Abstract
Background and objectives
Recent epidemiologic studies have provided evidence for an association between nephrolithiasis and cardiovascular disease, although the underlying mechanism is still unclear. Vascular calcification (VC) is a strong predictor of cardiovascular morbidity and the hypothesis explored in this study is that VC is more prevalent in calcium kidney stone formers (KSFs). The aims of this study were to determine (1) whether recurrent calcium KSFs have more VC and osteoporosis compared with controls and (2) whether hypercalciuria is related to VC in KSFs.
Design, setting, participants, & measurements
This is a retrospective, matched case-control study that included KSFs attending an outpatient nephrology clinic of the Royal Free Hospital (London, UK) from 2011 to 2014. Age- and sex-matched non-stone formers were drawn from a list of potential living kidney donors from the same hospital. A total of 111 patients were investigated, of which 57 were KSFs and 54 were healthy controls. Abdominal aortic calcification (AAC) and vertebral bone mineral density (BMD) were assessed using available computed tomography (CT) imaging. The prevalence, severity, and associations of AAC and CT BMD between KSFs and non-stone formers were compared.
Results
Mean age was 47±14 years in KSFs and 47±13 in non-stone formers. Men represented 56% and 57% of KSFs and non-stone formers, respectively. The prevalence of AAC was similar in both groups (38% in KSFs versus 35% in controls, P=0.69). However, the AAC severity score (median [25th percentile, 75th percentile]) was significantly higher in KSFs compared with the control group (0 [0, 43] versus 0 [0, 10], P<0.001). In addition, the average CT BMD was significantly lower in KSFs (159±53 versus 194 ±48 Hounsfield units, P<0.001). A multivariate model adjusted for age, sex, high BP, diabetes, smoking status, and eGFR confirmed that KSFs have higher AAC scores and lower CT BMD compared with non-stone formers (P<0.001 for both). Among stone formers, the association between AAC score and hypercalciuria was not statistically significant (P=0.86).
Conclusions
This study demonstrates that patients with calcium kidney stones suffer from significantly higher degrees of aortic calcification than age- and sex-matched non-stone formers, suggesting that VC may be an underlying mechanism explaining reported associations between nephrolithiasis and cardiovascular disease. Moreover, bone demineralization is more prominent in KSFs. However, more data are needed to confirm the possibility of potentially common underlying mechanisms leading to extraosseous calcium deposition and osteoporosis in KSFs.
Keywords: vascular calcification, kidney stones, cardiovascular
Introduction
Nephrolithiasis is a common healthcare problem and has traditionally been recognized as no more than an isolated and painful condition with few long-term clinical sequelae in most patients. However, over the last decade, a number of large-scale epidemiologic studies have provided evidence for an association between nephrolithiasis and systemic conditions such as the metabolic syndrome, hypertension, CKD, and cardiovascular disease (CVD) (1–4). As a result, the concept of nephrolithiasis as a systemic disease is becoming increasingly referred to in the medical literature.
The observations on the increased CVD morbidity in stone formers are of great interest, although the underlying mechanism is unclear and largely underexplored. A recent study demonstrated that young kidney stone formers (KSFs) have a higher prevalence of subclinical atherosclerosis based on increased carotid intima-media wall thickness measured by B-mode ultrasonography (5). Fabris et al. went on to confirm the possible relationship between nephrolithiasis and premature atherosclerosis. In their study, a higher pulse wave velocity in KSFs was demonstrated, suggesting an increased stiffness of small arteries, presumably as a consequence of vascular calcification (VC) (6).
VC is a strong predictor of cardiovascular morbidity and mortality and is associated with increasing age, hypertension, diabetes, dyslipidemia, CKD, and reduced bone mass (7,8). A number of reports have disclosed an inverse relationship between bone mineral density (BMD) and abnormal arterial stiffness, related, in part, to vessel calcifications (8,9). In addition, numerous epidemiologic studies revealed that VC coexists with bone loss, suggesting a relationship between osteoporosis and atherosclerosis (8,10). Interestingly, this phenomenon is significantly more pronounced in women (8). However, causality of this association has not been definitively demonstrated.
As in patients with VC, bone demineralization is a frequent finding in KSFs, and fasting hypercalciuria appears as the only biologic factor associated with low BMD in these patients (11). Thus, both VC and nephrolithiasis can be defined as extraosseous sites of abnormal calcium deposition and both seem to be associated with bone demineralization. On the basis of this evidence, we hypothesized that early atherosclerosis, presumably related to increased VC, might be more prominent in KSFs and could be an explanation for increased CVD burden.
Abdominal aorta calcification (AAC) measured by computed tomography (CT) scanning is considered as a strong predictor of cardiovascular (CV)-related morbidity or death and was used in our study as a measure of VC burden in KSFs and healthy control patients. The aims of this study were to (1) determine whether recurrent KSFs have more VC and more osteoporosis compared with healthy controls and (2) to determine whether hypercalciuria has any relationship to VC in KSFs.
Materials and Methods
Participants
This is a retrospective, matched case-control study. The study population was drawn from an adult kidney stone outpatient nephrology clinic of the Royal Free Hospital (London, UK). All patients with a confirmed diagnosis of recurrent calcium nephrolithiasis underwent a thorough clinical evaluation by a nephrologist and urologist, including a noncontrast CT scan of the abdomen and the pelvis that was done specifically to look for stones in the kidneys, ureters, and bladder (CT KUB), and a full metabolic evaluation. Only patients in whom CT scans were performed due to reasons unrelated to the study purpose and as a part of their routine care were included. Only patients with calcium stones were included in the analyses. Patients with stone types other than calcium (e.g., uric acid, cystine, infection-related stones, primary hyperoxaluria, distal renal tubular acidosis, indinavir stones, etc.) were excluded.
Age- and sex-matched non–stone formers were drawn from a list of potential living kidney donors from the same hospital. These patients underwent appropriate pretransplant evaluation including contrast-enhanced abdominal CT in all patients as part of their predonor transplant workup. Thirteen of 54 potential living donors who underwent the full pretransplant screening did not donate a kidney: five were found to be ineligible (diabetes mellitus, hypertension, low eGFR, or a combination of the above), two declined surgery, and the others had unknown reasons.
Written consent was not obtained from the individual patients because the study is based on observational data collected as part of routine clinical care. Local ethics committee approval was obtained.
Data Collection
Demographic and clinical variables were collected retrospectively by reviewing the patients’ computerized records. The following parameters were recorded in the KSF group: age, sex, weight, comorbidities if any (history of heart failure, CVD, diabetes, hypertension, hyperlipidemia, peripheral vascular disease, cerebral vascular accident), and smoking history. Laboratory data included the full metabolic panel: serum sodium, potassium, calcium, phosphorus magnesium, glucose, urea, uric acid, creatinine, bicarbonate, albumin, parathyroid hormone (PTH), 25(OH)vitamin D. In addition, the 24-hour urine collection was analyzed for volume, creatinine, sodium, potassium, calcium, phosphorus, magnesium, oxalate, citrate, uric acid, and biochemical analysis of stone material when available. eGFR was estimated in all patients by the four-component Modification of Diet in Renal Disease equation.
In the control group, demographic parameters and biochemical laboratory data were collected for analyses; however, 24-hour urinary collections and serum PTH and 25(OH)vitamin D levels were not done in this group.
Definitions of Urinary Metabolic Abnormalities in KSFs
Hypercalciuria was defined as urine calcium excretion >7.5 mmol/d for men and 6.5 mmol/d for women. Hyperoxaluria was defined as urine oxalate excretion >0.5 mmol/d. Hyperuricosuria was defined as urine uric acid excretion >4.8 mmol/d for men and 4.5 mmol/d for women. Hypocitraturia was defined as urine citrate excretion <1.52 mmol/d.
A stone was considered to be made of a single type (i.e., a “pure” stone) if >95% of the stone weight was represented by a single constituent. In general, for a constituent to be considered in the stone composition, it had to represent ≥5% of the stone weight. Biochemical analysis of stone material was performed by Fourier transformed mid-infrared spectroscopy.
CT Imaging for Measurement of VC and BMD
We retrospectively accessed the CT images for both aortic and spine measurements using a standard radiology picture archiving and communication system workstation, with images viewed in soft tissue and bone windows. Measurement of aortic calcification by CT permits the graded quantification of calcified lesions. For bone density measurement, CT has an advantage over posterior-anterior dual x-ray absorptiometry (DXA) assessment, in which calcium deposits in the aorta could be mistakenly interpreted as a falsely high BMD.
The abdominal aorta was visualized by noncontrast CT KUB in KSFs and by precontrast abdominal CT in the control group. The slice thickness was 2 or 5 mm in all CT scans. Calcification was considered to be present if an area ≥1 mm2 in the aortic wall displayed a density ≥130 Hounsfield units (HU). The cross-section of the abdominal aorta was divided into 10 segments radially, with each segment representing 10% of the aortic circumference. A segment containing an aortic wall with calcification in any section was defined as having aortic calcification. The number of calcified segments was counted and then summed to get a percentage score. Each CT slice was scored individually for circumference calcified, in between the celiac axis and the aortic bifurcation, and was determined to be mildly, moderately, or severely calcified, where mild is ≤10% of circumference, moderate is 11%–50%, and severe is ≥51%. To aid comparative scoring, we devised a severity multiplier to yield an overall severity score, an AAC score. We assigned each category a severity factor, ×5 for mild, ×30 for moderate, and ×75 for severe. These values are representative of the average percentage of circumference calcified in each category. The number of slices in the three severity categories was given as percentages of the total aorta calcified, to eliminate errors from different number of slices per patients, and was then multiplied with the respective factor to get an overall AAC severity score.
This approach was adapted from Yasui et al. and Leckstroem et al. (12,13). The scoring technique was independently verified in the previous study and the presence or absence of AAC was compared with interobserver and intraobserver variability. In this study, the Pearson’s product moment correlation coefficient for the AAC severity scores in a sample of 12 patients was r=0.99 for interobserver variability and r=0.99 for intraobserver variability (13).
Trained observers determined the degree of calcification subjectively. Manual scoring was used to avoid artifacts induced by movement, which are not registered reliably by the automated software. The research physician was not blinded to each patient’s allocation group when reviewing the CT scans for the aortic and spine measurements.
We assessed vertebral BMD by placing a single oval click-and-drag region of interest over an area of vertebral body trabecular bone and then measuring CT attenuation in HU, with lower HU (lower attenuation) representing less-dense bone at the L1 level; this process is identical to that used for measuring CT attenuation for other clinical conditions (e.g., adrenal adenomas, renal lesion enhancement, and fatty liver assessment). We avoided placing the region of interest near areas that would distort the BMD measurement (posterior venous plexus; focal heterogeneity or lesion, including compression fracture; and imaging-related artifacts). An L1 CT-attenuation threshold of 160 HU is 90% sensitive and a threshold of 110 HU is >90% specific for distinguishing osteoporosis from osteopenia and normal BMD. A threshold of 135 HU results in a balanced sensitivity and specificity of approximately 75% for each and was used to distinguish osteoporosis from osteopenia and normal BMD in our patients. This method was retrieved from a recently published study of the diagnostic accuracy of a simple BMD screening method for adults who have undergone abdominal CT imaging for other clinical indications (14).
In addition, we assessed the presence of vertebral compression fractures by using sagittal CT views of the lumbar spine by utilizing the Genant visual semiquantitative method (15), a widely accepted way of assessing vertebral fractures on conventional radiography that can be easily applied to sagittal CT images. We counted only obvious moderate (grade 2, 25%–40% loss of height) or severe (grade 3, >40% loss of height) compression deformities to avoid ambiguity related to more subjective borderline or mild compression deformities.
Statistical Analyses
Data are expressed as the mean±SD, except for parameters that are not normally distributed, which are shown as the median and interquartile range (25th percentile, 75th percentile). The nominal and categorical variables were compared using the chi-squared likelihood ratio or Fisher’s exact test.
To analyze the association between kidney stones and AAC score, four regression models were considered including Poisson, negative binomial, and their zero-inflated versions and the model with the best (smallest) Akaike information criterion was selected. Crude and adjusted zero-inflated negative binomial regression models were then selected. Linear regression models were used to analyze differences in CT BMD between groups. A two-sided P value <0.05 was considered to represent statistically significant differences. Correlation between AAC and CT BMD was evaluated using Spearman’s rank correlation coefficient. All statistical analyses were performed with Stata software (version 12.1; StataCorp, College Station, TX).
Results
We investigated 111 patients, of which 57 had a confirmed diagnosis of recurrent nephrolithiasis and 54 were age- and sex-matched controls. Baseline clinical characteristics of the study patients are presented in Table 1. The mean age was 47±14 years in KSFs and 47±13 in non–stone formers. Men represented 56% and 57% of the patients in KSFs and non–stone formers, respectively. Race was not characterized. The prevalence of diabetes and hypertension was higher in KSFs compared with non-stone formers (10% versus 2%, P=0.07; and 35% versus 9%, P=0.002, respectively).
Table 1.
Baseline clinical characteristics of the patients in the two study groups
| Clinical Characteristic | KSFs (n=57) | Non-KSFs (n=54) | P Value |
|---|---|---|---|
| Age, yr | 47±14 | 47±13 | 0.82 |
| Men | 32 (56) | 31 (57) | 0.89 |
| Comorbiditiesa | |||
| Systemic hypertension | 17 (35) | 5 (9) | 0.002 |
| Diabetes mellitus | 5 (10) | 1 (2) | 0.07 |
| Smokingb | 4 (12)b | 16 (30) | 0.06 |
| Laboratory results | |||
| Sodium, mEq/L | 141±2 | 143±2 | 0.02 |
| Potassium, mEq/L | 4.3±0.3 | 4.4±0.3 | 0.20 |
| Calcium, mg/dl | 9.4±0.4 | 9.6±0.4 | 0.26 |
| Phosphorus, mg/dl | 3.1±0.6 | 3.4±0.6 | 0.04 |
| Magnesium, mg/dl | 0.84±0.12 | ||
| Uric acid, mg/dl | 5.5±1.3 | ||
| Urea nitrogen, mg/dl | 16±5.9 | 14.8±2.8 | 0.58 |
| Creatinine, mg/dl | 1±0.4 | 0.9±0.1 | 0.45 |
| eGFR, ml/min–1 per 1.73 m–2 | 91±22 | 91±15 | 0.31 |
| Bicarbonate, mEq/L | 26±2 | 25±2 | 0.02 |
| Glucose, mg/dl | 88±18 | 93±18 | 0.08 |
| Albumin, g/L | 46±3 | 47±3 | 0.66 |
| Parathyroid hormone (range, 1.6–6.9 pmol/L) | 4.3 (3.1, 6.9) | ||
| 25(OH)vitamin D, nmol/L | 43 (28, 64) | ||
| 24-h urine collection (n=36) | |||
| Volume, liters | 2.0±0.9 | ||
| Sodium mEq/d | 156±46 | ||
| Potassium, mEq/d | 69±20 | ||
| Calcium, mg/d | 280 (124, 400) | ||
| Phosphorus, mg/d | 806±270 | ||
| Oxalate, mg/d | 30±7.2 | ||
| Uric acid, mg/d | 584±150 | ||
| Citrate, mg/d | 420 (360, 670) | ||
| Creatinine, mg/d | 1144±352 |
Data are presented as the mean ± SD, n (%), or median (25th percentile, 75th percentile). KSF, kidney stone former.
Comorbidity data were available in 50 KFSs.
Smoking history was available in 24 KFSs.
There were no clinically significant differences in laboratory parameters between KSFs and control patients. Both creatinine and eGFR were similar between groups (84±38 versus 80±13 µmol/L, P=0.45; and 91±22 versus 91±15 ml/min per 1.73 m2, P=0.31, in KSFs and non-KSFs, respectively).
Among KSFs, 32 patients (56%) had hypercalciuria, four suffered from hyperuricosuria, and one patient had hyperoxaluria. Decreased urinary citrate excretion was detected in six patients.
Stone composition was known in six KSFs: Four patients had calcium oxalate and two calcium phosphate stones. An additional three patients had nephrocalcinosis, suggesting either calcium oxalate or calcium phosphate stones or both.
Unadjusted analysis of the prevalence of AAC and osteoporosis in KSFs and non-KSFs (controls) is presented in Table 2.
Table 2.
Incidence of vascular calcification and osteoporosis in KSFs and healthy non-stone formers as detected by the unadjusted analyses
| Group | KSFs (n=57) | Non-KSFs (n=54) | P Value |
|---|---|---|---|
| Patients with AAC | 22/57 (38) | 19/54 (35) | 0.69 |
| Men | 9/31 (28) | 9/31(29) | |
| Women | 12/23 (52) | 10/23 (43) | |
| Median AAC scorea | 0 (0, 43) | 0 (0, 10) | <0.001 |
| Patients with osteoporosisb | 16/51 (31) | 9/54 (17) | 0.11 |
| Men | 5 (9.8) | 4 (14) | |
| Women | 7 (14) | 5 (9) | |
| Average CT BMD, HU | 159±53 | 194±48 | <0.001 |
Data are presented as n (%) unless otherwise indicated. AAC, abdominal aortic calcification; CT, computed tomography; BMD, bone mineral density; HU, Hounsfield unit.
AAC score, which was not normally distributed, is shown as the median (25th percentile, 75th percentile).
Osteoporosis is defined as CT attenuation <135 HU at the L1 level. In the KSFs, data were available in 51 patients.
The prevalence of AAC was similar in both groups (38% in KSFs versus 35% in controls, P=0.69). Six of 22 (27%) KSFs with VC were severely calcified (had ≥51% of aortic circumference involved) and nine (41%) had moderate calcification (11%–50% of circumference calcified). Overall, 68% of KSFs had moderate to severe AAC. In addition, 80% of KSFs had a proximal pattern of AAC distribution (located in the proximal two-thirds of the abdominal aorta).
Of 19 patients with VC in the control group, three and two patients had moderate and severe calcification, respectively (overall 26% of controls had moderate to severe AAC). Twelve controls (63%) had AAC detected in only one or two last slices of the abdominal aorta just above the iliac bifurcation.
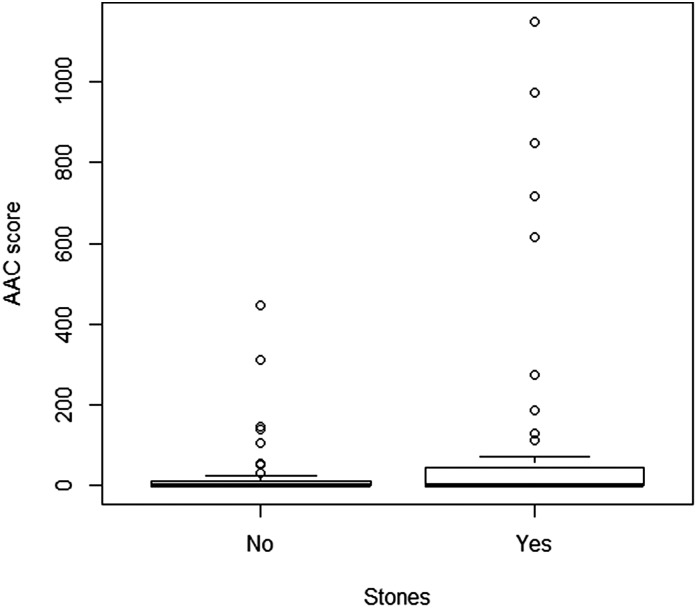
The AAC severity score (presented as the median [25th percentile, 75th percentile]) was significantly higher in KSFs compared with the control group (0 [0, 43] versus 0 [0, 10], P<0.001) (Table 2).
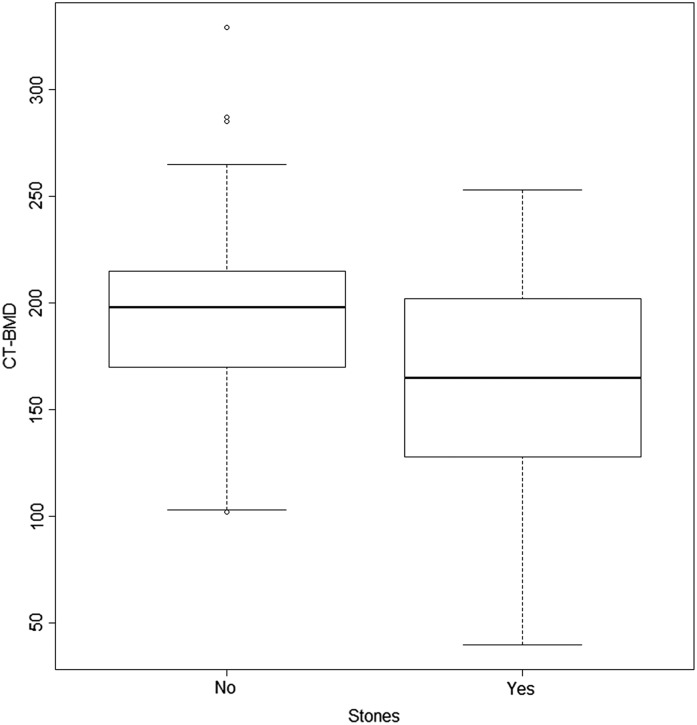
The prevalence of osteoporosis was not statistically significantly different in both groups, whereas the average CT BMD was significantly lower in KSFs (159±53 HU versus 194±48 HU, P<0.001).
Table 3 shows results of linear regression models for the estimated difference in AAC or CT BMD between KSFs and non-stone formers. Both crude analyses and statistical adjustment for age, sex, high BP, diabetes, smoking, and eGFR found that KSFs had higher AAC scores and lower CT BMD as compared with controls (P<0.001 for both).
Table 3.
Results of regression models for the estimated difference in AAC or CT BMD between KSFs and non-stone formers
| Analysis | Difference (95% Confidence Interval) | Akaike Information Criterion | P Value |
|---|---|---|---|
| AAC | |||
| Univariate | 3.56 units (3.39 to 3.78) | 631.03 | 0.01 |
| Multivariate | 3.78 units (3.53 to 4.06) | 445.88 | <0.001 |
| CT BMD | |||
| Univariate | −34.58 HU (−53.85 to −15.32) | 1110.70 | <0.001 |
| Multivariate | −35.88 HU (−55.95 to −15.82) | 870.63 | <0.001 |
Multivariate models are adjusted for age, sex, high BP, diabetes, smoking status, and eGFR. The r2 statistics are not presented because the model for AAC is a zero-inflated negative binomial and does not allow r2 computation. The Akaike information criterion is presented as a similar measure of fit.
Figures 1 and 2 represent distribution of AAC and osteoporosis in KSFs and non-KSFs.
Figure 1.
Distribution of AAC score in KSFs and healthy controls. The plot shows the distribution of AAC score in participants with and without stones. In the multivariate model adjusted for age, sex, high BP, diabetes, smoking status, and eGFR, the relationship between AAC severity score and kidney stone disease remained significant (P<0.001). The bottom and top of the box represent the first and third quartiles, the line represents the second quartile (median), the whiskers represent 1.5 times the interquartile range of the first and third quartiles, and the circles represent any values lying outside the whiskers. AAC, aortic calcification; KSF, kidney stone former.
Figure 2.
Distribution of CT BMD in KSFs and healthy controls. The plot shows the distribution of CT BMD in participants with and without stones. In the multivariate model adjusted for age, sex, high BP, diabetes, smoking status and eGFR, the relationship between osteoporosis and kidney stone disease remained significant (P<0.001). The bottom and the top of the box represent the first and third quartiles, the line represents the second quartile (median), the whiskers represent 1.5 times the interquartile range of the first and third quartiles, and the circles represent any values lying outside the whiskers. BMD, bone mineral density; CT, computed tomography.
In the multivariate model adjusted for age, sex, high BP, diabetes, smoking status, and eGFR, higher AAC scores and lower CT BMD in KSFs compared with controls remained statistically significant (P<0.001 for both). Including an interaction product for stones and sex in the model to test for an effect of sex was not significant for both AAC (P=0.94) and CT BMD (P=0.31).
A correlation between AAC and CT BMD was evaluated. A higher AAC score was strongly correlated with lower BMD in the whole study group (−0.56, P<0.001), in KSFs (−0.52, P<0.001), and in non–stone formers (−0.60, P<0.001).
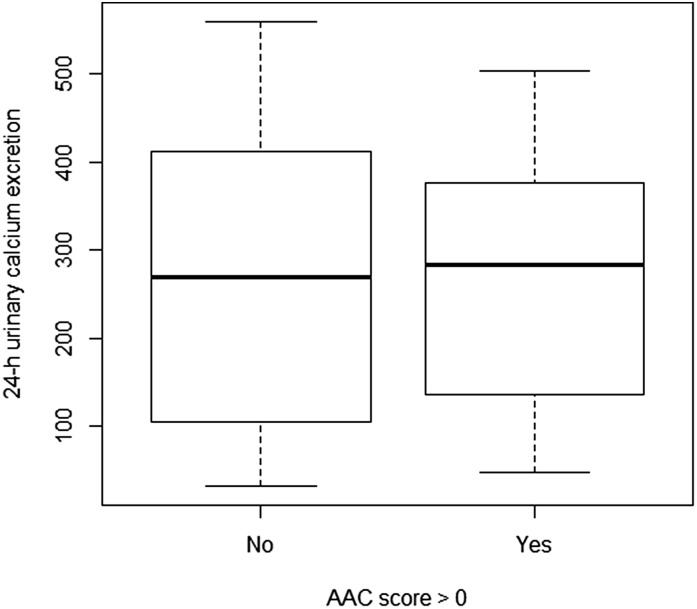
Figure 3 demonstrates distribution of urinary excretion of calcium among KSFs with and without VC. Urinary excretion of calcium was roughly similar among patients with and without an AAC score >0 (median 280 [120, 400] versus 288 [140, 360], P=0.86).
Figure 3.
Distribution of urinary excretion of calcium among KSFs with and without vascular calcification. Urinary excretion of calcium was roughly similar among patients with and without an AAC score >0 (median 280 mg/d [120, 400] versus 288 mg/d [140, 360], P=0.86).The bottom and the top of the box represent the first and third quartiles, the line represents the second quartile (median), and the whiskers represent 1.5 times the interquartile range of the first and third quartiles.
Discussion
Our study reveals several interesting findings in patients with kidney stones, which may provide a possible explanation for the underlying pathogenic mechanisms leading to previously reported associations between nephrolithiasis and CVD. First, and most importantly, we were able to demonstrate that KSFs had significantly higher AAC severity scores compared with age- and sex-matched non-KSFs, whereas the prevalence of AAC was similar in the two groups. Thus, a substantial prevalence of VC can be found in a relatively healthy middle-aged population, especially if a highly sensitive technology such as a CT scan is used to detect it. This finding is in line with another British study in which AAC prevalence, patterns, and severity in 93 live donors were assessed using the same methodology for AAC scoring. In this study, 29 of 93 patients (31%) had radiologically detectable AAC, and the majority had mild calcification (13). Similarly to this study, the prevalence of VC in our study was 35% in control patients and most of them had mild AAC with a distal distribution pattern (located mainly above the iliac bifurcation), whereas both a more extensive and proximal pattern of AAC was demonstrated in KSFs. Although the association between severity of AAC and adverse CV outcomes and death is well established, the clinical significance of distal versus more proximal AAC distribution is unknown and is worth further exploring in future studies.
Not unexpectedly, we found a higher prevalence of comorbidities such as diabetes, hypertension, and dyslipidemia in KSFs, whereas kidney function was similar between the groups. Although all of the comorbidities mentioned earlier are associated with increased incidence of VC, multivariate analyses adjusted for all potential confounders confirmed that kidney stone disease is independently associated with advanced forms of VC in KSFs compared with non-KSFs. In addition, lower BMD was clearly demonstrated in KSFs compared with the control group. However, neither VC nor osteoporosis was associated with the presence of hypercalciuria in KSFs.
VC has been found to coexist with bone loss in numerous epidemiologic studies, and low bone density has been associated with aortic calcification, as detected by conventional radiography (8–10), and with surrogate markers of subclinical vascular disease (8,11). VC is an active, regulated process that is induced by the transformation of vascular smooth muscle cells into osteoblast-like cells that are able of expressing various bone matrix and skeletal regulatory proteins, including osteocalcin, bone sialoprotein, osteonectin, collagen I, alkaline phosphatase, Msx-2, and Cbfa-1 (7,8). This process results in the development of tissue that is histomorphologically indistinguishable from bone in the blood vessel wall. It is not clear, however, whether the association between osteoporosis and VC is causal. Regardless of the underlying mechanism, our study provides evidence that VC and bone demineralization coexist in KSFs, suggesting the possibility of common underlying pathways leading to increased extraosseous calcium depositions in kidneys and blood vessels.
Several authors worldwide have evaluated the relationship between nephrolithiasis and CVD. In 2010, Rule et al. studied 5081 incident stone formers and 14,144 matched controls and found that stone formers have a 38% increased risk for myocardial infarction (MI) (2). The risk remained elevated even when adjusted for other risk factors such as hypertension, diabetes, obesity, dyslipidemia, and even CKD. A year later, Domingos and Serra performed a study in >23,000 adult individuals from the Fourth Portuguese National Health Survey and showed that stone formers have a higher prevalence of MI and stroke compared with non–stone formers (3). After adjustment for the presence of diabetes and hypertension, nephrolithiasis remained, at least in women, an earlier and independent risk factor for MI later in life. In a prospective study of 45,748 men and 196,357 women from the United States, Ferraro et al. demonstrated that among women, those with a reported history of kidney stones had a significantly higher adjusted risk of CVD than those without a history of kidney stones (4). Finally, in a recent cohort study of 3,195,452 people, the occurrence of a kidney stone was associated with a higher risk of acute MI, percutaneous transluminal coronary angioplasty/coronary artery bypass grafting, and stroke (16).
Although all of the studies cited above demonstrate a consistent association between nephrolithiasis and CVD, they do not disclose an underlying mechanism. According to our study, higher severity of AAC, which is a known and strong predictor of coronary and cerebrovascular morbidity and death, may be such a mechanism (7).
The use of CT technology for both aortic and spine measurements in our study provides a significant benefit over conventional radiographs, which are less precise and do not permit the graded quantification available with a CT scan. For bone density measurement, DXA assessment is less precise in patients with AAC, in whom calcium deposits in the aorta interfere with the precise measurement of BMD. CT measurement of BMD also allows for three-dimensional volumetric assessment, so that variations in bone size are less important than in two-dimensional areal DXA.
Our study has some limitations. First, demographic and clinical variables were collected retrospectively by reviewing the patients’ computerized records. Therefore, we cannot exclude known or unknown confounding factors entirely as an explanation for our results. However, data collection was uniform in all patients and the same research physicians have completed all datasheets. Second, the control group in our study consisted of potential living kidney donors who may be “healthier” than the general population. Despite this, we were able to match the study and control groups by age and sex, and then adjust for the presence of diabetes, hypertension, dyslipidemia, CKD, and smoking, which are known as the most powerful risk factors for VC. Moreover, the detailed medical history (from both the patients’ transplant physician and general practitioner) as well as CT scans of the kidneys and urinary tract were available to us to exclude kidney stone disease in the control group. Third, confounding is likely to persist and the multiple adjustments are not able to fully eliminate the potential for bias. In particular, the higher prevalence of hypertension in stone formers indicates an increased CVD risk that could be independent of stone-forming processes. Fourth, the research physician was not blinded to each patient’s allocation group when reviewing the CT scans for the aortic and spine measurements; however, the same trained researcher determined the degree of calcification and CT BMD using a scoring technique that has been independently verified in previous studies. Fifth, the data on PTH and vitamin D levels in the healthy controls were not available for us. Sixth, as described in the Materials and Methods, the CT scan parameters were not absolutely identical in the KSFs and the living kidney donor population, because both noncontrast and contrast-enhanced CT scans were performed in living donors, whereas KSFs had just noncontrast-enhanced CT scans. However, AAC is impossible to access in contrast-enhanced CT scans; therefore, these films in living donors were not reviewed. Moreover, a noncontrast-enhanced 2- to 5-mm sliced CT scan of the abdomen and pelvis was performed in both groups in the same hospital using the same technique, and optimal visualization of the abdominal aorta in between the celiac axis and the aortic bifurcation was obtained in all patients.
Although our study cannot prove direct causality, this is the first study to our knowledge to provide controlled evidence for a possible role of vessel calcification and associated osteoporosis in CV morbidity among KSFs. Our findings may serve as a useful basis for future prospective trials exploring the potential benefit of therapeutically targeting the bones and cardiovascular system in KSFs, as part of their routine management to mitigate CVD morbidity and mortality.
In summary, we evaluated a KSF group and a control group and focused specifically on the prevalence and severity of aortic calcification and osteoporosis. Although kidney stone disease was associated with a higher burden of comorbidities, multivariate analysis showed that severity of VC and osteoporosis is significantly higher in KSFs, suggesting a possible common underlying mechanism for all three: stones, VC, and osteoporosis. In addition, our findings raise several important questions that may be relevant to the care of KSFs. Existing CT KUB can be a useful tool for assessment of aortic calcification and BMD, along with kidney stone number and distribution. However, considerably more data will be needed to determine whether monitoring by CT KUB would be beneficial and cost-effective in patients with kidney stones, particularly in light of the increased radiation exposure. However, because many KSFs undergo a diagnostic or review CT KUB, it would seem reasonable to take this opportunity to also assess for aortic calcification and BMD.
Moreover, preliminary experimental and clinical evidence suggests that therapeutic strategies aimed at targeting osteoporosis may have a favorable effect on both the metabolic urinary profile and VC (17,18). However, future prospective intervention trials will be needed to test the hypothesis that KSFs are a population at high risk of CVD and that this can be reduced by measures aimed at identifying and reducing VC and decreased BMD.
Disclosures
R.J.U. is currently on secondment as a chief scientist with AstraZeneca Cardiovascular & Metabolic Diseases Innovative Medicines and Early Development Science Unit (Mölndal, Sweden).
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial, “Stones, Bones, and Cardiovascular Groans,” on pages 174–176.
References
- 1.Lange JN, Mufarrij PW, Wood KD, Holmes RP, Assimos DG: The association of cardiovascular disease and metabolic syndrome with nephrolithiasis. Curr Opin Urol 22: 154–159, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rule AD, Roger VL, Melton LJ, 3rd, Bergstralh EJ, Li X, Peyser PA, Krambeck AE, Lieske JC: Kidney stones associate with increased risk for myocardial infarction. J Am Soc Nephrol 21: 1641–1644, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Domingos F, Serra A: Nephrolithiasis is associated with an increased prevalence of cardiovascular disease. Nephrol Dial Transplant 26: 864–868, 2011 [DOI] [PubMed] [Google Scholar]
- 4.Ferraro PM, Taylor EN, Eisner BH, Gambaro G, Rimm EB, Mukamal KJ, Curhan GC: History of kidney stones and the risk of coronary heart disease. JAMA 310: 408–415, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reiner AP, Kahn A, Eisner BH, Pletcher MJ, Sadetsky N, Williams OD, Polak JF, Jacobs DR, Jr, Stoller ML: Kidney stones and subclinical atherosclerosis in young adults: The CARDIA study. J Urol 185: 920–925, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fabris A, Ferraro PM, Comellato G, Caletti C, Fantin F, Zaza G, Zamboni M, Lupo A, Gambaro G: The relationship between calcium kidney stones, arterial stiffness and bone density: Unraveling the stone-bone-vessel liaison [published online ahead of print September 30, 2014]. J Nephrol doi:10.1007/s40620-014-0146-0 [DOI] [PubMed] [Google Scholar]
- 7.Bastos Gonçalves F, Voûte MT, Hoeks SE, Chonchol MB, Boersma EE, Stolker RJ, Verhagen HJ: Calcification of the abdominal aorta as an independent predictor of cardiovascular events: A meta-analysis. Heart 98: 988–994, 2012 [DOI] [PubMed] [Google Scholar]
- 8.Schulz E, Arfai K, Liu X, Sayre J, Gilsanz V: Aortic calcification and the risk of osteoporosis and fractures. J Clin Endocrinol Metab 89: 4246–4253, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Toussaint ND, Lau KK, Strauss BJ, Polkinghorne KR, Kerr PG: Associations between vascular calcification, arterial stiffness and bone mineral density in chronic kidney disease. Nephrol Dial Transplant 23: 586–593, 2008 [DOI] [PubMed] [Google Scholar]
- 10.Kiel DP, Kauppila LI, Cupples LA, Hannan MT, O’Donnell CJ, Wilson PW: Bone loss and the progression of abdominal aortic calcification over a 25 year period: The Framingham Heart Study. Calcif Tissue Int 68: 271–276, 2001 [DOI] [PubMed] [Google Scholar]
- 11.Letavernier E, Traxer O, Daudon M, Tligui M, Hubert-Brierre J, Guerrot D, Sebag A, Baud L, Haymann JP: Determinants of osteopenia in male renal-stone-disease patients with idiopathic hypercalciuria. Clin J Am Soc Nephrol 6: 1149–1154, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yasui T, Itoh Y, Bing G, Okada A, Tozawa K, Kohri K: Aortic calcification in urolithiasis patients. Scand J Urol Nephrol 41: 419–421, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Leckstroem DC, Bhuvanakrishna T, McGrath A, Goldsmith DJ: Prevalence and predictors of abdominal aortic calcification in healthy living kidney donors. Int Urol Nephrol 46: 63–70, 2014 [DOI] [PubMed] [Google Scholar]
- 14.Pickhardt PJ, Pooler BD, Lauder T, del Rio AM, Bruce RJ, Binkley N: Opportunistic screening for osteoporosis using abdominal computed tomography scans obtained for other indications. Ann Intern Med 158: 588–595, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Genant HK, Wu CY, van Kuijk C, Nevitt MC: Vertebral fracture assessment using a semiquantitative technique. J Bone Miner Res 8: 1137–1148, 1993 [DOI] [PubMed] [Google Scholar]
- 16.Alexander RT, Hemmelgarn BR, Wiebe N, Bello A, Samuel S, Klarenbach SW, Curhan GC, Tonelli M, Alberta Kidney Disease Network : Kidney stones and cardiovascular events: A cohort study. Clin J Am Soc Nephrol 9: 506–512, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arrabal-Polo MA, Arias-Santiago S, de Haro-Muñoz T, Lopez-Ruiz A, Orgaz-Molina J, Gonzalez-Torres S, Zuluaga-Gomez A, Arrabal-Martin M: Effects of aminobisphosphonates and thiazides in patients with osteopenia/osteoporosis, hypercalciuria, and recurring renal calcium lithiasis. Urology 81: 731–737, 2013 [DOI] [PubMed] [Google Scholar]
- 18.McCarty MF, DiNicolantonio JJ: The molecular biology and pathophysiology of vascular calcification. Postgrad Med 126: 54–64, 2014 [DOI] [PubMed] [Google Scholar]