Abstract
Background and objectives
While ESRD from lupus nephritis (ESLN) increased in the United States after the mid-1990s and racial disparities were apparent, current trends are unknown.
Design, setting, participants, & measurements
Retrospective US Renal Data System data (n=1,557,117) were used to calculate standardized incidence ratios (standardized to 1995–1996) and outcomes of ESLN (n=16,649). For events occurring after initiation of RRT, follow-up ended on June 30, 2011.
Results
Overall ESLN rates (95% confidence intervals [95% CIs]) in 1995–1996 were 3.1 (2.9 to 3.2) cases per million per year. Rates were higher for subgroups characterized by African-American race (11.1 [95% CI, 10.3 to 11.9]); other race (4.9 [95% CI, 4.0 to 5.8]); female sex (4.9 [95% CI, 4.6 to 5.2]); and ages 20–29 years (4.9 [95% CI, 4.4 to 5.4]), 30–44 years (4.6 [95% CI, 4.2 to 5.0]), and 45–64 years (4.0 [95% CI, 3.7 to 4.4]). Standardized incidence ratios for the overall population in subsequent biennia were 1.19 (1.14 to 1.24) in 1997–1998, 1.17 (1.12 to 1.22) in 1999–2000, 1.17 (1.12 to 1.22) in 2001–2002, 1.21 (1.16 to 1.26) in 2003–2004, 1.18 (1.13 to 1.23) in 2005–2006, 1.16 (1.11 to 1.21) in 2007–2008, and 1.05 (1.01 to 1.09) in 2009–2010, respectively. During a median (interquartile range) follow-up of 4.4 (6.3) years, 42.6% of patients with ESLN died, 45.3% were listed for renal transplant, and 28.7% underwent transplantation. Patients with ESLN were more likely than matched controls to be listed for and to undergo transplantation, and mortality rates were similar. Among patients with ESLN, African Americans were less likely to undergo transplantation (adjusted hazard ratio, 0.54 [0.51 to 0.58]) and more likely to die prematurely (adjusted hazard ratio, 1.23 [1.17 to 1.30]).
Conclusions
While ESLN appears to have stopped increasing in the last decade, racial disparities in outcomes persist.
Keywords: end-stage renal disease, lupus nephritis, epidemiology and outcomes
Introduction
GN, which can be expected in 40%–75% of patients with SLE, has ominous implications (1,2). In this regard, therapeutic approaches to lupus nephritis have evolved substantially in the last three decades, and experimental evidence supports the use of many agents in both the induction and maintenance of remission (3). While a wide array of agents (including cyclophosphamide [3–6], systemic corticosteroids [3,4], mycophenolate mofetil [7–9], azathioprine [10,11], and calcineurin inhibitors [12]) have been assessed, trial designs have generally relied on changes in urinary protein and GFR as primary outcomes, as opposed to ESRD (13). As management of lupus nephritis has continued to evolve throughout the past decade, and a previous study showed that the burden of ESRD from lupus nephritis increased between the mid-1990s and early-2000s in several important subsets of the United States population (14), up-to-date information appears warranted. We therefore set out to enumerate national trends in end-stage lupus nephritis in a more contemporary framework (15–18).
Materials and Methods
Objectives
The principal objectives of this study were to evaluate trends in standardized incidence ratios (SIRs) of SLE necessitating RRT in the United States between 1995 and 2010. Secondary objectives included comparing rates of listing for renal transplant, undergoing renal transplantation, and death in matched patients with and without SLE and calculating hazards ratios for these outcomes among patients with SLE.
Participants
In this retrospective study, we used US Renal Data System standard analysis files to study United States patients who initiated maintenance RRT between 1995 and 2010 (n=1,557,117). Baseline characteristics at initiation of RRT were determined from the Centers for Medicare & Medicaid (CMS) Medical Evidence Report (form CMS-2728). By federal requirement, this form must be submitted for all new maintenance RRT patients in the United States, and resultant data are housed in the US Renal Data System Medevid95 and Medevid05 files. The Medical Evidence Report has changed twice in the past two decades, in 1995 and 2005. Unlike previous iterations, the 2005 version includes information about predialysis nephrologist care and vascular access at initiation of hemodialysis. In both versions, one of 82 causes is entered as the primary cause of ESRD, with identical options in the 1995 and 2005 versions. For this study, cases of ESRD from SLE were those with the primary cause of ESRD listed as “Lupus erythematosus, (SLE nephritis)” in the Medical Evidence Report. Dates of death and first renal transplant were obtained from the Patients file, and first waitlisting for transplant was determined from the Waitlist_ki and Waitlist_kp files.
Statistical Analyses
US Census data were used for population denominators for the years examined, with age in 5-year increments (15,17). The CKD-Epidemiology Collaboration equation was used to estimate GFR at initiation of RRT. The Poisson distribution was used to compute incidence rates of ESRD from SLE that necessitated RRT. For computation of SIRs, expected incidence rates were calculated by applying incidence rates in 1995–1996 to each individual permutation of age, sex, and race to the corresponding subgroup of the United States population in subsequent 2-year periods. Chi-squared analysis was used for unadjusted comparisons of patients with and without ESRD from SLE, and logistic regression was used for adjusted comparisons.
For comparisons of clinical outcomes of patients with and without SLE, patients were matched according to year of inception of RRT, age (in 1-year intervals), sex, race, and ethnicity. Poisson regression was used to compute incidence rates and adjusted hazards ratios for events occurring after initiation of RRT, with follow-up ending on June 30, 2011. SAS software, version 9.1.3 (SAS Institute, Inc., Cary, NC) was used for data analysis.
Results
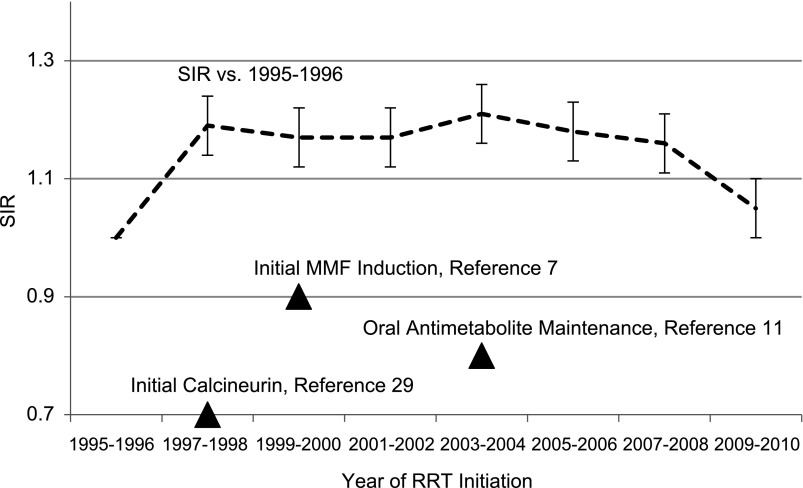
In 1995–1996, 1635 patients began RRT as a result of lupus nephritis, a rate (95% confidence interval [95% CI]) of 3.1 (2.9 to 3.2) cases per million per year (Table 1); rates exceed 3.1 cases per million per year for subgroups characterized by African-American race (11.1 [10.3 to 11.9]); other race (4.9 [4.0 to 5.8]; female sex, 4.9 [4.6 to 5.2], and ages 20–29 years (4.9 [4.4 to 5.4], 30–44 years (4.6 [4.2 to 5.0]), and 45–64 years (4.0 [3.7 to 4.4]). When incidence rates were standardized to 1995–1996 by age, sex, and race, SIRs (95% CI) for subsequent biennia were 1.19 (1.14 to 1.24), 1.17 (1.12 to 1.22), 1.17 (1.12 to 1.22), 1.21 (1.16 to 1.26), 1.18 (1.13 to 1.23), 1.16 (1.11 to 1.21), and 1.05 (1.01 to 1.09), respectively. The SIR rose noticeably for 1997–1998 (SIR, 1.19); however, no statistically significant rise in SIRs was observed between successive biennia thereafter, although all SIRs in the overall population continued to exceed 1 (Figure 1, Table 1). By the 2009–2010 biennium, SIRs were >1 overall (1.05 [1.01 to 1.1]) and for subgroups characterized by age 20–29 years (1.19 [1.09 to 1.29]), 30–44 years (1.17 [1.09 to 1.26]), and African-American race (1.1 [1.03 to 1.17]). However, although SIRs initially rose, they subsequently declined, and by 2009–2010 they did not differ between 1995–1996 and 2009–2010 for subgroups characterized by age younger than 20 years (1.07 [0.89 to 1.25]), 65 years or older (0.87 [0.72 to 1.02]), male sex (1.05 [0.94 to 1.16]), female sex (1.04 [1.0 to 1.09]), white race (1.03 [0.96 to 1.10]), and other race (0.89 [0.76 to 1.02]).
Table 1.
Standardized incidence ratios of end-stage lupus nephritis requiring RRT, 1995–2010
| Variable | Rate (95% CI) | SIR (95% CI) | ||||||
|---|---|---|---|---|---|---|---|---|
| 1995–1996 (O/E, 1635/1635) | 1997–1998 (O/E, 2005/1690) | 1999–2000 (O/E, 2042/1746) | 2001–2002 (O/E, 2101/1803) | 2003–2004 (O/E, 2249/1855) | 2005–2006 (O/E, 2246/1909) | 2007–2008 (O/E, 2268/1961) | 2009–2010 (O/E, 2103/2010) | |
| All | 3.1 (2.9 to 3.2) | 1.19 (1.14 to 1.24)a | 1.17 (1.12 to 1.22)a | 1.17 (1.12 to 1.22)a | 1.21 (1.16 to 1.26)a | 1.18 (1.13 to 1.23)a | 1.16 (1.11 to 1.21)a | 1.05 (1.01 to 1.1)b |
| Age | ||||||||
| <20 yr | 0.7 (0.6 to 0.8) | 0.84 (0.67 to 1.01) | 1.2 (1.00 to 1.40) | 1.2 (1.002 to 1.40)b | 1.41 (1.20 to 1.62)a | 1.30 (1.10 to 1.50)a | 1.17 (0.98 to 1.36) | 1.07 (0.89 to 1.25) |
| 20–29 yr | 4.9 (4.4 to 5.4) | 1.21 (1.10 to 1.32)a | 1.12 (1.01 to 1.23)b | 1.14 (1.03 to 1.25)c | 1.27 (1.16 to 1.38) | 1.30 (1.19 to 1.41)a | 1.31 (1.20 to 1.42)a | 1.19 (1.09 to 1.29)a |
| 30–44 yr | 4.6 (4.2 to 5.0) | 1.24 (1.15 to 1.33)a | 1.25 (1.16 to 1.34)a | 1.18 (1.09 to 1.27)a | 1.27 (1.18 to 1.36)a | 1.19 (1.10 to 1.28)a | 1.27 (1.18 to 1.36)a | 1.17 (1.09 to 1.26)a |
| 45–64 yr | 4.0 (3.7 to 4.4) | 1.12 (1.02 to 1.22)c | 1.07 (0.98 to 1.16) | 1.12 (1.03 to 1.21)a | 1.08 (1.0 to 1.16)b | 1.06 (0.98 to 1.14) | 0.96 (0.89 to 1.04) | 0.88 (0.81 to 0.95)a |
| ≥65 yr | 1.9 (1.6 to 2.2) | 1.41 (1.20 to 1.62)a | 1.27 (1.08 to 1.47)c | 1.28 (1.09 to 1.48)c | 1.18 (1.0 to 1.37) | 1.17 (0.99 to 1.35) | 1.11 (0.94 to 1.28) | 0.87 (0.72 to 1.02) |
| Sex | ||||||||
| Male | 1.1 (1.0 to 1.3) | 1.21 (1.09 to 1.33)a | 1.11 (0.99 to 1.23) | 1.13 (1.02 to 1.25)b | 1.32 (1.20 to 1.44)a | 1.21 (1.09 to 1.33)a | 1.16 (1.05 to 1.27)a | 1.05 (0.94 to 1.16) |
| Female | 4.9 (4.6 to 5.2) | 1.18 (1.12 to 1.24)a | 1.18 (1.12 to 1.24)a | 1.17 (1.11 to 1.23)a | 1.19 (1.14 to 1.25)a | 1.17 (1.12 to 1.22)a | 1.16 (1.11 to 1.21)a | 1.04 (1.0 to 1.09) |
| Race | ||||||||
| White | 1.7 (1.6 to 1.8) | 1.19 (1.11 to 1.27)a | 1.14 (1.07 to 1.22)a | 1.16 (1.09 to 1.24)a | 1.12 (1.05 to 1.19)a | 1.13 (1.06 to 1.20)a | 1.14 (1.07 to 1.21)a | 1.03 (0.96 to 1.10) |
| African American | 11.1 (10.3 to 11.9) | 1.22 (1.14 to 1.30)a | 1.2 (1.13 to 1.28)a | 1.19 (1.12 to 1.26)a | 1.31 (1.24 to 1.39)a | 1.23 (1.16 to 1.30)a | 1.23 (1.16 to 1.32)a | 1.1 (1.03 to 1.17)a |
| Other | 4.9 (4.0 to 5.8) | 0.98 (0.81 to 1.15) | 1.17 (0.99 to 1.35) | 1.05 (0.89 to 1.21) | 1.12 (0.96 to 1.28) | 1.14 (0.99 to 1.30) | 0.89 (0.76 to 1.02) | 0.89 (0.76 to 1.02) |
Standardized to 1995–1996, by age, sex, and race. Parameter estimates are rates per million per year or standardized incidence ratios (SIRs) with 95% confidence intervals (95% CIs) in parentheses. With PE denoting point estimate; confidence limit (CL); observed incidence rate (Obs); expected incidence rate from age-, sex-, and race-specific rates seen in 1995–1996 (Exp), standardized incidence ratios were calculated as [PEObs/PEExp] ([5% CLObs/PEExp]−[95% CLObs/PEExp]). P values refer to comparisons of observed rates and expected rates (O/E) when rates seen in the biennium used for standardization were applied to the years under consideration. P value ≥0.05 unless otherwise indicated.
P<0.001.
0.01≤P<0.05.
0.001≤P<0.01.
Figure 1.
Trends in the burden of end-stage lupus nephritis in the United States, with incidence ratios standardized (by age, sex, and race) against the 1995–1996 biennium. Dark triangles indicate the chronology of notable randomized intervention studies. Error bars refer to 95% confidence intervals around the standardized incidence ratios (SIRs). MMF, mycophenolate mofetil.
After adjustment for age, sex, race, and ethnicity, associations of ESRD from SLE at baseline (Table 2) included age 20–29 years; female sex; African-American race; other race; Hispanic ethnicity; absence of ischemic heart disease, peripheral vascular disease, diabetes, smoking, alcohol abuse, and drug abuse; catheters for hemodialysis; longer duration of nephrologist care; higher eGFR; lower body mass index; and lower serum albumin and hemoglobin levels. Ranked by magnitude, adjusted odds ratios for SLE were highest for female sex (6.13 [95% CI, 5.89 to 6.38]) and African-American race (1.88 [1.81 to 1.95] versus white) and were lowest for age 65 years or older (0.03 [0.02 to 0.03] versus younger than 20 years), age 45–64 years (0.12 [0.12 to 0.13]), diabetes (0.12 [0.12 to 0.13]), drug abuse (0.34 [0.28 to 0.4]), alcohol abuse (0.39 [0.31 to 0.48]), peripheral vascular disease (0.47 [0.43 to 0.51]), body mass index ≥30 kg/m2 (0.53 [0.51 to 0.55]), nephrology care for ≤12 months (0.59 [0.56 to 0.62]), and ischemic heart disease (0.63 [0.59 to 0.67]).
Table 2.
Characteristics at initiation of RRT (n=1,557,117), end-stage lupus nephritis
| Variable | ESLN (%) | Logistic Regression | ||
|---|---|---|---|---|
| Yes (n=16,650) | No (n=1,540,467) | Reference | AOR for ESLN (95% CI) | |
| Year of first RRT | ||||
| 1995–2000 | 34.1 | 31.3 | 2001–2005 | 0.97 (0.93 to 1.01) |
| 2001–2005 | 32.9 | 32.6 | 2001–2005 | 1 (Reference) |
| 2006–2010 | 33 | 36.1 | 2001–2005 | 0.94 (0.9 to 0.98) |
| Age | ||||
| <20 yr | 6.7 | 1.2 | <20 | 1 (Reference) |
| 20–29 yr | 22.8 | 2.5 | <20 | 1.46 (1.36 to 1.57) |
| 30–44 yr | 35.1 | 10.5 | <20 | 0.56 (0.52 to 0.6) |
| 45–64 yr | 27.9 | 36.6 | <20 | 0.12 (0.12 to 0.13) |
| ≥65 yr | 7.6 | 49.3 | <20 | 0.03 (0.02 to 0.03) |
| Female | 81.7 | 44.8 | Male | 6.13 (5.89 to 6.38) |
| Race/ethnicity | ||||
| White | 43.4 | 65.5 | White | 1 (Reference) |
| African American | 48.6 | 28.5 | White | 1.88 (1.81 to 1.95) |
| Other | 8 | 6 | White | 1.43 (1.34 to 1.52) |
| Hispanic | 17.2 | 12.9 | Non-Hispanic | 1.43 (1.37 to 1.5) |
| Ischemic heart disease | 6.2 | 24.3 | Absent | 0.63 (0.59 to 0.67) |
| Peripheral vascular disease | 3.2 | 14.1 | Absent | 0.47 (0.43 to 0.51) |
| Cerebrovascular disease | 5.1 | 9.2 | Absent | 1.06 (0.99 to 1.14) |
| Diabetes | 8.8 | 50.2 | Absent | 0.12 (0.12 to 0.13) |
| Smoking | 3.9 | 5.6 | Absent | 0.67 (0.62 to 0.72) |
| Alcohol abuse | 0.5 | 1.5 | Absent | 0.39 (0.31 to 0.48) |
| Drug abuse | 0.8 | 1.1 | Absent | 0.34 (0.28 to 0.4) |
| Initial mode of RRT | ||||
| Hemodialysis | 85.7 | 90.5 | Hemodialysis | 1 (Reference) |
| Peritoneal dialysis | 11.5 | 7.8 | Hemodialysis | 0.96 (0.91 to 1.01) |
| Transplant | 2.8 | 1.7 | Hemodialysis | 0.8 (0.73 to 0.88) |
| Initial hemodialysis access | ||||
| Fistula | 7.8 | 13.9 | Fistula | 1 (Reference) |
| Graft | 2.8 | 3.6 | Fistula | 1.04 (0.86 to 1.26) |
| Catheter | 89.4 | 82.5 | Fistula | 1.29 (1.16 to 1.42) |
| Nephrology care≤ 12 mo | 68.5 | 75.8 | >12 | 0.59 (0.56 to 0.62) |
| eGFR>15 ml/min per 1.73 m2 | 10.7 | 10.3b | ≤15 | 1.26 (1.19 to 1.32) |
| Body mass index≥ 30 kg/m2 | 22.4 | 30.5 | <30 | 0.53 (0.51 to 0.55) |
| Serum albumin< 3.5 g/dl | 73 | 64.7 | ≥3.5 | 1.46 (1.4 to 1.52) |
| Serum hemoglobin≥ 9 g/dl | 58.1 | 71.3 | <9 | 0.87 (0.84 to 0.9) |
Parameter estimates are presented as column percentages or odds ratios with 95% CIs in parentheses. For logistic regression, all variables in the first column were used as candidate variables, while age, sex, race, and ethnicity were used as adjustment variables, provided they were not also the candidate variable, in which case they were removed as adjustment variables. Data fields for predialysis vascular access for hemodialysis and predialysis nephrology care were available only in the 2005 version of the Medical Evidence Report. Missing data were as follows: initial hemodialysis access, 63.3%; prior nephrology care, 59.4%; eGFR, 1.5%; body mass index, 4.7%; serum albumin, 25.2%; serum hemoglobin, 11.1%. ESLN, end-stage lupus nephritis; AOR, adjusted (for age, sex, race, ethnicity) odds ratio.
Median (interquartile range) follow-up was 4.4 (6.3) years, during which time 45.3% of patients with lupus nephritis were listed for transplant, 28.7% underwent transplantation, and 42.6% died. Compared with matched controls with ESRD from other conditions, patients with lupus nephritis had similar mortality (7.9 [95% CI, 7.8 to 8.1] versus 7.9 [7.8 to 8.1] per 100 person-years), higher rates of listing for transplant (14.6 [14.3 to 15.0] versus 10.8 [10.5 to 11.1] per 100 person-years), and higher rates of undergoing transplantation (7.9 [7.7 to 8.1] versus 6.8 [6.6 to 7.0] per 100 person-years) (Table 3). Regarding subgroups, mortality rates for patients with lupus nephritis were higher for ages younger than 20 and 20–29 years and male sex; rates were lower for ages 30–44 and 45–64 years and non-Hispanic white race/ethnicity (Table 3). Rates of listing for transplant were higher for patients with SLE in all subgroups except age younger than 20 years and race/ethnicity categorized as other (Table 3). Rates of transplant were higher for patients with SLE in all subgroups except ages younger than 20 and 20–29 years, male sex, and race/ethnicity categorized as Hispanic and other (Table 3).
Table 3.
Rates of death, listing for transplant, and renal transplantation in patients with end-stage lupus nephritis (n=16,434 [98.7%]) and an equal number of matched control patients with ESRD from other causes
| Variable | Death | Listing for Transplant | Transplantation | |||
|---|---|---|---|---|---|---|
| Cases | Controls | Cases | Controls | Cases | Controls | |
| All | 7.9 (7.8 to 8.1) | 7.9 (7.8 to 8.1)a | 14.6 (14.3 to 15) | 10.8 (10.5 to 11.1) | 7.9 (7.7 to 8.1) | 6.8 (6.6 to 7) |
| Year of first RRT | ||||||
| 1995–2000 | 7.8 (7.5 to 8.1) | 7.5 (7.2 to 7.7)a | 12.2 (11.7 to 12.7) | 9.6 (9.2 to 10) | 8.5 (8.2 to 8.9) | 7.3 (7 to 7.6) |
| 2000–2005 | 8.0 (7.7 to 8.3) | 8.4 (8 to 8.7)a | 14.9 (14.3 to 15.5) | 10.7 (10.2 to 11.2) | 7.7 (7.4 to 8.1) | 6.8 (6.5 to 7.2) |
| 2006–2010 | 8.0 (7.8 to 8.2) | 8.0 (7.8 to 8.2)a | 14.9 (14.6 to 15.3) | 10.9 (10.6 to 11.2) | 7.9 (7.6 to 8.1) | 6.7 (6.5 to 6.9) |
| Age | ||||||
| <20 yr | 5.1 (4.6 to 5.7) | 2.3 (2 to 2.6) | 19.8 (18.3 to 21.5) | 18.4 (17 to 19.9)a | 11.3 (10.3 to 12.3) | 16 (14.8 to 17.3) |
| 20–29 yr | 4.8 (4.5 to 5.1) | 4.2 (4 to 4.5)c | 17.4 (16.6 to 18.3) | 14.7 (14 to 15.5) | 9.6 (9.1 to 10.2) | 9.4 (8.9 to 9.9)a |
| 30–44 yr | 6.0 (5.8 to 6.3) | 6.9 (6.6 to 7.2) | 16.4 (15.8 to 17.0) | 11.0 (10.5 to 11.5) | 8.1 (7.8 to 8.5) | 6.3 (6 to 6.6) |
| 45–64 yr | 11.8 (11.3 to 12.3) | 13.1 (12.6 to 13.6) | 11.5 (10.9 to 12.1) | 7.3 (6.9 to 7.8) | 6.4 (6.0 to 6.8) | 3.8 (3.5 to 4.1) |
| ≥65 yr | 30.1 (28.3 to 32) | 30 (28.2 to 31.9)a | 2.8 (2.3 to 3.5) | 1.6 (1.2 to 2.2)c | 1.7 (1.3 to 2.2) | 0.9 (0.6 to 1.3)c |
| Sex | ||||||
| Male | 8.4 (7.9 to 8.8) | 7.4 (7 to 7.8)c | 14.2 (13.4 to 15.1) | 11.5 (10.8 to 12.2) | 8.6 (8.1 to 9.2) | 7.8 (7.3 to 8.4)a |
| Female | 7.8 (7.6 to 8.1) | 8.1 (7.9 to 8.3)a | 14.7 (14.3 to 15.1) | 10.7 (10.4 to 11) | 7.7 (7.5 to 8) | 6.5 (6.3 to 6.8) |
| Race/ethnicity | ||||||
| Non-Hispanic white | 9.1 (8.7 to 9.4) | 10 (9.6 to 10.4) | 12.1 (11.5 to 12.7) | 9.8 (9.3 to 10.3) | 10.4 (9.9 to 11) | 9.2 (8.8 to 9.8)c |
| Non-Hispanic African American | 8.8 (8.5 to 9.1) | 8.4 (8.2 to 8.7)a | 15.2 (14.7 to 15.8) | 9.8 (9.4 to 10.2) | 6.3 (6.0 to 6.6) | 5 (4.8 to 5.3) |
| Hispanic | 5.1 (4.7 to 5.4) | 4.8 (4.5 to 5.2)a | 16.1 (15.2 to 17) | 13.7 (13 to 14.6) | 8.6 (8.0 to 9.2) | 8.2 (7.6 to 8.7)a |
| Other | 5.0 (4.5 to 5.6) | 4.4 (3.9 to 4.9)a | 17.8 (16.3 to 19.5) | 16.9 (15.5 to 18.6)a | 8.7 (7.8 to 9.6) | 8.5 (7.7 to 9.5)a |
Rates are per 100 person-years, with 95% confidence intervals in parentheses. Causes of ESRD in controls were diabetes mellitus type 2, 26%; unspecified renal failure, 22.1%; diabetes mellitus type 1, 9.6%; FSGS, 6.6%; GN (histologically not examined), 6.5%; other causes, 29.2%. Factors used for matching were year of inception of RRT, age (in 1-year intervals), sex, race, and ethnicity. P<0.001 unless otherwise stated.
P≥0.05.
0.001=P<0.01.
Table 4 shows adjusted hazards ratios for death, listing for transplant, and undergoing transplantation for patients with ESRD from lupus nephritis. Factors associated with higher mortality risk included older age, African-American race, non-Hispanic ethnicity, peripheral vascular disease, diabetes, smoking, alcohol and drug abuse, hemodialysis for RRT, hemodialysis with nonfistula vascular access, nephrologist care for 12 months or less, higher eGFR, and low serum albumin and hemoglobin levels. Listing for renal transplant was associated with initiation of RRT after 2005; younger age; African-American race; Hispanic ethnicity; lack of vascular disease, diabetes, and alcohol and drug abuse; peritoneal dialysis for RRT; fistulas for hemodialysis; longer duration of nephrologist care; lower eGFR and body mass index; and higher serum albumin and hemoglobin levels. Undergoing renal transplantation was associated with initiation of RRT before 2001; younger age; male sex; white race; non-Hispanic ethnicity; lack of vascular disease, diabetes, and alcohol and drug abuse; peritoneal dialysis for RRT; fistulas for hemodialysis; longer duration of nephrologist care; lower eGFR and body mass index; and higher serum albumin levels.
Table 4.
Adjusted hazards ratios for outcomes on RRT, patients with end-stage lupus nephritis (n=16,650)
| Characteristic | Reference | Death (42.6%) | Listing for Transplant (45.3%) | Transplantation (28.7%) |
|---|---|---|---|---|
| Year of first RRT | ||||
| 1995–2000 | 2001–2005 | 1.04 (0.99 to 1.1)a | 0.8 (0.76 to 0.85) | 1.08 (1.01 to 1.15)b |
| 2006–2010 | 2001–2005 | 0.98 (0.92 to 1.05)a | 1.31 (1.23 to 1.39) | 0.89 (0.82 to 0.96)c |
| Age | ||||
| <20 yr | <20 | 0.93 (0.83 to 1.05)a | 0.88 (0.8 to 0.96)c | 0.85 (0.76 to 0.94)c |
| 20–29 yr | <20 | 1.14 (1.01 to 1.27)b | 0.84 (0.77 to 0.91) | 0.72 (0.65 to 0.8) |
| 30–44 yr | <20 | 2.22 (1.98 to 2.48) | 0.59 (0.54 to 0.65) | 0.53 (0.48 to 0.6) |
| 45–64 yr | <20 | 5.75 (5.08 to 6.51) | 0.15 (0.12 to 0.19) | 0.12 (0.09 to 0.16) |
| Female | Male | 0.98 (0.92 to 1.04)a | 0.99 (0.93 to 1.06)a | 0.91 (0.85 to 0.98)b |
| Race/ethnicity | ||||
| African American | White | 1.23 (1.17 to 1.3) | 1.11 (1.05 to 1.18) | 0.54 (0.51 to 0.58) |
| Other | White | 0.84 (0.76 to 0.93) | 1.13 (1.03 to 1.23)c | 0.69 (0.62 to 0.76) |
| Hispanic ethnicity | Non-Hispanic | 0.82 (0.76 to 0.88) | 1.08 (1.01 to 1.16)b | 0.7 (0.65 to 0.76) |
| Ischemic heart disease | Absent | 1.53 (1.41 to 1.65) | 0.83 (0.73 to 0.95)c | 0.74 (0.62 to 0.87) |
| Peripheral vascular disease | Absent | 1.75 (1.57 to 1.94) | 0.85 (0.71 to 1.02)a | 0.77 (0.62 to 0.97)b |
| Cerebrovascular disease | Absent | 1.65 (1.51 to 1.81) | 0.74 (0.64 to 0.85) | 0.72 (0.61 to 0.85) |
| Diabetes | Absent | 1.43 (1.32 to 1.54) | 0.79 (0.71 to 0.88) | 0.66 (0.57 to 0.76) |
| Smoking | Absent | 1.63 (1.47 to 1.81) | 0.65 (0.56 to 0.76) | 0.65 (0.54 to 0.78) |
| Alcohol abuse | Absent | 1.77 (1.35 to 2.31) | 0.31 (0.17 to 0.56) | 0.36 (0.18 to 0.73)c |
| Drug abuse | Absent | 2.28 (1.83 to 2.85) | 0.27 (0.16 to 0.44) | 0.16 (0.06 to 0.38) |
| Initial mode of RRT | ||||
| Peritoneal dialysis | Hemodialysis | 0.79 (0.74 to 0.86) | 1.42 (1.32 to 1.52) | 1.57 (1.46 to 1.69) |
| Transplant | Hemodialysis | 0.12 (0.09 to 0.17) | — | — |
| Initial hemodialysis access | ||||
| Graft | Fistula | 1.89 (1.33 to 2.7) | 0.64 (0.45 to 0.9)b | 0.39 (0.22 to 0.69)c |
| Catheter | Fistula | 1.8 (1.44 to 2.25) | 0.75 (0.63 to 0.9)c | 0.61 (0.49 to 0.76) |
| Nephrology care≤ 12 mo | >12 | 1.46 (1.3 to 1.65) | 0.8 (0.73 to 0.87) | 0.63 (0.56 to 0.72) |
| eGFR>15 ml/min per 1.73 m2 | ≤15 | 1.38 (1.27 to 1.49) | 0.63 (0.58 to 0.69) | 0.5 (0.44 to 0.56) |
| Body mass index≥ 30 kg/m2 | <30 | 1.01 (0.95 to 1.07)a | 0.88 (0.83 to 0.94) | 0.75 (0.7 to 0.81) |
| Serum albumin< 3.5 g/dl | ≥3.5 | 1.67 (1.57 to 1.79) | 0.92 (0.86 to 0.97)c | 0.78 (0.72 to 0.83) |
| Serum hemoglobin≥ 9 g/dl | <9 | 0.94 (0.89 to 0.99)b | 0.93 (0.88 to 0.98)c | 1.01 (0.95 to 1.08)a |
Hazards ratios are adjusted for age, sex, race, and Hispanic ethnicity and presented with 95% CIs in parentheses; all variables in the first column were used as candidate variables, and age, sex, race, and ethnicity were used as adjustment variables, provided they were not also the candidate variable, in which case they were removed as adjustment variables. P<0.001 unless otherwise stated.
P≥0.05.
0.01≤P<0.05.
0.001≤P<0.01.
Discussion
We observed that incidence rates of ESRD from lupus nephritis stopped rising between 1995 and 2010 and may have declined in some important subgroups during the past decade. Female sex and African-American race were predominant and catheters were the primary initial vascular access for hemodialysis. Compared with matched controls, patients with lupus nephritis were more likely to be listed for renal transplant, more likely to undergo transplantation, and equally likely to die during the observation period. Regarding associations of these outcomes in lupus nephritis, it was notable that mortality risk was higher with African-American race (versus white), considering that white race is associated with higher rates in the overall ESRD population of the United States; although African-American patients were more likely to be listed for transplant, they were half as likely as their white counterparts to undergo transplantation.
Costenbader and colleagues examined similar issues in the United States from 1995 to 2006 and found that standardized incidence rates of ESRD from lupus nephritis increased among patients aged 5–39 years and among African Americans (14). Unlike in our study, standardized incidence rates for the overall population were not reported, and participants younger than age 5 years were excluded; after an initial rise, a plateau effect was apparent in demographic subgroups traditionally at highest risk. For example, for African Americans, SIRs were 12.80 per million (95% CI, 11.92 to 13.74) for 1995–1997, 15.04 (14.12 to 16.03) for 1998–2000, 15.24 (14.33 to 16.21) for 2001–2003, and 15.55 (14.64 to 16.51) for 2004–2006. A similar biphasic trend was seen for female sex; corresponding rates were 5.54 (5.19 to 5.92), 6.40 (6.03 to 6.80), 6.42 (6.05 to 6.81), and 6.52 (6.15 to 6.91), respectively. While we also showed that risk rose in the late 1990s, our study suggests that a sustained plateau effect has been apparent for over a decade. Although the decline in risk for 2009–2010 is encouraging, whether it is part of a longer-term phenomenon remains to be determined.
We found many meaningful race-related outcome disparities. ESRD from lupus nephritis did not decline in non-Hispanic African Americans, and African-American race was associated with higher mortality and lower likelihood of renal transplantation compared with white race. The reasons for this are unclear but may reflect the described higher incidence, prevalence, and severity of lupus nephritis in African-American patients, with possible contributions from disparities in access to medical care and a genetic predisposition (19,20). Several recent studies have examined the issue of race in patients with ESRD from lupus nephritis. Devlin and colleagues examined incident patterns of initial mode of RRT between 1995 and 2006 in the United States and reported that African Americans were less likely to use peritoneal dialysis or preemptive transplantation as initial RRT (21). Nee and colleagues examined renal transplant outcomes in recipients with lupus nephritis between 1995 and 2006 and reported increased risks of graft loss and death in African Americans; although income levels were associated with graft loss and death in African Americans, such an association was absent for other races (22). Paralleling our findings in the overall population, Hiraki and colleagues reported that African-American pediatric patients with ESRD from lupus nephritis in the United States were less likely to undergo transplantation and more likely to die prematurely than white patients (23).
While it is tempting to hypothesize that the salutary trends in lupus nephritis–related RRT may reflect more widespread use of treatments with proven efficacy for proximate outcomes, the nonexperimental design of our study precludes a definitive answer. Attempting to ascribe these temporal trends to a particular intervention is difficult; however, outlining the chronology of major developments in treatment may be helpful. Controlled trials of intravenous cyclophosphamide, corticosteroids, and azathioprine in patients with lupus nephritis began in the 1970s and continued through the 1980s (3). While efforts to refine these regimens continued in the 1990s and 2000s, cyclophosphamide-related toxicity prompted study of alternative agents (4–6). The US Food and Drug Administration approved mycophenolate for the prevention of renal allograft rejection in 1995, after which studies in lupus nephritis began to emerge (24,25). The first notable study comparing mycophenolate to cyclophosphamide and azathioprine in patients with lupus nephritis was reported in 2000, and subsequent studies suggested superiority in remission induction with an improved safety profile (7,9,26). In 2004, Contreras et al. found that oral mycophenolate or azathioprine was more effective and safer than long-term intravenous cyclophosphamide in maintaining remission (11). Use of these oral therapies may have become more widespread following this study (26–28). Although randomized trials of calcineurin inhibitors have reported some success in lupus nephritis beginning in 1998, their main role appears to be as part of a multitarget therapy (12,28,29).
In theory, declines in the population-level burden of SLE, the risk of lupus nephritis among patients with SLE, and the risk of ESRD among patients with lupus nephritis could all lead to the apparently salutary trends seen in this study. Unfortunately there are no published SLE-specific population denominators in the United States over the same period (14). Difficulties in defining this denominator include inability to identify milder cases or account for sampling disparities in race/ethnicity and access to medical care (30). Furthermore, the possibility that detection of SLE may have improved makes incidence rate trends difficult to define (19,31,32). There is an analogous lack of data from large representative lupus nephritis cohorts on trends in progression to ESRD over the study period.
Although the likelihood of death among patients with lupus nephritis declined over the study period, listing for renal transplant did not change and the likelihood of undergoing transplantation appeared to fall. Encouragingly, several potentially modifiable associations of adverse outcomes were seen in this study, including length of nephrology care before initiation of RRT, smoking, substance abuse, body mass index, mode of RRT, and vascular access for hemodialysis.
Our study has several limitations, including a retrospective registry-based design and lack of information on renal pathologic findings, severity of disease, and medication in the general population. Despite its limitations, our study provides some useful information. While research efforts to develop alternative efficacious treatments with fewer adverse effects (13,33) are clearly needed, particularly for treatment-resistant disease, it is encouraging that rates of RRT from lupus nephritis are no longer accelerating and may be declining.
Disclosures
None.
Acknowledgments
The authors thank Chronic Disease Research Group colleagues Beth Forrest for regulatory assistance, Delaney Berrini for manuscript preparation, and Nan Booth, MSW, ELS, for manuscript editing.
The data reported here have been supplied by the US Renal Data System. The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as an official policy or interpretation of the US Government.
The Irish Nephrology Society research bursary funded Dr. Donal Sexton. This work was supported by the Chronic Disease Research Group, Minneapolis Medical Research Foundation.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Mok CC, Kwok RC, Yip PS: Effect of renal disease on the standardized mortality ratio and life expectancy of patients with systemic lupus erythematosus. Arthritis Rheum 65: 2154–2160, 2013 [DOI] [PubMed] [Google Scholar]
- 2.Cameron JS: Lupus nephritis. J Am Soc Nephrol 10: 413–424, 1999 [DOI] [PubMed] [Google Scholar]
- 3.Austin HA, 3rd, Klippel JH, Balow JE, le Riche NG, Steinberg AD, Plotz PH, Decker JL: Therapy of lupus nephritis. Controlled trial of prednisone and cytotoxic drugs. N Engl J Med 314: 614–619, 1986 [DOI] [PubMed] [Google Scholar]
- 4.Boumpas DT, Austin HA, 3rd, Vaughn EM, Klippel JH, Steinberg AD, Yarboro CH, Balow JE: Controlled trial of pulse methylprednisolone versus two regimens of pulse cyclophosphamide in severe lupus nephritis. Lancet 340: 741–745, 1992 [DOI] [PubMed] [Google Scholar]
- 5.Levey AS, Lan SP, Corwin HL, Kasinath BS, Lachin J, Neilson EG, Hunsicker LG, Lewis EJ: Progression and remission of renal disease in the Lupus Nephritis Collaborative Study. Results of treatment with prednisone and short-term oral cyclophosphamide. Ann Intern Med 116: 114–123, 1992 [DOI] [PubMed] [Google Scholar]
- 6.Houssiau FA, Vasconcelos C, D’Cruz D, Sebastiani GD, Garrido Ed ER, Danieli MG, Abramovicz D, Blockmans D, Mathieu A, Direskeneli H, Galeazzi M, Gül A, Levy Y, Petera P, Popovic R, Petrovic R, Sinico RA, Cattaneo R, Font J, Depresseux G, Cosyns JP, Cervera R: Immunosuppressive therapy in lupus nephritis: The Euro-Lupus Nephritis Trial, a randomized trial of low-dose versus high-dose intravenous cyclophosphamide. Arthritis Rheum 46: 2121–2131, 2002 [DOI] [PubMed] [Google Scholar]
- 7.Chan TM, Li FK, Tang CS, Wong RW, Fang GX, Ji YL, Lau CS, Wong AK, Tong MK, Chan KW, Lai KN, Hong Kong-Guangzhou Nephrology Study Group : Efficacy of mycophenolate mofetil in patients with diffuse proliferative lupus nephritis. N Engl J Med 343: 1156–1162, 2000 [DOI] [PubMed] [Google Scholar]
- 8.Houssiau FA, D’Cruz D, Sangle S, Remy P, Vasconcelos C, Petrovic R, Fiehn C, de Ramon Garrido E, Gilboe IM, Tektonidou M, Blockmans D, Ravelingien I, le Guern V, Depresseux G, Guillevin L, Cervera R, MAINTAIN Nephritis Trial Group : Azathioprine versus mycophenolate mofetil for long-term immunosuppression in lupus nephritis: Results from the MAINTAIN Nephritis Trial. Ann Rheum Dis 69: 2083–2089, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ginzler EM, Dooley MA, Aranow C, Kim MY, Buyon J, Merrill JT, Petri M, Gilkeson GS, Wallace DJ, Weisman MH, Appel GB: Mycophenolate mofetil or intravenous cyclophosphamide for lupus nephritis. N Engl J Med 353: 2219–2228, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Grootscholten C, Ligtenberg G, Hagen EC, van den Wall Bake AW, de Glas-Vos JW, Bijl M, Assmann KJ, Bruijn JA, Weening JJ, van Houwelingen HC, Derksen RH, Berden JH, Dutch Working Party on Systemic Lupus Erythematosus : Azathioprine/methylprednisolone versus cyclophosphamide in proliferative lupus nephritis. A randomized controlled trial. Kidney Int 70: 732–742, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Contreras G, Pardo V, Leclercq B, Lenz O, Tozman E, O’Nan P, Roth D: Sequential therapies for proliferative lupus nephritis. N Engl J Med 350: 971–980, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Austin HA, 3rd, Illei GG, Braun MJ, Balow JE: Randomized, controlled trial of prednisone, cyclophosphamide, and cyclosporine in lupus membranous nephropathy. J Am Soc Nephrol 20: 901–911, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Favas C, Isenberg DA: B-cell-depletion therapy in SLE—what are the current prospects for its acceptance? Nat Rev Rheumatol 5: 711–716, 2009 [DOI] [PubMed] [Google Scholar]
- 14.Costenbader KH, Desai A, Alarcón GS, Hiraki LT, Shaykevich T, Brookhart MA, Massarotti E, Lu B, Solomon DH, Winkelmayer WC: Trends in the incidence, demographics, and outcomes of end-stage renal disease due to lupus nephritis in the US from 1995 to 2006. Arthritis Rheum 63: 1681–1688, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.U.S. Department of Commerce: U.S. Census Bureau. 2013. Available at: http://www.census.gov/. Accessed October 14, 2014
- 16.U.S. Department of Health and Human Services, Centers for Medicare & Medicaid Services: End Stage Renal Disease Medical Evidence Report Medicare Entitlement and/or Patient Registration: Form CMS-2728-U3. . 2006. Available at: http://www.cms.gov/Medicare/CMS-Forms/CMS-Forms/downloads/cms2728.pdf. Accessed October 14, 2014
- 17.Centers for Disease Control and Prevention: Hispanic or Latino populations. 2014. Available at: http://www.cdc.gov/minorityhealth/populations/REMP/hispanic.html. Accessed October 14, 2014
- 18.Contreras G, Lenz O, Pardo V, Borja E, Cely C, Iqbal K, Nahar N, de La Cuesta C, Hurtado A, Fornoni A, Beltran-Garcia L, Asif A, Young L, Diego J, Zachariah M, Smith-Norwood B: Outcomes in African Americans and Hispanics with lupus nephritis. Kidney Int 69: 1846–1851, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Somers EC, Marder W, Cagnoli P, Lewis EE, DeGuire P, Gordon C, Helmick CG, Wang L, Wing JJ, Dhar JP, Leisen J, Shaltis D, McCune WJ: Population-based incidence and prevalence of systemic lupus erythematosus: The Michigan Lupus Epidemiology and Surveillance program. Arthritis Rheum (Munch) 66: 369–378, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Freedman BI, Langefeld CD, Andringa KK, Croker JA, Williams AH, Garner NE, Birmingham DJ, Hebert LA, Hicks PJ, Segal MS, Edberg JC, Brown EE, Alarcón GS, Costenbader KH, Comeau ME, Criswell LA, Harley JB, James JA, Kamen DL, Lim SS, Merrill JT, Sivils KL, Niewold TB, Patel NM, Petri M, Ramsey-Goldman R, Reveille JD, Salmon JE, Tsao BP, Gibson KL, Byers JR, Vinnikova AK, Lea JP, Julian BA, Kimberly RP, Lupus Nephritis–End-Stage Renal Disease Consortium : End-stage renal disease in African Americans with lupus nephritis is associated with APOL1. Arthritis Rheum (Munch) 66: 390–396, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Devlin A, Waikar SS, Solomon DH, Lu B, Shaykevich T, Alarcón GS, Winkelmayer WC, Costenbader KH: Variation in initial kidney replacement therapy for end-stage renal disease due to lupus nephritis in the United States. Arthritis Care Res (Hoboken) 63: 1642–1653, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nee R, Jindal RM, Little D, Ramsey-Goldman R, Agodoa L, Hurst FP, Abbott KC: Racial differences and income disparities are associated with poor outcomes in kidney transplant recipients with lupus nephritis. Transplantation 95: 1471–1478, 2013 [DOI] [PubMed] [Google Scholar]
- 23.Hiraki LT, Lu B, Alexander SR, Shaykevich T, Alarcón GS, Solomon DH, Winkelmayer WC, Costenbader KH: End-stage renal disease due to lupus nephritis among children in the US, 1995-2006. Arthritis Rheum 63: 1988–1997, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Van Bruggen MC, Walgreen B, Rijke TP, Berden JH: Attenuation of murine lupus nephritis by mycophenolate mofetil. J Am Soc Nephrol 9: 1407–1415, 1998 [DOI] [PubMed] [Google Scholar]
- 25.Glicklich D, Acharya A: Mycophenolate mofetil therapy for lupus nephritis refractory to intravenous cyclophosphamide. Am J Kidney Dis 32: 318–322, 1998 [DOI] [PubMed] [Google Scholar]
- 26.Hogan J, Schwenk MH, Radhakrishnan J: Should mycophenolate mofetil replace cyclophosphamide as first-line therapy for severe lupus nephritis? Kidney Int 82: 1256–1260, 2012 [DOI] [PubMed] [Google Scholar]
- 27.Morris HK, Canetta PA, Appel GB: Impact of the ALMS and MAINTAIN trials on the management of lupus nephritis. Nephrol Dial Transplant 28: 1371–1376, 2013 [DOI] [PubMed] [Google Scholar]
- 28.Appel GB: New and future therapies for lupus nephritis. Cleve Clin J Med 79: 134–140, 2012 [DOI] [PubMed] [Google Scholar]
- 29.Fu LW, Yang LY, Chen WP, Lin CY: Clinical efficacy of cyclosporin a neoral in the treatment of paediatric lupus nephritis with heavy proteinuria. Br J Rheumatol 37: 217–221, 1998 [DOI] [PubMed] [Google Scholar]
- 30.Bartels CM, Ramsey-Goldman R: Editorial: Updates in US systemic lupus erythematosus epidemiology: Tales of two cities. Arthritis Rheum (Munch) 66: 242–245, 2014 [DOI] [PubMed] [Google Scholar]
- 31.Lim SS, Bayakly AR, Helmick CG, Gordon C, Easley KA, Drenkard C: The incidence and prevalence of systemic lupus erythematosus, 2002-2004: The Georgia Lupus Registry. Arthritis Rheum (Munch) 66: 357–368, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McCarty DJ, Manzi S, Medsger TA, Jr, Ramsey-Goldman R, LaPorte RE, Kwoh CK: Incidence of systemic lupus erythematosus. Race and gender differences. Arthritis Rheum 38: 1260–1270, 1995 [DOI] [PubMed] [Google Scholar]
- 33.Davies RJ, Sangle SR, Jordan NP, Aslam L, Lewis MJ, Wedgwood R, D’Cruz DP: Rituximab in the treatment of resistant lupus nephritis: Therapy failure in rapidly progressive crescentic lupus nephritis. Lupus 22: 574–582, 2013 [DOI] [PubMed] [Google Scholar]