Abstract
Background and objectives
Survival, symptom burden, and quality of life (QOL) are uncertain for elderly patients with advanced CKD managed without dialysis. We examined these outcomes in patients managed with renal supportive care without dialysis (RSC-NFD) and those planned for or commencing dialysis.
Design, setting, participants, & measurements
In this prospective observational study, symptoms were measured using the Memorial Symptom Assessment Scale and the Palliative care Outcomes Scale - Symptoms (renal) inventory and QOL was measured using the Short Form-36 survey. This study comprised 273 predialysis patients who had usual nephrology care and 122 nondialysis pathway patients who also attended a renal supportive care clinic adding the skills of a palliative medicine team. A further 72 patients commenced dialysis during this period without attending either clinic.
Results
Nondialysis patients were older than the predialysis group (82 versus 67 years; P<0.001) but had similar eGFR at the first clinic visit (16 ml/min per 1.73 m2; P=0.92). Of the predialysis patients, 92 (34%) commenced dialysis. Compared with the RSC-NFD group, the death rate was lower in the predialysis group who did not require dialysis (hazard ratio, 0.23; 95% confidence interval, 0.12 to 0.41] and in those requiring dialysis (0.30; 0.13 to 0.67) but not in dialysis patients who had not attended the predialysis clinic (0.60; 0.35 to 1.03). Median survival in RSC-NFD patients was 16 (interquartile range, 9, 37) months and 32% survived >12 months after eGFR fell below 10 ml/min per 1.73 m2. For the whole group, age, serum albumin, and eGFR <15 ml/min per 1.73 m2 were associated with poorer survival. Of the nondialysis patients, 57% had stable or improved symptoms over 12 months and 58% had stable or improved QOL.
Conclusions
Elderly patients who choose not to have dialysis as part of shared decision making survive a median of 16 months and about one-third survive 12 months past a time when dialysis might have otherwise been indicated. Utilizing the skills of palliative medicine helps provide reasonable symptom control and QOL without dialysis.
Keywords: dialysis, elderly patients, renal supportive care, palliative care
Introduction
Perhaps the most difficult clinical decision faced by nephrologists today is whether their elderly patient (arbitrarily defined as age >70 years) with advanced CKD is likely to benefit from dialysis (1). Half of all dialysis patients in Australia are aged >65 years and 26% are aged ≥75 years (2). Similarly, the fastest-growing incident group receiving dialysis comprises individuals aged >75 years in the United States (3) and 65 or older years in the United Kingdom (4).
Unfortunately, survival of elderly dialysis patients is generally worse than that for most cancers (5–7). The most common cause of death on dialysis in Australia appears to be withdrawal for psychosocial or progressive medical reasons, particularly for patients aged >65 years (2), suggesting that the initial choice of treatment may have been suboptimal for many patients. Their symptom burden on dialysis is high (8), quality of life (QOL) is often poor, and the social effects of dialysis on elderly individuals are great, particularly for those in nursing homes (9).
Rosansky stated that “the decision to initiate dialysis should be a joint decision made by patients and their nephrologists, after full disclosure of the potential harms and benefits of dialysis vs. non-dialysis management” (10). Nephrologists may struggle to have these conversations (11), partly due to the relative lack of data concerning the likely trajectory of patients when dialysis is not utilized.
To aid these discussions, we examined the survival of elderly patients not planned for dialysis and whether they could be provided with reasonable QOL and control of their symptoms.
Materials and Methods
We conducted a single-center prospective observational study in patients with CKD (stages 4/5) between March 2009 and March 2013, including the Strengthening the Reporting of Observational Studies in Epidemiology recommendations for reporting observational studies (12), approved by the South Eastern Sydney Human Research Ethics Committee (HREC/10/STG/121). Patients gave written informed consent.
Patients were recruited consecutively between March 2009 and March 2013 from the time that they attended the clinic or commenced dialysis as follows: (1) patients referred to a predialysis clinic with a planned future dialysis pathway, (2) patients referred to the renal supportive care (RSC) clinic planned for a nondialysis pathway (RSC-NFD), and (3) additional patients who commenced dialysis during this time period without attending the predialysis clinic either due to late presentation with ESRD (the stage when dialysis or transplantation is required to sustain life) or failure to attend despite referral. These patients are referred to as the “other dialysis” group.
Renal Clinics
The predialysis clinic is an education clinic where patients learn about ESRD and the process of dialysis, and is staffed by a senior nephrology nurse; patients’ progress is tracked after the first visit to this clinic and their dialysis plans are discussed when their eGFR falls below 15 ml/min per 1.73 m2. Patients receive usual nephrology care from their individual nephrologist in addition to attending this education clinic.
The RSC clinic is staffed by a palliative care specialist and a senior renal/palliative care nurse; additional support is provided by a dietician and social worker as required. At this clinic, a formal symptom inventory, the Palliative care Outcome Scale - Symptoms (POS-S) (renal) inventory, is collected at each visit and care is focused on management of symptoms as well as advance care planning, discussions with patients and their families about the likely trajectory of a nondialysis pathway, and nursing support between clinics to assist patients and their families. The RSC clinic caters to a largely elderly population; on occasion, visits are conducted at the patient’s home with further support by phone calls between visits. This clinic is provided separately from and in addition to the patient’s usual nephrology care.
The decision to recommend dialysis or a nondialysis pathway was that of the individual nephrologist in conjunction with the patient and his or her family, aiming for a shared decision (13–15).
Measures
Baseline data included age, sex, comorbidities, diabetes, smoking status, dementia, height, weight and body mass index, primary renal diagnosis, eGFR calculated by the Modification of Diet in Renal Disease formula, creatinine, albumin, hemoglobin, calcium, phosphate, nutritional status using the subjective global assessment scale (16), and planned mode of dialysis. Comorbidities included ischemic heart disease (IHD) or cardiac failure (CHF), cerebrovascular or peripheral vascular disease, chronic liver or lung disease, diabetes, and dementia; these data were obtained from the patients’ medical records and from discussions with their nephrologist. Cause of death was established from medical records and/or discussion with the patient’s physician.
Symptoms were assessed in all patients using the validated Memorial Symptom Assessment Scale (MSAS-SF) survey (17), as well as by the POS-S (renal) form only for those in the RSC-NFD group. QOL was assessed in all patients using Short Form-36 (SF-36) (18). Patients in the “other dialysis” group had no assessment of symptom burden or QOL because they did not attend either clinic predialysis.
eGFR was repeated every 6 months, along with repeat assessment of symptom burden by the MSAS-SF and QOL by the SF-36 survey tool.
Statistical Analyses
The primary outcomes of interest were as follows: (1) survival (time to death and the percentage 12-month survival), and potential confounders and effect modifiers were examined including age ≥75 years, survival from the time eGFR fell below 15 ml/min per 1.73 m2, and survival according to comorbidities; (2) maintenance or improvement of symptoms over 6 and 12 months; and (3) maintenance or improvement of QOL over 6 and 12 months.
Data were collected on patients only after they had decided which clinical pathway they would pursue.
Continuous variables were compared using t tests or Mann–Whitney tests as appropriate. Data are presented as the mean (SD) or median (interquartile range [IQR]); median values could not be computed for survival in the predialysis group. Categorical variables were compared using chi-squared testing. Survival was estimated by the Cox proportional hazards model adjusted for age, sex, diabetes, and IHD. Baseline factors potentially influencing survival were tested using logistic regression in univariable analysis and those significant by step-wise multivariable analysis (19). We included comorbidities as variables to test for influence on survival because both Murtagh et al. (20) and the Renal Physicians Association (RPA) recommendations (13) listed “high comorbidity score,” particularly IHD, as a factor portending poor survival. Change in symptoms or QOL over time were categorized as stable, improved, or worse and were compared by chi-squared testing. The level of significance was 0.05 and analyses were undertaken using SAS statistical software (version 9.2).
Results
We studied 467 patients, 37% of which were women, including 122 on a nondialysis pathway (RSC-NFD) and 273 who attended the predialysis clinic, 92 (34%) of whom underwent dialysis (n=55 on hemodialysis and n=37 on peritoneal dialysis; mean age 63 years) after an average of 9 months (Figure 1). A further 72 patients (mean age 69 years) who had not attended the predialysis clinic (“other dialysis” group) also commenced dialysis in this period (n=45 on hemodialysis and n=27 on peritoneal dialysis). Thirty-three patients (11%) who initially attended the predialysis clinic with the intent of having dialysis changed their mind after dialysis education and were managed in the RSC clinic. Data were not collected on any patient until the individual had decided upon his or her clinical pathway. Only two (1.6%) patients in the RSC-NFD group were converted to a dialysis pathway.
Figure 1.
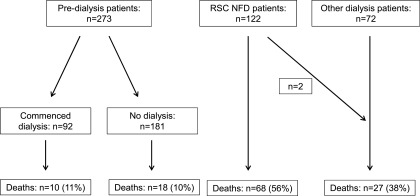
Pathways of the 467 patients, showing the course of those who commenced in the predialysis clinic (n=273), those in the RSC-NFD group clinic (n=122), and the “other dialysis” group (n=72). RSC-NFD, renal supportive care not planned for dialysis.
Comparison of Baseline Data
RSC-NFD patients were on average 15 years older than the predialysis group (82 versus 67 years; P<0.001) but had similar initial eGFR (16 ml/min per 1.73 m2; P=0.92) (Table 1). They also had more dementia and two or more comorbidities (57% versus 40%; P=0.001). Only 34% were well nourished, compared with 63% of the predialysis group (P=0.01). There were no differences in the proportion with diabetes or corrected calcium, phosphate, parathyroid hormone, or serum albumin; however, patients in the RSC-NFD group had lower hemoglobin and more vascular disease. Mean eGFR was 9 (4) ml/min per 1.73 m2 in patients commencing either hemodialysis or peritoneal dialysis.
Table 1.
Baseline characteristics at the first clinic visit in patients planned for dialysis (predialysis) and patients in the RSC-NFD group
| Variable | RSC-NFD (n=122) | Predialysis (n=273) | P Value | ||
|---|---|---|---|---|---|
| Mean (SD) or Proportion | n | Mean (SD) or Proportion | n | ||
| Age (yr) | 82 (9) | 122 | 67 (14) | 273 | <0.001 |
| eGFR (ml/min per 1.73 m2) | 16 (9) | 122 | 16 (7) | 273 | 0.92 |
| Weight (lb) | 160 (34) | 112 | 182 (44) | 256 | <0.001 |
| Height (cm) | 162 (10) | 108 | 167 (9) | 249 | <0.001 |
| BMI | 27.7 (5.5) | 108 | 29.1 (6.2) | 249 | 0.06 |
| Creatinine (mg/dl) | 3.9 (1.7) | 122 | 3.7 (1.5) | 273 | 0.37 |
| Hemoglobin (g/dl) | 10 (1.6) | 122 | 11.3 (1.8) | 270 | 0.04 |
| Albumin (g/dl) | 3.4 (0.6) | 119 | 3.5 (0.7) | 253 | 0.10 |
| Corrected Ca (mg/dl) | 9.2 (0.07) | 117 | 9.2 (0.07) | 267 | 0.62 |
| PO4 (mg/dl) | 4.7 (1.2) | 118 | 4.7 (1.2) | 266 | 0.95 |
| PTH (pg/ml) | 200 (145) | 62 | 218 (218) | 150 | 0.47 |
| Women | 45 | 55 | 33 | 90 | 0.2 |
| CKD group (stage) | 0.32 | ||||
| 4 | 57 | 69 | 57 | 155 | |
| 5 | 43 | 53 | 43 | 118 | |
| Diabetes | 53 | 64 | 52 | 141 | 0.88 |
| Clinical dementia | 11.5 | 14 | 0.4 | 1 | <0.001 |
| Comorbidities (n) | |||||
| ≥1 | 89 | 109 | 70 | 190 | <0.001 |
| ≥2 | 57 | 70 | 40 | 108 | 0.001 |
| ≥3 | 38 | 46 | 18 | 48 | <0.001 |
| Current or former smoker | 25 | 34 | 41 | 112 | 0.03 |
| Nutritional status | |||||
| A | 34 | 11 | 63 | 109 | 0.01 |
| B | 63 | 20 | 36 | 61 | |
| C | 3 | 1 | 1 | 2 | |
| Race | 0.01 | ||||
| Caucasian | 90 | 109 | 75 | 205 | |
| Asian | 4 | 5 | 14 | 37 | |
| ATSI | 0 | 0 | 0.4 | 1 | |
| Pacific | 3 | 4 | 4 | 10 | |
| Arabic | 3 | 4 | 7 | 20 | |
| Primary renal diagnoses | <0.001 | ||||
| Renal vascular disease | 57 | 70 | 27 | 74 | |
| Diabetes mellitus | 29 | 30 | 33 | 91 | |
| Polycystic kidneys | 1 | 1 | 6 | 17 | |
| GN | 4 | 5 | 20 | 55 | |
| Cause unknown | 0 | 0 | 1 | 2 | |
| Other | 8 | 10 | 13 | 34 | |
BMI, body mass index; PTH, parathyroid hormone; ATSI, Aboriginal and Torres Straight Islander; RSC-NFD, renal supportive care clinic not planned for dialysis.
Survival
Median follow-up was 16 months (IQR, 7–26) in the predialysis group (n=273) and 10 months (IQR, 4–21) in the RSC-NFD group. Within the predialysis group, death rates were similar among those who progressed to dialysis (11%) and those who did not (10%). Death rates were higher in the RSC-NFD group than in predialysis patients (56% versus 11%; P<0.001) or the “other dialysis” group (56% versus 38%; P<0.001).
Mean adjusted patient survival was 33 months (95% confidence interval [95% CI], 32 to 34) in the predialysis group (n=273), 34% of whom started dialysis, and 20 months (95% CI, 17 to 23) in the RSC-NFD group (n=122) (P<0.001) (Figures 2 and 3). Median survival was 16 months (IQR, 7, 39) in the RSC-NFD group.
Figure 2.
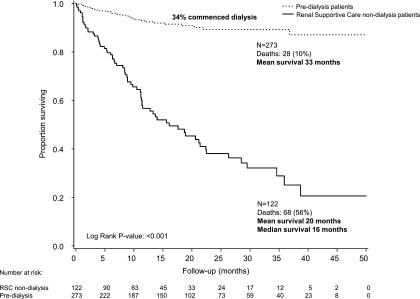
Survival in patients in the predialysis (n=273) or RSC-NFD (n=122) groups. Time zero is from first attendance at the predialysis or renal supportive care clinic after a decision had been made to pursue dialysis or not.
Figure 3.
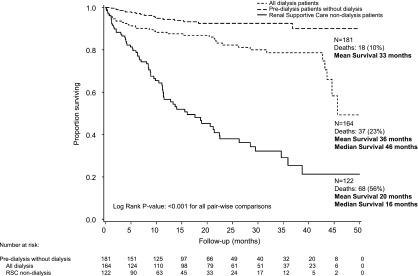
Survival in patients who remained in the predialysis clinic without receiving dialysis (n=181) compared with all of those receiving dialysis in this period (n=164) and patients in the RSC-NFD group (n=122). Time zero is from first attendance at the predialysis or renal supportive care clinic after a decision had been made to pursue dialysis or not. For the “all dialysis” group, this time point includes the time at first dialysis for the 72 patients who had not attended the predialysis clinic.
Survival was greater within the predialysis group than the RSC-NFD group both for those who required dialysis (hazard ratio [HR] for death, 0.30; 95% CI, 0.13 to 0.67; P=0.003; n=92) and for those who did not require dialysis (HR for death, 0.23; 95% CI, 0.12 to 0.41; P<0.001; n=181). Survival for dialysis patients who had not attended the predialysis clinic was not statistically different from that of the RSC-NFD group (HR for death, 0.60; 95% CI, 0.35 to 1.03; P=0.06; n=72). Cause of death was primarily renal failure in patients in the RSC-NFD group and cardiac failure in the predialysis group (Table 2). The five “renal” deaths in the planned dialysis group were in patients who decided at the end of their illness not to proceed with dialysis despite that having been their intended pathway until then. For patients aged >75 years, mean survival was 19 months (95% CI, 16 to 22) for the RSC-NFD group (n=105), 31 months (95% CI, 28 to 34) for the predialysis group (n=92), and 34 months (95% CI, 29 to 39) for all dialysis patients (n=55) (P<0.001).
Table 2.
Cause of death in patients in the RSC-NFD and predialysis groups
| Cause of Death | RSC–NFD (n=68) | Predialysis (n=28) |
|---|---|---|
| Renal | 42 (62) | 5 (18) |
| Cardiac | 9 (13) | 13 (46) |
| Malignancy | 8 (12) | 4 (14) |
| Sepsis | 2 (3) | 2 (7) |
| Other | 7 (10) | 4 (14) |
Data are presented as n (%). The distribution of causes of death differs between the two groups (p=0.001).
One-year survival was 93% in the predialysis group, 53% in the RSC-NFD group, and 81% in the “other dialysis” group. Within the RSC-NFD group, 13 of 41 patients (32%) with an eGFR<10 ml/min per 1.73 m2 survived longer than 12 months.
Over the time of this study, 11 (7%) dialysis patients withdrew from dialysis (all from the “other dialysis” group), which constituted 30% of deaths in dialysis patients.
Mean survival from eGFR<15 ml/min per 1.73 m2 was 13 months (95% CI, 9 to 16) in the RSC-NFD patients (n=53) and 20 months (95% CI, 19 to 21) in the predialysis group (n=118; P<0.001). For patients who reached an eGFR<15 ml/min per 1.73 m2 and were aged >75 years, adjusted risk of death remained higher in the RSC-NFD group (n=63) than in the predialysis group (n=45) (HR, 4.4; 95% CI, 2.21 to 8.88; P<0.001). Similarly, those in the RSC-NFD group with eGFR<15 ml/min per 1.73 m2 with two or more comorbidities (n=37) had higher risk of death than patients in the predialysis group (n=58) (HR, 6.1; 95% CI, 2.9 to 12.5; P<0.001). However, there was no statistically significant survival difference between all dialysis patients (n=18) and the RSC-NFD group (n=52) when analysis was restricted to patients aged >75 years who had two or more comorbidities, at least one of which was CHF or IHD (HR for death, 0.48; 95% CI, 0.21 to 1.09; P=0.08).
Factors Associated with Survival
In univariable analysis of all patients, factors significantly related to survival were age, weight, body mass index, hemoglobin, serum albumin, corrected calcium, serum creatinine, having any comorbidity, and being in the RSC-NFD group. In multivariable analysis, there was a small influence of age and lower serum albumin, a larger influence of eGFR<15 ml/min per 1.73 m2, and the main factor associated with lower survival was choosing to follow a RSC-NFD pathway (odds ratio, 6.4; 95% CI, 3.0 to 13.5; P<0.001).
The presence of two or more comorbidities at baseline was associated with a higher death rate in the combined dialysis group (HR, 2.30; 95% CI, 1.17 to 4.54; P=0.02) but not in the RSC-NFD group (HR, 1.16; 95% CI, 0.71 to 1.88; P=0.56). Within RSC-NFD patients, those with CHF (n=44) had a higher death risk than those without (HR, 1.7; 95% CI, 1.04 to 2.8; P=0.03) but IHD did not increase risk (P=0.11).On the other hand, IHD conveyed a worse outcome in all dialysis patients (HR, 4.9; 95% CI, 1.3 to 4.9; P=0.01).
Symptom Control
The response rate for the MSAS-S survey form was 49% in the predialysis group and 55% in the RSC-NFD patients, with no statistically significant differences in baseline demographics between those who returned surveys and those who did not (Supplemental Table 1). The number of symptoms and the symptom score were greater at the initial visit in the RSC-NFD patients although patients in the predialysis group had a substantial number of symptoms (Table 3).
Table 3.
Symptoms and QOL at the initial clinic visit and change in symptoms and QOL over time in the RSC-NFD and predialysis groups
| Predialysis | RSC-NFD | P Value | |
|---|---|---|---|
| Symptoms | 133 | 65 | |
| No. of symptoms at first visit (MSAS), mean (SD) | 9.1 (5.3) | 12.2 (5.6) | <0.001 |
| Score >20 at first visit (MSAS) | 44 (33%) | 37 (57%) | 0.001 |
| QOL | |||
| Physical composite (SF-36) | 137 | 63 | |
| Score at first visit; mean (SD) | 38 (11) | 29 (8) | <0.001 |
| Mental composite (SF-36) | |||
| Score at first visit; mean (SD) | 50 (10) | 46 (12) | 0.06 |
| QOL status | 49 | 19 | |
| Change of physical composite score over 12 mo | 0.12 | ||
| Stable | 2 (4%) | 3 (16%) | |
| Improved | 20 (41%) | 4 (21%) | |
| Worse | 27 (55%) | 12 (63%) | |
| Change of mental composite score over 12 mo | 0.78 | ||
| Stable | 1 (2%) | 1 (5%) | |
| Improved | 26 (53.1%) | 10 (53%) | |
| Worse | 22 (44.9%) | 8 (42%) | |
| MSAS symptom status | |||
| Change of MSAS symptoms score from initial visit to 6 mo | 84 | 45 | |
| Stable | 6 (7%) | 3 (8%) | 0.88 |
| Improved | 32 (38%) | 16 (42%) | |
| Worse | 46 (55%) | 19 (50%) | |
| Change of MSAS symptoms score from initial visit to 12 mo | 48 | 21 | 0.12 |
| Stable | 5 (10%) | 1 (5%) | |
| Improved | 15 (31%) | 12 (57%) | |
| Worse | 28 (58%) | 8 (38%) | |
| POS-S symptom status | 78 | ||
| Change of POS-S (renal) score over 6 mo | |||
| Stable | 3 (4%) | ||
| Improved | 48 (62%) | ||
| Worse | 27 (35%) | ||
| Change of POS-S (renal) score over 12 mo | 69 | ||
| Stable | 3 (4%) | ||
| Improved | 49 (71%) | ||
| Worse | 17 (25%) |
Data are n unless otherwise stated. The overall initial survey response rate was 51% for both the MSAS and SF-36 forms. Missing data from follow-up visits were mostly due to deaths or else failure to return the voluntary MSAS or SF-36 forms. Data for symptom assessment using the POS-S form in the RSC-NFD group were more complete as this was conducted at the time of their clinic visit. QOL, quality of life; MSAS, Memorial Symptom Assessment Scale; SF-36, Short Form-36.
MSAS assessments were available for comparison with baseline after 6 months in 84 patients (31%) in the predialysis group and 45 patients (33%) of the RSC-NFD group; corresponding data at 12 months were 48 patients (18%) and 23 patients (17%), respectively. Symptoms were stable or improved in 41%–56% of patients over time and there was no difference in the proportion achieving stability or improvement between groups (Table 3).
Data concerning symptom control were available in 98 of 122 patients within the RSC-NFD group as assessed by the POS-S (renal) assessment. There was no significant difference in the initial symptom score between those who died in the following 6 months [mean score 20 (10); n=14] and those who did not [mean score 17 (10); n=84; P=0.24]. Over two thirds of patients in the RSC-NFD group achieved improvement in their symptom burden by 6 and 12 months (Table 3).
QOL
The response rate for the SF-36 form was 51% in the predialysis group and 56% in the RSC-NFD patients, with no statistically significant differences in baseline demographics between those who returned surveys and those who did not. Physical but not mental health QOL was worse in those in the RSC-NFD group than in the predialysis group at the initial visit (Table 3).
Physical QOL was maintained or improved after 12 months in under half of each group, whereas mental health QOL was maintained or improved in just over half of each group (Table 3). There was no correlation between stable/improved or worsening symptoms or QOL and stable or worsening eGFR.
Discussion
In this prospective observational study, we found that elderly patients with advanced CKD managed via a nondialysis pathway that includes renal supportive care as well as usual nephrology care survived a median of 16 months with a 53% 1-year survival from the time of referral to a RSC clinic with mean eGFR of 16 ml/min per 1.73 m2. During this time, the majority of patients had improvement in their symptoms by utilizing the skills of a palliative care team. These patients had a lower survival than (younger) patients attending the predialysis clinic but there was no significant difference in their adjusted survival compared with dialysis patients who had not attended the predialysis clinic. Considerations in recommending dialysis to elderly patients have been addressed by the RPA (13) and others (1,5,21–26). Because our nephrologists broadly use these considerations, it is not surprising that patients managed in the RSC clinic were not only older but also had more comorbidities and poorer nutrition. A major factor related to survival was being referred to the RSC clinic; presumably, this finding relates to the broader and less tangible considerations of nephrologists, these patients, and their families in choosing to be managed without dialysis.
Survival
The survival of a median 16 months and 53% 12 month survival in our RSC-NFD group is within the range reported by O’Connor et al. (27) and others (20,28–33). Hussain and colleagues (34) retrospectively analyzed outcomes of 172 patients aged >70 years managed via a nondialysis pathway and 269 who were planned for dialysis. They found similar survival as we did in those managed without dialysis and noted that although overall survival was greater in those planned for or receiving dialysis, this advantage was lost for patients aged >80 years if they had poor functional status or high comorbidity. Murtagh et al. (20) and Chandna et al. (29) previously showed in retrospective analyses that survival was a median of 18–21 months in conservatively managed patients and that significant comorbidity offset the survival advantage of dialysis. However, we still observed a survival advantage in patients selecting dialysis provided that they had been through our predialysis program. The survival advantage for all dialysis patients seems to be lost for patients aged >75 years with two or more comorbidities if at least one of these was heart disease, similar to the findings of Murtagh et al. (20), although the number of dialysis patients in this category was small.
To the best of our knowledge, this is the largest study with prospectively collected data and defined outcomes in patients managed without dialysis; our mean survival of 20 months is similar to that of Wong et al. (33), who studied 79 patients from the time a decision was made not to pursue a dialysis pathway. Not surprisingly, this survival is less than that of the general Australian population who at age 82 years can be expected to live a further 7.5 years for men and 8.9 years for women (Australian Bureau of Statistics, 2011 3302.1.55.001, Life Tables, 2008–2010). An important observation in our study was that about one third of our elderly patients managed without dialysis survived past 12 months from an eGFR below 10 ml/min per 1.73 m2, a time when most would be considered in need of dialysis (35).
A further finding of interest is that patients who commenced dialysis without attending the predialysis clinic had a higher death rate than those who had attended the clinic and this subgroup accounted for all dialysis patients who withdrew at a later stage, suggesting the importance of predialysis education and support.
Symptom Control and QOL
A few studies have addressed symptoms in elderly dialysis or nondialysis patients with CKD and have shown a high symptom burden in all cases, often analogous to the burden carried by patients with cancer (8,36). O’Connor and Kumar (27) found only six studies of symptom burden or QOL in patients managed without dialysis, with a mean 7–17 symptoms, similar to our mean of 12. We are not aware of previous prospective studies addressing the capacity to maintain or improve this symptom burden in patients managed without dialysis. We found that although patients who chose a nondialysis pathway had a worse symptom burden than others at their initial clinic visit, it was possible (utilizing the skills of palliative care) to maintain and even improve symptom control in the majority of patients. This is a key finding because many elderly patients and their families wish to consider not only survival but also whether they will have their symptoms controlled as part of their decision to pursue a dialysis or nondialysis pathway.
In one study of 30 elderly patients treated without dialysis and 124 planned or starting dialysis, QOL was similar between the groups but satisfaction with life fell after dialysis initiation (37). This study was similar to ours in that survival and QOL were assessed prospectively. Patients in that study were managed by a multidisciplinary team, although a palliative care physician was not included and symptom burden was not formally assessed. QOL was examined more extensively than in our study; it was clear that QOL was not improved by dialysis but that median survival was longer. The important finding in both studies is that a RSC program offers the capacity to aid symptom control or QOL.
The limitations of this study include the following: it is not a randomized controlled trial, symptom and QOL survey rates were roughly 50%–55% at baseline, and there was an expected bias toward poorer survival in the nondialysis pathway patients. Furthermore, the relatively short follow-up (for a survival study) also makes it difficult to compare groups because the predialysis group was “selected” according to likely good short-term survival and the RSC-NFD group was selected according to likely poorer survival. A further issue is that we used single eGFR at the various time points; it is therefore possible that these may not have accounted for variability in eGFR, but this is generally what happens in real-world clinical care. The survey return rates were low but similar to other studies (38) and there were no major differences in patient characteristics between those who did and did not return surveys. Because we did not reassess symptom burden or QOL in predialysis clinic patients after they had commenced dialysis, we are likely to have biased these results in favor of the predialysis cohort. The drop-off in survey form returns over time also limits interpretation to some extent, although this was inevitable in the RSC group with high mortality rates. However, the symptom burden was similar at the initial clinic visit in those who died within 6 months and those who survived past this time, and the improvement in symptom control within the RSC-NFD group is not likely to be a function of selection of less symptomatic patients who survived longer.
The strengths of the study are the prospectively defined outcomes, its size (including about 20% of all RSC-NFD patients in the literature to date), the comparator groups being not just dialysis patients but also those planned for dialysis, and the prospective assessment of the capacity to control symptoms and QOL.
The attitude toward accepting patients for dialysis has come a long way since 1984, when almost half of United Kingdom nephrologists would not have dialyzed a 50-year-old man with IHD (39). Today, the decision regarding whether to recommend dialysis to an elderly patient remains a difficult one.
Nevertheless, some messages are becoming clearer. Elderly patients with CKD with significant comorbidities not receiving dialysis can anticipate a median survival of 16 months, or just over 50% likelihood of surviving 12 months, with one third surviving >1 year from a stage when they would normally have started dialysis. Importantly, we have shown that nondialysis care does not mean imminent death and these patients’ symptoms can be managed and QOL maintained with the added expertise of palliative care.
It is recognized that a wide range of factors are taken into account by elderly patients when considering not to start dialysis, including their concerns about suffering (40). Having a formal system of engaging these patients with both usual nephrology and palliative care services from early in the course of their CKD allows for stabilization or improvement of symptoms in the majority. It also allows for some patients (about 11% in this study) who were originally planning dialysis to change to a well supported nondialysis pathway and for <2% to change back to dialysis. It is hoped that our findings will be of use in helping clinicians, patients, and their families reach a shared decision about the appropriateness of dialysis for elderly patients.
Disclosures
M.A.B. received a grant from Amgen Australia, which was used to support employment of G.K.C. for the conduct of this research and to pay for independent statistical advice.
Supplementary Material
Acknowledgments
Statistical analysis was provided by statisticians at the George Institute, Sydney, Australia.
This research was supported by a grant from Amgen Australia. The company had no role in the study design; in the collection, analysis, or interpretation of data; in the writing of the report; or in the decision to submit the article for publication. Researchers were independent from the funding body.
The authors are willing to share all data upon individual request, consequent upon local ethics committee approval to do so.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.03330414/-/DCSupplemental.
See related editorial, “Four Plus Forty-Four: Hours to Modify, Theirs to Enjoy,” on pages 169–171.
References
- 1.Swidler MA: Geriatric renal palliative care. J Gerontol A Biol Sci Med Sci 67: 1400–1409, 2012 [DOI] [PubMed] [Google Scholar]
- 2.Australian and New Zealand Dialysis and Transplant Registry : ANZDATA 35th Annual Report, Adelaide, South Australia, ANZDATA, 2012 [Google Scholar]
- 3.US Renal Data System: Incidence, prevelance, patient characteristics, and treatment modalities, 2013. Available at: http://www.usrds.org/2013/pdf/v2_ch1_13.pdf. Accessed October 22, 2014
- 4.UK Renal Registry: UK RRT Incidence in 2011: National and centre-specific analyses, 2011. Available at: http://www.renalreg.org/wp-content/uploads/2014/09/Chapter_1.pdf. Accessed October 22, 2014
- 5.Brown MA, Crail SM, Masterson R, Foote C, Robins J, Katz I, Josland E, Brennan F, Stallworthy EJ, Brennan F, Siva B, Crail S, Brennan F, Siva B, Brennan F, Brown M, Miller C, Urban AK, Sajiv C, Stallworthy EJ, Glavish RN, May S, Langham R, Walker R, Fassett RG, Morton RL, Crail SM, Stewart C, Brennan F, Phipps L, Walker R, Healy H, Berquier I, Crail SM, Australian and New Zealand Society of Nephrology : ANZSN renal supportive care 2013: Opinion pieces [corrected]. Nephrology (Carlton) 18: 401–454, 2013 [DOI] [PubMed] [Google Scholar]
- 6.Hutchison AJ, Smith CP, Brenchley PEC: Pharmacology, efficacy and safety of oral phosphate binders. Nat Rev Nephrol 7: 578–589, 2011 [DOI] [PubMed] [Google Scholar]
- 7.Foote C, Ninomiya T, Gallagher M, Perkovic V, Cass A, McDonald SP, Jardine M: Survival of elderly dialysis patients is predicted by both patient and practice characteristics. Nephrol Dial Transplant 27: 3581–3587, 2012 [DOI] [PubMed] [Google Scholar]
- 8.Murtagh FEM, Addington-Hall JM, Edmonds PM, Donohoe P, Carey I, Jenkins K, Higginson IJ: Symptoms in advanced renal disease: A cross-sectional survey of symptom prevalence in stage 5 chronic kidney disease managed without dialysis. J Palliat Med 10: 1266–1276, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Kurella Tamura M, Covinsky KE, Chertow GM, Yaffe K, Landefeld CS, McCulloch CE: Functional status of elderly adults before and after initiation of dialysis. N Engl J Med 361: 1539–1547, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rosansky SJ: The sad truth about early initiation of dialysis in elderly patients. JAMA 307: 1919–1920, 2012 [DOI] [PubMed] [Google Scholar]
- 11.Schell JO, Patel UD, Steinhauser KE, Ammarell N, Tulsky JA: Discussions of the kidney disease trajectory by elderly patients and nephrologists: A qualitative study. Am J Kidney Dis 59: 495–503, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP, STROBE Initiative : Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. BMJ 335: 806–808, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Renal Physicians Association : Shared Decision Making in the Appropriate Initiation of and Withdrawal from Dialysis, 2nd Ed., Rockville, MD, Renal Physicians Association, 2010 [Google Scholar]
- 14.Moss AH: Revised dialysis clinical practice guideline promotes more informed decision-making. Clin J Am Soc Nephrol 5: 2380–2383, 2010 [DOI] [PubMed] [Google Scholar]
- 15.Moss AH, Ganjoo J, Sharma S, Gansor J, Senft S, Weaner B, Dalton C, MacKay K, Pellegrino B, Anantharaman P, Schmidt R: Utility of the “surprise” question to identify dialysis patients with high mortality. Clin J Am Soc Nephrol 3: 1379–1384, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Visser R, Dekker FW, Boeschoten EW, Stevens P, Krediet RT: Reliability of the 7-point subjective global assessment scale in assessing nutritional status of dialysis patients. Adv Perit Dial 15: 222–225, 1999 [PubMed] [Google Scholar]
- 17.Chang VT, Hwang SS, Feuerman M, Kasimis BS, Thaler HT: The memorial symptom assessment scale short form (MSAS-SF). Cancer 89: 1162–1171, 2000 [DOI] [PubMed] [Google Scholar]
- 18.McHorney CA, Ware JE, Jr, Raczek AE, Psychometric and Clinical Tests of Validity in Measuring Physical and Mental Health Constructs : The MOS 36-Item Short-Form Health Survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Med Care 31: 247–263, 1993 [DOI] [PubMed] [Google Scholar]
- 19.Hosmer DW, Lemeshow S: Applied Logistic Regression, 2nd Ed., Hoboken, NJ, John Wiley & Sons Inc, 2000 [Google Scholar]
- 20.Murtagh FEM, Marsh JE, Donohoe P, Ekbal NJ, Sheerin NS, Harris FE: Dialysis or not? A comparative survival study of patients over 75 years with chronic kidney disease stage 5. Nephrol Dial Transplant 22: 1955–1962, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Dasgupta I, Rayner HC: In good conscience—Safely withholding dialysis in the elderly. Semin Dial 22: 476–479, 2009 [DOI] [PubMed] [Google Scholar]
- 22.Swidler M: Considerations in starting a patient with advanced frailty on dialysis: Complex biology meets challenging ethics. Clin J Am Soc Nephrol 8: 1421–1428, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Crail S, Walker R, Brown M, Renal Supportive Care Working Group : Renal supportive and palliative care: Position statement. Nephrology (Carlton) 18: 393–400, 2013 [DOI] [PubMed] [Google Scholar]
- 24.Brennan F, Brown M: An ethical approach to dialysis—An alliance of nephrology, palliative medicine and ethics. QJM 106: 397–400, 2013 [DOI] [PubMed] [Google Scholar]
- 25.Williams ME: Tough choices: Dialysis, palliative care, or a third option for elderly ESRD. Semin Dial 25: 633–639, 2012 [DOI] [PubMed] [Google Scholar]
- 26.Brown EA: Non-dialysis therapy: A better policy than dialysis followed by withdrawal? Semin Dial 25: 26–27, 2012 [DOI] [PubMed] [Google Scholar]
- 27.O’Connor NR, Kumar P: Conservative management of end-stage renal disease without dialysis: A systematic review. J Palliat Med 15: 228–235, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carson RC, Juszczak M, Davenport A, Burns A: Is maximum conservative management an equivalent treatment option to dialysis for elderly patients with significant comorbid disease? Clin J Am Soc Nephrol 4: 1611–1619, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chandna SM, Da Silva-Gane M, Marshall C, Warwicker P, Greenwood RN, Farrington K: Survival of elderly patients with stage 5 CKD: comparison of conservative management and renal replacement therapy. Nephrol Dial Transplant 26: 1608–1614, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ellam T, El-Kossi M, Prasanth KC, El-Nahas M, Khwaja A: Conservatively managed patients with stage 5 chronic kidney disease—Outcomes from a single center experience. QJM 102: 547–554, 2009 [DOI] [PubMed] [Google Scholar]
- 31.Joly D, Anglicheau D, Alberti C, Nguyen AT, Touam M, Grünfeld JP, Jungers P: Octogenarians reaching end-stage renal disease: Cohort study of decision-making and clinical outcomes. J Am Soc Nephrol 14: 1012–1021, 2003 [DOI] [PubMed] [Google Scholar]
- 32.Smith C, Da Silva-Gane M, Chandna S, Warwicker P, Greenwood R, Farrington K: Choosing not to dialyse: Evaluation of planned non-dialytic management in a cohort of patients with end-stage renal failure. Nephron Clin Pract 95: c40–c46, 2003 [DOI] [PubMed] [Google Scholar]
- 33.Wong CF, McCarthy M, Howse MLP, Williams PS: Factors affecting survival in advanced chronic kidney disease patients who choose not to receive dialysis. Ren Fail 29: 653–659, 2007 [DOI] [PubMed] [Google Scholar]
- 34.Hussain JA, Mooney A, Russon L: Comparison of survival analysis and palliative care involvement in patients aged over 70 years choosing conservative management or renal replacement therapy in advanced chronic kidney disease. Palliat Med 27: 829–839, 2013 [DOI] [PubMed] [Google Scholar]
- 35.Kidney Disease Improving Global Outcomes : 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Chapter 5: Referral to specialists and models of care. Kidney Int Suppl 3: 112–119, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kane PM, Vinen K, Murtagh FE: Palliative care for advanced renal disease: A summary of the evidence and future direction. Palliat Med 27: 817–821, 2013 [DOI] [PubMed] [Google Scholar]
- 37.Da Silva-Gane M, Wellsted D, Greenshields H, Norton S, Chandna SM, Farrington K: Quality of life and survival in patients with advanced kidney failure managed conservatively or by dialysis. Clin J Am Soc Nephrol 7: 2002–2009, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Klemenc-Ketis Z, Smogavec M, Softic N, Kersnik J: Health-related quality of life: A population based study from Slovenia. Cent Eur J Public Health 19: 7–12, 2011 [DOI] [PubMed] [Google Scholar]
- 39.Challah S, Wing AJ, Bauer R, Morris RW, Schroeder SA: Negative selection of patients for dialysis and transplantation in the United Kingdom. Br Med J (Clin Res Ed) 288: 1119–1122, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Visser A, Dijkstra GJ, Kuiper D, de Jong PE, Franssen CF, Gansevoort RT, Izaks GJ, Jager KJ, Reijneveld SA: Accepting or declining dialysis: considerations taken into account by elderly patients with end-stage renal disease. J Nephrol 22: 794–799, 2009 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


