Abstract
Background and objectives
Improvements in agricultural practices in Croatia have reduced exposure to consumption of aristolochic acid-contaminated flour and development of endemic (Balkan) nephropathy. Therefore, it was hypothesized that Bosnian immigrants who settled in an endemic area in Croatia 15–30 years ago would be at lower risk of developing endemic nephropathy because of reduced exposure to aristolochic acid. To test this hypothesis, past and present exposure to aristolochic acid, proximal tubule damage as a hallmark of endemic nephropathy, and prevalence of CKD in Bosnian immigrants were analyzed.
Design, setting, participants, & measurements
In this cross-sectional observational study from 2005 to 2010, 2161 farmers were divided into groups: indigenous inhabitants from endemic nephropathy and nonendemic nephropathy villages and Bosnian immigrants; α-1 microglobulin-to-creatinine ratio >31.5 mg/g and eGFR<60 ml/min per 1.73 m2 were considered to be abnormal.
Results
CKD and proximal tubule damage prevalence was significantly lower in Bosnian immigrants than inhabitants of endemic nephropathy villages (6.9% versus 16.6%; P<0.001; 1.3% versus 7.3%; P=0.003, respectively); 20 years ago, Bosnian immigrants observed fewer Aristolochia clematitis in cultivated fields (41.9% versus 67.8%) and fewer seeds among wheat seeds (6.1% versus 35.6%) and ate more purchased than homemade bread compared with Croatian farmers from endemic nephropathy villages (38.5% versus 14.8%, P<0.001). Both Croatian farmers and Bosnian immigrants observe significantly fewer Aristolochia plants growing in their fields compared with 15–30 years ago. Prior aristolochic acid exposure was associated with proximal tubule damage (odds ratio, 1.64; 95% confidence interval, 1.04 to 2.58; P=0.02), whereas present exposure was not (odds ratio, 1.31; 95% confidence interval, 0.75 to 2.30; P=0.33). Furthermore, immigrant status was an independent negative predictor of proximal tubule damage (odds ratio, 0.40; 95% confidence interval, 0.19 to 0.86; P=0.02).
Conclusions
Bosnian immigrants and autochthonous Croats residing in endemic areas are exposed significantly less to ingestion of aristolochic acid than in the past. The prevalence of endemic nephropathy and its associated urothelial cancers is predicted to decrease over time.
Keywords: CKD, interstitial fibrosis, proximal tubule, albuminuria
Introduction
Endemic (Balkan) nephropathy (EN) is a chronic tubulointerstitial nephropathy associated with otherwise rare upper tract urothelial carcinoma (UTUC) reported exclusively in rural areas along the larger tributaries of the Danube river in Bosnia, Bulgaria, Croatia, Romania, and Serbia that affects genetically predisposed individuals exposed to aristolochic acid in the diet (1–9). EN has not been reported in individuals residing <15 years in an endemic area; therefore, it is believed that the intake of an environmental toxin over this period of time is required for the disease to develop. Although a number of agents has been considered as potential causes (10–13), EN/UTUC ultimately was shown to be an environmental form of aristolochic acid nephropathy (12,14).
The consumption of Aristolochia herbs in the practice of traditional Chinese medicine is a major cause of aristolochic acid nephropathy worldwide (15–17). In contrast, EN involves long-term, low-dose ingestion of the toxin through home-baked bread prepared from flour contaminated with seeds of Aristolochia clematitis (14,18,19). A study performed on Ukrainian immigrants (Ukraine has no patients with EN) who settled in Croatia in the early 20th century provided the first solid evidence that lifestyle plays an important role in this environmental disease (7). Over time, the risks of developing EN among Ukrainian settlers in endemic areas became similar to those of native Croats, whereas those who settled in nonendemic areas proved not to be at increased risk for EN (7).
In recent decades, improved harvesting and milling technologies have essentially eliminated the contamination of wheat grain with Aristolochia seeds, thus decreasing exposure to aristolochic acid in home-baked bread and consequently, reducing the prevalence of EN in many but not all endemic areas (19–22). Recent emigration of residents from nonendemic areas of Bosnia, where presumably, they were not previously exposed to aristolochic acid, into the Croatian endemic area after improvements in agricultural practices provided an opportunity to test the hypothesis that environmental exposure to this toxin is reduced, which would be reflected in unaltered kidney function of immigrants. Here, we investigate the prevalence of CKD and proximal tubule damage (PTD) in Croatian endemic areas, comparing the subgroup of Bosnian immigrants (BoENs) with long-term residents.
Materials and Methods
This cross-sectional observational study conducted in Croatia from 2005 to 2010 included 2822 inhabitants from nine endemic and three nonendemic villages. The overall participation rates in the endemic and nonendemic villages were 67.2% and 73.7%, respectively.
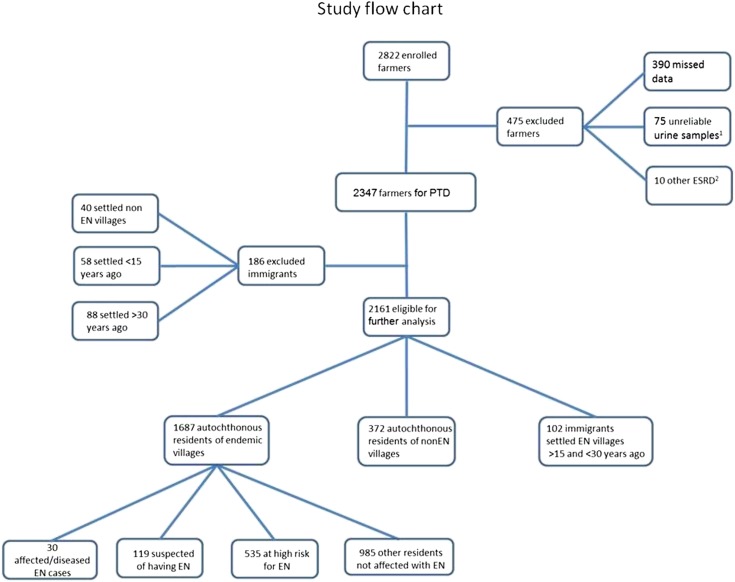
Of 2822 enrolled farmers, 2161 were eligible for additional analyses (Figure 1). Participants from EN villages were classified using the consensus diagnostic criteria (23) into four groups: affected participants (EN diseased), participants suspected of having EN, high-risk participants for EN, and other participants (Figure 1, Table 1). Because we wanted to test whether diminished exposure to aristolochic acid that took place a few decades ago reflects in today’s unaltered kidney function, we were particularly focused on a subgroup of BoENs that settled after agricultural changes took place and lived in the EN area for >15 years, which is considered a long enough period to ingest cumulative toxic doses of aristolochic acid if exposure was present.
Figure 1.
A study flow diagram. Proximal tubule damage (PTD) defined as α-1 microglobulin level corrected for urine creatinine >31.5 mg/g. EN, endemic nephropathy. 1Specific gravity <1.002 or >1.030. 2ESRD caused by other kidney diseases (one patient with nephrolithiasis, one patient with adult polycystic kidney disease, one patient with planocellular cancer, two patients with diabetic nephropathy, two patients with nephroangiosclerosis, one patient with rheumatoid arthritis, and two patients with chronic pyelonephritis).
Table 1.
Criteria for diagnosis of endemic nephropathy and classification of villagers from endemic areas
| Diagnostic Criteria | Affected Patients (Diseased) | Suspected of Having EN | At High Risk | Others | |||
|---|---|---|---|---|---|---|---|
| (a) Residence in an endemic household>15 yr/positive family history | + | + | + | + | + | + | − |
| (b) Tubular proteinuria | + | + | + | − | − | − | − |
| (c) Anemia | + | − | − | + | − | − | − |
| (d) Decreased eGFR | + | − | − | + | − | − | − |
| (e) UTUC | − | + | − | − | + | − | − |
Tubular proteinuria indicates α-1 microglobulin level corrected for urine creatinine >31.5 mg/g and α-1 microglobulin level corrected for urine albumine to urine creatinine ratio≥0.91. Anemia indicates hemoglobin<12.0 g/dl for men and women ages >50 years old and <11.0 g/dl for women ages <50 years old. Decreased eGFR indicates eGFR<60 ml/min per 1.73 m2. Affected/diseased indicates a+b+c+d or a+b+c. Suspected of having endemic nephropathy (EN) indicates a+b, a+c+d, or a+e. At high risk indicates a. Others indicates other villagers without any relation to EN. UTUC, upper tract urothelial cancer (23). ACR indicates albumin-to-creatinine ratio.
Study interviewers recruited adult participants by going door to door in the target villages. Informed consent was obtained from each participant who completed an extensive survey and provided a spot urine and fasting blood sample. All participants underwent ultrasound evaluation during the field work. Renal ultrasound examination was done using a Siemens Sonoline SI 250 ultrasound machine with a sector probe of 3.5 MHz by study personnel (i.e., three physicians: N.L, T.T., and M.L.) who were blinded to participants’ origins and clinical and laboratory data.
All study personnel were trained to collect survey and clinical information in a standardized manner. Weight and height were measured, and body mass index (BMI) was calculated. BP was measured on consecutive visits; each time, three measurements were obtained for the nondominant arm with an Omron M6 device as per European Society of Hypertension/European Cardiology Society guidelines (24).
Hypertension was defined as BP≥140/90 mmHg and/or the use of antihypertensive drugs. Diabetes was defined as fasting blood glucose >126 mg/dl and/or the use of antidiabetic drugs. CKD was defined as an eGFR<60 ml/min per 1.73 m2; CKD stages were classified according to the recent Kidney Disease Improving Global Outcomes guidelines (25). PTD was assessed according to α-1 microglobulin level corrected for urine creatinine (α-1CR), and PTD was considered if α-1CR values were >31.5 mg/g.
A survey was used to collect demographic data along with information regarding medical and family histories, dietary practices, and environmental exposures, with an emphasis on possible exposure to aristolochic acid. Specific questions were asked about wheat farming and grain-milling practices, observations of A. clematitis plants, sources of wheat and flour, and bread consumption to establish a history of aristolochic acid exposure. Importantly, traditional farming and lifestyle practices began to change several decades ago, and many of these are no longer maintained. Thus, at the time of the survey, questions focused on both current and traditional practices 20–30 years ago. Visual cues were used to increase the sensitivity of recall about A. clematitis. Specifically, 20×25-cm cards were developed showing common weeds growing in the region, including Ambrosia artemisiifolia, Agropyron repens, A. clematitis, Brassica napus, Cirsium lanceolatum, Convolvulus arvensis, Echinochloa crus-galli, Equisetum arvense, Galium aparine, Lolium spp., Mentha arvensis, Papaver rhoeas, Sorghum halepense, and Sorghum saccharatumpers. During the survey interview, participants were shown photographs of local plants and asked to identify and name the plant and if they recalled seeing them grow in meadows, gardens, and wheat fields. After seeing photographs of the specific weeds, participants also were asked to provide the traditional name of the weed used in the region. Recognition of the weed was considered positive if the traditional name of the weed matched the Latin binominal name. Participants also were asked to identify seeds from the plants. Aristolochic acid exposure was considered positive if the Aristolochia plant was observed in the farming fields and/or seeds of Aristolochia were among wheat seeds.
Fasting blood and urine samples were collected on the day of interview and stored on dry ice for transfer to the University Hospital Center Zagreb. Urinary α-1 microglobulin and albumin concentrations were determined using latex-enhanced immunonephelometry on a Behring Nephelometer II (Dade Behring, Marburg, Germany). Hemoglobin was determined with a Cell Dyn 1800 Hematology Analyzer (Abbott, Santa Clara, CA), and glucose was determined by ultraviolet photometry with hexokinase (AU 2700; Olympus, Tokyo, Japan). Serum and urine creatinine concentrations were measured by continuous photometry with alkyl picrate (AU 2700; Olympus). Kidney function was assessed by estimation of the GFR using the CKD Epidemiology Collaboration formula (26).
This study was approved by the Ethical Boards of the School of Medicine, University of Zagreb, the Croatian National Institute of Public Health, and General Hospital Dr. Josip Benčević.
Statistical analyses were performed using STATISTICA software, version 10. Variance homogeneity was tested by Lindeman’s test before the analysis of correlation and between-group differences. Normality of distribution was tested by using the Kolmogorov–Smirnov test. None of the variables came from normally distributed populations. Differences between groups for independent variables were analyzed using the Kruskal–Wallis test. Differences in the prevalence of individual conditions were tested using the chi-squared test. Logistic regression models were used to evaluate the association between prior and present aristolochic acid exposure, residence in an EN village, positive household history for EN, and immigrant status with PTD (α-1CR>31.5 mg/g). For the multivariate logistic model, all indigenous residents and all immigrants were included in the analysis. Odds ratios and 95% confidence intervals were estimated for each of the independent variables with adjustment for variables that can influence PTD (age, sex, BMI, history of arterial hypertension, and diabetes mellitus). Statistically significant differences were defined as a P value <0.05 in all analyses.
Results
Table 2 summarizes the demographic and clinical characteristics of the study participants. Baseline characteristics were similar in terms of age, sex, BMI, BP, and prevalence of hypertension and diabetes. Immigrants resided in the endemic area for significantly shorter periods of time than other study participants (P<0.001). The prevalence of CKD (percentage) was higher in endemic villages compared with nonendemic villages and BoENs (16.6 versus 9.1 versus 6.9; P<0.001). Distribution of CKD stages (Table 3) shows that CKD stage ≥3a was significantly higher in EN villages (P=0.001), whereas no difference between BoENs and residents of control nonendemic villages was observed (P<0.99).
Table 2.
Baseline clinical and biologic data of enrolled subjects
| Clinical and Biological Data | Endemic Villages (n=1687) | Control Nonendemic Villages (n=372) | Bosnian Immigrantsa (n=102) | P value |
|---|---|---|---|---|
| Age, yr | 50 (18–91) | 53 (18–90)b | 52 (20–86) | 0.02 |
| Sex (men), % | 40.4 | 40.9 | 37.3 | 0.80 |
| Living in village, yr | 41 (24–55) | 45 (26–60) | 18 (15–21)b,c | <0.001 |
| Family history of EN (positive), % | 37.2 | 1.1b | 2.0b | <0.001 |
| Height, cm | 168 (161–175) | 167 (160–175) | 167 (160–174) | 0.40 |
| Weight, kg | 76 (66–88) | 79 (68–89) | 74 (64–87) | 0.09 |
| Body mass index, kg/m2 | 26.87 (23.71–30.74) | 27.66 (24.30–31.25) | 26.59 (23.81–29.91) | 0.07 |
| Systolic BP, mmHg | 135 (120–154) | 138 (123.5–159.8) | 137 (126–156) | 0.07 |
| Diastolic BP, mmHg | 81 (75–90) | 81 (75–92) | 82 (76–90) | 0.77 |
| Hypertension, % | 41.2 | 44.6 | 43.8 | 0.48 |
| Diabetes, % | 6.1 | 9.2 | 7.9 | 0.09 |
| CKD, % | 16.6 | 9.1 | 6.9 | <0.001 |
Bosnian immigrants who settled in the EN area 15–30 years ago. Values are expressed as medians and interquartile ranges (25th–75th; minimum to maximum for age) or percentage if indicated.
Significant difference (P<0.05) with endemic villages group.
Significant difference (P<0.05) with control nonendemic villages group.
Table 3.
Frequency of CKD stages in endemic and nonendemic villages and Bosnian immigrants who settled EN villages 15–30 years ago
| CKD Stage | Endemic Villages (%; n=1687) | Control Nonendemic Villages (%; n=372) | Bosnian Immigrants (%; n=102) |
|---|---|---|---|
| 1 | 1.3 | 1.9 | 1.0 |
| 2 | 4.2 | 5.9 | 4.9 |
| 3a | 9.4 | 7.5 | 4.9 |
| 3b | 4.6 | 1.3 | 1.0 |
| 4 | 1.4 | 0.0 | 1.0 |
| 5 | 1.2 | 0.3 | 0.0 |
As shown in Table 4, values for urine albumine to urine creatinine ratio, hemoglobin level, and urine specific gravity were comparable among all three subgroups (all with P>0.05). eGFR was higher among BoENs compared with the two indigenous groups (P=0.003), whereas the shortest kidneys were found in participants living in endemic villages (P<0.001). More farmers with biomarkers of kidney function above the cutoff for abnormal values resided in endemic villages compared with farmers in nonendemic villages and BoENs. However, there were no differences between BoENs and autochthonous residents of nonendemic villages.
Table 4.
Markers of kidney function in autochthonous villagers and Bosnian immigrants who settled EN villages 15–30 years ago
| Laboratory Parameter | Endemic Villages (n=1687) | Control Nonendemic Villages (n=372) | Bosnian Immigrants (n=102) | P value |
|---|---|---|---|---|
| α-1CR, mg/g | 5.98 (3.75–10.53) | 7.39 (4.74–12.47)a | 7.04 (4.66–11.17) | <0.001 |
| α-1CR>31.5 mg/g, N (%) | 82 (7.3) | 10 (0.3)a | 1 (1.3)a | 0.003 |
| ACR, mg/g | 5.29 (3.51–10.68) | 5.68 (3.87–10.61) | 5.58 (3.95–9.64) | 0.30 |
| ACR>30 mg/g, N (%) | 189 (15.8) | 38 (11.4)a | 7 (6.7)a | 0.05 |
| Serum creatinine, mg/dl | 1.09 (0.97–1.25) | 1.05a (0.94–1.21) | 0.98 (0.9–1.12)a,b | <0.001 |
| eGFR, ml/min per 1.73 m2 | 80 (67–94) | 81 (70–92) | 88 (73–100)a,b | 0.003 |
| eGFR<60 ml/min per 1.73 m2, N (%) | 280 (16.6) | 34 (9.1)a | 7 (6.9)a | <0.001 |
| Hemoglobin, g/dl | 13.7 (12.9–14.7) | 14 (13–14.8) | 13.8 (13–14.7) | 0.13 |
| Urine specific gravity | 1.015 (1.010–1.021) | 1.015 (1.010–1.020) | 1.016 (1.011–1.022) | 0.22 |
| Left kidney length, mm | 109 (100–117) | 113 (108–119)a | 112 (103–120) | <0.001 |
| Right kidney length, mm | 109 (101–117) | 114 (106–120)a | 114 (102–118) | <0.001 |
Values are expressed as medians and interquartile ranges (25th–75th). α-1CR, α-1 microglobulin level corrected for urine creatinine; ACR, urine albumine to urine creatinine ratio.
Significant difference (P<0.05) with endemic villages group.
Significant difference (P<0.05) with control nonendemic villages group.
According to the diagnostic criteria for EN (Table1), among autochthonous EN villagers, there were 30 affected and 119 suspected participants, whereas 535 participants were at high risk for EN (Figure 1). Importantly, EN was not diagnosed in immigrants who settled in the endemic area between 15 and 30 years ago, a period when agricultural practices affecting consumption of wheat grain had begun to change. Exposure to aristolochic acid among the various groups was estimated using parameters similar to those in a related study (19). Twenty years ago, BoENs had fewer wheat fields than indigenous Croatian farmers (P<0.001), and their fields were less frequently flooded—a factor related to the growth of Aristolochia (P<0.01) (Table 5). Immigrants and farmers from the nonendemic area observed fewer Aristolochia plants in their fields (P<0.001) as well as fewer Aristolochia seeds among wheat seeds (P<0.001) than farmers from endemic villages. Interestingly, there were no differences between BoENs and Croatian farmers from nonendemic areas with respect to the same observations. At the time, immigrants baked bread using their own flour less often than native Croatian farmers (P=0.001). Presently, observations of Aristolochia plants in farming fields as well as Aristolochia seeds among wheat seeds are comparable between the three groups (P>0.05 for all), and participants from EN villages less frequently observe Aristolochia plants in their farming fields and Aristolochia seeds among wheat than they did in the past (67.8% versus 40.0%; 35.6% versus 8.3%; P<0.001 for both). Farmers from all groups reported that the amount of bread ingested today and 20 years ago is comparable (P=0.30). However, native Croatian farmers bake bread using homemade flour less often than previously, choosing to purchase bread in stores (P=0.04).
Table 5.
Exposure to aristolochic acid on the basis of differences in farming practices and dietary habits presently and 15–30 years ago
| Question/Answer | Past (15–30 years ago) N (%) | Present N (%) | ||||||
|---|---|---|---|---|---|---|---|---|
| EN | Non-EN | Bosnian Immigrant | P value | EN | Non-EN | Bosnian Immigrant | P value | |
| Did/do you have farming fields? | ||||||||
| Yes | 886 (91.2) | 190 (88.8) | 46 (73)a,b | <0.001 | 1119 (81.1) | 214 (77.3) | 63 (78.8) | 0.32 |
| Did/do you grow wheat in your farming fields? | ||||||||
| Yes | 838 (95.1) | 180 (94.7) | 36 (76.6)a,b | <0.001 | 789 (71) | 156 (73.6) | 29 (45.3)a,b | <0.001 |
| Were/are farming fields flooded? | ||||||||
| Yes | 388 (45.4) | 97 (51.3) | 10 (24.4)a,b | <0.01 | 277 (27.5) | 74 (35.6)a | 19 (34.5) | 0.05 |
| Did/do you observe Aristolochia plants in your fields? | ||||||||
| Yes | 581 (67.8) | 98 (51.9)a | 18 (41.9)a | <0.001 | 418 (40) | 78 (37.9) | 26 (41.9) | 0.79 |
| Did/do you observe Aristolochia seeds among wheat seeds? | ||||||||
| Yes | 285 (35.6) | 25 (14.5)a | 2 (6.1)a | <0.001 | 68 (8.3) | 10 (6) | 2 (4.7) | 0.45 |
| Did/do you bake bread? | ||||||||
| Yes | 916 (92.6) | 206 (95.4) | 50 (74.6)a,b | 0.001 | 553 (42.5) | 105 (39) | 43 (55.1)a,b | 0.04 |
| If yes, where did/do you get the flour? | ||||||||
| Grind wheat at home | 114 (12.4) | 16 (7.8) | 9 (18) | <0.001 | 14 (2.6) | 2 (2) | 2 (4.8) | 0.001 |
| Miller | 770 (84.1) | 176 (86.3) | 28 (56) | 410 (77.4) | 63 (62.4) | 23 (54.8) | ||
| Store | 32 (3.5) | 12 (5.9) | 13 (26) | 106 (20) | 36 (35.6) | 17 (40.5) | ||
| Did/do you buy bread rather than bake? | ||||||||
| Yes | 147 (14.8) | 20 (9.3) | 25 (38.5)a,b | <0.001 | 1191 (86.2) | 231 (83.1) | 62 (75.6)a,b | 0.02 |
| Did you eat more or less bread 20 years ago compared with today? | ||||||||
| More than now | 628 (64.3) | 140 (65.1) | 36 (57.1) | 0.30 | ||||
| Less than now | 79 (8.1) | 19 (8.8) | 10 (5.9) | |||||
| About the same | 269 (27.6) | 56 (26) | 17 (27) | |||||
Significant difference (P<0.05) with endemic villages group.
Significant difference (P<0.05) with control nonendemic villages group.
History of prior aristolochic acid exposure (>30 years ago), residence in an EN village, and family household history of EN were positively associated with PTD (Table 6). On the contrary, present aristolochic acid exposure and time period of exposure 30–15 years ago were not related to PTD. Furthermore, immigrant status was identified to be an independent negative predictor of PTD, regardless of time periods of exposure (odds ratio, 0.40; 95% confidence interval, 0.19 to 0.86; P=0.02).
Table 6.
Independent predictors of proximal tubule damage
| Independent Predictor | OR (95% CI) | P value |
|---|---|---|
| Living in EN village (yes) | 3.97 (1.78 to 8.85) | 0.001 |
| Family/household history for EN (positive) | 2.74 (1.77 to 4.24) | <0.001 |
| Past aristolochic acid exposure (yes) | 1.64 (1.04 to 2.58) | 0.03 |
| Time of exposure 30–15 yr ago (yes) | 1.59 (0.46 to 5.42) | 0.57 |
| Present aristolochic acid exposure (yes) | 1.31 (0.75 to 2.30) | 0.33 |
| Immigrant status (yes) | 0.40 (0.19 to 0.86) | 0.02 |
Proximal tubule damage defined as α-1 microglobulin level corrected for urine creatinine >31.5 mg/g. Covariates in the multivariate logistic regression models included age, sex, body mass index, hypertension, and diabetes. OR, odds ratio; 95% CI, 95% confidence interval.
Discussion
The main finding of this survey is that the prevalence of CKD and tubular proteinuria in Bosnians who immigrated to Croatian endemic areas is lower compared with native residents, whereas no differences were detected in these parameters between BoENs and the nonendemic Croatian rural population. Because of changes in lifestyle and agricultural practices, exposure to aristolochic acid in the diet is reduced compared with several decades ago; thus, a gradual decrease in the prevalence of EN is predicted. The prevalence of CKD is higher in Croats residing in the endemic area compared with BoENs and Croats residing in control villages. Furthermore, the high prevalence of CKD in endemic areas (16.6%) is greater than that reported in the majority of other geographic regions worldwide (27–35). Importantly, it is essential to follow such patients not only with the aim of postponing progression to ESRD but also, because as many as 50% of patients with EN are likely to eventually develop UTUC (1,4,17,36–38). In nonendemic villages, 9.1% of villagers had CKD stage ≥3, similar to estimates in other countries (27,29,31–33,35). The higher prevalence of CKD in endemic areas is attributed to prior exposure to aristolochic acid (14). Presently, the prevalence of both affected and suspected patients is lower than previously reported, and a shift to older ages is observed (1,22,39,40).
We failed to detect any participants with EN among BoENs. Additionally, the prevalence of CKD and PTD in BoENs is the same as that observed in residents of nonendemic villages. Diagnosis of UTUC was made in eight farmers from endemic villages, and all of them were diagnosed and classified as patients with EN, whereas none of the suspected or high-risk participants for EN had UTUC. Additionally, UTUC was not diagnosed in any farmers from nonendemic villages or among BoENs. These data support the hypothesis that exposure of this population to aristolochic acid is significantly diminished. Using logistic regression analyses, we observed that immigrant status is an independent protective variable for PTD. In contrast, positive family/household history for EN, residence in an EN village, and past aristolochic acid exposure were significantly positive predictors. Present exposure to aristolochic acid was not associated with PTD, confirming our hypothesis. In the group of BoENs who were excluded from this analysis, because they settled in the Croatian endemic area >30 years ago, two farmers were diagnosed as EN affected, and three farmers were diagnosed as EN suspected. This observation is in line with our hypothesis, because these farmers moved to the endemic area before improvement in agricultural practices occurred. Indeed, these individuals showed the same risk for developing EN as autochthonous Croats and the same risk as Ukrainians who immigrated to this area previously. Thus, our results are consistent with those of an earlier study investigating EN in Ukrainian immigrants (7).
According to a report from 1985, the prevalence of patients with EN and those suspected of having EN among Ukrainians who settled in endemic villages was the same as the prevalence in autochthonous Croatian villagers (10.5% versus 12.1%; P>0.05), whereas EN was not reported in Ukrainians who settled in nonendemic villages (7). In our survey, we observed a lower prevalence of EN in autochthonous Croats than Ceović et al. (7) found 40 years ago (1.8% versus 12.1%). Both Ukrainian immigrants and BoENs settled in the same endemic area; therefore, the two studies seem comparable. In the past, the presence of aristolochic acid in the environment coupled with agricultural practices in the endemic area led to similar risk factors for EN among Ukrainian immigrants and autochthonous farmers. Presently, advances in agriculture practices contribute to decreased contamination of flour with aristolochic acid and therefore, decreased risks of exposure. This conclusion is confirmed with our observations of BoENs who settled in this area after those changes occurred. In the last three decades, farmers began using modern combines, which improved the separation of weed seeds; importantly, wheat grain now is processed in large common mills. Thus, despite the continued presence of A. clematitis in farming fields, the risk of contamination of wheat grain with aristolochic acid is unlikely to occur. Furthermore, villagers rarely prepare their own flour or bake their own bread as in the past, because the majority of farmers now purchase bread in bakeries or stores. Results from this survey conducted in a general rural population are in concordance with data obtained from patients with EN undergoing dialysis or surgery for aristolochic acid–induced UTUC (12,14,19).
This study has several important strengths. It is the first report on the overall prevalence of CKD in an endemic area and the largest epidemiologic study to evaluate early renal damage specific to EN. Additionally, we report that exposure of residents of the endemic area to aristolochic acid has significantly decreased in the last two to three decades.
Our study also has certain limitations inherent to a cross-sectional observational study design. The survey was conducted in a single endemic focus. Thus, although our results are statistically significant, similar studies should be performed in other endemic areas. Recall bias could be questioned. However, we did not ask participants to recognize only the A. clematitis but also, several other common weeds as well. The issue of participation bias could be also raised. Nevertheless, we recruited participants on the door-to-door basis, and participation rate was good and without differences among groups. Also, we determined α-1CR and urine albumine to urine creatinine ratio in a single spot urine sample. However, recent studies suggest that similar measurements of α-1CR are a reliable marker for EN (23,25,39–45) and other chronic tubulointerstitial diseases, such as cadmium nephropathy (46–49). Most authors believe that α-1 microglobulin has advantages over β-2 microglobulin. Stefanović et al. (43) found that both α-1 and β-2 microglobulin are significant predictors for differentiation of EN from healthy participants and other kidney diseases. Interestingly, analyses odds ratios and P values were much more in favor of α-1 than β-2 microglobulin. Other works evaluated diagnostic criteria for the diagnosis of EN and concluded that α-1 microglobulinuria significantly discriminated EN from other kidney diseases (50,51). Finally, in our cohort with >2000 enrolled farmers, we obtained the same results (42). Accordingly, α-1CR was included in the latest consensus EN document prepared by leading experts in the field (23). CKD was diagnosed using a single measurement of eGFR; however, in most epidemiologic studies of this kind, only a single measurement is obtained.
In conclusion, our results are fully consistent with the hypothesis that chronic dietary exposure to aristolochic acid is the cause of EN (9,12,14,18,19). We also confirm the relationship between reduced exposure to aristolochic acid in endemic areas and less PTD. Additionally, we confirm that present exposure to aristolochic acid is reduced. Although we predict additional decreases in the prevalence of EN, the prevalence of CKD remains high in endemic areas, underscoring the need for screening and strict follow-up of suspected patients. Finally, our research highlights the important role of environment and lifestyle on renal disease. It is also an example of how human behavior, knowledge, and advances in technology can positively influence the natural course of an environmental disease.
Disclosures
None.
Acknowledgments
This research was supported by the Ministry of Science of the Republic of Croatia (108-0000000-0329), Croatian Foundation for Science Grant 04/38, and National Institute of Environmental Health Sciences Grant ES-04068.
Footnotes
B.J. and I.V.L. contributed equally to this work.
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial, “Preventing Aristolochic Acid Nephropathy,” on pages 167–168.
References
- 1.Čeović S, Miletić-Medved M: Epidemiological features of endemic nephropathy in focal area of Brodska Posavina, Croatia. In: Endemic Nephropathy in Croatia, edited by Čvorišćec D, Čeović S, Stavljenić-Rukavina A, Zagreb, Croatia, Academia Croatica Scientiarum Medicarum, 1996, pp 7–21. [Google Scholar]
- 2.Đukanović L, Radovanović Z: Balkan endemic nephropathy. In: Clinical Nephrotoxins: Renal Injury from Drugs and Chemicals, edited by de Broe ME, Porter GA, Bennett WM, Verpooten GA, Kluwer Academic Publishers, 2003, pp 587–601 [Google Scholar]
- 3.Imamović G, Trnančević S, Mesić E, Stipančić Ž: Endemic (Balkan) nephropathy in Bosnia and Herzegovina: Current status. Coll Antropol 30[Suppl 1]: 41, 2006 [Google Scholar]
- 4.Stefanović V, Radovanović Z: Balkan endemic nephropathy and associated urothelial cancer. Nat Clin Pract Urol 5: 105–112, 2008 [DOI] [PubMed] [Google Scholar]
- 5.Gluhovschi G, Margineanu F, Velciov S, Gluhovschi C, Bob F, Petrica L, Bozdog G, Trandafirescu V, Modalca M: Fifty years of Balkan endemic nephropathy in Romania: Some aspects of the endemic focus in the Mehedinti county. Clin Nephrol 75: 34–48, 2011 [PubMed] [Google Scholar]
- 6.Dimitrov PS, Simeonov VA, Stein AD: Balkan endemic nephropathy in Vratza, Bulgaria, 1964-1987: An epidemiologic analysis of population-based disease registers. Eur J Epidemiol 17: 847–853, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ceović S, Hrabar A, Radonić M: An etiological approach to Balkan endemic nephropathy based on the investigation of two genetically different populations. Nephron 40: 175–179, 1985 [DOI] [PubMed] [Google Scholar]
- 8.Ceović S, Hrabar A, Sarić M: Epidemiology of Balkan endemic nephropathy. Food Chem Toxicol 30: 183–188, 1992 [DOI] [PubMed] [Google Scholar]
- 9.Grollman AP, Jelaković B: Role of environmental toxins in endemic (Balkan) nephropathy. October 2006, Zagreb, Croatia. J Am Soc Nephrol 18: 2817–2823, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Long DT, Voice TC: Role of exposure analysis in solving the mystery of Balkan endemic nephropathy. Croat Med J 48: 300–311, 2007 [PMC free article] [PubMed] [Google Scholar]
- 11.Voice TC, Long DT, Radovanović Z, Atkins JL, McElmurry SP, Niagolova ND, Dimitrov P, Petropoulos EA, Ganev VS: Critical evaluation of environmental exposure agents suspected in the etiology of Balkan endemic nephropathy. Int J Occup Environ Health 12: 369–376, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Grollman AP, Shibutani S, Moriya M, Miller F, Wu L, Moll U, Suzuki N, Fernandes A, Rosenquist T, Medverec Z, Jakovina K, Brdar B, Slade N, Turesky RJ, Goodenough AK, Rieger R, Vukelić M, Jelaković B: Aristolochic acid and the etiology of endemic (Balkan) nephropathy. Proc Natl Acad Sci U S A 104: 12129–12134, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Broe ME: Chinese herbs nephropathy and Balkan endemic nephropathy: Toward a single entity, aristolochic acid nephropathy. Kidney Int 81: 513–515, 2012 [DOI] [PubMed] [Google Scholar]
- 14.Jelaković B, Karanović S, Vuković-Lela I, Miller F, Edwards KL, Nikolić J, Tomić K, Slade N, Brdar B, Turesky RJ, Stipančić Ž, Dittrich D, Grollman AP, Dickman KG: Aristolactam-DNA adducts are a biomarker of environmental exposure to aristolochic acid. Kidney Int 81: 559–567, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vanherweghem JL, Debelle F, Muniz-Martinez MC, Nortier J: Aristolochic acid nephropathy after Chinese herb remedies. In: Clinical Nephrotoxins, edited by De Broe ME, Porter GA, Benett WM, Verpooten GA, 2nd Ed., Dordrecht, The Netherlands, Kluwer, 2003, pp 579–603 [Google Scholar]
- 16.Vanherweghem JL, Tielemans C, Abramowicz D, Depierreux M, Vanhaelen-Fastre R, Vanhaelen M, Dratwa M, Richard C, Vandervelde D, Verbeelen D, Jadoul M: Rapidly progressive interstitial renal fibrosis in young women: Association with slimming regimen including Chinese herbs. Lancet 341: 387–391, 1993 [DOI] [PubMed] [Google Scholar]
- 17.Nortier JL, Martinez MC, Schmeiser HH, Arlt VM, Bieler CA, Petein M, Depierreux MF, De Pauw L, Abramowicz D, Vereerstraeten P, Vanherweghem JL: Urothelial carcinoma associated with the use of a Chinese herb (Aristolochia fangchi). N Engl J Med 342: 1686–1692, 2000 [DOI] [PubMed] [Google Scholar]
- 18.Ivić M: Etiology of endemic nephropathy. Lijec Vjesn 91: 1273–1281, 1969 [PubMed] [Google Scholar]
- 19.Hranjec T, Kovač A, Kos J, Mao W, Chen JJ, Grollman AP, Jelaković B: Endemic nephropathy: The case for chronic poisoning by aristolochia. Croat Med J 46: 116–125, 2005 [PubMed] [Google Scholar]
- 20.Cvitković A, Vuković-Lela I, Edwards KL, Karanović S, Jurić D, Cvorišćec D, Fuček M, Jelaković B: Could disappearance of endemic (Balkan) nephropathy be expected in forthcoming decades? Kidney Blood Press Res 35: 147–152, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Čukuranović R, Petrović B, Čukuranović Z, Stefanović V: Balkan endemic nephropathy: A decreasing incidence of the disease. Pathol Biol (Paris) 48: 558–561, 2000 [PubMed] [Google Scholar]
- 22.Bukvić D, Marić I, Arsenović A, Janković S, Djukanović L: Prevalence of Balkan endemic nephropathy has not changed since 1971 in the Kolubara region in Serbia. Kidney Blood Press Res 30: 117–123, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Jelaković B, Nikolić J, Radovanović Z, Nortier J, Cosyns JP, Grollman AP, Bašić-Jukić N, Belicza M, Bukvić D, Čavaljuga S, Cvorišćec D, Cvitković A, Dika Z, Dimitrov P, Dukanović L, Edwards K, Ferluga D, Fuštar-Preradović L, Gluhovschi G, Imamović G, Jakovina T, Kes P, Leko N, Medverec Z, Mesić E, Miletić-Medved M, Miller F, Pavlović N, Pasini J, Pleština S, Polenaković M, Stefanović V, Tomić K, Trnačević S, Vuković Lela I, Štern-Padovan R: Consensus statement on screening, diagnosis, classification and treatment of endemic (Balkan) nephropathy. Nephrol Dial Transplant 29: 2020–2027, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mancia G, De Backer G, Dominiczak A, Cifkova R, Fagard R, Germano G, Grassi G, Heagerty AM, Kjeldsen SE, Laurent S, Narkiewicz K, Ruilope L, Rynkiewicz A, Schmieder RE, Boudier HA, Zanchetti A, ESH-ESC Task Force on the Management of Arterial Hypertension : 2007 ESH-ESC Practice Guidelines for the Management of Arterial Hypertension: ESH-ESC Task Force on the Management of Arterial Hypertension. J Hypertens 25: 1751–1762, 2007 [DOI] [PubMed] [Google Scholar]
- 25.KDIGO : Chapter 1: Definition and classification of CKD. Kidney Int Suppl (2011) 3: 19–62, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J, CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) : A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, Van Lente F, Levey AS: Prevalence of chronic kidney disease in the United States. JAMA 298: 2038–2047, 2007 [DOI] [PubMed] [Google Scholar]
- 28.Brown WW, Peters RM, Ohmit SE, Keane WF, Collins A, Chen SC, King K, Klag MJ, Molony DA, Flack JM: Early detection of kidney disease in community settings: The Kidney Early Evaluation Program (KEEP). Am J Kidney Dis 42: 22–35, 2003 [DOI] [PubMed] [Google Scholar]
- 29.Cirillo M, Del Giudice L, Bilancio G, Franzese MD, Chiricone D, De Santo NG: Early detection of chronic kidney disease: Epidemiological data on renal dysfunction. G Ital Nefrol 25: 690–693, 2008 [PubMed] [Google Scholar]
- 30.Hallan SI, Coresh J, Astor BC, Asberg A, Powe NR, Romundstad S, Hallan HA, Lydersen S, Holmen J: International comparison of the relationship of chronic kidney disease prevalence and ESRD risk. J Am Soc Nephrol 17: 2275–2284, 2006 [DOI] [PubMed] [Google Scholar]
- 31.Nitsch D, Felber Dietrich D, von Eckardstein A, Gaspoz JM, Downs SH, Leuenberger P, Tschopp JM, Brändli O, Keller R, Gerbase MW, Probst-Hensch NM, Stutz EZ, Ackermann-Liebrich U, SAPALDIA team : Prevalence of renal impairment and its association with cardiovascular risk factors in a general population: Results of the Swiss SAPALDIA study. Nephrol Dial Transplant 21: 935–944, 2006 [DOI] [PubMed] [Google Scholar]
- 32.Viktorsdottir O, Palsson R, Andresdottir MB, Aspelund T, Gudnason V, Indridason OS: Prevalence of chronic kidney disease based on estimated glomerular filtration rate and proteinuria in Icelandic adults. Nephrol Dial Transplant 20: 1799–1807, 2005 [DOI] [PubMed] [Google Scholar]
- 33.Otero A, de Francisco A, Gayoso P, García F, EPIRCE Study Group : Prevalence of chronic renal disease in Spain: Results of the EPIRCE study. Nefrologia 30: 78–86, 2010 [DOI] [PubMed] [Google Scholar]
- 34.Süleymanlar G, Utaş C, Arinsoy T, Ateş K, Altun B, Altiparmak MR, Ecder T, Yilmaz ME, Çamsari T, Başçi A, Odabas AR, Serdengeçti K: A population-based survey of Chronic REnal Disease In Turkey—the CREDIT study. Nephrol Dial Transplant 26: 1862–1871, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ong-Ajyooth L, Vareesangthip K, Khonputsa P, Aekplakorn W: Prevalence of chronic kidney disease in Thai adults: A national health survey. BMC Nephrol 10: 35, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Su T, Zhang L, Li X, Zuo L, Zhang P, Wang H: Regular use of nephrotoxic medications is an independent risk factor for chronic kidney disease—results from a Chinese population study. Nephrol Dial Transplant 26: 1916–1923, 2011 [DOI] [PubMed] [Google Scholar]
- 37.Mesić E, Lukić L, Dolenec L, Petković N, Stipancić Z, Resić H, Trnacević S, Halilbasić A: Balkan endemic nephropathy in Bosnia and Herzegovina—renal registry report. Med Arh 60: 240–242, 2006 [PubMed] [Google Scholar]
- 38.Tsvetan D: Balkan endemic nephropathy in Bulgaria. Facta universitatis Facta Univ Ser Mech Autom Control Robot 9: 7–14, 2002 [Google Scholar]
- 39.Miletić-Medved M, Jelaković B, Bistrović D, Leko N, Marić Z: Epidemiologic characteristics of endemic nephropathy in Croatia in 2005. Acta Med Croatica 61: 141–148, 2007 [PubMed] [Google Scholar]
- 40.Miletić-Medved M, Domijan AM, Peraica M: Recent data on endemic nephropathy and related urothelial tumors in Croatia. Wien Klin Wochenschr 117: 604–609, 2005 [DOI] [PubMed] [Google Scholar]
- 41.Cvorisćec D: Early diagnosis of endemic nephropathy. Clin Chim Acta 297: 85–91, 2000 [DOI] [PubMed] [Google Scholar]
- 42.Dika Ž: Evaluation of diagnostic criteria for endemic nephropathy, PhD thesis, Zagreb, Croatia, University of Zagreb School of Medicine, 2012 [Google Scholar]
- 43.Stefanović V, Djukanović L, Cukuranović R, Bukvić D, Ležaić V, Marić I, Ogrizovic SS, Jovanović I, Vlahovic P, Pešić I, Djordjević V: Beta2-microglobulin and alpha1-microglobulin as markers of Balkan endemic nephropathy, a worldwide disease. Ren Fail 33: 176–183, 2011 [DOI] [PubMed] [Google Scholar]
- 44.Yu H, Yanagisawa Y, Forbes MA, Cooper EH, Crockson RA, MacLennan IC: Alpha-1-microglobulin: An indicator protein for renal tubular function. J Clin Pathol 36: 253–259, 1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Itoh Y, Cooper EH, Stavlienić A, Čvoriščec D: Urinary alpha 1 microglobulin levels in surveys of Balkan endemic nephropathy. Biomed Pharmacother 40: 341–344, 1986 [PubMed] [Google Scholar]
- 46.Ikeda M, Ezaki T, Tsukahara T, Moriguchi J, Furuki K, Fukui Y, Ukai SH, Okamoto, Sakurai H: Critical evaluation of alpha1- and beta2-microglobulins in urine as markers of cadmium-induced tubular dysfunction. Biometals 17: 539–541, 2004 [DOI] [PubMed] [Google Scholar]
- 47.Ikeda M, Ezaki T, Tsukahara T, Moriguchi J, Furuki K, Fukui Y, Ukai H, Okamoto S, Sakurai H: Reproducibility of urinary cadmium, alpha1-microglobulin, and beta2-microglobulin levels in health screening of the general population. Arch Environ Contam Toxicol 48: 135–140, 2005 [DOI] [PubMed] [Google Scholar]
- 48.Kido T, Honda R, Yamada Y, Tsuritani I, Ishizaki M, Nogawa K: alpha 1-Microglobulin determination in urine for the early detection of renal tubular dysfunctions caused by exposure to cadmium. Toxicol Lett 24: 195–201, 1985 [DOI] [PubMed] [Google Scholar]
- 49.Chia KS, Tan AL, Chia SE, Ong CN, Jeyaratnam J: Renal tubular function of cadmium exposed workers. Ann Acad Med Singapore 21: 756–759, 1992 [PubMed] [Google Scholar]
- 50.Djukanović L, Marić I, Marinković J, Ignjatović S, Bukvić D: Evaluation of criteria for the diagnosis of Balkan endemic nephropathy. Ren Fail 29: 607–614, 2007 [DOI] [PubMed] [Google Scholar]
- 51.Djukanović L, Marinković J, Marić I, Lezaić V, Dajak M, Petronić D, Matić M, Bukvić D: Contribution to the definition of diagnostic criteria for Balkan endemic nephropathy. Nephrol Dial Transplant 23: 3932–3938, 2008 [DOI] [PubMed] [Google Scholar]