Abstract
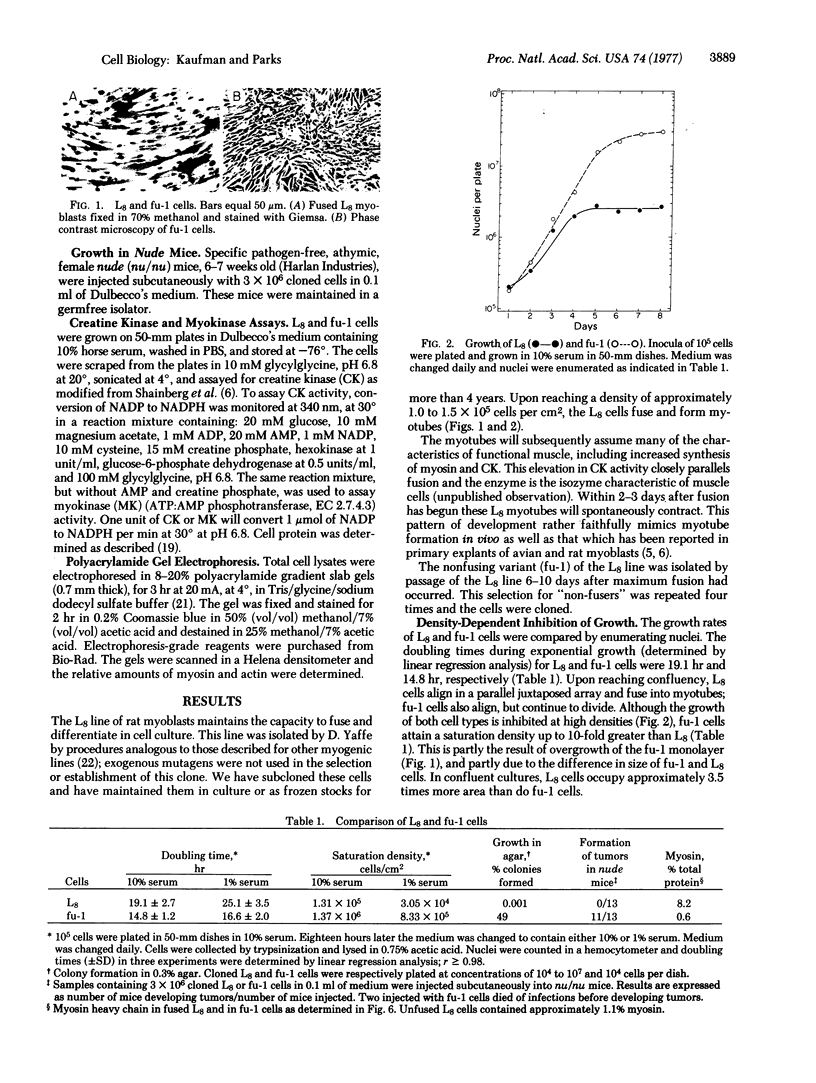
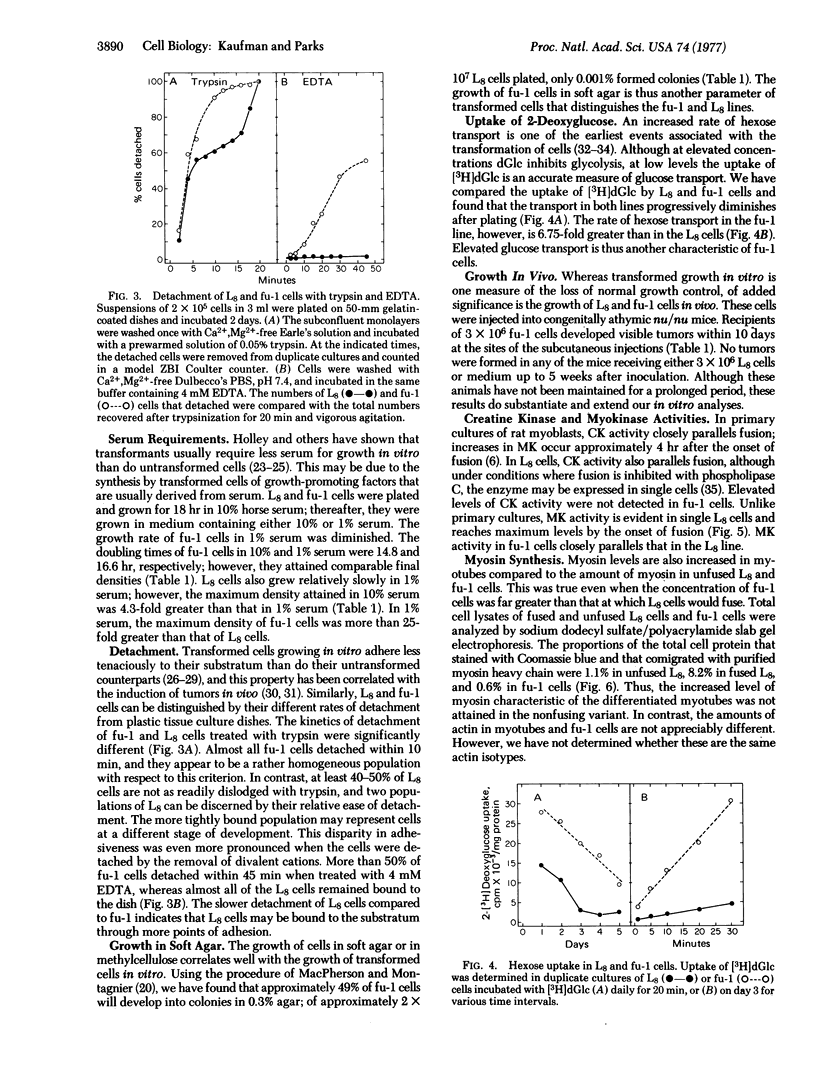
A nonfusing variant, fu-1, of the L8 line of rat myoblasts was isolated and characterized with respect to its growth in vitro and developmental properties. Comparative analyses of density-dependent inhibition of growth, serum requirements, cell adhesiveness, colony formation in soft agar, and hexose transport in L8 and fu-1 cells support the conclusion that the fu-1 cells are transformed. In addition, fu-1, but not L8, cells promote the development of tumors in athymic nude mice. fu-1 cells also do not make increased levels of creatinekinase (ATP:creatine N-phosphotransferase, EC 2.7.3.2) or myosin and they express an endogenous type-C virus. Both L8 and fu-1 cells express myokinase (ATP:AMP phosphotransferase, EC 2.7.4.3) activities in single cells. In contrast to fu-1 cells, the parent L8 line has increased creatine kinase and myosin after fusion and spontaneously contracts; expression of an endogenous virus could not be detected in these cells. These results suggest that loss of the ability to differentiate normally is associated with the loss of the normal control of cell division of myoblasts grown in vitro and in vivo.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bischoff R., Holtzer H. Mitosis and the processes of differentiation of myogenic cells in vitro. J Cell Biol. 1969 Apr;41(1):188–200. doi: 10.1083/jcb.41.1.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulbecco R. Topoinhibition and serum requirement of transformed and untransformed cells. Nature. 1970 Aug 22;227(5260):802–806. doi: 10.1038/227802a0. [DOI] [PubMed] [Google Scholar]
- Emerson C. P., Jr, Beckner S. K. Activation of myosin synthesis in fusing and mononucleated myoblasts. J Mol Biol. 1975 Apr 25;93(4):431–447. doi: 10.1016/0022-2836(75)90238-7. [DOI] [PubMed] [Google Scholar]
- Engel W. K., Askanas V. Letter: Overlooked avian oncornavirus in cultured muscle--functionally significant? Science. 1976 Jun 18;192(4245):1252–1253. doi: 10.1126/science.192.4245.1252-a. [DOI] [PubMed] [Google Scholar]
- Fambrough D., Rash J. E. Development of acetylcholine sensitivity during myogenesis. Dev Biol. 1971 Sep;26(1):55–68. doi: 10.1016/0012-1606(71)90107-2. [DOI] [PubMed] [Google Scholar]
- Fischbach G. D., Cohen S. A. The distribution of acetylcholine sensitivity over uninnervated and innervated muscle fibers grown in cell culture. Dev Biol. 1973 Mar;31(1):147–162. doi: 10.1016/0012-1606(73)90326-6. [DOI] [PubMed] [Google Scholar]
- Fiszman M. Y., Fuchs P. Temperature-sensitive expression of differentiation in transformed myoblasts. Nature. 1975 Apr 3;254(5499):429–431. doi: 10.1038/254429a0. [DOI] [PubMed] [Google Scholar]
- Freedman V. H., Shin S. I. Cellular tumorigenicity in nude mice: correlation with cell growth in semi-solid medium. Cell. 1974 Dec;3(4):355–359. doi: 10.1016/0092-8674(74)90050-6. [DOI] [PubMed] [Google Scholar]
- HOLTZER H., MARSHALL J. M., Jr, FINCK H. An analysis of myogenesis by the use of fluorescent antimyosin. J Biophys Biochem Cytol. 1957 Sep 25;3(5):705–724. doi: 10.1083/jcb.3.5.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatanaka M., Hanafusa H. Analysis of a functional change in membrane in the process of cell transformation by Rous sarcoma virus; alteration in the characteristics of sugar transport. Virology. 1970 Aug;41(4):647–652. doi: 10.1016/0042-6822(70)90429-0. [DOI] [PubMed] [Google Scholar]
- Hatanaka M., Huebner R. J., Gilden R. V. Alterations in the characteristics of sugar uptake by mouse cells transformed by murine sarcoma viruses. J Natl Cancer Inst. 1969 Nov;43(5):1091–1096. [PubMed] [Google Scholar]
- Holley R. W., Kiernan J. A. "Contact inhibition" of cell division in 3T3 cells. Proc Natl Acad Sci U S A. 1968 May;60(1):300–304. doi: 10.1073/pnas.60.1.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzer H., Biehl J., Yeoh G., Meganathan R., Kaji A. Effect of oncogenic virus on muscle differentiation. Proc Natl Acad Sci U S A. 1975 Oct;72(10):4051–4055. doi: 10.1073/pnas.72.10.4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes R. O., Martin G. S., Shearer M., Critchley D. R., Epstein C. J. Viral transformation of rat myoblasts: effects on fusion and surface properties. Dev Biol. 1976 Jan;48(1):35–46. doi: 10.1016/0012-1606(76)90043-9. [DOI] [PubMed] [Google Scholar]
- KONIGSBERG I. R. Some aspects of myogenesis in vitro. Circulation. 1961 Aug;24:447–457. doi: 10.1161/01.cir.24.2.447. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- MACPHERSON I., MONTAGNIER L. AGAR SUSPENSION CULTURE FOR THE SELECTIVE ASSAY OF CELLS TRANSFORMED BY POLYOMA VIRUS. Virology. 1964 Jun;23:291–294. doi: 10.1016/0042-6822(64)90301-0. [DOI] [PubMed] [Google Scholar]
- Martin G. S., Venuta S., Weber M., Rubin H. Temperature-dependent alterations in sugar transport in cells infected by a temperature-sensitive mutant of Rous sarcoma virus. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2739–2741. doi: 10.1073/pnas.68.11.2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlie J. P., Sobel A., Changeux J. P., Gros F. Synthesis of acetylcholine receptor during differentiation of cultured embryonic muscle cells. Proc Natl Acad Sci U S A. 1975 Oct;72(10):4028–4032. doi: 10.1073/pnas.72.10.4028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mintz B., Baker W. W. Normal mammalian muscle differentiation and gene control of isocitrate dehydrogenase synthesis. Proc Natl Acad Sci U S A. 1967 Aug;58(2):592–598. doi: 10.1073/pnas.58.2.592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill M., Strohman R. C. Changes in DNA polymerase activity associated with cell fusion in cultures of embryonic muscle. J Cell Physiol. 1969 Feb;73(1):61–68. doi: 10.1002/jcp.1040730109. [DOI] [PubMed] [Google Scholar]
- OYAMA V. I., EAGLE H. Measurement of cell growth in tissue culture with a phenol reagent (folin-ciocalteau). Proc Soc Exp Biol Med. 1956 Feb;91(2):305–307. doi: 10.3181/00379727-91-22245. [DOI] [PubMed] [Google Scholar]
- Paterson B., Strohman R. C. Myosin synthesis in cultures of differentiating chicken embryo skeletal muscle. Dev Biol. 1972 Oct;29(2):113–138. doi: 10.1016/0012-1606(72)90050-4. [DOI] [PubMed] [Google Scholar]
- Prives J., Silman I., Amsterdam A. Appearance and disappearance of acetycholine receptor during differentiation of chick skeletal muscle in vitro. Cell. 1976 Apr;7(4):543–550. doi: 10.1016/0092-8674(76)90204-x. [DOI] [PubMed] [Google Scholar]
- Richler C., Yaffe D. The in vitro cultivation and differentiation capacities of myogenic cell lines. Dev Biol. 1970 Sep;23(1):1–22. doi: 10.1016/s0012-1606(70)80004-5. [DOI] [PubMed] [Google Scholar]
- Risser R., Pollack R. A nonselective analysis of SV40 transformation of mouse 3T3 cells. Virology. 1974 Jun;59(2):477–489. doi: 10.1016/0042-6822(74)90457-7. [DOI] [PubMed] [Google Scholar]
- STOCKDALE F. E., HOLTZER H. DNA synthesis and myogenesis. Exp Cell Res. 1961 Sep;24:508–520. doi: 10.1016/0014-4827(61)90450-5. [DOI] [PubMed] [Google Scholar]
- STREHLER B. L., KONIGSBERG I. R., KELLEY J. E. PLOIDY OF MYOTUBE NUCLEI DEVELOPING IN VITRO AS DETERMINED WITH A RECORDING DOUBLE BEAM MICRO-SPECTROPHOTOMETER. Exp Cell Res. 1963 Nov;32:232–241. doi: 10.1016/0014-4827(63)90098-3. [DOI] [PubMed] [Google Scholar]
- Shainberg A., Yagil G., Yaffe D. Alterations of enzymatic activities during muscle differentiation in vitro. Dev Biol. 1971 May;25(1):1–29. doi: 10.1016/0012-1606(71)90017-0. [DOI] [PubMed] [Google Scholar]
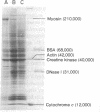
- Shields R., Pollock K. The adhesion of BHK and PyBHK cells to the substratum. Cell. 1974 Sep;3(1):31–38. doi: 10.1016/0092-8674(74)90034-8. [DOI] [PubMed] [Google Scholar]
- Shin S. I., Freedman V. H., Risser R., Pollack R. Tumorigenicity of virus-transformed cells in nude mice is correlated specifically with anchorage independent growth in vitro. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4435–4439. doi: 10.1073/pnas.72.11.4435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H. S., Scher C. D., Todaro G. J. Induction of cell division in medium lacking serum growth factor by SV40. Virology. 1971 May;44(2):359–370. doi: 10.1016/0042-6822(71)90267-4. [DOI] [PubMed] [Google Scholar]
- Stockdale F. E. Changing levels of DNA polymerase activity during the development of skeletal muscle tissue in vivo. Dev Biol. 1970 Mar;21(3):462–474. doi: 10.1016/0012-1606(70)90136-3. [DOI] [PubMed] [Google Scholar]
- Stoker M., O'Neill C., Berryman S., Waxman V. Anchorage and growth regulation in normal and virus-transformed cells. Int J Cancer. 1968 Sep 15;3(5):683–693. doi: 10.1002/ijc.2910030517. [DOI] [PubMed] [Google Scholar]
- Sytkowski A. J., Vogel Z., Nirenberg M. W. Development of acetylcholine receptor clusters on cultured muscle cells. Proc Natl Acad Sci U S A. 1973 Jan;70(1):270–274. doi: 10.1073/pnas.70.1.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarikas H., Schubert D. Regulation of adenylate kinase and creatine kinase activities in myogenic cells. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2377–2381. doi: 10.1073/pnas.71.6.2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong P. K., Yuen P. H., Kaufman S. J. Induction of syncytia by Moloney murine leukemia virus in myoblasts defective in differentiation. J Virol. 1977 Jan;21(1):319–327. doi: 10.1128/jvi.21.1.319-327.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaffe D. Cellular aspects of muscle differentiation in vitro. Curr Top Dev Biol. 1969;4:37–77. doi: 10.1016/s0070-2153(08)60480-9. [DOI] [PubMed] [Google Scholar]