Abstract
RATIONALE
Carbohydrates are highly variable in structure owing to differences in their anomeric configurations, monomer stereochemistry, inter-residue linkage positions and general branching features. The separation of carbohydrate isomers poses a great challenge for current analytical techniques.
METHODS
The isomeric heterogeneity of disaccharide ions and monosaccharideglycolaldehyde product ions evaluated using electrospray traveling wave ion mobility mass spectrometry (Synapt G2 high definition mass spectrometer) in both positive and negative ion modes investigation.
RESULTS
The separation of isomeric disaccharide ions was observed but not fully achieved based on their mobility profiles. The mobilities of isomeric product ions, the monosaccharide-glycolaldehydes, derived from different disaccharide isomers were measured. Multiple mobility peaks were observed for both monosaccharide-glycolaldehyde cations and anions, indicating that there was more than one structural configuration in the gas phase as verified by NMR in solution. More importantly, the mobility patterns for isomeric monosaccharide-glycolaldehyde product ions were different, which enabled partial characterization of their respective disaccharide ions. Abundant disaccharide cluster ions were also observed. The Results showed that a majority of isomeric cluster ions had different drift times and, moreover, more than one mobility peak was detected for a number of specific cluster ions.
CONCLUSIONS
It is demonstrated that ion mobility mass spectrometry is an advantageous method to assess the isomeric heterogeneity of carbohydrate compounds. It is capable of differentiating different types of carbohydrate ions having identical m/z values as well as multiple structural configurations of single compounds.
Introduction
Mass spectrometry and tandem mass spectrometric methods[1-5] have long been used to analyze a wide variety of compounds, where ions having specific m/z values can be isolated and their dissociation patterns acquired using collisional activation, photon absorption, or other methods more specific to individual classes of molecules. However, as the complexity of molecular mixtures increases, the number of potential analytes that are isomeric in nature (having ions with identical m/z values) increases to a point where one question cannot be ignored. Is the dissociation pattern observed for a selected precursor ion m/z value really derived from just one molecule? The same argument applies to multiple dissociation steps, even using a pure precursor molecule, where potentially different fragmentation pathways might give rise to isomeric sets of product ions. Can any product ion at a specific m/z value potentially be a mixture of isomers derived from a single precursor ion?
In the same vein, if molecules can exist in more than one configuration in the gas phase, as occurs, for example, with sugars as cyclic, interconvertible α or β anomers, or lipids having cis or trans double bonds, how might one reproducibly assess their presence, or even be aware of their existence, solely through selection of m/z values? In many cases, predicted and observed product ions may also be identical in which case the presence (or absence) of isomers simply cannot be assessed based on classical dissociation spectra. While precursor ion heterogeneity can often be addressed through upfront separation techniques such as gas chromatography, liquid chromatography, or capillary electrophoresis, for example, these separation techniques can be moderately time-consuming. Moreover, there is no guarantee that compounds will not coincidentally co-migrate using any separation system. Furthermore, proving the isomeric heterogeneity and detailed structures of product ions in the gas phase is not trivial and is far less tractable. For these transiently-isolated ionic species, the most reproducible and unique physical evidence for their structures may be obtained through ion spectroscopy. Depending on the nature of the analytes, relatively high resolution spectra have been acquired for ions in the electronic (UV) and infrared electromagnetic regimes,,[6,7] and the microwave region,[8] and Raman spectroscopy has been carried out on performed on ions soft-landed on surfaces.[9] However, the ability to assess and define mixtures of isomeric product ions at the same time, particularly unknown species, is more limited using these methods, depending on the nature of the ions being analyzed.
Carbohydrates exemplify a classically difficult group of molecules to analyze where ions are frequently mixtures of isomers, and product ions are suspected also to be so. They are involved in numerous biological activities through their interactions with proteins.[10-14] More than half of human proteins are glycosylated.[15] Unlike proteins and nucleic acids, carbohydrates are indirect products of gene expression and are biosynthesized through a consecutive series of enzymes.[16,17] Sequencing methods developed for the proteome and genome cannot be applied for studies of carbohydrate structure. Their structural diversity and heterogeneity arise from the large number of isomers possible,[18-20] varying in anomeric configuration, and differing in linkage positions and branching. Differentiation of isomeric carbohydrate species poses a grand challenge for analytical techniques.
While mass spectrometry (MS)[21-24] has been extensively used for carbohydrate structure analysis, even with multiple isolation/dissociation steps carried out on tandem MS instruments, the product ion spectra resulting from isomeric precursor mixtures complicate product ion patterns and limit absolute structural identification of unknown saccharides. Liquid[25-27] and gas chromatography[28, 29] are the most common separation methods employed for resolving carbohydrate isomers prior to mass spectral analysis, but both these methods limit sample throughput, are complex with multiple analytical sample preparation steps, and can be difficult to reproduce with precision among laboratories. Because stationary phases of chromatographic columns differ from batch to batch it is not possible to assign absolute retention times to specific saccharides; even relative retention times vary from column to column. Moreover, sample derivatization, especially for gas chromatography, is often needed to enhance volatilization, but can decrease resolution in complex mixtures and limit the size of molecules being analyzed. One approach to decrease analysis time, increase resolving power and improve reproducibility has been to incorporate ion mobility spectrometry (IMS)[30-33] as the separation process prior to mass spectrometry. Rather than differences in equilibrium between two phases, IMS separation is based on the physical size of the ion, providing hard experimental data related to the structure of the ion. Similar to mass spectrometry where ion separation is based on the mass-to-charge ratio (m/z) of the ion, IMS separation is based on the size-to-charge ratio (Ω/z) where size is measured as the ion's cross section, Ω. An ion's cross section is characteristic of its size and it can be measured reproducibly from time-to-time, instrument-to-instrument and laboratory-to-laboratory. As with mass, cross section is an intrinsic physical property of an ion and the buffer gas through which the ion migrates. In ion mobility experiments, the measured drift time is directly proportional to the cross section. When the separation capability of IMS is combined with mass identification, stereo-structural differences of carbohydrate compounds can be resolved by ion mobility mass spectrometry (IMMS).[34] IMMS has been applied to the separation and detection of various complex biological samples in the areas now delineated as metabolomics,[35, 36] proteomics[37, 38] and glycomics.[39, 40]
Collision-induced dissociation of mass-selected glycan ions is often used to aid in the identification of the precursor ion. During CID processes, dissociation at glycosidic bonds and/or cross ring cleavages generate substructures such as monosaccharides, the most basic unit, and disaccharides, the smallest substructure still containing a linkage between two sugars. Many of these product ions are isomeric and their stereochemistry cannot be distinguished by mass spectrometry. Knowing the cross section of each of these product ions and the specific patterns of product ion mobility profiles can add important, reproducible physical information in addition to their measured m/z values in assigning their stereochemistry, thereby leading to a more confident identification of the parent oligosaccharide. It has been demonstrated that isomeric monosaccharide methyl glycosides with subtle structural differences can be distinguished using IMMS.[41, 42] Ion mobility differentiation of disaccharide isomers has also been shown.[43-45] However, current applications of IMMS to the separation of small carbohydrate structural isomers have been mainly focused on precursor ions.[39, 40, 43-45] Ion mobility methods for identifying isomeric carbohydrate product ions derived from isomeric precursor ions have not been investigated.
Bendiak and colleagues[18, 46, 47] have reported that 2-, 4- and 6-linked disaccharides dissociated to generate product ions that were established through chemical synthesis to be monosaccharide-glycolaldehydes. According to their findings, the product ion spectra of monosaccharide-glycolaldehyde anions were dependent on their stereochemistries, which enabled the anomeric configurations and stereochemistries of 20 isomeric variants to be assigned (all hexopyranoses and common N-acetylhexosamines). However, should these ions be derived from two or more branches in a larger branched oligosaccharide, two or more monosaccharide-glycolaldehyde isomers might well be present as an isomeric mixture, which would confound their assignments solely based on dissociation patterns. Furthermore, these compounds were found to have up to three structural variants in aqueous solution as assessed by NMR, an open-chain form and two hemiacetals with cyclization of the aldehyde with the OH-2.[18] However, to date, no information has been presented as to whether these molecules exist in more than one configuration in the gas phase, when ionized in either negative or positive modes. In this study, 2-, 4- and 6-linked disaccharides were used as representative examples and traveling wave ion mobility mass spectrometry was employed to evaluate the heterogeneity in configuration of isomeric ions as well as their structurally informative product ions, the monosaccharide-glycolaldehydes, in both positive and negative modes.
Experimental
Chemicals and solvents
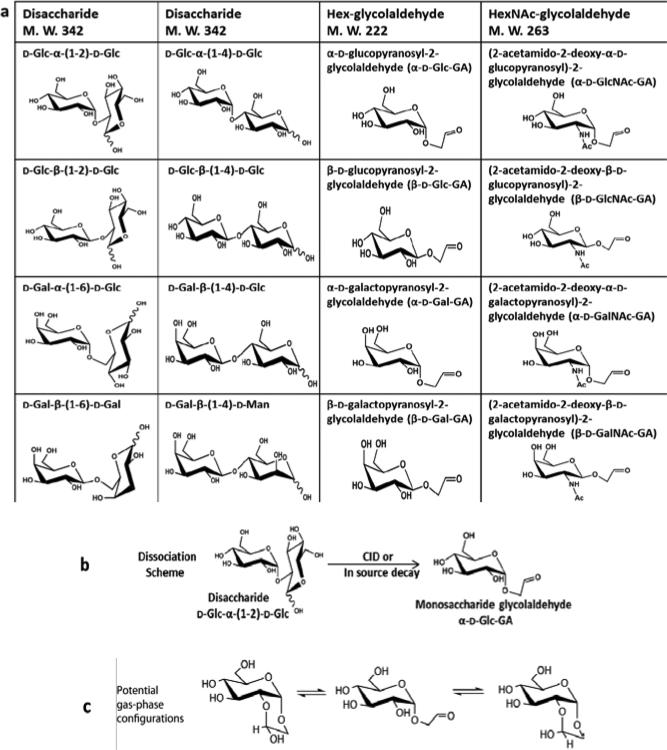
Disaccharide isomers investigated in this study include D-Glc-α-(1-2)-D-Glc, D-Glc-β-(1-2)-D-Glc, D-Glc-α-(1-4)-D-Glc, D-Glc-β-(1-4)-D-Glc, D-Gal-α-(1-6)-D-Glc, D-Gal-β-(1-6)-D-Gal, D-Gal-β-(1-4)-D-Glc and D-Gal-β-(1-4)-D-Man, (Glc denotes glucose, Gal denotes galactose and Man denotes mannose). Their structures and nomenclature are shown in Table 1a. All the disaccharides are reducing sugars (having an -OH group at the anomeric carbon in more than one stereochemical configuration or ring form) and had either 2-, 4- or 6-linkages. Also contained in Table 1a are eight monosaccharide-glycolaldehyde synthetic standards. There are four isomeric Hex-glycolaldehydes (M. W. 222) including α-D-glucopyranosyl-2-glycolaldehyde (α-D-Glc-GA), β-D-glucopyranosyl-2-glycolaldehyde (β-D-Glc-GA), α-D-galactopyranosyl-2-glycolaldehyde (α-D-Gal-GA) and β-D-galactopyranosyl-2-glycolaldehyde (β-D-Gal-GA). The counterparts to these molecules containing the 2-acetamido-2-deoxy group were the HexNAc-glycolaldehydes (M. W. 263) including (2-acetamido-2-deoxy-α-D-glucopyranosyl)-2-glycolaldehyde (α-D-GlcNAc-GA), (2-acetamido-2-deoxy-β-D-glucopyranosyl)-2-glycolaldehyde (β-D-GlcNAc-GA), (2-acetamido-2-deoxy-α-D-galactopyranosyl)-2-glycolaldehyde (α-D-GalNAc-GA) and (2-acetamido-2-deoxy-β-D- galactopyranosyl)-2-glycolaldehyde (β-D-GalNAc-GA) which were also examined by IMMS. Dissociation of 2-, 4- and 6-linked disaccharide ions yields product ions of monosaccharideglycolaldehydes.[18, 46] A dissociation scheme having sodiated or deprotonated D-Glc-α-(1-2)-DGlc as a precursor ion and sodiated or deprotonated α-Glc-GA as a product ion is shown in Table 1b. It was demonstrated[18] that this type of product ion is composed of an intact non-reducing sugar and a two carbon aglycon derived from the reducing sugar, for 2-, 4- and 6-linked disaccharides. All the disaccharides and NH4Cl were purchased from Sigma Chemical Co. (St. Louis, MO, USA) and used without modification. Monosaccharide-glycolaldehyde standards were synthesized as described previously.[18, 46, 47] LC-MS grade solvents of methanol and water were purchased from Fisher Scientific Inc. (Pittsburgh, PA, USA) and an equal volume mixture of methanol and water was used as electrospray ionization (ESI) solvent. A final concentration of 10 μM was prepared for all the sugar samples using the ESI solvent for the positive mode study, and the concentration was increased to 20 μM for negative mode measurements. In order to investigate the influence of chloride adduction on the ion mobility separation for disaccharides, 4 μL of a stock solution of NH4Cl (10 mM in ESI solvent) was added to 1 mL of a 20 μM disaccharide solution to enhance the formation of chloride adducts, resulting in a molar ratio of salt: sugar of 2:1 in each sample. Due to the potential reaction between ammonia and the aldehyde group of reducing sugars to form a Schiff base, the samples containing NH4Cl were prepared freshly prior to analysis.
Table 1.
a. Structures and nomenclature for isomeric disaccharides and monosaccharide-glycolaldehydes. b. The dissociation scheme from D-Glc-α-(1-2)-D-Glc to α-D-Glc-GA. c. The potential gas-phase configurations of α-D-Glc-GA.-Note: the species shown in Tables 1b and 1c can be either [M+Na]+ ions or [M-H]- ions.
|
Ion mobility mass spectrometry measurements
All the experiments were performed on a Synapt G2 high definition mass spectrometer (Waters Corp., Manchester, UK). It is a hybrid quadrupole/ion mobility/orthogonal time of flight mass spectrometer.[48] The traveling wave ion mobility device that is incorporated into the instrument employs dynamic inhomogeneous electric fields under reduced pressures. The effects on mobility of these unique instrumental parameters have been thoroughly described.[49-51] A trap and a transfer cell are located in front of and after the ion mobility separator, respectively. Collision induced dissociation (CID) can be initiated in either or both cells by elevating the collision energy (CE). A wave height of 40 V and a wave velocity of 650 m/s were utilized for ion mobility separation in both positive and negative modes. Nitrogen was used as a drift gas at a flow rate of 90 mL/min, resulting in a pressure of ~3.5 mbar in the ion mobility device. Two types of experiments were performed in this study. (1) To obtain mobility profiles of precursor ions and their corresponding cluster ions, no CE was used in the trap and transfer cells. All the ions passed through the trap cell, were evaluated by traveling wave IMS directly and then transferred to the TOF mass analyzer. (2) To investigate the mobility of the product ions derived from disaccharides, a disaccharide precursor ion was first selected by the quadrupole, and the appropriate CE was applied in the trap cell to induce fragmentation. Mobility separation of all the product ions occurred sequentially in the traveling wave ion mobility unit of the instrument and all the ions then travelled through the transfer cell and were measured by the TOF analyzer. The ESI voltages were 3.2 KV and 2.25 KV for the positive and negative ion modes, respectively. Nitrogen was used as the desolvation gas at 200°C with a flow rate of 600 L/hr. Samples were injected using a syringe pump (Chemyx Inc., Stafford, TX, USA) at a flow rate of 3 μL/min and the data was acquired for 3 min for all the analytes. Masslynx V4.1 (Waters Corp.,) was used to collect and process the data. More instrumental parameters are summarized in Table S-1 (Supporting Information).
Results and Discussion
Separation of isomeric monosaccharide-glycolaldehyde standards
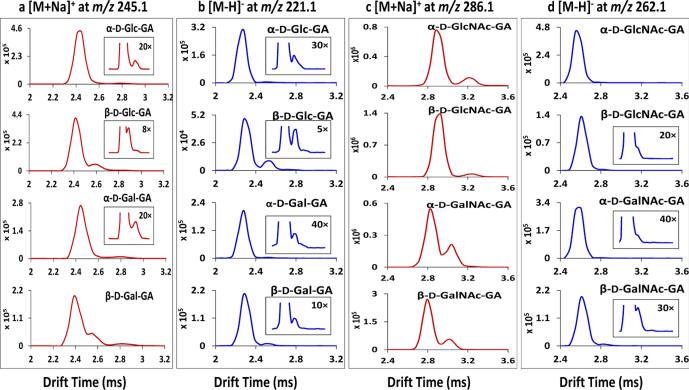
Monosaccharide-glycolaldehyde product ions are structurally informative and their mobility spectra can be used to assign/determine the stereochemistries of 20 structural variants.[18, 46, 47] Figure. 1 displays the traveling wave ion mobility spectra of the monosaccharide-glycolaldehyde standards used in this study. The inserted window accompanying each compound represents the spectrum with the intensity scale magnified as labeled. Figure. 1a shows the mobility profiles of sodiated α-D-Glc-GA, β-D-Glc-GA, α-D-Gal-GA and β-D-Gal-GA at m/z 245.1 in the positive mode. The major mobility peaks for these isomers showed similar drift times near 2.45 ms. However, multiple mobility peaks were detected for all the individual monosaccharide-glycolaldehydes;, for example, three mobility peaks were observed for β-D-Glc-GA and β-D-Gal-GA, and two peaks were seen for α-D-Glc-GA and α-D-Gal-GA. This indicates that there is more than one gas-phase stereo-structure for each Hex-glycolaldehyde. As mentioned, monosaccharide-glycolaldehydes may exist in multiple isomeric acyclic / cyclic forms in the gas phase through internal hemiacetal formation between the glycolaldehyde carbonyl group and the sugar 2-hydroxyl group (Table 1 panel c), and this is supported by NMR spectroscopy of the compounds in aqueous solution.[18] The mobility spectra of Hex-glycolaldehyde anions at m/z 221.1 are shown in Fig. 1b. There was no obvious resolution among the isomeric Hex-glycolaldehyde anions and more than one mobility peak was found for all the standards. Figure. 1c displays the ion mobility spectra of the [M+Na]+ ions of HexNAc-glycolaldehydes at m/z 286.1. Partial separation was achieved for epimer pairs such as α-D-GlcNAc-GA and α- D-GalNAc-GA, but no differentiation was detected for anomer pairs of α- and β-D-GlcNAc-GA and α- and β-D-GalNAc-GA. Again, more than one mobility peak was detected for single compounds: two baseline- separated mobility peaks were observed for α- and β-D-GlcNAc-GA (2.9 and 3.2 ms), and two partially resolved peaks were found for α- and β-D-GalNAc-GA (2.8 and 3.0 ms). Mobility evaluation of the [M-H]− ions of the HexNAc-glycolaldehydes at m/z 262.1 is presented in Fig. 1d. In addition to the predominant mobility peak, another extremely low intensity peak was partially resolved from the major peak for specific m/z 262.1 ions. For HexNAc-glycolaldehydes, better ion mobility separation and sensitivity especially for the low abundance structural variants were obtained using cations rather than anions. The reason for the additional ion mobility peaks for the HexNAc-GA compounds is not clear, because these compounds cannot cyclize at the sugar 2-position like the Hex-GA compounds; possibly the sodium can reside at more than one location for the sodiated compounds. Overall, ion mobility showed its unique separation capability to evaluate the isomeric heterogeneity of these monosaccharide-glycolaldehyde ions in the gas phase; multiple structural configurations were resolved on the millisecond timescale. These configurations are impossible to assess or even to know exist using mass spectrometric techniques that rely solely on dissociation patterns of precursor ions of selected m/z values, although other gas-phase spectroscopy techniques may be able to provide some information about isomeric components.[6-9]
Figure 1.
(a) Traveling wave ion mobility separation (TWIMS) of Hex-glycolaldehyde standards in the positive mode as [M+Na]+ ions at m/z 245.1. (b) TWIMS of Hex-glycolaldehyde standards in the negative mode as [M-H]− ions at m/z 221.1. (c) TWIMS of HexNAc-glycolaldehyde standards in the positive mode as [M+Na]+ ions at m/z 286.1. (d) TWIMS of HexNAc-glycolaldehyde standards in the negative mode as [M-H]− ions at m/z 262.1.
Isomeric disaccharide precursor and product ion separation
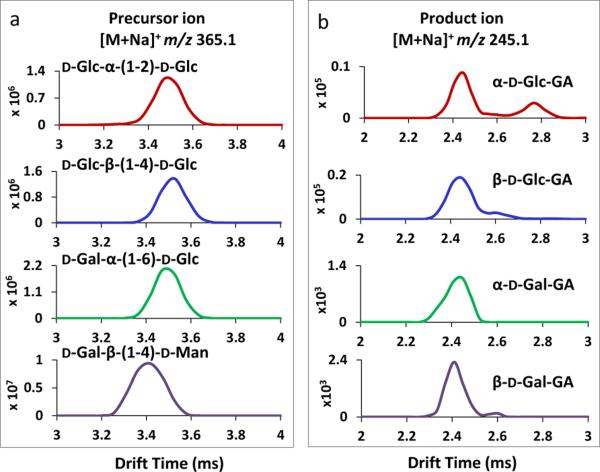
Fig 2a displays the traveling wave ion mobility spectra of isomeric disaccharide ions of D-Glc-α-(1-2)-D-Glc, D-Glc-β-(1-4)-D-Glc, D-Gal-α-(1-6)-D-Glc and D-Gal-β-(1-4)-D-Man, in the positive mode as [M+Na]+ ions at m/z 365.1. Identical drift times (3.5 ms) were observed for D-Glc-α-(1-2)-D-Glc, D-Glc-β-1-4-D-Glc and D-Gal-α-(1-6)-DGlc ions; their collision cross section variations were too small to be differentiated by IMS. A drift time value of 3.4 ms was obtained for D-Gal-β-(1-4)-D-Man, making it partially resolved from the other isomers. The traveling wave ion mobility resolving power[49] obtained was ~30-40 in this study and the separation can be improved by using IMS with higher resolving power. The mobility separation of the monosaccharide-glycolaldehyde product ions of α-D Glc-GA, β-D-Glc-GA, α-D-Gal-GA and β-D-Gal-GA, derived from the disaccharide isomers in Fig. 2a, is shown in Fig. 2b. This was achieved by selecting specific precursor ions using the quadrupole and applying 30 eV collision energy (CE) in the trap cell prior to the traveling wave ion mobility measurement. More than one mobility peak was observed for the majority of sodiated monosaccharide-glycolaldehyde product ions at m/z 245.1 and the drift time values matched with those of their respective synthetic standards shown in Fig. 1a. The Drift time variation is ± 0.02 ms in this study. However, some mobility peaks of low abundance were not observed for m/z 245.1 product ions in comparison with the mobility spectra acquired from standards;, for example, isomeric mobility peaks near 2.8 ms were missing for the product ions of α-D-Gal-GA and β-D-Gal-GA. This was probably due to the overall low intensity (~ thousand counts) obtained for these ions, making the structural variants with even lower abundance not detectable. These minor peaks were at most a few percent in relative abundance (Fig. 1a) and they might have been observable with longer acquisition times. It is also possible that the ratio of cyclic configurations and open chain forms of monosaccharide-glycolaldehydes obtained directly after dissociation of a disaccharide in the gas phase is different from that resulting from electrospray of the standards already equilibrated in aqueous solution, for a number of reasons. For instance, the extent of ionization of individual configurations of monosaccharide-glycolaldehydes from solution might be different from those arising by disaccharide dissociation. Also, the rate at which the open-chain and cyclic forms may interconvert in the gas phase could be dependent upon the energy input into the precursor ion, which differs among different disaccharide linkages. An open-chain carbonyl form has been shown to exist in varying proportions for different monosaccharides in the gas phase using variable-wavelength infrared photodissociation in the carbonyl stretch region.[52] Thus, the proportions of different configurations for the glycosyl-glycolaldehydes derived from disaccharide dissociation could depend on kinetic barriers to achieving a true thermodynamic equilibrium among configurations in the gas phase. Although little separation was achieved for the major peaks of different isomeric monosaccharide-glycolaldehyde product ions, the mobility patterns of this specific product ion, derived from different disaccharide isomers, were different, and this enabled the differentiation of these isomeric product ions. For instance α-D-Glc-GA and β-D-Glc-GA (Fig. 2b) showed distinct patterns, one with a more abundant secondary peak near 2.8 ms and one with a secondary peak near 2.6 ms. Note that only four selected disaccharides are shown in all the Figures in the manuscript, and the corresponding data for other disaccharide compounds are included in the Supporting Information.
Figure 2.
(a) TWIMS of isomeric disaccharide [M+Na]+ ions in ions at m/z 365.1. The disaccharide ions displayed were sodiated adducts of D-Glc-α-(1-2)-D-Glc, D-Glc-β-(1-4)-D-Glc, D-Gal-α-(1-6)-D-Glc and D-Gal-β-(1-4)-D-Man. (b) TWIMS of Hex-glycolaldehyde product ions derived from disaccharide ions shown in (a) in positive mode as [M+Na]+ ions at m/z 245.1. The corresponding product ions generated were sodiated adducts of α-D-Glc-GA, β-D-Glc-GA, α-D-Gal-GA and β-D-Gal-GA.
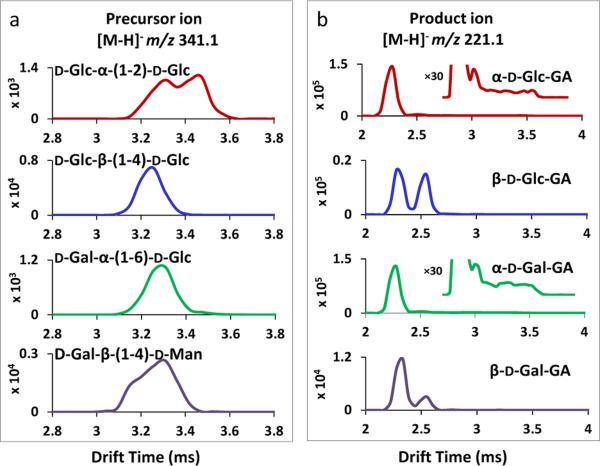
Isomeric disaccharides were also examined by traveling wave IMMS in the negative ion mode. The mobility spectra of D-Glc-α-(1-2)-D-Glc, D-Glc-β-(1-4)-D-Glc, D-Gal-α-(1-6)-D-Glc and D-Gal-β-(1-4)-D-Man, as [M-H]− precursor ions at m/z 341.1, are shown in Fig. 3a. The four disaccharide isomers were not fully differentiated;, however, two partially resolved mobility peaks were observed for D-Glc-α-(1-2)-D-Glc and D-Gal-β-(1-4)-D-Man. This could be attributed to the reducing end of disaccharides, where the -OH group at the anomeric carbon could exist in either the α or β pyranose configuration or even the aldehyde open chain form,[52] resulting in multiple isomeric forms for a single molecule to be resolved by IMS. In this study, Hexglycolaldehyde product anions were observed in abundance along with the precursor ions without any CE applied (see mass spectrum in Fig. 4b below). This was observed for all the disaccharide isomers and matched with previous findings that disaccharides having 2-, 4-, and 6-linkages frequently yield monosaccharide-glycolaldehyde product anions.[18] These monosaccharide-glycolaldehyde product anions evidently resulted from in-source fragmentation. Figure .3b displays the traveling wave ion mobility spectra of Hex-glycolaldehyde product ions in the negative mode as [M-H]− ions at m/z 221.1, derived from the disaccharide precursor ions shown in Fig. 3a. The product ions generated were deprotonated α-D-Glc-GA, β-D-Glc-GA, α-D-Gal-GA and β-D-Gal-GA. Two isomeric mobility peaks were detected for all four product anions and the mobility values were consistent with those of the respective standards in Fig. 1b. The relative abundance of the two configurational states of m/z 221.1 varied among the product ions having different stereochemistries;, for example, the two peaks were of approximately equal intensity for β-D-Glc-GA, while the configurational isomer having faster mobility was the predominant peak for the other product ions. In addition, increasing the intensity scale 30-fold for the product ion mobility spectra derived from the precursor anions of D-Glc-α-(1-2)-D-Glc and D-Gal-α-(1-6)-D-Glc (see enlarged insets in Fig. 3b) revealed a tailing of drift times as far back as the precursor ion mobility peaks themselves near 3.6 ms. This indicates that the disaccharide precursor ions partially dissociated to give monosaccharide-glycolaldehyde product ions en route along the ion mobility drift tube. This can occur either by auto-dissociation of metastable precursor ions above their dissociation threshold or from collision energy imparted to precursors very near or at their threshold of dissociation during impact with the drift gas. It also suggests that ion mobility could serve as a potential tool for real time reaction monitoring. Only 2- and 6-linked disaccharides showed the phenomenon of auto-dissociation in the ion mobility drift tube and there was no observable mobility tailing detected for m/z 221.1 ions produced from 4-linked disaccharides. The experimental conditions used were the same;, thus, as well as linkage position, other structural differences among disaccharide isomers may also contribute to the results that were observed. In summary, negative ions of isomeric monosaccharide-glycolaldehydes derived from isomeric disaccharide precursor ions showed different mobility patterns, which yielded unique information about their precursor ions that is complementary to the direct mass spectral dissociation patterns of the m/z 221.1 product ions themselves.[18]
Figure 3.
(a) TWIMS of isomeric disaccharide [M-H]− ions m/z 341.1. The disaccharide ions displayed were deprotonated D-Glc-α-(1-2)-D-Glc, D-Glc-β-(1-4)-D-Glc, D-Gal-α-(1-6)-D-Glc and D-Gal-β-(1-4)-D-Man. (b) TWIMS of Hex-glycolaldehyde product ions derived from disaccharides ions shown in (a) in the negative mode as [M-H]− ions at m/z 221.1. The corresponding product ions generated deprotonated α-D-Glc-GA, β-D-Glc-GA, α-D-Gal-GA and β-D-Gal-GA.
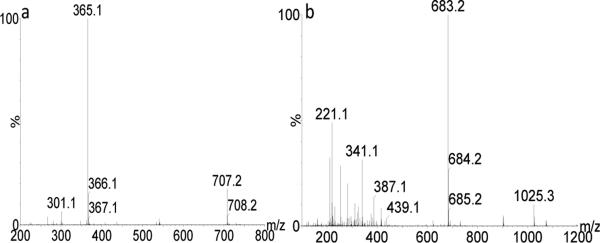
Figure 4.
(a) The mass spectrum of D-Glc-α-(1-2)-D-Glc in the positive mode, showing [M+Na]+ ions at m/z 365.1 and as [2M+Na]+ ions at m/z 707.2. (b) The mass spectrum of D-Gal-α-(1-6)-D-Glc in the negative mode, showing [M-H]− ions at m/z 341.1, [2M-H]− ions at m/z 683.2 and [3M-H]− ions at m/z 1025.3. The ion at m/z 221.1 represented a product ion derived from spontaneous in-source dissociation of the disaccharide in the negative mode. Other disaccharides had essentially the same observed ions.
Mobility separation of isomeric disaccharide cluster ions
In addition to the ions formed from a single molecule, disaccharide cluster ions were typically observed at the same time in the m/z spectrum. Figure. 4 shows the mass spectra of the disaccharides D-Glc-α-(1-2)-D-Glc in the positive mode (Fig. 4a) and D-Gal-α-(1-6)-D-Glc in the negative mode (Fig. 4b). Besides In addition to the major [M+Na]+ ion at m/z 365.1, a sodiated cluster ion having two disaccharide molecules [2M+Na]+ at m/z 707.2 was observed. In the negative mode, three related ions were found: the the ion [M-H]− ion at m/z 341.1, a cluster ion [2M-H]− at m/z 683.2 and a cluster ion [3M-H]− at m/z 1025.3. All other disaccharides showed the same ion clusters in both positive and negative ion modes but the relative intensities of different ion species varied. A cluster ion at m/z 683.2 was detected at higher abundance than the single disaccharide anion at m/z 341.1; this might be caused in part by preferential in-source fragmentation of m/z 341.1 ions to give product ions such as the m/z 221.1 ion, as shown in the mass spectrum (Fig. 4b). Sonoda et al.[53] studied the carbohydrate clustering effect in aqueous solution using fructose as an example. They found that carbohydrates tend to form hydrogen bonded clusters on increasing concentration. The concentration of disaccharides in this investigation (10 μM positive mode or 20 μM negative mode) was much smaller than those numbers (1-5 M) reported in the study by Sonada et al.[53] The formation of disaccharide clusters perhaps occurs during the ionization (desolvation) process where the solvents start to evaporate and solutes transit from scattered “isolated” molecules to H-bonded clusters in less diluted solutions. Moreover, the multiple –OH groups on disaccharides could enhance the formation of cooperative hydrogen-bonded networks compared with fructose. Cluster ions having up to three disaccharide molecules were found in the negative mode but not in the positive mode, and this could in part be due to the relatively higher concentrations that were used for electrospray of the molecules in the negative mode. The occurrence of cluster ions can be eliminated by decreasing the sample concentrations. The mobilities of isomeric disaccharide cluster ions were determined and are reported here for the first time.
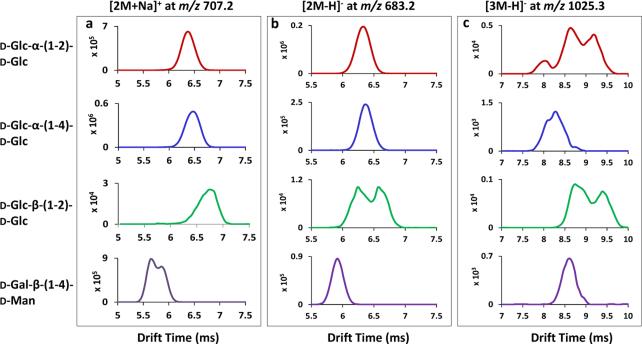
Traveling wave ion mobility spectra of cluster cations and anions, using D-Glc-α-(1-2)-DGlc, D-Glc-α-(1-4)-D-Glc, D-Glc-β-(1-2)-D-Glc and D-Gal-β-(1-4)-D-Man as examples, are shown in Fig. 5. For the [2M+Na]+ cluster ion at m/z 707.2, the central drift time values obtained were 6.4 ms, 6.4 ms, 6.8 ms and 5.7 ms for the different disaccharide isomers displayed in Fig. 5a. Mobility measurements of cluster ions could aid in the differentiation of disaccharide isomers. For example, D-Glc-α-(1-2)-D-Glc and D-Gal-β-(1-4)-D-Man were only slightly separated as sodium adducts based on mobility spectra of single molecule precursor ions (Fig. 2a), but were baseline resolved as their respective cluster cations (6.4 and 5.7 ms, Fig. 5a). In addition, two partially resolved mobility peaks were observed for the dimeric clusters of D-Gal-β-(1-4)-D-Man, indicating that there are at least two isomeric forms / structure variants / stereochemical isomers. In the negative ion mode, based on the mobility spectra of the dimeric [2M-H]− ions at m/z 683.2 (Fig. 5b), D-Gal-β-(1-4)-D-Man having a drift time of 5.9 ms was resolved from the other isomers. Two mobility peaks were detected for D-Glc-β-(1-2)-D-Glc. Multiple mobility peaks were frequently observed for specific cluster ions and the mobility profiles of the [3M-H]− ions at m/z 1025.3 are shown in Fig 5c: three clearly separated peaks for D-Glc-α-(1-2)-D-Glc, three barely resolved peaks for D-Glc-α-1-4-D-Glc and two partially distinguished peaks for D-Glc-β-1-2-D-Glc. This is the first time that the structural heterogeneity of sugar cluster ions, using reducing disaccharides as examples, has been investigated by ion mobility. Multiple mobility peaks for a single cluster ion may result from differences (α, β) at the reducing end of disaccharides, as discussed above, or from different clustering interactions - the geometry disaccharides adopt to form the clusters through various intermolecular interactions. In general, more mobility peaks were resolved for disaccharide cluster ions in than for the ions formed from single molecules. This may be explained in part by more widely differing sizes and shapes of clusters due to stereochemically-dependent interactions that enable them to be more easily separated by IMS. The mobility data of cluster ions could also serve as a further analytical property for the identification of sugar stereo- and linkage isomers.
Figure 5.
Traveling wave ion mobility separation (TWIMS) of isomeric disaccharide cluster ions of (a) [2M+Na]+ ions at m/z 707.2 (b) [2M-H]− ions at m/z 683.2 and (c) [3M-H]− ions at m/z 1025.3. The disaccharides used are labeled at the left in the same order from top to bottom for all three panels.
Mobility separation of disaccharides as chloride adducts and chloride adduct clusters
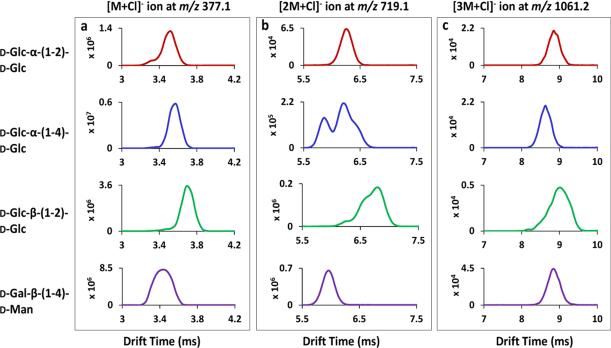
Disaccharide chloride adducts were observed upon adding an NH4Cl solution to each sample. The ions observed were the [M+Cl]− ion at m/z 377.1, the [2M+Cl]− ion at m/z 719.1 and the [3M+Cl]− ion at m/z 1061.2. Their representative mass spectra are included in the Supporting Information (Fig. S-4). The mobility spectra of disaccharide chloride adducts for D-Glc-α-(1-2)-D-Glc, D-Glc-α-(1-4)-D-Glc, D-Glc-β-(1-2)-D-Glc and D-Gal-β-(1-4)-D-Man are displayed in Fig. 6. Partial mobility separation was achieved for the [M+Cl]− ions among these isomers, as shown in Fig. 6a. An additional mobility peak having faster mobility was detected with very low abundance for all the disaccharides except D-Gal-β-(1-4)-D-Man. Figure. 6b shows the ion mobility separation of isomeric [2M+Cl]− ions, where three mobility peaks were partially resolved for both D-Glc-α-(1-4)-D-Glc and D-Glc-β-(1-2)-D-Glc. Compared with the [2M-H]− cluster ions in Fig. 5b, more isomeric forms were differentiated as chloride adduct clusters. However, this scenario was reversed for the [3M+Cl]− ions, as shown in Fig. 6c, where one peak was found for all the disaccharides, with drift time of 8.8 ms, 8.7 ms, 9.0 ms and 8.8 ms; fewer mobility peaks were differentiated for specific [3M+Cl]− ions than for their respective [3M-H]− ions (Fig. 5c). The structural configuration(s) of disaccharide cluster ions induced by the addition of chloride is expected to be different from that of cluster ions having lost one proton. The collision cross section area differences among multiple isomeric forms of a single cluster species may become either larger or smaller upon the adduction of a Cl− ion as demonstrated by IMS (compare Figs. 5b and c with Fig. 6b and c). In addition, it is worth noting that no separation method has infinite resolution. Due to the relatively low resolving power of the Synapt G2 mass spectrometer, there is always the possibility that some isomeric structural variants could coincidentally co-elute as one ion mobility peak, such as the broad peak detected for the [M+Cl]− ion of D-Gal-β-(1-4)-D-Man (Fig. 6a) and the [3M+Cl]− ion of D-Glc-β-(1-2)-D-Glc (Figure 6c). The results shown in Fig. 6 indicate that different anions can influence the ion mobility separation of carbohydrate structural isomers, both for ions formed from single molecules and for clusters ions. However, no specific rules can be concluded as the structures of specific clusters are unknown. Further investigations need to be performed on more negative ion forms (such as [M+I]−, [M+Br]− and [M+NO3]- ions) to more fully understand the mobility characteristics observed here.
Figure 6.
(a) TWIMS of the selected disaccharides (a) for the [M+Cl]− ion at m/z 377.1; (b) for the [2M+Cl]− ion at m/z 719.1 and (c) for the [3M+Cl]− ion at m/z 1061.2. The isomeric disaccharides shown at the left are in the same order from top to the bottom for all three panels.
Conclusions
The isomeric heterogeneity of disaccharide ions, product ions dissociated from disaccharide ions and disaccharide cluster ions was evaluated using traveling wave ion mobility mass spectrometry in both the positive and negative ion modes. Partial mobility separation was achieved for isomeric ions having the same m/z values but differing in anomeric configurations, linkage positions (2-, 4- and 6-linked), or monomer components. The mobilities of structurally informative, isomeric product ions derived from disaccharides, the monosaccharide-glycolaldehydes, were measured and compared. More than one isomeric mobility peak was obtained for specific monosaccharide-glycolaldehyde product ions derived from disaccharide ions, which appear to correspond to the multiple structural isomers reported in previous studies.[18, 46, 47] Moreover, the mobility distributions of this product ion were different among disaccharide isomers, which enables the separation of isomeric product ions and provides another physical property for the characterization of isomeric disaccharides. Both 2- and 6-linked reducing disaccharide ions dissociated to yield Hexglycolaldehyde anions within the ion mobility drift tube as demonstrated by the mobility spectra. Differentiation of isomeric cluster ions was also observed by IMS; a majority of cluster ions showed multiple mobility peaks, which may be due either to alternate (α, β) reducing end structures of the disaccharides and/or the three-dimensional geometry of their interactions. Furthermore, mobility separation of isomeric carbohydrate ions including precursor and cluster ions could be altered by using different anions, such as chloride adducts in this investigation.
The results demonstrated that IMMS is an advantageous method to assess the isomeric heterogeneity of carbohydrate compounds. The analysis is fast, sensitive and convenient, able to resolve isomers and differentiate multiple structural configurations of single compounds. While this study was carried out with small carbohydrate ions and their product ions, the much broader question as to whether ions of any molecule might give rise to physically separable sets of isomeric product ions can now be addressed. Whether such product ions arise through alternate dissociation pathways, or as a result of them having more than one configuration, ion mobility spectrometry/mass spectrometry as described herein may provide important information to assess their presence and to determine their cross-sectional areas as a bona fide, reproducible physical property unique to each isomeric product ion and the specific gas through which it migrates.
Supplementary Material
Acknowledgements
This work was supported in part by the National Institutes of Health with grant # 5R33RR020046.
References
- 1.Paul W, Reinhard HP, von Zahn U. The electric mass filter as a mass spectrometer and isotope separator. Phys. 1958;152:143. [Google Scholar]
- 2.Hager JW. A new linear ion trap mass spectrometer. Rapid Commun. Mass Spectrom. 2002;16:512. doi: 10.1002/rcm.1020. [DOI] [PubMed] [Google Scholar]
- 3.Yost RA, Enke CG, McGilvery DC, Smith D, Morrison JD. High efficiency collision-induced dissociation in an rf-only quadrupole. Int. J. Mass Spectrom.. Ion Phys. 1979;30:127. [Google Scholar]
- 4.Yergey AL, Coorssen JR, Backlund PS, Blank PS, Humphrey GA, Zimmerberg J, Campbell JM, Vestal ML. De novo sequencing of peptides using MALDI/TOF-TOF. J. Am Soc. Mass Spectrom. 2002;13:784. doi: 10.1016/S1044-0305(02)00393-8. [DOI] [PubMed] [Google Scholar]
- 5.Marshall AG. Milestones in FT-ICR MS technique development. Int. J. Mass Spectrom. 2000;200:331. [Google Scholar]
- 6.Rizzo TR, Stearns JA, Boyarkin OV. Spectroscopic studies of cold, gas-phase biomolecular ions. Int. Rev. Phys. Chem. 2009;28:481. [Google Scholar]
- 7.Eyler JR. Infrared multiphoton dissociation spectroscopy of ions in penning traps. Mass Spectrom. Rev. 2009;28:448. doi: 10.1002/mas.20217. [DOI] [PubMed] [Google Scholar]
- 8.McCarthy MC, Thaddeus P. High-resolution rotational spectroscopy of the carbon chain anions C3N−, C4H− and C4D−. J. Chem. Phys. 2008;129:054314. doi: 10.1063/1.2960626. [DOI] [PubMed] [Google Scholar]
- 9.Cyriac J, Wleklinski M, Li G, Gao L, Cooks RG. In situ Raman spectroscopy of surfaces modified by ion soft landing. Analyst. 2012;137:1363. doi: 10.1039/c2an16163j. [DOI] [PubMed] [Google Scholar]
- 10.Raman R, Raguram S, Venkataraman G, Paulson JC, Sasisekharan R. Glycomics: an integrated systems approach to structure-function relationships of glycans. Nat. Methods. 2005;2:817. doi: 10.1038/nmeth807. [DOI] [PubMed] [Google Scholar]
- 11.Ratner DM, Adams EW, Disney MD, Seeberger PH. Tools for glycomics: mapping interactions of carbohydrates in biological systems. ChemBioChem. 2004;5:1375. doi: 10.1002/cbic.200400106. [DOI] [PubMed] [Google Scholar]
- 12.Rudd PM, Elliott T, Cresswell P, Wilson IA, Dwek RA. Glycosylation and the immune system. Science. 2001;291:2370. doi: 10.1126/science.291.5512.2370. [DOI] [PubMed] [Google Scholar]
- 13.An HJ, Kronewitter SR, de Leoz MLA, Lebrilla CB. Glycomics and Disease Markers. Curr. Opin. Chem. Biol. 2009;13:601. doi: 10.1016/j.cbpa.2009.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kam RKT, Poon TCW. The potentials of glycomics in biomarker discovery. Clin. Proteom. 2008;4:67. [Google Scholar]
- 15.Apweiler R, Hermjakob H, Sharon N. On the frequency of protein glycosylation, as deducted from analysis of the SWISS-PROT database. Biochim. Biophys. Acta. 1999;1473:4. doi: 10.1016/s0304-4165(99)00165-8. [DOI] [PubMed] [Google Scholar]
- 16.Iwasaki H, Zhang Y, Tachibana K, Gotoh M, Kikuchi N, Kwon Y-D, Togayachi A, Kudo T, Kubota T, Narimatsu H. Initiation of O-glycan synthesis in IgA1 hinge region is determined by a single enzyme, UDP-N-acetyl-α-D-galactosamine: polypeptide N- acetylgalactosaminyltransferase 2. J. Biol. Chem. 2003;278:5613. doi: 10.1074/jbc.M211097200. [DOI] [PubMed] [Google Scholar]
- 17.Kim M-J, Hennen WJ, Sweers HM, Wong C-H. Enzymes in carbohydrate synthesis: N-acetylneuraminic acid aldolase catalyzed reactions and preparation of N-acetyl-2-deoxy-D-neuraminic acid derivatives. J. Am. Chem. Soc. 1988;110:6481. [Google Scholar]
- 18.Fang TT, Bendiak B. The stereochemical dependence of unimolecular dissociation of monosaccharide-glycolaldehyde anions in the gas phase: a basis for assignment of the stereochemistry and anomeric configuration of monosaccharides in oligosaccharides by mass spectrometry via a key discriminatory product ion of disaccharide fragmentation, m/z 221. J. Am. Chem. Soc. 2007;129:9721. doi: 10.1021/ja0717313. [DOI] [PubMed] [Google Scholar]
- 19.Martensson S, Levery SB, Fang TT, Bendiak B. Neutral core oligosaccharides of bovine submaxillary mucin: Use of lead tetraacetate in the cold for establishing branch positions. Eur. J. Biochem. 1998;258:603. doi: 10.1046/j.1432-1327.1998.2580603.x. [DOI] [PubMed] [Google Scholar]
- 20.Fenn LS, Mclean JA. Structural resolution of carbohydrate positional and structural isomers based on gas-phase ion mobility-mass spectrometry. Phys. Chem. Chem. Phys. 2011;13:2196. doi: 10.1039/c0cp01414a. [DOI] [PubMed] [Google Scholar]
- 21.Zaia J. Mass spectrometry and the emerging field of glycomics. J. Chem. Biol. 2008;15:881. doi: 10.1016/j.chembiol.2008.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morelle W, Michalski JC. Glycomics and mass spectrometry. Curr. Pharm. Des. 2005;11:2615. doi: 10.2174/1381612054546897. [DOI] [PubMed] [Google Scholar]
- 23.Morelle W, Michalski JC. The mass spectrometric analysis of glycoproteins and their glycan structures. Curr. Anal. Chem. 2005;1:29. [Google Scholar]
- 24.Reinhold VN, Reinhold BB, Costello CE. Carbohydrate molecular weight profiling, sequence, linkage, and branching data: ES-MS and CID. Anal. Chem. 1995;67:1772. doi: 10.1021/ac00107a005. [DOI] [PubMed] [Google Scholar]
- 25.Chu CS, Ninonuevo MR, Clowers BH, Perkins PD, An HJ, Yin H, Killeen K, Miyamoto S, Grimm R, Lebrilla CB. Profile of native N-linked glycan structures from human serum using high performance liquid chromatography on a microfluidic chip and time-of-flight mass spectrometry. Proteomics. 2009;9:1939. doi: 10.1002/pmic.200800249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ninonuevo M, An H, Yin H, Killeen K, Grimm R, Ward R, German B, Lebrilla C. Nanoliquid chromatography-mass spectrometry of oligosaccharides employing graphitized carbon chromatography on microchip with a high-accuracy mass analyzer. Electrophoresis. 2005;26:3641. doi: 10.1002/elps.200500246. [DOI] [PubMed] [Google Scholar]
- 27.Pabst M, Bondili JS, Stadlmann J, Mach L, Altmann F. Mass + retention time = structure: a strategy for the analysis of N-glycans by carbon LC-ESI-MS and its application to fibrin N-glycans. Anal. Chem. 2007;79:5051. doi: 10.1021/ac070363i. [DOI] [PubMed] [Google Scholar]
- 28.Karlsson H, Carlstedt I, Hansson GC. The use of gas chromatography and gas chromatography-mass spectrometry for the characterization of permethylated oligosaccharides with molecular mass up to 2300. Anal. Biochem. 1989;182:438. doi: 10.1016/0003-2697(89)90620-9. [DOI] [PubMed] [Google Scholar]
- 29.Thomsson KA, Hinojosa-Kurtzberg M, Axelsson KA, Domino SE, Lowe JB, Gendler SJ, Hansson GC. Intestinal mucins from cystic fibrosis mice show increased fucosylation due to an induced Fucα1-2 glycosyltransferase. Biochem. J. 2002;367:609. doi: 10.1042/BJ20020371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eiceman GA, Karpas Z. Ion mobility spectrometry. 2nd edn Taylor and Francis Group, LLC; Boca Raton: 2005. [Google Scholar]
- 31.Eiceman GA. Ion-mobility spectrometry as a fast monitor of chemical composition. Trends Anal. Chem. 2002;21:259. [Google Scholar]
- 32.Ewing RG, Atkinson DA, Eiceman GA, Ewing GJ. A critical review of ion mobility spectrometry for the detection of explosives and explosive related compounds. Talanta. 2001;54:515. doi: 10.1016/s0039-9140(00)00565-8. [DOI] [PubMed] [Google Scholar]
- 33.Kanu AB, Hill HH., Jr Identity confirmation of drugs and explosives in ion mobility spectrometry using a secondary drift gas. Talanta. 2007;73:692. doi: 10.1016/j.talanta.2007.04.058. [DOI] [PubMed] [Google Scholar]
- 34.Kanu AB, Dwivedi P, Tam M, Matz L, Hill HH., Jr Ion mobility-mass spectrometry. J. Mass Spectrom. 2008;43:1. doi: 10.1002/jms.1383. [DOI] [PubMed] [Google Scholar]
- 35.Dwivedi P, Wu P, Klopsch SJ, Puzon GJ, Xun L, Hill HH., Jr Metabolic profiling by ion mobility mass spectrometry (IMMS). Metabolomics. 2008;4:63. [Google Scholar]
- 36.Kaplan K, Dwivedi P, Davidson S, Yang Q, Tso P, Siems W, Hill HH., Jr Monitoring dynamic changes in lymph metabolome of fasting and fed rats by electrospray ionization-ion mobility mass spectrometry (ESI-IMMS). Anal. Chem. 2009;81:7944. doi: 10.1021/ac901030k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McLean JA, Ruotolo BH, Gillig KJ, Russell DH. Ion mobility–mass spectrometry: a new paradigm for proteomics. Int. J. Mass Spectrom. 2005;240:301. [Google Scholar]
- 38.Valentine SJ, Plasencia MD, Liu X, Krishnan M, Naylor S, Udseth HR, Smith RD, Clemmer DE. Toward Plasma Proteome Profiling with Ion Mobility-Mass Spectrometry. J. Proteome Res. 2006;5:2977. doi: 10.1021/pr060232i. [DOI] [PubMed] [Google Scholar]
- 39.Isailovic D, Kurulugama RT, Plasencia MD, Stokes ST, Kyselova Z, Goldman R, Mechref Y, Novotny MV, Clemmer DE. Profiling of human serum glycans associated with liver cancer and cirrhosis by IMS-MS. J. Proteome Res. 2008;7:1109. doi: 10.1021/pr700702r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Plasencia MD, Isailovic D, Merenbloom SI, Mechref Y, Clemmer DE. Resolving and assigning N-linked glycan structural isomers from ovalbumin by IMS-MS. J. Am. Soc. Mass Spectrom. 2008;19:1706. doi: 10.1016/j.jasms.2008.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dwivedi P, Bendiak B, Clowers BH, Hill HH., Jr Rapid resolution of carbohydrate isomers by electrospray ionization ambient pressure ion mobility spectrometry-time-of-flight mass spectrometry (ESI-APIMS-TOFMS). J. Am. Soc. Mass Spectrom. 2007;18:1163. doi: 10.1016/j.jasms.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 42.Li H, Giles K, Bendiak B, Kaplan K, Siems WF, Hill HH., Jr Resolving structural isomers of monosaccharide methyl glycosides using drift tube and traveling wave ion mobility mass spectrometry. Anal. Chem. 2012;84:3231. doi: 10.1021/ac203116a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhu M, Bendiak B, Clowers BH, Hill HH., Jr Ion mobility-mass spectrometry analysis of isomeric carbohydrate precursor ions. Anal. Bioanal. Chem. 2009;394:1853. doi: 10.1007/s00216-009-2865-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Clowers BH, Dwivedi P, Steiner WE, Hill HH., Jr Separation of sodiated isobaric disaccharides and trisaccharides using electrospray ionization-atmospheric pressure ion mobility- time of flight mass spectrometry. J. Am. Soc. Mass Spectrom. 2005;16:660. doi: 10.1016/j.jasms.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 45.Sunyoung L, Valentine SJ, Reilly JP, Clemmer DE. Analyzing a mixture of disaccharides by IMS-VUVPD-MS. Int. J. Mass Spectrom. 2012;309:161. doi: 10.1016/j.ijms.2011.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bendiak B, Fang TT. Assignment of the stereochemistry and anomeric configuration of structurally informative product ions derived from disaccharides: infrared photodissociation of glycosyl-glycolaldehydes in the negative ion mode. Carbohyd. Res. 2010;345:2390. doi: 10.1016/j.carres.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 47.Fang TT, Zirrolli J, Bendiak B. Differentiation of the anomeric configuration and ring form of glucosyl-glycolaldehyde anions in the gas phase by mass spectrometry: isomeric discrimination between m/z 221 anions derived from disaccharides and chemical synthesis of m/z 221 standards. Carbohyd. Res. 2007;342:217. doi: 10.1016/j.carres.2006.11.021. [DOI] [PubMed] [Google Scholar]
- 48.Pringle SD, Giles K, Wildgoose JL, Williams JP, Slade SE, Thalassinos K, Bateman RH, Bowers MT, Scrivens JH. An investigation of the mobility separation of some peptide and protein ions using a new hybrid quadrupole/travelling wave IMS/oa-ToF instrument. Int. J. Mass Spectrom. 2007;261:1. [Google Scholar]
- 49.Shvartsburg AA, Smith RD. Fundamentals of traveling wave ion mobility spectrometry. Anal. Chem. 2008;80:9689. doi: 10.1021/ac8016295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Giles K, Wildgoose JL, Langridge DJ, Campuzano I. A method for direct measurement of ion mobilities using a travelling wave ion guide. Int. J. Mass Spectrom. 2010;298:10. [Google Scholar]
- 51.Giles K, Pringle SD, Worthington KR, Little D, Wildgoose JL, Bateman RH. Applications of a travelling wave-based radio-frequency only stacked ring ion guide. Rapid Commun. Mass Spectrom. 2004;18:2401. doi: 10.1002/rcm.1641. [DOI] [PubMed] [Google Scholar]
- 52.Brown DJ, Stefan SE, Berden G, Steill JD, Oomens J, Eyler JR, Bendiak B. Direct evidence for the ring opening of monosaccharide anions in the gas phase: photodissociation of aldohexoses and aldohexoses derived from disaccharides using variable-wavelength infrared irradiation in the carbonyl stretch region. Carbohyd. Res. 2011;346:2469. doi: 10.1016/j.carres.2011.06.018. [DOI] [PubMed] [Google Scholar]
- 53.Sonoda MT, Skaf MS. Carbohydrate clustering in aqueous solutions and the dynamics of confined water. J. Phys. Chem. B. 2007;111:11948. doi: 10.1021/jp0749120. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.