Abstract
Background and objectives
Behavioral stage of change (SoC) algorithms classify patients’ readiness for medical treatment decision-making. In the precontemplation stage, patients have no intention to take action within 6 months. In the contemplation stage, action is intended within 6 months. In the preparation stage, patients intend to take action within 30 days. In the action stage, the change has been made. This study examines the influence of SoC on dialysis modality decision-making.
Design, setting, participants, & measurements
SoC and relevant covariates were measured, and associations with dialysis decision-making were determined. In-depth interviews were conducted with 16 patients on dialysis to elicit experiences. Qualitative interview data informed the survey design. Surveys were administered to adults with CKD (eGFR≤25 ml/min/1.73 m2) from August, 2012 to June, 2013. Multivariable logistic regression modeled dialysis decision-making with predictors: SoC, provider connection, and dialysis knowledge score.
Results
Fifty-five patients completed the survey (71% women, 39% white, and 59% black), and median annual income was $17,500. In total, 65% of patients were in the precontemplation/contemplation (thinking) and 35% of patients were in the preparation/maintenance (acting) SoC; 62% of patients had made dialysis modality decisions. Doctors explaining modality options, higher dialysis knowledge scores, and fewer lifestyle barriers were associated with acting versus thinking SoC (all P<0.02). Patients making modality decisions had doctors who explained dialysis options (76% versus 43%), were in the acting versus the thinking SoC (50% versus 10%), had higher dialysis knowledge scores (1.4 versus 0.5), and had lower eGFR (13.9 versus 16.8 ml/min/1.73 m2; all P<0.05). In adjusted analyses, dialysis knowledge was significantly associated with decision-making (odds ratio, 4.2; 95% confidence interval, 1.4 to 12.9; P=0.01), and SoC was of borderline significance (odds ratio, 5.8; 95% confidence interval, 1.0 to 32.6; P=0.05). The model C statistic was 0.87.
Conclusions
Dialysis decision-making was associated with SoC, dialysis knowledge, and physicians discussing treatment options. Future studies determining ways to assist patients with CKD in making satisfying modality decisions are warranted.
Keywords: chronic renal insufficiency, clinical nephrology, dialysis, ESRD
Introduction
Home dialysis therapies (peritoneal dialysis [PD] and home hemodialysis [HD]) have equivalent outcomes (1–6) and are more cost-effective than in-center HD (IHD) (7–9). Although kidney transplantation is ideal for patients with ESRD, many patients must make dialysis modality decisions because of low organ availability. Time and provider support are necessary for patients to process their dialysis options. In one study, about one half of patients were undecided about modality choice, despite receiving predialysis nephrology care education (10). This suggests the need to better understand the dialysis decision-making process. Patients frequently start dialysis with a modality that they did not select (11). Timely dialysis modality decisions may prevent unnecessary hospitalizations for urgent dialysis starts.
Patients might hesitate to decide because of fear of depending on dialysis or “magical thinking”—thinking about dialysis may make it happen. Stage of behavior change (SoC) has been shown to affect patients’ ability to make medical treatment-related decisions and take actions on the basis of those decisions (12). In the precontemplation stage, patients have no intention to take action within the next 6 months. In the contemplation stage, there is the intention to take action in the next 6 months. In the preparation stage, there is intent to take action in the next 30 days. In the action stage, the change has been made. Patients progressing one SoC during the first 1 month of treatment can double their chances of action (13). The SoC model has been used in multiple disciplines to aid patients in making difficult decisions about many conditions, including renal transplantation (14–20). The aims of this study were to (1) develop an SoC tool to measure patient readiness for dialysis modality decision-making, (2) determine associations between SoC and dialysis modality decision-making, and (3) determine additional factors associated with both SoC and dialysis decision-making.
Materials and Methods
Study Design
This is a mixed methods study (qualitative and quantitative) to measure SoC for dialysis modality decision-making. In phase 1, the original survey was drafted. In phase 2, in-depth semistructured interviews were conducted with patients who were already on dialysis. The qualitative interviews elicited a range of patient experiences and perspectives. In phase 3, on the basis of these qualitative results and a literature review, the survey was updated by encompassing concepts/measures expected to associate with dialysis decision-making. The survey was pilot tested using five cognitive interviews, and minor modifications were made to improve the clarity and comprehensiveness of questions. The final survey (Supplemental Appendix) was administered to patients with late-stage CKD (defined as at least two eGFR values ≤25 ml/min per 1.73 m2 in 12 months).
In-Depth Interviews
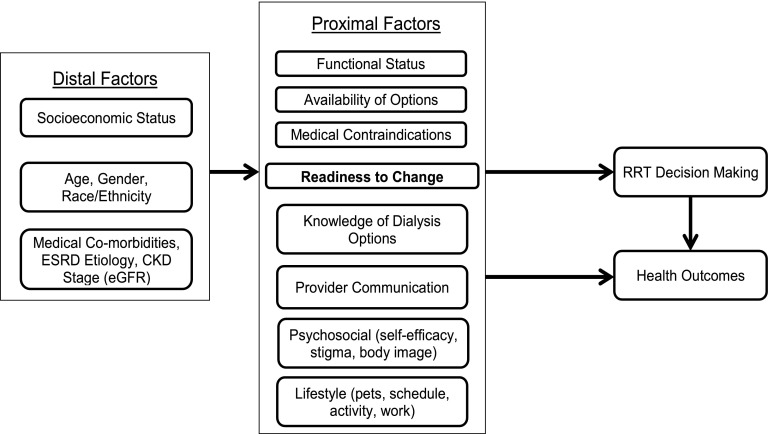
The purpose of the in-depth interviews was to identify themes that were not captured by the investigators in the first survey draft. A semistructured interview guide was created (available on request) to ensure that interviewers carefully elicited patient experiences (the conceptual framework is shown in Figure 1). Patients ages ≥18 years with ESRD who were on any dialysis modality and could complete the interview in English were eligible to participate. Patients were recruited from the MetroHealth Medical Center and the Cleveland Clinic (both in Cleveland, Ohio).
Figure 1.
Conceptual model for dialysis decision-making. The figure shows factors felt to influence patients' dialysis modality decision making.
A sample size of five to 25 participants is recommended for investigating shared experiences (21). We anticipated that 15–20 participants would be sufficient to meet sampling requirements for theoretical saturation (21–23). Interviews were audio recorded, deidentified, and transcribed. A variant of the thematic constant comparative approach was used to identify themes in the interviews (24,25). Iterative rounds of coding and team meetings were used to generate a coding dictionary of patient-reported, dialysis decision-making themes, and these themes were compared with the concepts measured in the draft survey; 25 questions (four categories) were derived from themes uncovered in qualitative analysis and added to the survey. Topics included questions about helpfulness of peer support for decision-making, perceived pros/cons of decision-making, questions that comprised the four factors created (personal involvement in decision, provider connection, concerns about dialysis interference with family, social life, and activity, and ability to urinate), and complementary/alternative therapies.
Survey
The final surveys were administered to patients ages ≥18 years who had stages 4 or 5 CKD (two eGFR readings of ≤25 ml/min per 1.73 m2 within 1 year) and had not yet initiated dialysis. This time period was chosen, because at this level of kidney function, RRT options are reviewed, and kidney transplant programs allow for patient referral. Patients were excluded if they were not candidates for more than one dialysis modality. Factors that may exclude patients from home dialysis included homelessness, using street drugs, unable to sanitize their home, no electricity, nephrologist deemed ineligible, unable to give informed consent, or have dementia, serious memory problems, or untreated mental health diagnosis that interferes with ability to perform activities of daily living (26) Patients were recruited from nephrology and primary care clinics at the MetroHealth Medical Center using the electronic medical records to identify eligible patients. Surveys were administered by a research associate either by phone (34 of 55 participants) or in person during clinic visits (21 of 55 participants). Open-ended survey questions about decisional balance were coded by two authors in a manner similar to the in-depth interviews. For the two coders (S.P. and A.M.), inter-rater reliability (Cohen κ) was estimated. Institutional review board approval was obtained from the MetroHealth Medical Center, and all participants provided written informed consent.
Statistical Analyses
Multiple-item scales were examined using factor and reliability analyses and combined into composite scales. Exploratory factor analysis was conducted using principle axis factoring with an oblique (PROMAX) rotation. Scree plots and eigenvalues were examined to decide on the number of factors. Items with low primary factor loadings (<0.4) or high secondary loadings (>0.3) were eliminated. The questions measuring knowledge of dialysis modalities used a four-point Likert scale: none, a little, some, or a lot; responses were scored from zero to three, respectively, and a composite score for each patient was calculated that was the mean of the knowledge scores for all of the dialysis options. A similar process was used to create a lifestyle barrier to home dialysis score; patients who listed higher numbers of barriers had higher scores. CKD self-efficacy questions were also analyzed in this manner.
The main outcome was whether a patient made a dialysis decision. Other variables examined included age, sex, race, ethnicity, SoC, comorbid conditions, alcohol use (27), hernias, health literacy score (28), dialysis modality knowledge score, CKD knowledge, having someone to help with home dialysis, ability to lift a 2-kg (4.4-lb) bag, average distance that the caregiver lives from the patient, and time that the home dialysis helper could spend with the patient. A series of bivariate analyses examined associations between these variables and dialysis decision-making with SoC to assess readiness for decision-making. Proportions are reported for categorical data, means (SDs) are reported for normally distributed data, and medians (interquartile ranges) are reported for non-normally distributed data. For two-group continuous variable comparisons, two-sample Wilcoxon tests were used, and either chi-squared or Fisher exact tests were used for categorical variables. The Kruskal–Wallis test was used for comparing more than two groups. Multivariate analysis was conducted using logistic regression and limited to three covariates (SoC, dialysis mean composite knowledge score, and provider connection) given the small sample size. Other covariates, including age, sex, diabetes, and concern about travel time to a PD unit, were added in subsequent models. None of these subsequent models were superior to the initial model on the basis of nonsignificant P values.
Results
In-Depth Interviews
Sixteen patients completed in-depth interviews ascertaining what facilitated their dialysis decision-making (Table 1). Topics included knowledge of dialysis, involvement in the decision, talking to patients who had already decided about dialysis modality and those who had a transplant, having the doctor explain the different types of dialysis, and the ability to continue alternative medicine practices while on dialysis. One respondent described the importance of seeing different types of dialysis.
Table 1.
Baseline characteristics of patients included in the survey or the interview
| Patient Characteristics | Survey (n=55) | Interview (n=16) |
|---|---|---|
| Age (yr) | 58.3 (11.2) | 50.2 (8.5) |
| Sex (women) | 70.9% | 37.5% |
| Race | ||
| Caucasian | 38.9% | 37.5% |
| Black | 59.3% | 56.3% |
| Other | 1.9% | — |
| Hispanic/Latino | 3.6% | 6.3% |
| Annual household income (US$)a | 17,500 (12,500) | — |
| Health literacy score (3.0–15.0)a | 15.0 (3.0) | — |
| eGFR (ml/min per 1.73 m2) | 15.0 (4.4) | — |
| Ability to lift a 2-kg bag | 87.0% | — |
| Available person to help with home dialysis | 65.4% | — |
| Diabetes | 47.3% | — |
| Hypertension | 89.1% | — |
| Congestive heart failure | 25.4% | — |
| Coronary artery disease | 16.4% | — |
| Stroke | 16.4% | — |
| Psychiatric diagnosis | 18.2% | — |
| Psychiatric condition on treatment/in therapy | 60.0% | — |
| Hernia | 14.6% | — |
| Duration of care by a nephrologist (yr)a | 2 (5.5) | — |
| Stage of change | ||
| Precontemplation | 9.1% | — |
| Contemplation | 56.4% | — |
| Preparation | 7.3% | — |
| Action | 27.3% | — |
| Dialysis modality decision made | 61.8% | — |
| Modality chosen | ||
| Conventional hemodialysis | 82.4% | — |
| Peritoneal dialysis | 11.8% | — |
| Nocturnal hemodialysis | 5.9% | — |
| Time before survey that modality decision was made (mo)a | 16 (5) | — |
| Patients took educational reading material offered | 74.5% | — |
Baseline demographic and other clinical features of 55 patients included in the quantitative survey and 16 patients in the in-depth interviews are shown. Many more clinical characteristics were collected on the patients who took the survey. Proportions were reported for categorical variables, means (SDs) were reported for normally distributed numerical variables, and medians (interquartile ranges) were reported for non-normally distributed numerical variables. Data not present or collected are indicated by —. Among 16 interview patients, 50% were on in-center hemodialysis, 12.5% were on in-center nocturnal hemodialysis, and 37.5% were on peritoneal dialysis.
Variables for which medians (interquartile ranges) were calculated.
My mom, she would say “Hey, we should probably go and see this dialysis, just to see what’s involved.” At that time, it was either that or the hemo, being in the hospital 3 days out of the week, that’s too much of a drag. Where this has its pluses and its minuses, but it’s got a lot more pluses than it does minuses … I think I chose the right one by far.
In the interviews, patients expressed a range of preferences on being involved in the decision-making process from not wanting to be involved at all to wanting to know all of the details in the decision. Another respondent recommended that people faced with dialysis be more engaged.
Be better educated about your choices. Ask more questions about your choices, the type of dialysis you’re going to do. Just the fact that there’s more than one way of doing it I think would be a great thing to feed to the people and, yeah, everybody should do as much research as they can, whether it be online or with your doctor. I did not get that information when I started, and it would’ve been nice to have known I had other options.
Conversely, another subject discussed the importance of being involved in monitoring kidney disease.
Most of my information came from my doctor. He was really proactive in making sure that, when the time came for me to have to do dialysis, that I had all the information that I needed. He always kept me up-to-date on my numbers and what would happen at this particular stage and what I could do there.
Potential barriers to dialysis decision-making were elicited. One patient felt that the absence of noticeable physical symptoms of kidney failure and fears prevented the consideration of different dialysis options.
I was just very scared, ‘cause I didn’t feel different and Dr. X told me the signs for dialysis and I didn’t understand why because I didn’t throw up. I didn’t feel sicker, so I didn’t understand why, but he/she was just going on my blood work.
On the basis of these responses, we added several questions that asked participants about their interest in being more involved in dialysis decision making. These questions comprised the four factors in the factor analyses (Supplemental Table 1).
Survey Results
Factor analysis was performed to better understand 12 newly authored survey items. The analysis supported four factors: patient involvement in the decision-making process, provider connection (discussion of options and rating of provider engagement), ability to urinate, and social issues (dialysis affecting family, interfering with social life, and time concerns). In bivariate analyses, provider connection factor score was significantly associated with being in the thinking versus acting SoC (−0.17 versus 0.32; P=0.02), and there was borderline significance with dialysis decision-making (−0.26 versus 0.16; P=0.05).
The Cronbach α for the set of questions comprising the dialysis modality knowledge score was 0.91. The knowledge scores (25th, 75th percentiles) for patients in the thinking versus the acting SoC were lower (0.8 [0, 1.0] versus 1.4 [0.8, 2.0]; P=0.003). The lifestyle barriers (reasons for not choosing a home dialysis modality) were also combined into a score per patient. For the lifestyle barriers, Cronbach α was 0.71. Those in the thinking SoC reported more lifestyle barriers (higher scores) than patients in the acting SoC (1.1 [0.9, 1.9] versus 0.7 [0.4, 1.0]; P=0.001).
Lifestyle barriers that were cited more frequently in the thinking versus the acting SoC were feeling that home dialysis was too difficult, others’ opinions, and body image with the PD catheter (Supplemental Appendix). Variables significantly associated with SoC (Table 2) were doctors explaining modality options and whether a dialysis modality decision was made; 47.2% of patient in the thinking stage compared with 89.5% of patients in the action stage had decided on a modality at the time of survey (P=0.003). The Cronbach α for CKD self-efficacy was low at 0.39 and hence, felt not to comprise a reliable measure.
Table 2.
Knowledge score, stress, and modality decision made by stage of change
| Parameter (n=55) | Stage of Change | P Value | |
|---|---|---|---|
| Thinking (n=36) | Acting (n=19) | ||
| Dialysis knowledge scorea | 0.8 (1.0) | 1.4 (1.2) | 0.003 |
| eGFR (ml/min per 1.73 m2) | 15.5 (3.6) | 14.2 (5.5) | 0.41 |
| Doctors explaining modality options | 51.4% | 84.2% | 0.02 |
| Visiting a dialysis center | 29.4% | 36.8% | 0.76 |
| Took educational reading material | 72.7% | 77.8% | 0.75 |
| Effect of travel time to home dialysis unit on dialysis decision makinga | 3.0 (2.0) | 3.0 (1.0) | 0.08 |
| Dialysis modality decision made | 47.2% | 89.5% | 0.003 |
| Lifestyle barrier scorea | 1.14 (0.71) | 0.64 (0.57) | 0.001 |
| CKD self-efficacy score | 3.6 (0.5) | 3.7 (0.8) | 0.65 |
| Provider connection | −0.17 | 0.32 | 0.02 |
| Duration of nephrologist care (yr)a | 2.0 (5.0) | 4.0 (5.0) | 0.16 |
Dialysis knowledge score is the combined knowledge of all RRTs (higher score equates to more knowledge). Lifestyle barrier score is higher if more barriers are reported to making a dialysis decision. CKD self-efficacy is a set of questions designed to gauge a person’s confidence in making decisions about and managing their CKD (higher score means higher efficacy). Provider connection is the factor score from the factor analyses listed. Travel time refers to the time taken to travel between a patient’s home and the closest dialysis unit offering home dialysis being a factor in the modality decision. The first three scores represent composite scores, whereas travel time is a score for that single factor, and all scores are from factor analyses. Proportions were reported for categorical variables, means (SDs) were reported for normally distributed numerical variables, and medians (interquartile ranges) were reported for non-normally distributed numerical variables.
Variables for which medians (interquartile ranges) were calculated.
Fifty-five patients completed the survey. The mean age of participants was 58.3 (11.2) years; 70.9% were women, 38.9% were white, 59.3% were black, and 3.64% were of Hispanic/Latino ethnicity (Table 1). In total, 65.4% had someone who could assist with home dialysis, and 87% could lift a 2-kg (4.4-lb) bag. The median health literacy score was 15.0 (3.0; range=3.0–15.0; higher score indicated greater health literacy).
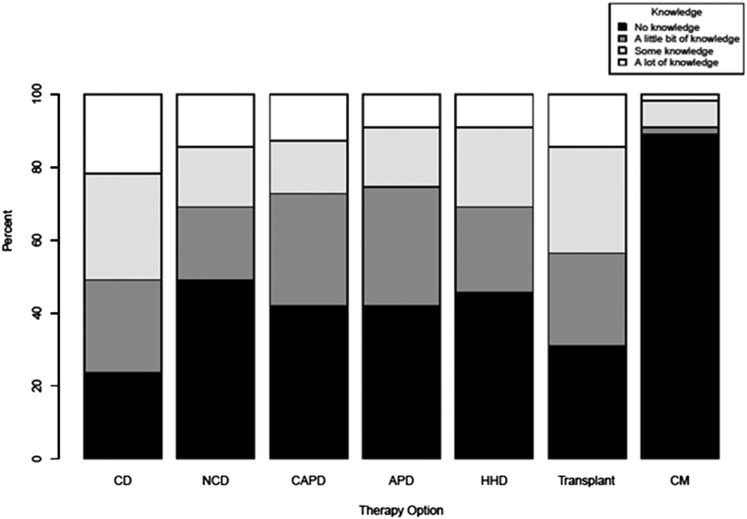
In total, 96% of patients acknowledged having CKD; 9.1% of patients were in the precontemplation SoC, 56.4% of patients were in the contemplation SoC, 7.2% of patients were in the preparation SoC, and 27.3% of patients were in the action SoC. To address the small sample size being spread across four SoC categories, we collapsed precontemplation/contemplation into a thinking stage and preparation/action into an action stage; 61.8% of patients had already made a modality decision when surveyed. None of the patients in the precontemplative SoC, 55% of the patients in the contemplation SoC (47.2% of patients in the thinking stage), 50% of the patients in the preparation SoC, and 100% of the patients in the action SoC (89.4% of patients in the acting stage) had decided on dialysis modality. Of the patients who made decisions, 82.4% chose IHD, 11.8% chose PD, and 5.9% chose nocturnal HD; 100% of patients were willing to consider dialysis if it were the only means to survive, and 58.2% were considering a renal transplant. Patient self-reported knowledge of the various dialysis modalities, transplant, and conservative management was quite varied (Figure 2).
Figure 2.
Patient self-reported knowledge distributions by RRT modality (n=55). APD, automated peritoneal dialysis (cycler); CAPD, continuous ambulatory peritoneal dialysis; CD, conventional in-center hemodialysis; CM, conservative management; HHD, home hemodialysis; NCD, nocturnal in-center dialysis; transplant, kidney transplantation.
CKD programs often use different methods to educate and familiarize patients with dialysis modality options (29); 57% and 63% of patients reported that talking with other patients on dialysis and after transplantation, respectively, would be very helpful, but only 40% and 32% of patients, respectively, had these opportunities. Similarly, 97% of patients said that visiting a dialysis center would assist in the decision, whereas only 63% of patients had visited. Thus, it is possible that patient/peer support could prove an effective mechanism for aiding patients in advancing SoC and decision-making.
Questions on decisional balance regarding the act of modality decision-making revealed that pros were living longer (26%), do not know (18%), starting dialysis (15%), someone to take care of them (15%), feeling better/more energy (15%), and better to plan for dialysis ahead (13%). Cons for deciding included needing to rely on others for care, scared, feeling sick, body image, needles, and space issues (35%), schedule (18%), against home therapy (15%), and transportation (9%). Frequent fears about deciding included uncertainty (18%), feeling tired and sick (18%), permanence (13%), death (13%), and time (13%) and inconvenience (13%) of doing/attending dialysis. Two respondents did not answer the open-ended questions. Raters were reliable, with Cohen κ=0.81 (P=0.03) (30).
The variables that were associated with making a dialysis decision (Table 3) were acting versus thinking SoC, doctors explaining dialysis options versus not (76% versus 43%; P=0.02), higher dialysis knowledge scores (2.3 [5.0] versus 0.2 [1.0]; range=0–3; P<0.001), and lower versus higher eGFR (13.9 [4.5] versus 16.8 [3.5] ml/min per 1.73 m2; P=0.04).
Table 3.
Stage of change, knowledge, and other factors by modality decision being made
| Parameter (n=55) | Modality Decision Made | P Value | |
|---|---|---|---|
| No (n=21) | Yes (n=34) | ||
| Age (yr) | 62 (10.8) | 56 (10.9) | 0.13 |
| Dialysis knowledge scorea | 0.2 (1.0) | 1.4 (1.2) | <0.01 |
| eGFR (ml/min per 1.73 m2) | 16.8 (3.5) | 13.9 (4.5) | 0.04 |
| Doctors explaining modality options | 42.9% | 75.8% | 0.02 |
| Visiting a dialysis center | 29.4% | 36.8% | 0.23 |
| Health literacy scorea | 15.0 (4.0) | 15.0 (3.0) | >0.99 |
| Lifestyle barrier scorea | 0.9 (0.5) | 1.1 (0.9) | 0.85 |
| Provider connection | −0.026 | 0.16 | 0.05 |
| Duration of nephrologist care (yr)a | 2.0 (4.5) | 2.3 (5.0) | 0.80 |
| Annual household income (US$)a | 17,500 (12,500) | 17,500 (12,500) | 0.48 |
| Travel time to home dialysis unita | 3.0 (1.0) | 3.0 (1.0) | 0.02 |
Dialysis knowledge score is the combined knowledge of all RRTs (higher score equates to more knowledge). Lifestyle barrier score is higher if more barriers are reported to making a dialysis decision. Provider connection is the factor score from the factor analyses listed. Travel time refers to the time taken to travel between a patient’s home and the closest dialysis unit offering home dialysis being a factor in the modality decision. The first two scores represent composite scores, whereas travel time is a score for that single factor, and all scores are from factor analyses. Health literacy score was derived using the scale reported by Wallace et al. (28). Proportions were reported for categorical variables, means (SDs) were reported for normally distributed numerical variables, and medians (interquartile ranges) were reported for non-normally distributed numerical variables.
Variables for which medians (interquartile ranges) were calculated.
In multivariate analyses, dialysis knowledge was significantly associated with dialysis decision-making, and SoC was of borderline significance, whereas provider connection was not significant (Table 4). The odds ratios for being in the acting versus thinking SoC was 5.8 (95% confidence interval, 1.0 to 32.6; P=0.05), and the odds ratio for dialysis knowledge was 4.2 (95% confidence interval, 1.4 to 12.9; P=0.01). The likelihood ratio test (P=0.49) was used to compare the two nested models, and the simpler three-covariate model was sufficient to explain the outcome (model P value<0.001; C statistic=0.87).
Table 4.
Multivariate model examining dialysis modality decision making
| Covariate (n=55) | Odds Ratio (95% Confidence Interval) | P Value |
|---|---|---|
| Dialysis knowledge score | 4.2 (1.4–12.9) | 0.01 |
| Stage of change | 5.8 (1.0–32.6) | 0.05 |
| Provider connection | 1.0 (0.4–2.2) | 0.95 |
Multivariate model of a few predictors of whether a dialysis modality decision is made. Dialysis knowledge score was significant, and stage of change was borderline significant (P=0.05). Provider connection was significant in bivariate analysis but not in the model.
Discussion
This study suggests that measurement of SoC for dialysis decision-making is a potentially useful tool in the care of patients with late-stage CKD. The proportion of patients having decided was higher at later SoC. There are wide ranges of lifestyle barriers to dialysis modality decision-making that vary according to a patient’s unique circumstances. Patients reporting more lifestyle barriers were less likely to be ready for dialysis decision-making. Education and knowledge influence both readiness to decide (SoC) and decision-making itself. SoC and doctors explaining dialysis options were also key factors in decision-making.
Our findings that patients at later SoC had more knowledge of the modalities and reported fewer lifestyle barriers are consistent with a previous study examining decision-making about renal transplantation. Patients in the action SoC had more knowledge, fewer decisional balance cons, and more self-efficacy (20,31). CKD self-efficacy was not associated with SoC or decision-making in our study; however, the sample size was small, and a more cohesive set of questions in that category may have shown different results.
Qualitative and quantitative findings indicated that health care providers have a strong influence on patient engagement and dialysis decision-making. It is plausible that provider connection was not significant in the multivariate analysis, because SoC was included in the model. In bivariate analyses, provider connection influenced both SoC and decision-making. Provider connection may influence decision-making through SoC, and hence, this may have nullified its effect. Joint decisions between patients and physicians were more likely in patients who received PD as opposed to IHD in one study (32). Davison et al. (33) reported that current processes of care do not consistently reflect preferences of patients or health professionals in terms of detailed prognostic information and shared decision-making. Concern about caregiver burden may also influence decision-making (34). These results point to the need for structured dialysis decision-making education tools that support providers in flexibly addressing the diverse lifestyle barriers, need for provider connection, and learning requirements of patients. This was shown in a unique discrete choice experiment study by Morton et al. (35), in which treatment time, travel restrictions, and life expectancy significantly influenced treatment choice.
Significantly more patients on PD versus IHD participated in educational incentives, including visual, verbal, and written information, DVDs, and visits to a renal unit (36). Golper et al. (37) discuss the importance of evaluating the effectiveness of specific instructional methods in patients with CKD, which is highlighted by the fact that 49% of patients who received such education had not made dialysis modality decisions (10). Future studies are warranted to examine predialysis education processes, including incorporation of more nephrologist involvement in the discussion of modality options, addressing patient-based cons of decision making and lifestyle barriers, peer support (including patient peers on various dialysis modalities, who chose conservative management, or who received a transplant), and visiting dialysis centers to observe different modality options. These empirically derived strategies could improve patient comfort and satisfaction with decision-making around RRT.
This study had several limitations. This was a cross-sectional study, and therefore, comments cannot be made regarding causation. Future prospective studies of larger sample sizes would allow for additional delineation of causal relationship among the variables examined in this study over time. We could not implement a uniform RRT/conservative management education program or assess conservative management in more detail, which might be done in future studies. Our sample size was small, and therefore, our multivariate modeling capability was limited along with the ability to perform subgroup analyses. Patients in our study were from a single metropolis, and therefore, barriers to dialysis decision-making may be different in other regions.
In conclusion, the SoC algorithm developed in this study seems to be a useful tool in measuring patients’ readiness for dialysis decision-making and correlated with dialysis decision making. Furthermore, the mechanism by which doctors explaining dialysis options influences decision-making may be through increasing patients’ readiness for decision-making or advancing the SoC from thinking to acting. Our results support the need for future studies to explore how to help patients move from the thinking to the acting SoC and ultimately, make timely modality decisions, and therefore, they are more likely to initiate their dialysis modality of choice nonurgently.
Disclosures
None.
Supplementary Material
Acknowledgments
The authors thank Catherine Firanek and Dr. Stephen Guest for help and support. The authors also thank Dr. Douglas Einstadter for providing a list of potential study subjects from our inclusion criteria. We thank Dr. Beth Piraino for her suggestions with our in-depth interview guide.
Baxter provided a grant for this study. Baxter contributed funds to supplement hospital funds after the study protocol had already been finalized. This study was supported, in parts, by National Institutes of Health Grants UL1-TR000439 and P60-MD00265.
A draft of the completed manuscript was sent to Baxter before submission for peer review. Baxter had no role in the study design or analyses of results.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.05560614/-/DCSupplemental.
References
- 1.Collins AJ, Hao W, Xia H, Ebben JP, Everson SE, Constantini EG, Ma JZ: Mortality risks of peritoneal dialysis and hemodialysis. Am J Kidney Dis 34: 1065–1074, 1999 [DOI] [PubMed] [Google Scholar]
- 2.Fenton SS, Schaubel DE, Desmeules M, Morrison HI, Mao Y, Copleston P, Jeffery JR, Kjellstrand CM: Hemodialysis versus peritoneal dialysis: a comparison of adjusted mortality rates. Am J Kidney Dis 30: 334–342, 1997 [DOI] [PubMed] [Google Scholar]
- 3.Mehrotra R, Kermah D, Fried L, Kalantar-Zadeh K, Khawar O, Norris K, Nissenson A: Chronic peritoneal dialysis in the United States: Declining utilization despite improving outcomes. J Am Soc Nephrol 18: 2781–2788, 2007 [DOI] [PubMed] [Google Scholar]
- 4.McFarlane PA, Bayoumi AM, Pierratos A, Redelmeier DA: The quality of life and cost utility of home nocturnal and conventional in-center hemodialysis. Kidney Int 64: 1004–1011, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Kooistra MP, Vos J, Koomans HA, Vos PF: Daily home haemodialysis in The Netherlands: effects on metabolic control, haemodynamics, and quality of life. Nephrol Dial Transplant 13: 2853–2860, 1998 [DOI] [PubMed] [Google Scholar]
- 6.Culleton BF, Walsh M, Klarenbach SW, Mortis G, Scott-Douglas N, Quinn RR, Tonelli M, Donnelly S, Friedrich MG, Kumar A, Mahallati H, Hemmelgarn BR, Manns BJ: Effect of frequent nocturnal hemodialysis vs conventional hemodialysis on left ventricular mass and quality of life: A randomized controlled trial. JAMA 298: 1291–1299, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Berger A, Edelsberg J, Inglese GW, Bhattacharyya SK, Oster G: Cost comparison of peritoneal dialysis versus hemodialysis in end-stage renal disease. Am J Manag Care 15: 509–518, 2009 [PubMed] [Google Scholar]
- 8.Goeree R, Manalich J, Grootendorst P, Beecroft ML, Churchill DN: Cost analysis of dialysis treatments for end-stage renal disease (ESRD). Clin Invest Med 18: 455–464, 1995 [PubMed] [Google Scholar]
- 9.Lee H, Manns B, Taub K, Ghali WA, Dean S, Johnson D, Donaldson C: Cost analysis of ongoing care of patients with end-stage renal disease: the impact of dialysis modality and dialysis access. Am J Kidney Dis 40: 611–622, 2002 [DOI] [PubMed] [Google Scholar]
- 10.Hanko J, Romann A, Taylor P, Copland M, Beaulieu M: Optimizing AVF creation prior to dialysis start: the role of predialysis renal replacement therapy choices. Nephrol Dial Transplant 27: 4205–4210, 2012 [DOI] [PubMed] [Google Scholar]
- 11.Liebman SE, Bushinsky DA, Dolan JG, Veazie P: Differences between dialysis modality selection and initiation. Am J Kidney Dis 59: 550–557, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prochaska JO, DiClemente CC: Stages and processes of self-change of smoking: Toward an integrative model of change. J Consult Clin Psychol 51: 390–395, 1983 [DOI] [PubMed] [Google Scholar]
- 13.Prochaska JO, Norcross JC, Diclemente CC: Applying the stages of change. Psychother Aust 19: 10–15, 2013 [Google Scholar]
- 14.Jones H, Edwards L, Vallis TM, Ruggiero L, Rossi SR, Rossi JS, Greene G, Prochaska JO, Zinman B, Diabetes Stages of Change (DiSC) Study : Changes in diabetes self-care behaviors make a difference in glycemic control: The Diabetes Stages of Change (DiSC) study. Diabetes Care 26: 732–737, 2003 [DOI] [PubMed] [Google Scholar]
- 15.Rakowski W, Ehrich B, Goldstein MG, Rimer BK, Pearlman DN, Clark MA, Velicer WF, Woolverton H, 3rd: Increasing mammography among women aged 40-74 by use of a stage-matched, tailored intervention. Prev Med 27: 748–756, 1998 [DOI] [PubMed] [Google Scholar]
- 16.Vallis M, Ruggiero L, Greene G, Jones H, Zinman B, Rossi S, Edwards L, Rossi JS, Prochaska JO: Stages of change for healthy eating in diabetes: Relation to demographic, eating-related, health care utilization, and psychosocial factors. Diabetes Care 26: 1468–1474, 2003 [DOI] [PubMed] [Google Scholar]
- 17.Westley C, Briggs LA: Using the stages of change model to improve communication about advance care planning. Nurs Forum 39: 5–12, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Fried TR, Redding CA, Robbins ML, Paiva A, O’Leary JR, Iannone L: Stages of change for the component behaviors of advance care planning. J Am Geriatr Soc 58: 2329–2336, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vogel RI, Petzel SV, Cragg J, McClellan M, Chan D, Dickson E, Jacko JA, Sainfort F, Geller MA: Development and pilot of an advance care planning website for women with ovarian cancer: A randomized controlled trial. Gynecol Oncol 131: 430–436, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Waterman AD, Robbins ML, Paiva AL, Hyland SS: Kidney patients’ intention to receive a deceased donor transplant: Development of stage of change, decisional balance and self-efficacy measures. J Health Psychol 15: 436–445, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Polkinghorne D: Phenomenological Research Methods in Psychology. Existential-Phenomenological Perspectives in Psychology, New York, Plenum Press, 1989, pp 41–60 [Google Scholar]
- 22.Onwuegbuzie AJ: A typology of mixed methods sampling designs in social science research. Qual Rep 12: 281–316, 2007 [Google Scholar]
- 23.Marshall C: Designing Qualitative Research, 4th Ed., 2006 [Google Scholar]
- 24.Boeije H: A purposeful approach to the constant comparative method in the analysis of qualitative interviews. Qual Quant 36: 391–409, 2002 [Google Scholar]
- 25.Bradley EH, Curry LA, Devers KJ: Qualitative data analysis for health services research: Developing taxonomy, themes, and theory. Health Serv Res 42: 1758–1772, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schatell D, Witten B: Method to Assess Treatment Choices for Home Dialysis (MATCH-D), 2009. Available at: http://www.homedialysis.org/match-d. Accessed September 20, 2014 [Google Scholar]
- 27.Bush K, Kivlahan DR, McDonell MB, Fihn SD, Bradley KA: The AUDIT alcohol consumption questions (AUDIT-C): An effective brief screening test for problem drinking. Ambulatory Care Quality Improvement Project (ACQUIP). Alcohol Use Disorders Identification Test. Arch Intern Med 158: 1789–1795, 1998 [DOI] [PubMed] [Google Scholar]
- 28.Wallace LS, Rogers ES, Roskos SE, Holiday DB, Weiss BD: Brief report: Screening items to identify patients with limited health literacy skills. J Gen Intern Med 21: 874–877, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Manns BJ, Taub K, Vanderstraeten C, Jones H, Mills C, Visser M, McLaughlin K: The impact of education on chronic kidney disease patients’ plans to initiate dialysis with self-care dialysis: A randomized trial. Kidney Int 68: 1777–1783, 2005 [DOI] [PubMed] [Google Scholar]
- 30.Landis JR, Koch GG: The measurement of observer agreement for categorical data. Biometrics 33: 159–174, 1977 [PubMed] [Google Scholar]
- 31.Bandura A: Self-efficacy: Toward a unifying theory of behavioral change. Psychol Rev 84: 191–215, 1977 [DOI] [PubMed] [Google Scholar]
- 32.Golper T: Patient education: can it maximize the success of therapy? Nephrol Dial Transplant 16[Suppl 7]: 20–24, 2001 [DOI] [PubMed] [Google Scholar]
- 33.Davison SN, Kromm SK, Currie GR: Patient and health professional preferences for organ allocation and procurement, end-of-life care and organization of care for patients with chronic kidney disease using a discrete choice experiment. Nephrol Dial Transplant 25: 2334–2341, 2010 [DOI] [PubMed] [Google Scholar]
- 34.Suri RS, Larive B, Hall Y, Kimmel PL, Kliger AS, Levin N, Tamura MK, Chertow GM, Frequent Hemodialysis Network (FHN) Trial Group : Effects of frequent hemodialysis on perceived caregiver burden in the Frequent Hemodialysis Network trials. Clin J Am Soc Nephrol 9: 936–942, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morton RL, Snelling P, Webster AC, Rose J, Masterson R, Johnson DW, Howard K: Dialysis modality preference of patients with CKD and family caregivers: a discrete-choice study. Am J Kidney Dis 60: 102–111, 2012 [DOI] [PubMed] [Google Scholar]
- 36.Chanouzas D, Ng KP, Fallouh B, Baharani J: What influences patient choice of treatment modality at the pre-dialysis stage? Nephrol Dial Transplant 27: 1542–1547, 2012 [DOI] [PubMed] [Google Scholar]
- 37.Golper TA, Saxena AB, Piraino B, Teitelbaum I, Burkart J, Finkelstein FO, Abu-Alfa A: Systematic barriers to the effective delivery of home dialysis in the United States: a report from the Public Policy/Advocacy Committee of the North American Chapter of the International Society for Peritoneal Dialysis. Am J Kidney Dis 58: 879–885, 2011 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.