0-8 | 0-8 | 0-8 |
| Mean (SD) | 0.91 (1.42) | 1.13 (1.54) | 1.42 (2.15) |
| Weight Preoccupation | |||
| Range (Max = 8) | 0-8 | 0-8 | 0-8 |
| Mean (SD) | 1.93 (2.14) | 2.02 (2.10) | 2.87 (2.60) |
| Eating Disorder Examination Questionnaire (EDE-Q) | |||
| Global Score | |||
| Range (Max = 6) | 0-3.6 | 0-4.4 | 0-4.5 |
| Mean (SD) | 0.57 (0.73) | 0.86 (1.01) | 1.26 (1.31) |
| % > clinical mean | 0.65 | 2.40 | 8.70 |
| Eating Disorder Examination Questionnaire (EDE-Q) | |||
| Dietary Restraint | |||
| Range (Max = 6) | 0-4.6 | 0-4.8 | 0-5.4 |
| Mean (SD) | 0.43 (0.77) | 0.55 (0.95) | 0.95 (1.28) |
| Shape Concerns | |||
| Range (Max = 6) | 0-5.5 | 0-5.8 | 0-5.8 |
| Mean (SD) | 0.69 (0.99) | 1.23 (1.38) | 1.64 (1.69) |
| Weight Concerns | |||
| Range (Max = 6) | 0-5.2 | 0-5.6 | 0-4.40 |
| Mean (SD) | 0.71 (0.99) | 1.02 (1.23) | 1.39 |
Note. 2D:4D = index (2D)/ring (4D) finger; Participants were females from same-sex twin pairs (see Methods).
Participants were recruited through birth records in collaboration with the Michigan Department of Community Health and the Michigan Bureau of Integration, Information, and Planning Services (for additional recruitment details, see Klump & Burt, 2006; Burt & Klump, 2013). Consistent with the recruitment region (see http://www.michigan.gov/mdch; Culbert et al., 2008), the majority of participants were White (87%). The remaining participants were Black/African American (6.8%), Asian (1.0%), American Indian/Alaska Native (0.5%), or Bi-racial (4.6%). Race/ethnicity was included as a covariate in analyses since, similar to prior research (e.g., Manning, Churchill, & Peters, 2007), mean 2D:4D finger-length ratios were significantly [F (1, 406) = 4.36, p = .04] higher in White participants [M (SD) = 0.97 (0.03)] than in other racial/ethnic groups [M (SD) = 0.96 (0.05)].
Variables
Disordered Eating Symptoms
Disordered eating was assessed with the Minnesota Eating Behaviors Survey (MEBS2; von Ranson, Klump, Iacono, & McGue, 2005) and the Eating Disorder Examination Questionnaire (EDE-Q; Fairburn & Beglin, 1994). The inclusion of both measures allowed us to determine whether findings replicate across disordered eating scales and to determine potential unique effects (e.g., variation in the magnitude of prenatal testosterone's effects) across symptoms. As expected, the MEBS and EDE-Q scales showed moderate-to-high correlations (mean r = .60, range r's = .38-.83), suggesting that many of the symptoms/constructs assessed by each of these scales show considerable overlap, yet unique variance is captured as well (i.e., shared variance across scales: r2 = .14-.69).
The MEBS is a 30-item true/false self-report questionnaire that was developed for use in children as young as 9 years old (von Ranson et al., 2005), and thus is suitable for use across the full age range of participants in this study. The MEBS consists of a total score and four subscales: binge eating (the tendency to think about and/or engage in binge eating), body dissatisfaction (dissatisfaction with one's body size and/or shape), compensatory behavior (the tendency to use or contemplate use of inappropriate compensatory behaviors, such as self-induced vomiting), and weight preoccupation (the tendency to be preoccupied with dieting, weight, and the pursuit of thinness). The EDE-Q is a self-report questionnaire that assesses disordered eating over the past 28 days, in terms of shape concerns (dissatisfaction and discomfort with one's body shape), weight concerns (preoccupation with weight and a desire to lose weight), eating concerns (preoccupation with food, eating in secret, and guilt about eating), and dietary restraint (restraint over eating and avoidance of eating). A global score is comprised of items across all subscales. Notably, younger participants (ages ≤ 14; n = 154) completed a modified version of the EDE-Q that was adapted for youth (e.g., simplification of descriptive terms and use of words appropriate for a 3rd grade literacy level; Goldschmidt, Doyle, & Wilfley, 2007). This adapted version is identical to the original EDE-Q in terms of general item content and computed scales. Higher scores on the MEBS and EDE-Q indicate more pathological eating attitudes and behaviors.
The MEBS and EDE-Q have demonstrated good psychometric properties in male and female samples (Goldschmidt et al., 2007; Lavender, DeYoung, & Anderson, 2010; Mardersian et al., unpublished data; Mond, Hay, Rodgers, Owen, & Beaumont, 2004a; von Ranson et al., 2005; Zehr, Culbert, Sisk, & Klump, 2007). In addition, the MEBS and EDE-Q have been shown to successfully discriminate between individuals with versus without an eating disorder (Aardoom, Dingemans, Slof Op't Landt, & van Furth, 2012; von Ranson et al., 2005), and the EDE-Q has demonstrated high correlations with scores attained via the Eating Disorder Examination interview (Binford, Le Grange, & Jellar, 2005; Carter, Aime, & Mills, 2001; Goldschmidt et al., 2007; Mond, Hay, Rodgers, Owen, & Beaumont, 2004b).
The MEBS and EDE-Q total/global scores, body weight and shape scales (MEBS: body dissatisfaction and weight preoccupation; EDE-Q: shape concerns and weight concerns), and EDE-Q dietary restraint were included in this study since these scales showed good internal consistency across all age groups (α's = .71-.96). Low internal consistencies were observed for the MEBS binge eating and compensatory behavior subscales (e.g., α's = .25-.79, mean α =.52 in some subgroups, particularly adolescents) and EDE-Q eating concerns subscale (e.g., α = .61 in early adolescents) in this study and prior studies (e.g., Culbert et al., 2013; Decaluwé & Braet, 2004; von Ranson et al., 2005). These scales were therefore not examined separately in analyses. However, similar to previous research (Klump, Keel, Sisk, & Burt, 2010), a composite score that summed the MEBS binge eating and compensatory behavior items exhibited more acceptable internal consistency (full sample: α = .70; each age group: α's = .68-.81, mean α =.72), and this composite score of “bulimic symptoms” was included in analyses.
Finger-length Ratios
Finger-length ratios [index finger (2D)/ring finger (4D)] were calculated from electronic computer screen caliper measurements (Iconico, Inc. software). Electronic screen calipers were positioned over scanned images of the hands, and the length of the 2nd (index) and 4th (ring) fingers were measured from the basal crease to the tip, to the nearest 0.01 centimeter. Importantly, computer analysis of 2D:4D ratios from hand scans has shown to be more reliable than other measurement methods (e.g., physical measurements, photocopies, printed scans; Allaway et al., 2009). It is also important to acknowledge that 2D:4D ratio values obtained from scanned images may be lower than ratios calculated from direct finger measurements (Manning, Fink, Neave, & Caswell, 2005); however, such effects would not unduly alter the findings of this study given that the same measurement method was used for all participants. Two trained research assistants independently measured all hand scans. Inter-rater reliability was excellent (all r's > .94), and thus, average right hand 2D:4D and left hand 2D:4D score were computed across raters. However, analyses included only right hand 2D:4D, as previous research suggests that the right hand is more responsive to prenatal testosterone exposure (see Breedlove, 2010).3
Age Groupings
Every effort was made to map the age groups used in this study onto the ages examined in previous work (e.g., early adolescence: Culbert et al., 2013; late adolescence: Baker et al., 2009; young adulthood: Culbert et al., 2008, Klump et al., 2006; Oinonen & Bird, 2012; Raevuori et al., 2008; Smith et al., 2010). The sample was divided into three age groups (see Table 1): early adolescence (ages 9-14: n = 154, M age ± SD = 11.44, ±1.69), late adolescence (ages 15-19: n = 209, M age ± SD = 17.23, ±1.04), and young adulthood (ages 20-23: n = 46, M age ± SD = 21.22, ±0.77). These age categories allowed us to explore whether prenatal testosterone's protective effects on disordered eating varies across the three key developmental periods.
Statistical Analyses
Data Preparation
Scores on the MEBS and EDEQ were prorated if participants were missing 10% or fewer of the items and coded as missing for a small number of participants missing more than 10% of the items on each scale (≤ 1% of total sample: MEBS: n's = 2-5, EDE-Q: n's = 1-7). The MEBS bulimic symptoms score and the EDE-Q dietary restraint, weight concerns, and global scores were log transformed prior to analyses to account for positive skew.
2D:4D Associations
Pearson correlations and partial correlations (i.e., controlling for race/ethnicity) were used to examine associations between disordered eating and 2D:4D finger-length ratios in early adolescence, late adolescence, and young adulthood. Positive 2D:4D-disordered eating correlations (i.e., that lower 2D:4D, which is indicative of higher prenatal testosterone, is associated with lower levels of disordered eating) in early adolescence and young adulthood, but negligible associations in late adolescence, would provide initial support for hypotheses that prenatal testosterone-disordered eating associations vary across development.
Mixed linear modeling was then used to directly examine developmental differences in 2D:4D-disordered eating associations. The non-independence of twin data was accounted for within mixed linear modeling analyses by nesting the lower-level unit (i.e., twin identification number) within an upper-level unit (i.e., family number that is shared by co-twins). Levels of disordered eating were predicted as a function of 2D:4D finger-length ratios (e.g., prenatal testosterone exposure) and age group (i.e., early adolescence, late adolescence, young adulthood), covarying race/ethnicity. 2D:4D was examined continuously and age group was dummy coded (i.e., two dummy coded variables to represent the three age groups, with late adolescence coded 0) in these regression models. Further, since mixed linear modeling provides unstandardized estimates of predictor effects, the continuous 2D:4D variable was standardized prior to analysis to allow for the interpretation of unstandardized coefficients as standardized coefficients and to ease interpretation of effect sizes.
Statistical models estimated the age group main effect, 2D:4D main effect, and Age group × 2D:4D interaction on levels of disordered eating, controlling for main effects of race/ethnicity. A main effect of 2D:4D would suggest that prenatal testosterone exposure predicts disordered eating, and a main effect of age group would suggest that levels of disordered eating vary by age. The interaction effect was of particular interest since a significant Age group × 2D:4D interaction would suggest that the influence of prenatal testosterone on disordered eating varies by age. Specifically, significant Age group × 2D:4D interactions were expected when comparing the early adolescent and young adulthood groups to the late adolescent group, as this pattern of results would suggest that prenatal testosterone exposure (i.e., 2D:4D finger-length ratio) predicts variation in levels of disordered eating symptoms in early adolescence and young adulthood but not late adolescence.
Results
Descriptive Statistics
Within each age group, many disordered eating attitudes and behaviors were endorsed by participants (see Table 1) and a number of participants scored above the clinical mean on the MEBS (i.e., total score M ≥ 15.55, von Ranson et al., 2005; see Table 1) and EDE-Q (i.e., global score M ≥ 3.46, Aardoom et al., 2012; see Table 1). These descriptive data indicate that the MEBS and EDE-Q scale distributions spanned a spectrum of severity and that this study had ample variability to detect associations between prenatal testosterone (i.e., 2D:4D) and disordered eating variables in each age group.
2D:4D Associations
Pearson correlations between 2D:4D finger-length ratios and disordered eating symptoms (see Table 2) suggested that higher levels of prenatal testosterone exposure (i.e., lower 2D:4D) are associated with lower levels of disordered eating symptoms in early adolescence (r's = .16-.25, with exception of dietary restraint) and young adulthood (r's = .23-.35), but not in late adolescence (r's = .00-.08). It is important to note that this interpretation is based on the magnitude of 2D:4D-disordered eating correlations, which were either similar or slightly higher in the young adulthood group as compared to the early adolescent group. Thus, the lower significance levels observed in the young adulthood group, relative to the early adolescent group, is likely due to smaller sample size.
Table 2. Pearson and Partial Correlations between 2D:4D and Disordered Eating by Age Group (Study 1).
| Early Adolescence (n = 154) |
Late Adolescence (n = 209) |
Young Adulthood (n = 46) |
|
|---|---|---|---|
|
|
|
|
|
| Variables | 2D:4D | 2D:4D | 2D:4D |
| Pearson Correlations | |||
| Minnesota Eating Behavior Survey (MEBS) | |||
| Total Score | .25** | -.004 | .33* |
| Bulimic Symptoms | .23** | .07 | .26† |
| Body Dissatisfaction | .19* | -.04 | .26† |
| Weight Preoccupation | .17* | -.04 | .26† |
| Eating Disorder Examination Questionnaire (EDE-Q) | |||
| Global Score | .16* | -.08 | .30* |
| Dietary Restraint | .004 | -.06 | .35* |
| Shape Concerns | .17* | -.07 | .24 |
| Weight Concerns | .21** | -.08 | .23 |
| Partial Correlations, controlling for Race/Ethnicity | |||
| Minnesota Eating Behavior Survey (MEBS) | |||
| Total Score | .25** | -.002 | .36* |
| Bulimic Symptoms | .27** | .11 | .30† |
| Body Dissatisfaction | .18* | -.04 | .30† |
| Weight Preoccupation | .18* | -.03 | .28† |
| Eating Disorder Examination Questionnaire (EDE-Q) | |||
| Global Score | .20* | -.01 | .30* |
| Dietary Restraint | .02 | .01 | .27† |
| Shape Concerns | .18* | -.07 | .22 |
| Weight Concerns | .23** | .01 | .27† |
Note: Early Adolescence = ages 9-14, Late Adolescence = ages 15-19, Young Adulthood = ages 20-23.
p < .10;
p < .05;
p < .01
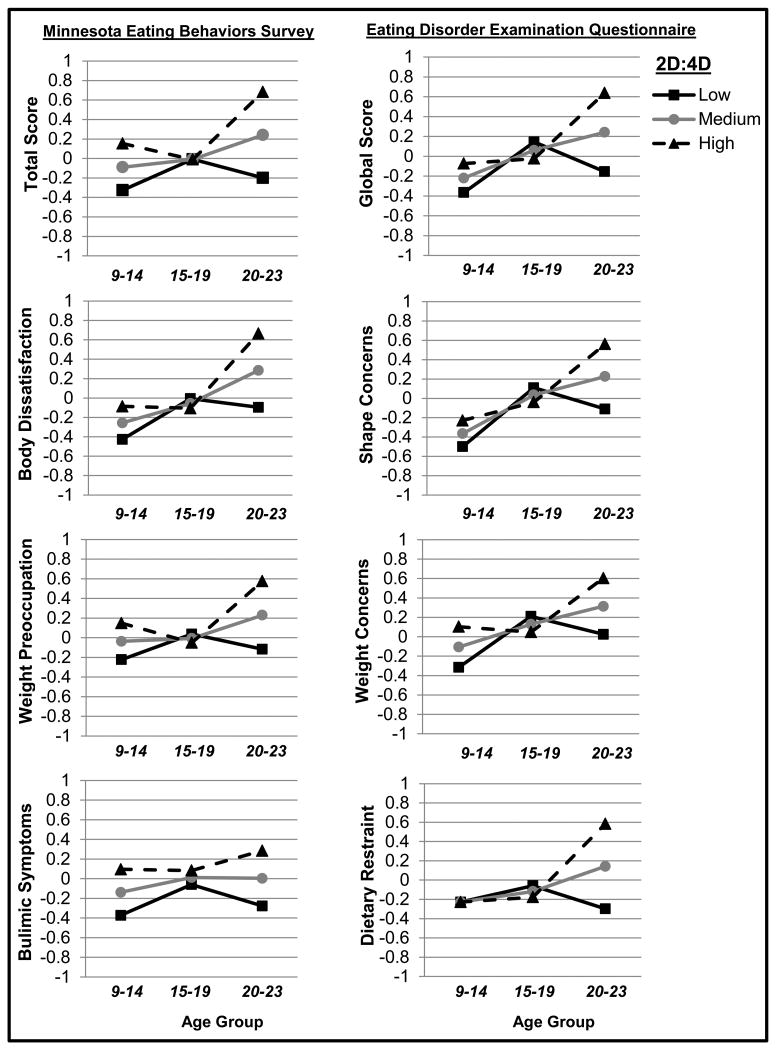
Results from mixed linear modeling analyses are presented in Table 3 and Figure 1. Age group × 2D:4D interactions supported hypotheses for most disordered eating symptoms (see Table 3 and Figure 1). Specifically, for total/global disordered eating scores and all body weight and shape symptoms, significant Age group × 2D:4D interactions were observed in comparisons between early adolescent and late adolescent groups (see Table 3, estimate for Age Group 1 × 2D:4D) as well as between young adulthood and late adolescent groups (see Table 3, estimate for Age Group 2 × 2D:4D). Thus, higher levels of prenatal testosterone exposure (i.e., lower 2D:4D ratio) predicted lower levels of overall disordered eating and attitudinal/cognitive symptoms in early adolescence and young adulthood, but not late adolescence (see Table 3 and Figure 1).
Table 3. 2D:4D by Age Group Interactions (Study 1).
| Statistical Estimates | ||
|---|---|---|
|
| ||
| Initial Model | ||
|
|
||
| Variables | b (S.E.) | p |
| Minnesota Eating Behavior Survey (MEBS) | ||
| Total Score | ||
| 2D:4D | -0.002 (0.06) | .98 |
| Age Group 1 | -0.07 (0.10) | .48 |
| Age Group 2 | 0.25 (0.17) | .15 |
| 2D:4D × Age Group 1 | 0.24 (0.11) | .03 |
| 2D:4D × Age Group 2 | 0.44 (0.17) | .01 |
| Body Dissatisfaction | ||
| 2D:4D | -0.05 (0.06) | .42 |
| Age Group 1 | -0.20 (0.10) | .06 |
| Age Group 2 | 0.34 (0.17) | <.05 |
| 2D:4D × Age Group 1 | 0.22 (0.11) | .04 |
| 2D:4D × Age Group 2 | 0.43 (0.17) | .01 |
| Bulimic Symptoms | ||
| 2D:4D | 0.07 (0.07) | .28 |
| Age Group 1 | -0.15 (0.11) | .15 |
| Age Group 2 | -0.01 (0.17) | .96 |
| 2D:4D × Age Group 1 | 0.16 (0.11) | .14 |
| 2D:4D × Age Group 2 | 0.21 (0.17) | .21 |
| Weight Preoccupation | ||
| 2D:4D | -0.04 (0.07) | .49 |
| Age Group 1 | -0.03 (0.11) | .77 |
| Age Group 2 | 0.24 (0.18) | .18 |
| 2D:4D × Age Group 1 | 0.23 (0.11) | .04 |
| 2D:4D × Age Group 2 | 0.39 (0.17) | .03 |
| Eating Disorder Examination Questionnaire (EDE-Q) | ||
| Global Score | ||
| 2D:4D | -0.08 (0.06) | .19 |
| Age Group 1 | -0.28 (0.10) | .008 |
| Age Group 2 | 0.18 (0.17) | .29 |
| 2D:4D × Age Group 1 | 0.23 (0.11) | .03 |
| 2D:4D × Age Group 2 | 0.48 (0.17) | .004 |
| Dietary Restraint | ||
| 2D:4D | -0.06 (0.06) | .35 |
| Age Group 1 | -0.11 (0.11) | .28 |
| Age Group 2 | 0.26 (0.17) | .13 |
| 2D:4D × Age Group 1 | 0.06 (0.11) | .61 |
| 2D:4D × Age Group 2 | 0.50 (0.17) | .004 |
| Shape Concerns | ||
| 2D:4D | -0.07 (0.06) | .24 |
| Age Group 1 | -0.40 (0.10) | .001 |
| Age Group 2 | 0.19 (0.17) | .28 |
| 2D:4D × Age Group 1 | 0.21 (0.11) | .05 |
| 2D:4D × Age Group 2 | 0.41 (0.17) | .02 |
| Weight Concerns | ||
| 2D:4D | -0.08 (0.06) | .21 |
| Age Group 1 | -0.24 (0.11) | .03 |
| Age Group 2 | 0.19 (0.17) | .28 |
| 2D:4D × Age Group 1 | 0.29 (0.11) | .008 |
| 2D:4D × Age Group 2 | 0.37 (0.17) | .03 |
Note: Early Adolescence = ages 9-14 (dummy coded: 1 in “Age Group 1” and 0 in “Age Group 2”), Late Adolescence = ages 15-19 (dummy coded: 0 in “Age Group 1” and “Age Group 2”); Young Adulthood = ages 20-23 (dummy coded: 0 in “Age Group 1” and 1 in “Age Group 2”). Since mixed linear modeling provides unstandardized estimates of predictor effects, the continuous 2D:4D variable was standardized prior to analysis to allow for the interpretation of unstandardized coefficients as standardized coefficients and to ease interpretation of effect sizes. Race/ethnicity was included as a covariate in these analyses but its effects were non-significant (all p's > .05).
Figure 1. Predicted Levels of Disordered Eating by 2D:4D in Each Age Group.
Y-axis values represent predicted standardized disordered eating scores, adjusted for covariates (i.e., race/ethnicity and MSUTR old/new study variables). Early adolescence = ages 9-14, late adolescence = ages 15-19, young adulthood = ages 20-23. Effects are plotted at 1 standard deviation above the 2D:4D mean (i.e., “High 2D:4D” = low prenatal testosterone exposure), at the 2D:4D mean (i.e., “Medium 2D:4D” = average prenatal testosterone exposure), and 1 standard deviation below the 2D:4D mean (i.e., “Low 2D:4D” = high prenatal testosterone exposure).
Mixed linear modeling results for dietary restraint and bulimic symptoms differed from the other disordered eating scales. Higher levels of prenatal testosterone exposure (i.e., lower 2D:4D ratio) predicted lower levels of dietary restraint in young adulthood only, i.e., not in early or late adolescence (i.e., see Table 3; Figure 1). In addition, although Pearson correlations (see Table 2) and the regression graph (see Figure 1) pointed to possible Age group × 2D:4D effects on bulimic symptoms, all mixed linear modeling main and interaction estimates were non-significant (see Table 3).
Results from Study 1 generally support the hypothesis that prenatal testosterone's protective effects (measured indirectly via 2D:4D) on several disordered eating symptoms differ across age. We aimed to replicate these results in Study 2 by using a different proxy measure (i.e., OS twin model) of variation in prenatal testosterone exposure.
Study 2: Comparisons of OS-F Twins
This study was approved by the Michigan State University Institutional Review Board and was conducted in compliance with national legislation. All participants provided written informed consent/assent.
Participants
Participants included 1,538 males and females from opposite-sex and same-sex twin pairs ages 9-30 (see Table 4) from the population-based Michigan State University Twin Registry (Klump & Burt, 2006; Burt & Klump, 2013). Participants had previously participated in the Twin Study of Hormones and Disordered Eating across Puberty (see Burt & Klump, 2013), Twin Study of Hormones and Behavior across the Menstrual Cycle (Klump et al., 2013), or Adult Twin Study of Behavioral Adjustment and Development (Culbert et al., 2008). Although a significant number of these twins (n = 1,015; 66% of total sample) were included in prior reports of prenatal testosterone's effects (via twin type comparisons) on disordered eating (i.e., Culbert et al., 2008; Culbert et al., 2013), these reports did not examine age differences in the protective effects of prenatal testosterone on disordered eating. Moreover, several newly assessed pairs (n = 57 opposite-sex twin pairs; n = 409 SS-F twins) have been added to the current sample. Because there were some mean differences on levels of disordered eating between the previously and newly assessed twins (i.e., new SS-F twins showed somewhat lower levels of disordered eating), all statistical models controlled for twin “sample” by using a “new” (coded 1) versus “old” (coded 0) study variable as a covariate in analyses (see Statistical Analyses).
Table 4. Descriptive Statistics (Study 2).
| Early Adolescence | Late Adolescence | Young Adulthood | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||||||
| Males | OS-F | SS-F | NT | Males | OS-F | SS-F | NT | Males | OS-F | SS-F | NT | |
| Sample Size (n) | 135 | 51 | 332 | 54 | 137 | 53 | 375 | 35 | 177 | 76 | 202 | 42 |
| Mean Age (SD) | 12.21 (1.30) | 12.34 (1.36) | 12.00 (1.62) | 12.86 (1.30) | 18.01 (1.71) | 17.51 (1.87) | 17.82 (1.26) | 18.11 (1.75) | 22.46 (2.31) | 22.70 (2.48) | 22.32 (2.00) | 21.56 (1.55) |
| Minnesota Eating Behavior Survey (MEBS) | ||||||||||||
| Total Score | ||||||||||||
| Range (Max = 30) | 0-19 | 0-18 | 0-24 | 0-24 | 0-21 | 0-28 | 0-29 | 1-21 | 0-19 | 0-19 | 0-28 | 2-21 |
| Mean (SD) | 4.44 | 4.59 | 5.60 | 6.81 | 4.01 | 8.21 | 6.30 | 8.21 | 3.76 | 7.03 | 8.44 | 9.89 |
| (4.59) | (4.72) | (5.17) | (5.37) | (4.03) | (6.35) | (5.57) | (5.13) | (4.16) | (5.77) | (6.35) | (5.16) | |
| % > clinical mean | 3.70 | 1.96 | 6.93 | 7.84 | 1.63 | 15.07 | 10.40 | 11.32 | 2.54 | 5.36 | 16.34 | 12.50 |
| Body Dissatisfaction | ||||||||||||
| Range (Max = 6) | 0-6 | 0-6 | 0-6 | 0-6 | 0-6 | 0-6 | 0-6 | 0-6 | 0-6 | 0-6 | 0-6 | 0-6 |
| Mean (SD) | 0.82 | 1.00 | 1.27 | 1.63 | 0.94 | 2.30 | 1.82 | 1.93 | 0.98 | 2.14 | 2.82 | 3.07 |
| (1.45) | (1.54) | (1.70) | (1.84) | (1.62) | (2.28) | (2.04) | (1.86) | (1.28) | (2.21) | (2.21) | (1.84) | |
| Bulimic Symptoms | ||||||||||||
| Range (Max = 13) | 0-8 | 0-8 | 0-9 | 0-6 | 0-8 | 0-11 | 0-12 | 0-9 | 0-7 | 0-8 | 0-12 | 0-6 |
| Mean (SD) | 1.30 | 1.42 | 1.22 | 1.36 | 1.17 | 2.08 | 1.48 | 2.22 | 1.02 | 1.84 | 2.02 | 2.24 |
| (1.69) | (1.84) | (1.68) | (1.54) | (1.42) | (2.48) | (1.88) | (1.70) | (1.28) | (2.02) | (2.35) | (1.87) | |
| Weight reoccupation | ||||||||||||
| Range (Max = 8) | 0-7 | 0-6 | 0-8 | 0-8 | 0-7 | 0-8 | 0-8 | 0-8 | 0-7 | 0-8 | 0-8 | 0-8 |
| Mean (SD) | 1.56 | 1.67 | 2.28 | 2.11 | 1.39 | 3.00 | 2.47 | 2.31 | 1.39 | 2.62 | 2.98 | 3.58 |
| (1.81) | (1.81) | (2.22) | (2.71) | (1.65) | (2.49) | (2.28) | (3.41) | (1.70) | (2.42) | (2.49) | (1.99) | |
| Eating Disorder Examination Questionnaire (EDE-Q) | ||||||||||||
| Global Score | ||||||||||||
| Range (Max = 6) | 0-4.3 | 0-3.4 | 0-4.7 | 0-4.6 | 0-2.6 | 0-4.6 | 0-5.2 | 0-5.1 | 0-3.8 | 0-4.4 | 0-5.0 | 0-4.0 |
| Mean (SD) | 0.64 | 0.73 | 0.84 | 1.10 | 0.64 | 1.55 | 1.12 | 1.42 | 0.68 | 1.29 | 1.45 | 1.73 |
| (0.81) | (0.87) | (0.98) | (1.17) | (0.72) | (1.22) | (1.16) | (1.08) | (0.87) | (1.15) | (1.20) | (1.29) | |
| % > clinical mean | 0.74 | 0.00 | 3.61 | 5.56 | 0.00 | 9.09 | 8.04 | 8.57 | 2.41 | 6.58 | 7.54 | 10.00 |
| Dietary Restraint | ||||||||||||
| Range (Max = 6) | 0-4.8 | 0-3.6 | 0-4.8 | 0-3.8 | 0-3.8 | 0-5.2 | 0-6.0 | 0-4.8 | 0-4.8 | 0-4.4 | 0-5.4 | 0-3.4 |
| Mean (SD) | 0.50 | 0.62 | 0.62 | 0.74 | 0.57 | 1.22 | 0.86 | 1.13 | 0.68 | 1.19 | 1.15 | 1.48 |
| (0.89) | (0.99) | (0.95) | (1.04) | (0.90) | (1.29) | (1.22) | (1.24) | (1.04) | (1.35) | (1.23) | (1.35) | |
| Shape Concerns | ||||||||||||
| Range (Max = 6) | 0-5.1 | 0-4.0 | 0-5.5 | 0-6.0 | 0-4.4 | 0-5.9 | 0-6.0 | 0-6.0 | 0-5.4 | 0-5.4 | 0-5.9 | 0-4.6 |
| Mean (SD) | 0.78 | 0.96 | 1.09 | 1.48 | 0.92 | 2.17 | 1.57 | 2.10 | 0.97 | 1.76 | 2.01 | 2.45 |
| (1.07) | (1.13) | (1.31) | (1.56) | (1.06) | (1.61) | (1.54) | (1.38) | (1.22) | (1.53) | (1.61) | (1.52) | |
| Weight Concerns | ||||||||||||
| Range (Max = 6) | 0-5.2 | 0-3.6 | 0-6.0 | 0-5.8 | 0-4.2 | 0-6.0 | 0-6.0 | 0-6.0 | 0-5.4 | 0-5.0 | 0-5.6 | 0-4.6 |
| Mean (SD) | 0.72 | 0.83 | 1.01 | 1.30 | 0.69 | 1.83 | 1.30 | 1.65 | 0.74 | 1.51 | 1.71 | 2.09 |
| (0.99) | (1.03) | (1.25) | (1.47) | (0.92) | (1.62) | (1.39) | (1.35) | (1.08) | (1.31) | (1.43) | (1.60) | |
Note. SS-F = same-sex female twins ; OS-F = opposite-sex female twins; NT = non-twin females reared with a brother.
Approximately 61% of twins in this study (n = 933) were recruited through birth records in collaboration with the Michigan Department of Community Health and the Michigan Bureau of Integration, Information, and Planning Services (for additional recruitment details, see Klump & Burt, 2006; Burt & Klump, 2013). The remaining twins (n = 605; 39%) were recruited through newspaper advertisements, flyers, or university registrar offices, as collaborations with the Michigan Department of Community Health and Michigan Bureau of Integration, Information, and Planning Services were not yet established at the time of recruitment. Notably, there were no significant differences in levels of disordered eating for twins recruited through birth records versus those recruited via other methods, adjusting for age (all p's > .05). Similar to Study 1 and the recruitment region (Culbert et al., 2008), the majority of twins (85%) were White and remaining twins were Black/African American (8%), Asian (2.1%), American Indian/Alaska Native (0.2%), or Bi-racial (4.7%).
In addition to the twins, we examined a sample of 131 non-twin females (ages 10-27) who were reared with at least one close-in-age biological brother (± 4 years of their own age) as an additional comparison group (see Table 4). The inclusion of non-twin females allowed us to confirm that differences in disordered eating across OS-F and other twin pairs were not due to socialization effects from being raised with a close-in-age brother. All of the non-twins have been included in prior reports (i.e., Culbert et al., 2008; Culbert et al., 2013), but again, these previously published manuscripts did not examine age differences in effects. Non-twin females were recruited in collaboration with the Michigan Department of Community Health and Michigan Bureau of Integration, Information, and Planning Services (n = 64; 49%) or through a volunteer university research subject pool (n = 67; 51%). As with the twin sample, there were no significant differences in levels of disordered eating for non-twin participants recruited through birth records versus those recruited via other methods, adjusting for age (all p's > .05). Most non-twin participants were also Caucasian (75.6%). The remaining non-twins were Black/African American (8.4%), Asian (3.1%), American Indian/Alaska Native (0.8%), or Bi-racial (12.2%).
Notably, similar to study 1, race/ethnicity was included as a covariate in analyses since mean levels of some disordered eating variables (e.g., body dissatisfaction, bulimic symptoms, dietary restraint; p's < .05) were significantly higher in White participants than other racial/ethnic groups.
Measures
Disordered Eating Symptoms
Identical to Study 1, disordered eating was assessed with the Minnesota Eating Behaviors Survey (MEBS; von Ranson et al., 2005) and the Eating Disorder Examination Questionnaire (EDE-Q; Fairburn & Beglin, 1994), with the youth-adapted version of the EDE-Q (Goldschmidt et al., 2007) administered to a subset of younger participants (9.23% of full sample). We again focused on scales showing adequate internal consistency in the full sample, both sexes, and across all ages (MEBS: α's = .73-.90; EDE-Q: α's = .70-.95) and only excluded the EDE-Q eating concerns scale from analyses (α's = .63-.81; α = .63 in males). The MEBS and EDE-Q scales were again moderately-to-highly correlated (males, mean r = .63, range r's = .33-.84; females, mean r = .69, range r's = .48-.87), but the percent overlap ranged from minimal to more substantial (r2 = 11-76% of variance shared).
Age Groupings
The sample was divided into three age groups (see Table 1) that were consistent with the groupings used in Study 1: early adolescence (ages 9-14: n = 572, M age ± SD = 12.16, ±1.52), late adolescence (ages 15-19: n = 600, M age ± SD = 17.85, ±1.47), and young adulthood (ages 20-30: n = 497, M age ± SD = 22.36, ±2.17).
Statistical Analyses
Data Preparation
Skewness and kurtosis were examined for all disordered eating variables. The MEBS bulimic symptoms score and the EDE-Q dietary restraint, weight concerns, and global scores were log transformed prior to analyses to reduce their positive skew. The MEBS and EDE-Q total scores were prorated if participants were missing 10% or fewer of the items. Scores were coded as missing for a small number of participants missing more than 10% of the items comprising MEBS (< 2% of total sample; n's = 19-32) or EDE-Q (2.3-4.5% of total sample; n's = 39-76) scales. Sample sizes therefore vary slightly across analyses.
Males from same-sex (monozygotic, n = 156, dizygotic, n = 109) and opposite-sex twin pairs were combined in analyses to minimize the overall number of comparisons and to maximize the sample size in the male group. Monozygotic and dizygotic twins from same-sex female twin pairs (monozygotic, n = 493, dizygotic, n = 416) were also combined to maximize the sample size in the SS-F group. Importantly, the aggregation of data in these ways is unlikely to have unduly influenced our results; males from same-sex and opposite-sex twin pairs showed no significant differences on most disordered eating variables (d's = .02-.29; mean d = .12)4. Mean levels of disordered eating also did not significantly differ by zygosity in males (p's > .05; d's = .02-.21, mean d = .11) or females (p's > .05; d's = .00-.09, mean d = .03) from same-sex twin pairs. Finally, findings did not differ when analyses used only males from same-sex twin pairs or males from opposite-sex twin pairs, or when using only monozygotic versus only dizygotic same-sex twins (data available upon request).
Twin Type Comparisons
Mixed linear modeling was used to examine whether prenatal testosterone's protective effects (measured indirectly via twin type comparisons) on disordered eating vary across age groups: early adolescence, late adolescence, and young adulthood. Mixed linear modeling is an ideal statistical method since the non-independence of dyadic twin data could be accounted for by nesting the lower-level unit (i.e., twin identification number) within an upper-level unit (i.e., family identification number that is shared by co-twins). Mean differences on the MEBS and EDE-Q scales were examined as a function of twin type (i.e., males, OS-F, SS-F, and female non-twins) and age group (i.e., early adolescence, late adolescence, young adulthood), covarying for twin sample (i.e., “new” versus “old” study data) and race/ethnicity. Elevated prenatal testosterone exposure was expected to be protective against disordered eating symptoms (i.e., OS-F would exhibit lower levels of disordered eating than other females) in all age groups except the late adolescent group.
Statistical models first tested the age group main effect, twin type main effect, and the interaction between age group × twin type on levels of disordered eating. In this model, a main effect of twin type would suggest that levels of disordered eating differ between twin type groups, and a main effect of age group would suggest that levels of disordered eating differ across age. A significant interaction was of particular interest since this would suggest that the influence of twin type on disordered eating varies by age. Notably, when there was a significant age group × twin type interaction, pairwise comparisons (i.e., OS-F twins versus other groups: males, SS-F twins, and non-twin females) were specified within the interaction models to identify the twin type groups that significantly differed from each other within each age group. Cohen's d was also computed to provide a standardized measure of effect sizes for twin type mean differences on disordered eating variables.
Results
Descriptive Statistics
A range of disordered eating attitudes and behaviors were endorsed by participants within each age group (see Table 4 and 6). A number of participants also scored above the clinical mean on the MEBS (i.e., total score M ≥ 15.55, von Ranson et al., 2005) and EDE-Q (i.e., global score M ≥ 3.46, Aardoom et al., 2012; see Table 4). Thus, similar to study 1, the MEBS and EDE-Q scales captured a spectrum of severity and this study had ample variability to detect the protective effects of prenatal testosterone (i.e., lower disordered eating in OS-F twins as compared to other females) on disordered eating symptoms in each age group.
Table 6. Twin Type Differences on Mean Levels of Disordered Eating in Each Age Group (Study 2).
| Main Effect Twin Type |
Pair-wise Comparisons | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| t-value | Cohen's d | ||||||||||
|
| |||||||||||
| Mean (S.E.) | OS-F | OS-F | |||||||||
| vs. | vs. | ||||||||||
| Males | OS-F | SS-F | NT | F (3, 1579-1636) | Males | SS-F | NT | Males | SS-F | NT | |
| Early Adolescence: Ages 9-14 | |||||||||||
| Minnesota Eating Behavior Survey (MEBS) | |||||||||||
| Total Score | 2.73 (0.50) | 2.91 (0.76) | 5.02 (0.32) | 5.25 (0.75) | 7.42*** | 0.22 | -2.64** | -2.28* | .03 | .36 | .42 |
| Body Dissatisfaction | 0.24 (0.18) | 0.44 (0.27) | 1.07 (0.11) | 1.11 (0.26) | 7.10*** | 0.67 | -2.23* | -1.84† | .10 | .32 | .35 |
| Bulimic Symptoms | 0.75 (0.18) | 0.86 (0.27) | 1.01 (0.11) | 0.85 (0.26) | 0.66 | 0.37 | -0.54 | 0.04 | .05 | .08 | .01 |
| Weight Preoccupation | 1.05 (0.21) | 1.17 (0.31) | 2.13 (0.13) | 2.24 (0.31) | 9.66*** | 0.38 | -2.93** | -2.55* | .05 | .41 | .47 |
| Eating Disorders Examination Questionnaire (EDE-Q) | |||||||||||
| Global Score | 0.29 (0.10) | 0.39 (0.15) | 0.73 (0.06) | 0.78 (0.15) | 7.46*** | 0.59 | -2.33* | -2.00* | .09 | .31 | .36 |
| Dietary Restraint | 0.15 (0.11) | 0.28 (0.16) | 0.50 (0.07) | 0.43 (0.16) | -- | -- | -- | -- | -- | -- | -- |
| Shape Concerns | 0.28 (0.13) | 0.47 (0.20) | 0.95 (0.08) | 1.03 (0.20) | 8.34*** | 0.84 | -2.26* | -2.06* | .13 | .33 | .38 |
| Weight Concerns | 0.33 (0.12) | 0.46 (0.19) | 0.89 (0.08) | 0.94 (0.19) | 8.01*** | 0.74 | -2.33* | -1.94† | .09 | .30 | .35 |
| Late Adolescence: Ages 15-19 | |||||||||||
| Minnesota Eating Behavior Survey (MEBS) | |||||||||||
| Total Score | 2.45 (0.49) | 6.68 (0.75) | 5.98 (0.30) | 6.91 (0.93) | 17.20*** | 5.03*** | 0.88 | -0.20 | .74 | .12 | .04 |
| Body Dissatisfaction | 0.42 (0.18) | 1.82 (0.27) | 1.71 (0.11) | 1.41 (0.32) | 15.50*** | 4.64*** | 0.39 | 1.02 | .67 | .05 | .21 |
| Bulimic Symptoms | 0.67 (0.17) | 1.59 (0.26) | 1.36 (0.10) | 1.77 (0.31) | 6.47*** | 3.13** | 0.83 | -0.46 | .47 | .12 | .10 |
| Weight Preoccupation | 0.92 (0.20) | 2.54 (0.31) | 2.40 (0.12) | 2.99 (0.39) | 17.80*** | 4.68*** | 0.43 | -0.95 | .70 | .06 | .20 |
| Eating Disorders Examination Questionnaire (EDE-Q) | |||||||||||
| Global Score | 0.31 (0.11) | 1.21 (0.16) | 1.09 (0.06) | 1.13 (0.18) | 18.29*** | 5.08*** | 0.75 | 0.08 | .71 | .10 | .07 |
| Dietary Restraint | 0.25 (0.11) | 0.88 (0.17) | 0.80 (0.06) | 0.85 (0.20) | -- | -- | -- | -- | -- | -- | -- |
| Shape Concerns | 0.46 (0.14) | 1.68 (0.21) | 1.53 (0.08) | 1.68 (0.24) | 18.60*** | 5.06*** | 0.66 | -0.02 | .75 | .10 | .00 |
| Weight Concerns | 0.33 (0.13) | 1.44 (0.20) | 1.26 (0.07) | 1.32 (0.22) | 17.67*** | 4.97*** | 0.67 | 0.08 | .73 | .13 | .09 |
| Young Adulthood: Ages 20-30 | |||||||||||
| Minnesota Eating Behavior Survey (MEBS) | |||||||||||
| Total Score | 2.08 (0.46) | 5.35 (0.64) | 7.36 (0.40) | 8.32 (0.82) | 36.31*** | 4.54*** | -2.85** | -2.99** | .56 | .35 | .54 |
| Body Dissatisfaction | 0.42 (0.17) | 1.58 (0.23) | 2.45 (0.14) | 2.55 (0.29) | 40.00*** | 4.51*** | -3.43*** | -2.73** | .58 | .43 | .49 |
| Bulimic Symptoms | 0.49 (0.16) | 1.29 (0.23) | 1.66 (0.14) | 1.74 (0.28) | 14.35*** | 3.21** | -1.48 | -1.30 | .39 | .19 | .23 |
| Weight Preoccupation | 0.88 (0.19) | 2.12 (0.26) | 2.67 (0.17) | 3.11 (0.35) | 24.78*** | 4.15*** | -1.88† | -2.38* | .51 | .23 | .43 |
| Eating Disorders Examination Questionnaire (EDE-Q) | |||||||||||
| Global Score | 0.33 (0.09) | 0.95 (0.13) | 1.24 (0.08) | 1.40 (0.17) | 29.64*** | 4.80*** | -2.05* | -1.99* | .54 | .25 | .40 |
| Dietary Restraint | 0.33 (0.10) | 0.84 (0.13) | 0.93 (0.08) | 1.15 (0.18) | -- | -- | -- | -- | -- | -- | -- |
| Shape Concerns | 0.48 (0.12) | 1.27 (0.17) | 1.70 (0.11) | 1.98 (0.23) | 27.98*** | 4.25*** | -2.32* | -2.62** | .51 | .28 | .48 |
| Weight Concerns | 0.34 (0.11) | 1.13 (0.15) | 1.48 (0.10) | 1.73 (0.21) | 32.05*** | 5.36*** | -1.72† | -1.90† | .57 | .25 | .45 |
Note. SS-F = same-sex female twins; OS-F = opposite-sex female twins; NT = non-twin females; df = degrees of freedom; S.E. = standard error; Statistical results for MEBS bulimic symptoms and EDE-Q dietary restraint, weight concerns, and global score used log-transformed scores, but untransformed means and standard errors are presented to ease interpretation. Main effects and pair-wise comparisons are not presented for dietary restraint given the non-significant Age Group × Twin Type interaction (see Table 5). Race/ethnicity and a variable representing “old” versus “new” MSUTR data (old = 0 vs. new = 1) were included as covariates, and both were significant predictors (p < .05) in all models.
p ≤ .10,
p < .05,
p < .01,
p < .001
Twin Type Comparisons
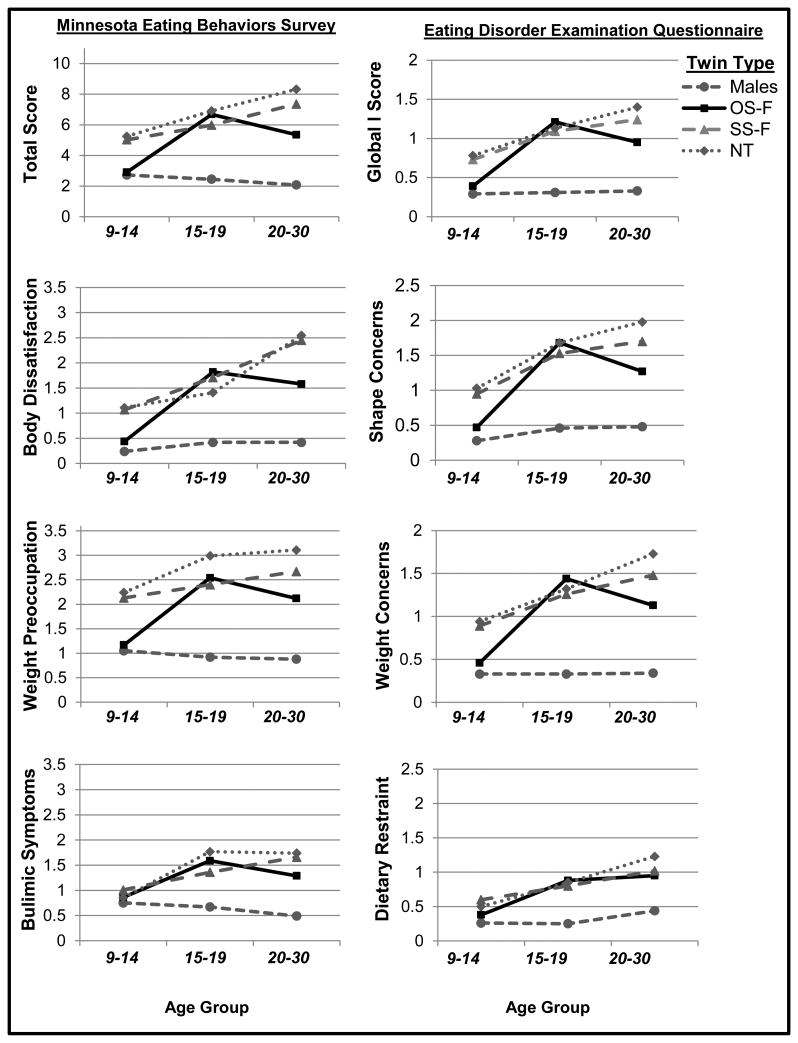
Mixed linear modeling models indicated a significant age group × twin type interaction (see Table 5) for nearly all disordered eating variables (with the exception of dietary restraint – see below). Pairwise comparisons within each age group suggested that prenatal testosterone's protective effects on disordered eating in OS-F twins differs across age, with effects generally present in early adolescence and young adulthood but not late adolescence (see Table 6 and Figure 2). Specifically, in early adolescence and young adulthood, OS-F twins scored lower on overall levels of disordered eating (i.e., total/global scores) and attitudinal/cognitive symptoms (e.g., body weight and shape concerns) than SS-F twins (d's =.20-.43), and scored similarly (d's = .03-.11 in early adolescence) or slightly higher (d's = .42-.58 in young adulthood) than the male twins. Moreover, the OS-F twins exhibited significantly lower disordered eating scores than the non-twin females raised with a brother during these same developmental stages (d's = .21-.53), suggesting that socialization effects of growing up with a brother are unlikely to account for lower levels of disordered eating in OS-F twins.
Table 5. Twin Type by Age Group Interactions (Study 2).
| Statistical Estimates | |||
|---|---|---|---|
|
| |||
| Initial Model | |||
|
|
|||
| Variables | F (df) | p | |
| Minnesota Eating Behavior Survey (MEBS) | |||
| Total Score | |||
| Twin Type | 47.43 (3, 1089) | <.001 | |
| Age Group | 11.24 (2, 1240) | <.001 | |
| Twin Type × Age Group | 4.47 (6, 1109) | <.001 | |
| Body Dissatisfaction | |||
| Twin Type | 47.28 (3, 1021) | <.001 | |
| Age Group | 26.39 (2, 1163) | <.001 | |
| Twin Type × Age Group | 4.81 (6, 1028) | <.001 | |
| Bulimic Symptoms | |||
| Twin Type | 15.77 (3, 1115) | <.001 | |
| Age Group | 9.95 (2, 1261) | <.001 | |
| Twin Type × Age Group | 2.97 (6, 1133) | .007 | |
| Weight Preoccupation | |||
| Twin Type | 44.01 (3, 1044) | <.001 | |
| Age Group | 7.07 (2, 1185) | .001 | |
| Twin Type × Age Group | 2.35 (6, 1065) | .03 | |
| Eating Disorder Examination Questionnaire (EDE-Q) | |||
| Global Score | |||
| Twin Type | 41.33 (3, 947) | <.001 | |
| Age Group | 16.77 (2, 1114) | <.001 | |
| Twin Type × Age Group | 3.08 (6, 950) | .005 | |
| Dietary Restraint | |||
| Twin Type | 19.21 (3, 1074) | <.001 | |
| Age Group | 16.72 (2, 1254) | <.001 | |
| Twin Type × Age Group | 1.25 (6, 1102) | .28 | |
| Shape Concerns | |||
| Twin Type | 45.91 (3, 940) | <.001 | |
| Age Group | 24.57 (2, 1107) | <.001 | |
| Twin Type × Age Group | 2.72 (6, 942) | .01 | |
| Weight Concerns | |||
| Twin Type | 42.18 (3, 911) | <.001 | |
| Age Group | 15.08 (2, 1081) | <.001 | |
| Twin Type × Age Group | 3.16 (6, 919) | .004 | |
Note: MEBS = Minnesota Eating Behaviors Survey; EDE-Q = Eating Disorders Examination Questionnaire. Race/ethnicity and a variable representing “old” versus “new” MSUTR data (old = 0 vs. new = 1) were included as covariates, and both were significant predictors (p < .05) in all models.
Figure 2. Mean Levels of Disordered Eating by Twin Type and Age Groups.
Early adolescence = ages 9-14, late adolescence = ages 15-19, young adulthood = ages 20-30. Y-axis values represent mean disordered eating scores, adjusted for covariates (i.e., race/ethnicity and MSUTR old/new study variables). OS-F = opposite-sex female twins; SS-F = same-sex female twins; NT = non-twin females.
By contrast, in late adolescence, there was no evidence for prenatal testosterone's protective effects on any disordered eating variable in OS-F twins. Mean levels of disordered eating symptoms did not significantly differ between OS-F twins, SS-F twins, or non-twin females, and the OS-F scores were significantly higher than those of males (see Table 6 and Figure 2). Taken together, these results strongly suggest that prenatal testosterone's effects vary by age, with protective effects on several disordered eating symptoms in early adolescence and young adulthood and negligible effects during late adolescence.
Notably, more variable results were observed for prenatal testosterone's protective effects on dietary restraint and bulimic symptoms. There was no clear evidence for a prenatal testosterone effect on dietary restraint in any age group, and prenatal testosterone's protective effects on bulimic symptoms were somewhat evident in young adulthood but were small in magnitude (e.g., OS-F vs. SS-F, d = .18; OS-F vs. non-twin females, d = .21; p-values were non-significant in pairwise comparisons).
Overall, similar to Study 1, findings from Study 2 suggest that prenatal testosterone's protective effects (measured indirectly via twin type) on disordered eating symptoms differ across age, with effects generally present during early adolescence and young adulthood but not during late adolescence.
Discussion
This series of studies aimed to reconcile prior inconsistent findings in the literature by examining whether age moderates prenatal testosterone's protective effects on disordered eating symptoms. Using two different biomarkers of prenatal testosterone exposure (i.e., 2D:4D finger-length ratios and twin type comparisons) and two well-validated measures of eating pathology (i.e., MEBS and EDE-Q), we found that prenatal testosterone's protective effects on overall levels of disordered eating symptoms and attitudinal/cognitive symptoms (e.g., weight preoccupation, body dissatisfaction) differ as a function of age. Specifically, the protective effects of prenatal testosterone on disordered eating tend to be evident in early adolescence and young adulthood but not in late adolescence. Overall, these data suggest that age-related effects may account for previous inconsistent results in the literature, and thus, point to the possibility of developmental windows of expression for the protective effects of prenatal testosterone on disordered eating risk.
The consistency in effects observed for total scores and attitudinal/cognitive symptoms suggests that prenatal testosterone's protective effects on these constructs are robust. However, it is important to note that more variable results were found for behavioral symptoms, including dietary restraint and bulimic symptoms. For example, 2D:4D analyses suggested that prenatal testosterone exerts protective effects on dietary restraint, but only in young adulthood, whereas there was no clear evidence for prenatal testosterone's effects on dietary restraint in any age group using the OS-F twin design. Further, 2D:4D analyses (i.e., correlations and Figure 1) pointed to the possibility that higher levels of prenatal testosterone may predict lower levels of bulimic symptoms in early adolescence and young adulthood but effects did not reach significance, and prenatal testosterone's protective effects on bulimic symptoms were somewhat evident using the OS-F twin design but only in young adulthood. The tendency to obtain more inconsistent results with behavioral symptoms of disordered eating is in line with prior research, as stronger and/or more consistent effects have been observed with total/global scores of disordered eating (Culbert et al., 2008; Culbert et al., 2013; Smith et al., 2010) or cognitive/attitudinal symptoms (Culbert et al., 2013; Oinonen & Bird, 2012; Raevuori et al., 2008) than behavioral symptoms or diagnoses (Lydecker et al., 2012; Quinton et al., 2011; Raevuori et al., 2008). Measurement issues may at least partially explain this pattern, as self-report measures of both dietary restraint (Stice, Fisher, & Lowe, 2004; Stice, Cooper, Schoeller, Tappe, & Lowe, 2007) and key components of bulimic symptoms (e.g., binge eating; Fairburn & Beglin, 1994) can be problematic (e.g., over-reporting; Fairburn & Beglin, 1994; Stice et al., 2004; Stice et al., 2007). The weaker psychometric properties of these scales (e.g., α's ≃ .70) relative to total/global scores and cognitive/attitudinal scales (e.g., α's > .80), also suggest that measurement issues may have contributed to inconsistent results and attenuated any age effects that might be present. Additional psychometric work and research using interview-based assessments and/or additional measures of these constructs is needed to confirm whether prenatal testosterone exerts protective effects on these symptoms, and if so, whether effects vary by age.
Nonetheless, the bulk of our data suggest that prenatal testosterone's protective effects on disordered eating show developmental differences for the majority of scales, and thus may account for previously discrepant findings in the literature. A lack of evidence for prenatal testosterone's protective effects on disordered eating during late adolescence is consistent with prior research showing no differences between OS-F and SS-F twins on levels of several disordered eating symptoms (e.g., overall levels of disordered eating, bulimic symptoms, drive for thinness) during this time period (i.e., Baker et al., 2009). However, in both early adolescence (Culbert et al., 2013) and young adulthood (e.g., Culbert et al., 2008; Klump et al., 2006; Smith et al., 2009), significant effects have been observed using the 2D:4D ratio or OS-F twin design. Interestingly, our findings may also help to explain the lack of evidence for prenatal testosterone's protective effects on lifetime prevalence rates of eating disorder diagnoses, particularly when measured in middle adulthood (e.g., Lydecker et al., 2013). Lifetime prevalence rates are cumulative and only index the history of having had an eating disorder diagnosis at some point during one's lifetime. Thus, lifetime prevalence rates are unable to document variation in new onset cases across developmental stages. Examining changes in eating disorder incidence across age would more precisely capture developmental (e.g., age-related) differences in the onset of new eating disorder cases.
While our findings are novel and point to possible developmental windows of expression for prenatal testosterone's protective effects on disordered eating, a lingering question remains: how or why might this occur? The specific mechanisms contributing to the observed age effects are currently unknown; however, the phenotypic expression of all traits involves complex interactions between genetic/biological influences and environmental factors. In the case of eating disorders, it may be that prenatal testosterone exerts protective effects on eating pathology, but these effects are “trumped” in females during late adolescence when a myriad of risk factors for eating disorders likely come on-line. Indeed, prior research shows increases in risk for disordered eating in females during early to late adolescence (Abebe et al., 2012a; Abebe et al., 2012b; Attie & Brooks-Gunn, 1989; Calam & Waller, 1998; Jones et al., 2001; Lewinsolm et al., 2000; Steinhusen et al., 2005; Stice et al., 1998; Stice et al., 2009; Stice et al., 2013) and that late adolescence is the peak period of risk for disordered eating symptoms and the onset of eating disorders (e.g., Abebe et al., 2012b; Lewinsolm et al., 2000; Steinhusen et al., 2005; Stice et al., 2009). These developmental data, in combination with the results from our studies, indirectly suggest that prenatal testosterone's protective effects on disordered eating may be most strongly expressed under “average” risk periods (i.e., during early adolescence and young adulthood) and attenuated during peak risk periods when other risk factors predominate.
Moving forward, it will be important to identify the risk factors that attenuate the protective effects of prenatal testosterone during the late adolescent period. One possibility is that new sources of environmental risk or age-specific environmental risk factors emerge during late adolescence, as demonstrated in biometric twin research (Klump, Burt, McGue, & Iacono, 2007; Wade et al., 2013), and serve to override or suppress prenatal testosterone's protective effects on disordered eating. Nonetheless, univariate biometric twin models estimate latent environmental effects and cannot identify the specific environmental factors at play unless these factors are also measured. Studies (e.g., biometric twin models) that directly explore developmental shifts in risk factors contributing to disordered eating symptoms will be an important next step. Potential candidates to explore as developmental moderators of prenatal testosterone's effects on disordered eating include factors that increase during late adolescence, e.g., pressures to develop autonomy, importance and pursuit of the thin ideal, and time spent with peers or dating partners (Stice et al., 2009; Stice, Ng, & Shaw, 2010; Field et al., 2001).
Another possibility is that the factors serving to attenuate the protective effects of prenatal testosterone on disordered eating during late adolescence may not be purely “environmental.” There are marked changes in neural systems and social and emotional processing during late adolescence (Jones et al., 2014; Somerville, 2013). Moreover, relative to pre-adolescents and young adults, striking behavioral changes that could contribute to heightened risk for disordered eating symptoms, e.g., elevations in social interaction and motivational drive for peer acceptance, occur during adolescence in a range of species (e.g., Douglas, Varlinskaya, & Spear, 2004; Jones et al., 2014; Somerville, 2013). Late adolescence is also a developmental period of heightened social sensitivity (Somerville et al., 2013), as adolescents show elevated levels of self-consciousness, activation of socioaffective neural circuitry, and autonomic arousal in response to perceived social evaluation as compared to pre-adolescents and young adults (Somerville et al., 2013). Speculatively, these non-linear patterns of developmental changes in neural, social, and emotional functioning could heighten vulnerability to disordered eating symptoms in females during late adolescence, over and above any protective effects of prenatal testosterone exposure. Future studies should aim to directly investigate whether these biopsychosocial risk mechanisms contribute to developmental shifts in prenatal testosterone-disordered eating associations.
Importantly, while our data are consistent with prior findings indicating protective effects of prenatal testosterone on disordered eating symptoms in early adolescence (Culbert et al., 2013) and young adulthood (e.g., Culbert et al., 2008, Klump et al., 2006, Smith et al., 2009) but not in late adolescence (Baker et al., 2009), our results could be viewed as inconsistent with findings from studies that examined eating disorder diagnoses (e.g., Quinton et al., 2008; Lydecker et al., 2013). As noted above, the use of lifetime prevalence rates that do not account for developmental effects may have hindered the ability to detect prenatal testosterone effects on eating disorder diagnoses; however, it is also possible that prenatal testosterone may exert protective effects on disordered eating symptoms but not clinical eating disorders and/or that the direction (risk vs. protection) of prenatal testosterone's effects may differ between eating disorder diagnoses. Nonetheless, such specificity effects are not strongly or consistently supported by current data. For example, findings from Quinton et al. (2011) indicated that elevated prenatal testosterone exposure may be protective against bulimia nervosa yet increase risk for anorexia nervosa, whereas other studies have pointed to opposite effects in anorexia nervosa, i.e., that low prenatal testosterone may increase risk for broadly defined anorexia nervosa (Raevuori et al., 2008), or failed to detect prenatal testosterone-eating disorder associations in clinical populations (Lydecker et al., 2013). In addition, disordered eating symptoms and clinical eating disorders fall on the same continuum (Mintz, O'Halloran, Mulholland, & Schneider, 1997; Tylka & Subich, 1999) – disordered eating symptoms are strong precursors to the later onset of an eating disorder (Jacobi et al., 2004). Several disordered eating symptoms (e.g., weight preoccupation, binge eating; American Psychiatric Association, 2013) are also shared across the eating disorders and there is high diagnostic cross-over between diagnoses (Eddy et al., 2008; Stice et al., 2009). Thus, mixed findings from studies that have examined clinical diagnoses (e.g., Quinton et al., 2011; Raevuori et al., 2008; Lydecker et al., 2013) may be better explained by low statistical power due to low prevalence rates (e.g., 0.3-3.5%; Hudson et al., 2007; Swanson et al., 2011) and/or the inability to account for possible age effects, rather than specificity of prenatal testosterone's effects on eating disorder diagnoses and/or disordered eating symptoms (Raevuori et al., 2014).
In addition, the current series of studies explored age differences in prenatal testosterone's effects on disordered eating, but whether additional factors also serve to enhance or attenuate prenatal testosterone's effects on disordered eating is largely unknown. Sexual orientation and autistic traits (e.g., high systemizing scores; social skills deficits) are two factors that have been linked to prenatal testosterone (e.g., via 2D:4D; Breedlove, 2010; De Bruin, De Nijs, Verheij, Verhagen, & Ferdinand, 2009; Manning, Baron-Cohen, Wheelwright, & Sanders, 2001) and eating pathology (Baron-Cohen et al., 2013; Jones & Morgan, 2010; Zucker et al., 2007) and could therefore moderate prenatal testosterone-disordered eating associations. Data to support such effects is somewhat limited. Higher prenatal testosterone (lower 2D:4D) has been associated with lower levels of disordered eating in males, irrespective of sexual orientation (Smith et al., 2009). Conversely, in women, 2D:4D-disordered eating associations have been shown to differ by sexual orientation; higher prenatal testosterone (i.e., lower 2D:4D) was associated with lower levels of disordered eating in heterosexual women but not lesbian/bisexual women (Oinonen et al., 2012). These data are important in suggesting that our prenatal testosterone-disordered eating findings may only be relevant to heterosexual girls/women, and the inability to adjust for sexual orientation in our studies, at least in females, could have attenuated the magnitude of 2D:4D-disordered eating associations (e.g., Study 1). However, given that ∼96.5% of our sample would be expected to be of heterosexual orientation (based on population estimates: Gates, 2011; Ward, Dahlhamer, Galinsky, & Joestl, 2014), it is unlikely that our results would be substantially altered. Future research should continue to investigate links between prenatal testosterone and disordered eating using large diverse samples (e.g., in terms of sexual orientation, race/ethnicity, etc.) to further delineate such effects. It may also be important for future studies to investigate whether autistic features alter the direction or magnitude of prenatal testosterone-disordered eating associations. Higher levels of prenatal testosterone (e.g., lower 2D:4D) have been associated with autistic features (e.g., De Bruin et al., 2009; Manning et al., 2001) and autistic features have been linked to anorexia nervosa (Zucker et al., 2007). Such effects have led some researchers to speculate that high prenatal testosterone may increase risk (rather than decrease risk) for anorexia nervosa (e.g., Quinton et al., 2011), but as noted above, current data do not provide strong support for this possibility (see mixed findings: Lydecker et al., 2013; Raevuori et al., 2008; Quinton et al., 2011). Additional studies that directly explore relationships between prenatal testosterone, autistic features, and eating pathology are needed. Moreover, since several social/interpersonal and cognitive deficits are known to occur as a consequence of the ill-state of anorexia nervosa (e.g., severe low weight), it may be beneficial for future studies to examine prenatal testosterone, autistic features, and eating pathology associations in individuals with a history of anorexia nervosa (e.g., weight restored and/or recovered) or via the use of dimensional measures of disordered eating in large community-based samples.
Several limitations of this study must be acknowledged. First, data were cross-sectional, and thus, this study cannot confirm that age-related differences in the magnitude of prenatal testosterone's effects directly reflect developmental changes. Longitudinal studies are needed to confirm the presence of within-person developmental shifts in prenatal testosterone's protective effects on disordered eating symptoms and to identify which putative risk factors may account for such changes. Second, finger-length ratios (i.e., 2D:4D) and twin type (e.g., OS-F twins) were used as indirect measures of prenatal testosterone exposure, given that direct measures of prenatal hormones are difficult to obtain. It is therefore important to reiterate that our methods are not completely synonymous with prenatal testosterone exposure and that other factors (e.g., genetic influences independent of hormone secretion; Gobrogge, Breedlove, & Klump, 2008) would also be expected to contribute to between-person variability in 2D:4D and phenotypic differences between females from opposite-sex versus same-sex twin pairs (Wallen, 2009). Nonetheless, to our knowledge, there is currently no other hypothesized mechanism that has been shown to account for as much variance in 2D:4D as prenatal hormone exposure (e.g., Lutchmaya et al., 2004; Zheng et al., 2011), and the general consistency of results across two different methodologies speaks to the potential strength of our findings. Future studies should, however, aim to replicate our effects using other models of prenatal testosterone exposure (e.g., girls with congenital adrenal hyperplasia) and continue to identify other biological factors (e.g., ovarian hormones, neurotransmitter systems; Klump et al., 2013; Zheng, 2009) that act independent of and/or in combination with prenatal hormone exposure to contribute to within-sex and between-sex differences in disordered eating.
Third, both studies used a community-based sample of twins that were not clinically screened for or diagnosed with an eating disorder, and thus it remains unknown whether findings generalize to clinical populations. Nonetheless, our findings likely have etiologic relevance. A wide range of disordered eating symptoms were examined, including those (e.g., body dissatisfaction, weight preoccupation) that are the strongest precursors to the development of eating disorders (Jacobi et al., 2004). However, it is also important to reiterate that capturing age moderation of prenatal testosterone's effects on eating disorder diagnoses would be challenging. Lifetime prevalence rates of eating disorder diagnoses, which have been used in past studies (e.g., Lydecker et al., 2012; Quinton et al., 2011; Raevuori et al., 2008), are cumulative estimates that do account for developmental changes in risk. Testing our hypotheses using clinical diagnoses would therefore require large sample sizes and/or the assessment of age-specific incidence or prevalence rates, from early adolescence to young adulthood.
Fourth, disordered eating symptoms were assessed with self-report questionnaires that are psychometrically valid and appropriate for use across the age spectrum of participants (e.g., ages 9-30). The use of self-report questionnaires allowed for the assessment of several key disordered eating symptoms; however, as noted above, the use of self-report measures in the assessment of dietary restraint and bulimic symptoms (e.g., binge eating) can be problematic, such as over-reporting of symptoms (Fairburn & Beglin, 1994; Stice et al., 2004; Stice et al., 2007). Future studies may benefit from utilizing additional methodological approaches, such as interview-based assessments and/or experimental eating behavior paradigms (e.g., feeding tests; Goldschmidt, Tanofsky-Kraff, & Wilfley, 2011; Zandian, Ioakimidis, Bergh, Leon, & Sodersten, 2011), as doing so may enhance our understanding of prenatal testosterone's protective effects on disordered eating behaviors, particularly for constructs (e.g., dietary restriction, loss of control) that are difficult to assess via self-report.
Finally, we were unable to investigate other developmental periods (e.g., < age 9 or ≥ 30 years), and thus, cannot rule out whether the expression of prenatal testosterone's effects on disordered eating symptoms also differ at other ages. Disordered eating disturbances are present in childhood/pre-adolescence (e.g., < age 9; Shapiro, Newcomb, & Burns Loeb, 1997; Wood, Becker, & Thompson, 1996) and later adulthood (e.g., > age 30; Gagne et al., 2012; Mangweth-Matzek et al., 2013), but symptom rates are relatively lower than other developmental periods. For example, disordered eating symptoms tend to increase in females from childhood to adolescence (e.g., during puberty; Ferreiro, Seoane, & Senra, 2011; Shapiro et al., 1997), and reductions in several disordered eating symptoms are observed in women across young adulthood (Healtherton, Mahamedi, Striepe, Field, & Keel, 1997) and into midlife (Keel, Baxter, Heatherton, & Joiner, 2007; Rizvi, Stice, & Agras, 1999). Thus, prenatal testosterone's protective effects on disordered eating may be evident during pre-adolescence and adulthood/mid-life since these are not “high risk” periods; however, an alternative possibility is that prenatal testosterone's protective effects on disordered eating are negligible during these developmental periods given that the magnitude of within-sex differences (i.e., individual differences) and between-sex differences (i.e., males versus females) on disordered eating are smaller. Additional research is needed to support these hypotheses and to fully elucidate developmental changes in prenatal testosterone's protective effects on disordered eating symptoms across the lifespan.
Acknowledgments
Funding/Support: The research was supported by the National Institute of Mental Health: 1R21-MH070542-01 (KLK, CLS), 1R03MH63851-01 (KLK), 1R01-MH0820-54 (KLK, SAB, CLS, PKK, MCN, SMB), 1R01-MH092377-01 (KLK, SAB, CLS), F31-MH084470 (KMC), T32-MH070343 (KMC), and T32-MH082761 (KMC); the Michigan State University Intramural Grants Program (71-IRGP-4831;KLK) and College of Social Science Faculty Initiatives Fund (KLK), Graduate Student Research Enhancement Award (KMC), and John Hurley Endowed Fellowship (KMC); the Hilda and Preston Davis Foundation (KMC); the Academy for Eating Disorders Student Research Grant (KMC); Blue Cross Blue Shield of Michigan Dissertation Research Award (1412.SAP; KMC); American Psychological Association Dissertation Research Award (KMC); American Psychological Foundation Clarence J. Rosecrans Scholarship (KMC). We thank Dr. Joel T. Nigg for his contribution to the data collection of this project via 1R21-MH070542-01 and Dr. Molly Nikolas for providing statistical/graphing consultation. The content is solely the responsibility of the authors and does not necessarily represent the official views of these granting agencies. All authors had full access to the data and take responsibility for the integrity of the data and accuracy of the data analysis.
Footnotes
A re-analysis of Raevuori et al. (2008) using mixed linear models to account for the non-independence of twin data resulted in an additional statistical trend (p = .09) towards lower mean levels of body dissatisfaction in OS-F twins as compared to SS-F twins (personal communication, January 2009).
The Minnesota Eating Behavior Survey (MEBS; previously known as the Minnesota Eating Disorder Inventory (M-EDI)) was adapted and reproduced by special permission of Psychological Assessment Resources, Inc., 16204 North Florida Avenue, Lutz, Florida 33549, from the Eating Disorder Inventory (collectively, EDI and EDI-2) by Garner, Olmstead, Polivy, Copyright 1983 by Psychological Assessment Resources, Inc. Further reproduction of the MEBS is prohibited without prior permission from Psychological Assessment Resources, Inc.
Post-hoc analyses were conducted using the left hand 2D:4D to ensure that our exclusion of the left hand did not unduly alter the conclusions of this study. Pearson correlations and mixed linear model results using the left hand 2D:4D were largely similar to those attained with the right hand, albeit somewhat weaker in magnitude (data available upon request).
Differences in prenatal testosterone exposure via in utero effects can be more difficult to detect in male animals and humans, as opposed to females, since all males are naturally exposed to high levels of testosterone during prenatal development (e.g., Miller, 1994; Ryan & Vandenbergh, 2002). Nonetheless, when mean differences (d's = .20-.29) on disordered eating variables were observed between males from same-sex versus opposite-sex twin pairs, effects were in the expected direction such that males from same-sex twin pairs (i.e., males who would presumably be exposed to higher levels of prenatal testosterone due to developing in utero with a male co-twin) exhibited lower levels of disordered eating than males from opposite-sex twin pairs (i.e., males who developed in utero with a female co-twin).
None of the authors have financial conflicts of interest.
Contributor Information
Kristen M. Culbert, Email: culbertk@msu.edu.
S. Marc Breedlove, Email: breedsm@msu.edu.
Cheryl L. Sisk, Email: sisk@msu.edu.
Pamela K. Keel, Email: keel@psy.fsu.edu.
Michael C. Neale, Email: neale@vcu.edu.
Steven M. Boker, Email: smb3u@virginia.edu.
S. Alexandra Burt, Email: burts@msu.edu.
Kelly L. Klump, Email: klump@msu.edu.
References
- Aardoom JJ, Dingemans AE, Slof Op'Landt MC, Van Furth EF. Norms and discriminative validity of the Eating Disorder Examination Questionnaire (EDE-Q) Eating Behaviors. 2012;13:305–309. doi: 10.1016/j.eatbeh.2012.09.002. [DOI] [PubMed] [Google Scholar]
- Abebe DS, Lien L, Torgersen L, von Soest T. Binge eating, purging and non-purging compensatory behaviours decrease from adolescence to adulthood: A population-based, longitudinal study. BMC Public Health. 2012a;12:32. doi: 10.1186/1471-2458-12-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abebe DS, Lien L, von Soest T. The development of bulimic symptoms from adolescence to young adulthood in females and males: A population-based longitudinal cohort study. International Journal of Eating Disorders. 2012b;45:737–745. doi: 10.1002/eat.20950. [DOI] [PubMed] [Google Scholar]
- Allaway HC, Bloski TG, Pierson RA, Lujan ME. Digit ratios (2D: 4D) determined by computer-assisted analysis are more reliable than those using physical measurements, photocopies, and printed scans. American Journal of Human Biology. 2009;21:365–370. doi: 10.1002/ajhb.20892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th. Washington DC: 2013. [Google Scholar]
- Arnold AP, Breedlove SM. Organizational and activational effects of sex steroids on brain and behavior: a reanalysis. Hormones and Behavior. 1985;19:469–498. doi: 10.1016/0018-506X(85)90042-X. [DOI] [PubMed] [Google Scholar]
- Attie I, Brooks-Gunn J. Development of eating problems in adolescent girls: A longitudinal study. Developmental Psychology. 1989;25:70. doi: 10.1037/0012-1649.25.1.70. [DOI] [Google Scholar]
- Baker JH, Girdler SS, Bulik CM. The role of reproductive hormones in the development and maintenance of eating disorders. Expert Review of Obstetrics & Gynecology. 2012;7:573–583. doi: 10.1586/eog.12.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker JH, Lichtenstein P, Kendler KS. Intrauterine testosterone exposure and risk for disordered eating. The British Journal of Psychiatry. 2009;194:375–376. doi: 10.1192/bjp.bp.108.054692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron-Cohen S, Jaffa T, Davies S, Auyeung B, Allison C, Wheelwright S. Do girls with anorexia nervosa have elevated autistic traits. Molecular Autism. 2013;4:24. doi: 10.1186/2040-2392-4-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binford RB, Le Grange D, Jellar CC. Eating Disorders Examination versus the Eating Disorders Examination-Questionnaire in adolescents with full and partial-syndrome bulimia nervosa and anorexia nervosa. International Journal of Eating Disorders. 2005;37:44–49. doi: 10.1002/eat.20062. [DOI] [PubMed] [Google Scholar]
- Berenbaum SA, Beltz AM. Sexual differentiation of human behavior: effects of prenatal and pubertal organizational hormones. Frontiers in Neuroendocrinology. 2011;32:183–200. doi: 10.1016/j.yfrne.2011.03.001. [DOI] [PubMed] [Google Scholar]
- Berenbaum SA, Bryk KK, Nowak N, Quigley CA, Moffat S. Fingers as a marker of prenatal androgen exposure. Endocrinology. 2009;150:5119–5124. doi: 10.1210/en.2009-0774. doi: http://dx.doi.org/10.1210/en.2009-0774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breedlove SM. Sexual differentiation of the human nervous system. Annual Review of Psychology. 1994;45:389–418. doi: 10.1146/annurev.ps.45.020194.002133. [DOI] [PubMed] [Google Scholar]
- Breedlove SM. Minireview: Organizational hypothesis: instances of the fingerpost. Endocrinology. 2010;151:4116–4122. doi: 10.1210/en.2010-0041. doi: http://dx.doi.org/10.1210/en.2010-0041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown WM, Hines M, Fane BA, Breedlove SM. Masculinized finger length patterns in human males and females with congenital adrenal hyperplasia. Hormones and Behavior. 2002;42:380–386. doi: 10.1006/hbeh.2002.1830. [DOI] [PubMed] [Google Scholar]
- Burt SA, Klump KL. The Michigan State University Twin Registry (MSUTR): an update. Twin Research and Human Genetics. 2013;16:344–350. doi: 10.1017/thg.2012.87. doi: http://dx.doi.org/10.1017/thg.2012.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calam R, Waller G. Are eating and psychosocial characteristics in early teenage years useful predictors of eating characteristics in early adulthood? A 7-year longitudinal study. International Journal of Eating Disorders. 1998;24:351–362. doi: 10.1002/(SICI)1098-108X(199812)24:4<351::AID-EAT2>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Carter JC, Aime AA, Mills JS. Assessment of bulimia nervosa: a comparison of interview and self-report questionnaire methods. International Journal of Eating Disorders. 2001;30:187–192. doi: 10.1002/eat.1071. [DOI] [PubMed] [Google Scholar]
- Cohen-Bendahan CC, Buitelaar JK, van Goozen SH, Cohen-Kettenis PT. Prenatal exposure to testosterone and functional cerebral lateralization: a study in same-sex and opposite-sex twin girls. Psychoneuroendocrinology. 2004;29:911–916. doi: 10.1016/j.psyneuen.2003.07.001. [DOI] [PubMed] [Google Scholar]
- Cohen-Bendahan CC, Buitelaar JK, van Goozen SH, Orlebeke JF, Cohen-Kettenis PT. Is there an effect of prenatal testosterone on aggression and other behavioral traits? A study comparing same-sex and opposite-sex twin girls. Hormones and Behavior. 2005;47:230–237. doi: 10.1016/j.yhbeh.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Culbert KM, Breedlove SM, Burt SA, Klump KL. Prenatal hormone exposure and risk for eating disorders: a comparison of opposite-sex and same-sex twins. Archives of General Psychiatry. 2008;65:329–336. doi: 10.1001/archgenpsychiatry.2007.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culbert KM, Breedlove SM, Sisk CL, Burt SA, Klump KL. The emergence of sex differences in risk for disordered eating attitudes during puberty: a role for prenatal testosterone exposure. Journal of Abnormal Psychology. 2013;122:420–432. doi: 10.1037/a0031791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bruin EI, De Nijs PF, Verheij F, Verhagen DH, Ferdinand RF. Autistic features in girls from a psychiatric sample are strongly associated with a low 2D: 4D ratio. Autism. 2009;13:511–521. doi: 10.1177/1362361309335720. [DOI] [PubMed] [Google Scholar]
- Decaluwé V, Braet C. Assessment of eating disorder psychopathology in obese children and adolescents: Interview versus self-report questionnaire. Behaviour Research and Therapy. 2004;42:799–811. doi: 10.1016/j.brat.2003.07.008. [DOI] [PubMed] [Google Scholar]
- Donohoe TP, Stevens R. Effects of ovariectomy, estrogen treatment and CI-628 on food inake and body weight in female rats treated neonatally with gonadal hormones. Physiology and Behavior. 1983;31:325–329. doi: 10.1016/0031-9384(83)90196-8. [DOI] [PubMed] [Google Scholar]
- Douglas LA, Varlinskaya EI, Spear LP. Rewarding properties of social interactions in adolescent and adult male and female rats: Impact of social versus isolate housing of subjects and partners. Developmental Psychobiology. 2004;45:153–162. doi: 10.1002/dev.20025. [DOI] [PubMed] [Google Scholar]
- Eddy K, Dorer D, Franko D, Tahilani K, Thompson-Brenner H, Herzog D. Diagnostic crossover in anorexia nervosa and bulimia nervosa: implications for DSM-V. American Journal of Psychiatry. 2008;165:245–250. doi: 10.1176/appi.ajp.2007.07060951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairburn CG, Beglin SJ. Assessment of eating disorders: interview or self-report questionnaire? International Journal of Eating Disorders. 1994;16:363–370. doi: 10.1002/1098-108X(199412)16:4<363::AID-EAT2260160405>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Ferreiro F, Seoane G, Senra C. A prospective study of risk factors for the development of depression and disordered eating in adolescents. Journal of Clinical Child & Adolescent Psychology. 2011;40:500–505. doi: 10.1080/15374416.2011.563465. [DOI] [PubMed] [Google Scholar]
- Gagne DA, Von Holle A, Brownley KA, Runfola CD, Hofmeier S, Branch KE, Bulik CM. Eating disorder symptoms and weight and shape concerns in a large web-based convenience sample of women ages 50 and above: Results of the gender and body image (GABI) study. International Journal of Eating Disorders. 2012;45:832–844. doi: 10.1002/eat.22030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gates GJ. How many people are lesbian, gay, bisexual, and transgender? Los Angeles, CA: The Williams Institute, UCLA School of Law; 2011. [Google Scholar]
- Gobrogge KL, Breedlove SM, Klump KL. Genetic and environmental influences on 2D: 4D finger length ratios: a study of monozygotic and dizygotic male and female twins. Archives of Sexual Behavior. 2008;37:112–118. doi: 10.1007/s10508-007-9272-2. [DOI] [PubMed] [Google Scholar]
- Goldschmidt AB, Doyle AC, Wilfley DE. Assessment of binge eating in overweight youth using a questionnaire version of the child eating disorder examination with instructions. International Journal of Eating Disorders. 2007;40:460–467. doi: 10.1002/eat.20387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldschmidt AB, Tanofsky-Kraff M, Wilfley DE. A laboratory-based study of mood and binge eating behavior in overweight children. Eating Behaviors. 2011;12:37–43. doi: 10.1016/j.eatbeh.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heatherton TF, Mahamedi F, Striepe M, Field AE, Keel P. A 10-year longitudinal study of body weight, dieting, and eating disorder symptoms. Journal of Abnormal Psychology. 1997;106:117–125. doi: 10.1037/0021-843X.106.1.117. [DOI] [PubMed] [Google Scholar]
- Hildebrandt T, Alfano L, Tricamo M, Pfaff DW. Conceptualizing the role of estrogens and serotonin in the development and maintenance of bulimia nervosa. Clinical Psychology Review. 2010;6:655–668. doi: 10.1016/j.cpr.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson JI, Hiripi E, Pope HG, Jr, Kessler RC. The prevalence and correlates of eating disorders in the National Comorbidity Survey Replication. Biological Psychiatry. 2007;61:348–358. doi: 10.1016/j.biopsych.2006.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobi C, Hayward C, de Zwaan M, Kraemer HC, Stewart A. Coming to terms with risk factors for eating disorders: application of risk terminology and suggestions for a general taxonomy. Psychological Bulletin. 2004;130:19–65. doi: 10.1037/0033-2909.130.1.19. [DOI] [PubMed] [Google Scholar]
- Jones JM, Bennett S, Olmsted MP, Lawson ML, Rodin G. Disordered eating attitudes and behaviours in teenaged girls: a school-based study. Canadian Medical Association Journal. 2001;165:547–552. [PMC free article] [PubMed] [Google Scholar]
- Jones W, Morgan J. Eating disorders in men: a review of the literature. Journal of Public Mental Health. 2010;9:23–31. doi: 10.5042/jpmh.2010.0326. [DOI] [Google Scholar]
- Jones RM, Somerville LH, Li J, Ruberry EJ, Powers A, Mehta N, Dyke J, Casey BJ. Adolescent-specific patterns of behavior and neural activity during social reinforcement learning. Cognitive, Affective, & Behavioral Neuroscience. 2014;14:683–697. doi: 10.3758/s13415-014-0257-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keel PK, Baxter MG, Heatherton TF, Joiner TE. A 20-year longitudinal study of body weight, dieting, and eating disorder symptoms. Journal of Abnormal Psychology. 2007;116:422. doi: 10.1037/0021-843X.116.2.422. [DOI] [PubMed] [Google Scholar]
- Keel PK, Forney KJ. Psychosocial risk factors for eating disorders. International Journal of Eating Disorders. 2013;46:433–439. doi: 10.1002/eat.22094. [DOI] [PubMed] [Google Scholar]
- Klump KL, Burt SA. The Michigan State University Twin Registry (MSUTR): genetic, environmental and neurobiological influences on behavior across development. Twin Research and Human Genetics. 2006;9:971–977. doi: 10.1375/183242706779462868. doi: http://dx.doi.org/10.1375/twin.9.6.971. [DOI] [PubMed] [Google Scholar]
- Klump KL, Burt SA, McGue M, Iacono WG. Changes in genetic and environmental influences on disordered eating across adolescence: a longitudinal twin study. Archives of General Psychiatry. 2007;64:1409–1415. doi: 10.1001/archpsyc.64.12.1409. [DOI] [PubMed] [Google Scholar]
- Klump KL, Gobrogge KL, Perkins P, Thorne D, Sisk CL, Breedlove SM. Preliminary evidence that gonadal hormones organize and activate disordered eating. Psychological Medicine. 2006;12:1–8. doi: 10.1017/S0033291705006653. doi: http://dx.doi.org/10.1017/S0033291705006653. [DOI] [PubMed] [Google Scholar]
- Klump KL, Keel PK, Racine SE, Burt SA, Neale M, Sisk CL, Boker S, Hu JY. The interactive effects of estrogen and progesterone on changes in emotional eating across the menstrual cycle. Journal of Abnormal Psychology. 2013;122:131–137. doi: 10.1037/a0029524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klump KL, Keel PK, Sisk C, Burt SA. Preliminary evidence that estradiol moderates genetic influences on disordered eating attitudes and behaviors during puberty. Psychological Medicine. 2010;40:1745–1753. doi: 10.1017/S0033291709992236. doi: http://dx.doi.org/10.1017/S0033291709992236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavender JM, De Young KP, Anderson DA. Eating Disorder Examination Questionnaire (EDE-Q): norms for undergraduate men. Eating Behaviors. 2010;11:119–121. doi: 10.1016/j.eatbeh.2009.09.005. [DOI] [PubMed] [Google Scholar]
- Lewinsohn PM, Striegel-Moore RH, Seeley JR. Epidemiology and natural course of eating disorders in young women from adolescence to young adulthood. Journal of American Academy of Child and Adolescent Psychiatry. 2000;39:1284–1292. doi: 10.1097/00004583-200010000-00016. [DOI] [PubMed] [Google Scholar]
- Lutchmaya S, Baron-Cohen S, Raggatt P, Knickmeyer R, Manning J. 2nd and 4th digit ratios, fetal testosterone and estradiol. Early Human Development. 2004;77:23–28. doi: 10.1016/j.earlhumdev.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Lydecker JA, Pisetsky EM, Mitchell KS, Thornton LM, Kendler KS, Reichborn-Kjennerud T, Lichtenstein P, Bulik CM, Mazzeo SE. Association between co-twin sex and eating disorders in opposite sex twin pairs: evaluations in North American, Norwegian, and Swedish samples. Journal of Psychosomatic Research. 2012;72:73–77. doi: 10.1016/j.jpsychores.2011.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madrid JA, Lopez-Bote C, Martin E. Effects of neonatal androgenization on the circadian rhythm of feeding behavior in rats. Physiology and Behavior. 1993;53:329–335. doi: 10.1016/0031-9384(93)90213-Y. [DOI] [PubMed] [Google Scholar]
- Malas M, Dogan S, Evcil EH, Desdicioglu K. Fetal development of the hands, digits, and digit ratios (2D:4D) Early Human Development. 2006;82:469–475. doi: 10.1016/j.earlhumdev.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Mangweth-Matzek B, Hoek HW, Rupp CI, Kemmler G, Pope HG, Kinzl J. The menopausal transition—A possible window of vulnerability for eating pathology. International Journal of Eating Disorders. 2013;46:609–616. doi: 10.1002/eat.22157. [DOI] [PubMed] [Google Scholar]
- Manning JT, Baron-Cohen S, Wheelwright S, Sanders G. The 2nd to 4th digit ratio and autism. Developmental Medicine & Child Neurology. 2001;43:160–164. doi: 10.1111/j.1469-8749.2001.tb00181.x. [DOI] [PubMed] [Google Scholar]
- Manning JT, Churchill AJ, Peters M. The effects of sex, ethnicity, and sexual orientation on self-measured digit ratio (2D: 4D) Archives of Sexual Behavior. 2007;36:223–233. doi: 10.1007/s10508-007-9171-6. [DOI] [PubMed] [Google Scholar]
- Manning JT, Fink B, Neave N, Caswell N. Photocopies yield lower digit ratios (2D: 4D) than direct finger measurements. Archives of Sexual Behavior. 2005;34:329–333. doi: 10.1007/s10508-005-3121-y. [DOI] [PubMed] [Google Scholar]
- Manning JT, Kilduff L, Cook C, Crewther B, Fink B. Digit ratio (2D:4D): a biomarker for prenatal sex steroids and adult sex steroids in challenge situations. Frontiers in Endocrinology. 2014;5:9. doi: 10.3389/fendo.2014.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning JT, Scutt D, Wilson J, Lewis-Jones DI. The ratio of 2nd to 4th digit length: a predictor of sperm numbers and concentrations of testosterone, luteinizing hormone and oestrogen. Human Reproduction. 1998;13:3000–3004. doi: 10.1093/humrep/13.11.3000. [DOI] [PubMed] [Google Scholar]
- Marderosian A, Wu Y, Culbert KM, Burt SA, Nigg JT, Klump KL. Psychometric properties of the Minnesota Eating Behaviors Survey in pre-adolescent and adolescent girls and boys unpublished Data. [Google Scholar]
- McFadden D. A masculinizing effect on the auditory systems of human females having male co-twins. Proceedings of the National Academy of Sciences of the United States of America USA. 1993;90:11900–11904. doi: 10.1073/pnas.90.24.11900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller EM. Prenatal sex hormone transfer: a reason to study opposite-sex twins. Personality and Individual Differences. 1994;17:511–529. doi: 10.1016/0191-8869(94)90088-4. [DOI] [Google Scholar]
- Mintz LB, O'Halloran MS, Mulholland AM, Schneider PA. Questionnaire for eating disorder diagnoses: reliability and validity of operationalizing DSM—IV criteria into a self-report format. Journal of Counseling Psychology. 1997;44:63–79. doi: http://dx.doi.org/10.1037/0022-0167.44.1.63. [Google Scholar]
- Mond JM, Hay PJ, Rodgers B, Owen C, Beaumont PJV. Temporal stability of the eating disorder examination questionnaire. International Journal of Eating Disorders. 2004;36:195–203. doi: 10.1002/eat.20017. [DOI] [PubMed] [Google Scholar]
- Mond JM, Hay PJ, Rodgers B, Owen C, Beaumont PJV. Validity of the eating disorder examination questionnaire (EDE-Q) in screening for eating disorders in community samples. Behavioral Research and Therapy. 2004b;42:551–567. doi: 10.1016/S0005-7967(03)00161-X. [DOI] [PubMed] [Google Scholar]
- Oinonen KA, Bird JL. Age at menarche and digit ratio (2D:4D): Relationships with body dissatisfaction, drive for thinness, and bulimia symptoms in women. Body Image. 2012;9:302–306. doi: 10.1016/j.bodyim.2011.12.003. [DOI] [PubMed] [Google Scholar]
- Okten A, Kalyoncu M, Yari N. The ratio of second- and fourth-digit lengths and congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Early Human Development. 2002;70:47–54. doi: 10.1016/S0378-3782(02)00073-7. [DOI] [PubMed] [Google Scholar]
- Field AE, Camargo CA, Taylor CB, Berkey CS, Roberts SB, Colditz GA. Peer, parent, and media influences on the development of weight concerns and frequent dieting among preadolescent and adolescent girls and boys. Pediatrics. 2001;107:54–60. doi: 10.1542/peds.107.1.54. [DOI] [PubMed] [Google Scholar]
- Presnell K, Bearman SK, Stice E. Risk factors for body dissatisfaction in adolescent boys and girls: A prospective study. International Journal of Eating Disorders. 2004;36:389–401. doi: 10.1002/eat.20045. [DOI] [PubMed] [Google Scholar]
- Quinton SJ, Smith AR, Joiner T. The 2nd to 4th digit ratio (2D: 4D) and eating disorder diagnosis in women. Personality and Individual Differences. 2011;51:402–405. doi: 10.1016/j.paid.2010.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raevuori A, Kaprio J, Hoek HW, Sihvola E, Rissanen A, Keski-Rahkonen A. Anorexia and bulimia nervosa in same-sex and opposite-sex twins: lack of association with twin type in a nationwide study of Finnish twins. American Journal of Psychiatry. 2008;165:1604–1610. doi: 10.1176/appi.ajp.2008.08030362. [DOI] [PubMed] [Google Scholar]
- Rizvi SL, Stice E, Agras WS. Natural history of disordered eating attitudes and behaviors over a 6-Year period. International Journal of Eating Disorders. 1999;26:406–413. doi: 10.1002/(SICI)1098-108X(199912)26:4<406::AID-EAT6>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Ryan BC, Vandenbergh JG. Intrauterine position effects. Neuroscience and Biobehavioral Reviews. 2002;26:665–678. doi: 10.1016/S0149-7634(02)00038-6. [DOI] [PubMed] [Google Scholar]
- Shapiro S, Newcomb M, Burns Loeb T. Fear of fat, disregulated-restrained eating, and body-esteem: prevalence and gender differences among eight-to ten-year-old children. Journal of Clinical Child Psychology. 1997;26:358–365. doi: 10.1207/s15374424jccp2604_4. [DOI] [PubMed] [Google Scholar]
- Slutske WS, Bascom EN, Meier MH, Medland SE, Martin NG. Sensation seeking in females from opposite-versus same-sex twin pairs: hormone transfer or sibling imitation? Behavior Genetics. 2011;41:533–542. doi: 10.1007/s10519-010-9416-3. [DOI] [PubMed] [Google Scholar]
- Smith AR, Hawkeswood SE, Joiner TE. The measure of a man: associations between digit ratio and disordered eating in males. International Journal of Eating Disorders. 2010;43:543–548. doi: 10.1002/eat.20736. [DOI] [PubMed] [Google Scholar]
- Somerville LH. The teenage brain sensitivity to social evaluation. Current directions in Psychological Science. 2013;22:121–127. doi: 10.1177/0963721413476512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville LH, Jones RM, Ruberry EJ, Dyke JP, Glover G, Casey BJ. The medial prefrontal cortex and the emergence of self-conscious emotion in adolescence. Psychological Science. 2013;24:1554–1562. doi: 10.1177/0956797613475633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhausen HC, Gavez S, Metzke CW. Psychosocial correlates, outcome, and stability of abnormal adolescent eating behavior in community samples of young people. International Journal of Eating Disorders. 2005;37:119–126. doi: 10.1002/eat.20077. [DOI] [PubMed] [Google Scholar]
- Stice E, Cooper JA, Schoeller DA, Tappe K, Lowe MR. Are dietary restraint scales valid measures of moderate-to long-term dietary restriction? Objective biological and behavioral data suggest not. Psychological Assessment. 2007;19:449–458. doi: 10.1037/1040-3590.19.4.449. [DOI] [PubMed] [Google Scholar]
- Stice E, Fisher M, Lowe MR. Are dietary restraint scales valid measures of acute dietary restriction? Unobtrusive observational data suggest not. Psychological Assessment. 2004;16:51–59. doi: 10.1037/1040-3590.16.1.51. [DOI] [PubMed] [Google Scholar]
- Stice E, Killen JD, Hayward C, Taylor CB. Age of onset for binge eating and purging during late adolescence: a found year survival analysis. Journal of Abnormal Psychology. 1998;107:671–675. doi: 10.1037/0021-843X.107.4.671. [DOI] [PubMed] [Google Scholar]
- Stice E, Marti CN, Rohde P. Prevalence, incidence, impairment, and course of the proposed DSM-5 eating disorder diagnoses in an 8-year prospective community study of young women. Journal of Abnormal Psychology. 2013;122:445. doi: 10.1037/a0030679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stice E, Marti CN, Shaw H, Jaconis M. An 8-year longitudinal study of the natural history of threshold, subtreshold, and partial eating disorders from a community sample of adolescents. Journal of Abnormal Psychology. 2009;118:587–597. doi: 10.1037/a0016481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stice E, Ng J, Shaw H. Risk factors and prodromal eating pathology. Journal of Child Psychology and Psychiatry. 2010;51:518–525. doi: 10.1111/j.1469-7610.2010.02212.x. [DOI] [PubMed] [Google Scholar]
- Striegel-Moore RH, Bulik CM. Risk factors for eating disorders. American Psychologist. 2007;62:181–198. doi: 10.1037/0003-066X.62.3.181. [DOI] [PubMed] [Google Scholar]
- Swanson SA, Crow SJ, Le Grange D, Swendsen J, Merikangas KR. Prevalence and correlates of eating disorders in adolescents: results from the national comorbidity survey replication adolescent supplement. Archives of General Psychiatry. 2011;68:714–723. doi: 10.1001/archgenpsychiatry.2011.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapp AL, Maybery MT, Whitehouse AJ. Evaluating the twin testosterone transfer hypothesis: a review of the empirical evidence. Hormones and Behavior. 2011;60:713–722. doi: 10.1016/j.yhbeh.2011.08.011. [DOI] [PubMed] [Google Scholar]
- Tylka TL, Subich LM. Exploring the construct validity of the eating disorder continuum. Journal of Counseling Psychology. 1999;46:268–276. doi: http://dx.doi.org/10.1037/0022-0167.46.2.268. [Google Scholar]
- von Ranson KM, Klump KL, Iacono WG, McGue M. The Minnesota Eating Behavior Survey: a brief measure of disordered eating attitudes and behaviors. Eating Behaviors. 2005;4:373–392. doi: 10.1016/j.eatbeh.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Wade TD, Hansell NK, Crosby RD, Bryant-Waugh R, Treasure J, Nixon R, Byrne S, Martin NG. A study of changes in genetic and environmental influences on weight and shape concern across adolescence. Journal of Abnormal Psychology. 2013;122:119. doi: 10.1037/a0030290. [DOI] [PubMed] [Google Scholar]
- Wallen K. Does finger fat produce sex differences in second to fourth digit ratios? Endocrinology. 2009;150:4819–4822. doi: 10.1210/en.2009-0986. doi: http://dx.doi.org/10.1210/en.2009-0986. [DOI] [PubMed] [Google Scholar]
- Ward BW, Dahlhamer JM, Galinsky AM, Joestl SS. National Health Statistics Report. Vol. 77. Hyattsville, MD: National Center for Health Statistics; 2014. Sexual orientation and health among U.S adults: national health interview survey, 2013. [PubMed] [Google Scholar]
- Wood KC, Becker JA, Thompson JK. Body image dissatisfaction in preadolescent children. Journal of Applied Developmental Psychology. 1996;17:85–100. doi: 10.1016/S0193-3973(96)90007-6. [DOI] [Google Scholar]
- Zandian M, Ioakimidis I, Bergh C, Leon M, Södersten P. A sex difference in the response to fasting. Physiology & Behavior. 2011;103:530–534. doi: 10.1016/j.physbeh.2011.04.009. [DOI] [PubMed] [Google Scholar]
- Zheng P. Neuroactive steroid regulation of neurotransmitter release in the CNS: action, mechanism and possible significance. Progress in Neurobiology. 2009;89:134–152. doi: 10.1016/j.pneurobio.2009.07.001. [DOI] [PubMed] [Google Scholar]
- Zheng Z, Cohn MJ. Developmental basis of sexually dimorphic digit ratios. Proceedings of the National Academy of Sciences. 2011;108:16289–16294. doi: 10.1073/pnas.1108312108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zehr JL, Culbert KM, Sisk CL, Klump KL. An association of early puberty with disordered eating and anxiety in a population of undergraduate women and men. Hormones and Behavior. 2007;52:427–435. doi: 10.1016/j.yhbeh.2007.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker NL, Losh M, Bulik CM, LaBar KS, Piven J, Pelphrey KA. Anorexia nervosa and autism spectrum disorders: guided investigation of social cognitive endophenotypes. Psychological Bulletin. 2007;133:976–1006. doi: 10.1037/0033-2909.133.6.976. doi: http://dx.doi.org/10.1037/0033-2909.133.6.976. [DOI] [PubMed] [Google Scholar]