Abstract
Background and objectives
Impairment of HDL function has been associated with cardiovascular events in patients with kidney failure. The protein composition of HDLs is altered in these patients, presumably compromising the cardioprotective effects of HDLs. This post hoc study assessed the relation of distinct HDL-bound proteins with cardiovascular outcomes in a dialysis population.
Design, setting, participants, & measurements
The concentrations of HDL-associated serum amyloid A (SAA) and surfactant protein B (SP-B) were measured in 1152 patients with type 2 diabetes mellitus on hemodialysis participating in The German Diabetes Dialysis Study who were randomly assigned to double-blind treatment of 20 mg atorvastatin daily or matching placebo. The association of SAA(HDL) and SP-B(HDL) with cardiovascular outcomes was assessed in multivariate regression models adjusted for known clinical risk factors.
Results
High concentrations of SAA(HDL) were significantly and positively associated with the risk of cardiac events (hazard ratio per 1 SD higher, 1.09; 95% confidence interval, 1.01 to 1.19). High concentrations of SP-B(HDL) were significantly associated with all-cause mortality (hazard ratio per 1 SD higher, 1.10; 95% confidence interval, 1.02 to 1.19). Adjustment for HDL cholesterol did not affect these associations.
Conclusions
In patients with diabetes on hemodialysis, SAA(HDL) and SP-B(HDL) were related to cardiac events and all-cause mortality, respectively, and they were independent of HDL cholesterol. These findings indicate that a remodeling of the HDL proteome was associated with a higher risk for cardiovascular events and mortality in patients with ESRD.
Keywords: dialysis, cardiovascular disease, lipids
Introduction
Numerous epidemiologic, clinical, and experimental studies showed an association between low HDLs and increased cardiovascular risk (1). The cardioprotective properties of HDLs are thought to include the efflux of cholesterol from macrophages, enhancement of endothelial function, antioxidative and anti-inflammatory activities (2–5). Emerging evidence suggests that the mere cholesterol content of HDL (HDL-C) is insufficient to estimate the cardioprotective functions of HDLs. Pharmacologic increase of HDL-C was not uniformly associated with a reduction of cardiovascular risk in several high-risk populations (6–9). Moreover, the ability of HDLs to promote cholesterol removal from macrophages was shown to be inversely associated with cardiovascular risk independent of HDL-C (10). These and other findings raised the hypotheses that merely increasing the cholesterol content of HDLs is not necessarily beneficial and does not reflect HDL functionality (11–16).
Patients with CKD harbor an exceptionally high cardiovascular risk (17,18). Despite a direct association of low HDL-C with reduced kidney function (19,20), the relation of HDL-C with cardiovascular events and mortality in CKD and ESRD is inconsistent. For example, a study found that HDL-C was predictive of incident myocardial infarction in Japanese patients on hemodialysis without history of cardiovascular disease (CVD) (21), whereas no association of HDL-C with all-cause or cardiovascular mortality was found in European patients with CKD (22,23). Importantly, a recent post hoc analysis of the cohort from The German Diabetes Dialysis Study (the 4D Study) has shown that HDL-C is not predictive of all-cause mortality or cardiovascular events in this population (23). Additionally, HDL functions are severely impaired in patients with ESRD and importantly, directly related to structural alterations of HDLs (24–27). Together, this evidence indicates that the effect of HDL-C is modulated by renal function and that the atheroprotective HDL particles may be rendered dysfunctional in the setting of CKD.
HDLs are a family of heterogeneous particles that vary 4-fold in cholesterol content (28,29) and carry a protein cargo of >50 different proteins (30,31). The protein composition of HDLs emerges as important determinant when investigating the association between HDL function or concentration and CVD (30,32). For example, individual proteins were found enriched or depleted in HDLs from patients with diseases that bear major cardiovascular risks, such as ESRD, coronary artery disease, or rheumatoid arthritis (25,26,31,33–35). Most consistently, serum amyloid A (SAA) is enriched in HDLs isolated from patients with these diseases compared with the HDLs from healthy individuals. Moreover, SAA accumulation in HDLs contributes to decreased protective functions of HDLs, including reduced anti-inflammatory properties (25,33). In addition, surfactant protein B (SP-B) was found highly enriched in HDLs from individuals with ESRD and coronary artery disease (25,32). These findings suggest that remodeling of the HDL proteome contributes to the cardiovascular risk in these diseases.
We hypothesized that the amount of SAA and SP-B bound to HDLs, which we termed SAA(HDL) and SP-B(HDL), respectively, from patients with kidney failure might affect clinical events or mortality. Therefore, we performed a post hoc analysis of the 4D Study by measurement of SAA(HDL) and SP-B(HDL) concentrations in patients with type 2 diabetes on hemodialysis and assessed their associations with defined cardiovascular end points and mortality.
Materials and Methods
Design, Setting, and Participants
Design and methods of the 4D Study have been reported previously (36–38). The 4D Study was a double-blinded, randomized, multicenter trial including 1255 patients with type 2 diabetes mellitus who were 18–80 years of age and had a previous duration of hemodialysis of <2 years. Between March of 1998 and October of 2002, patients were recruited and randomly assigned to receive either treatment with 20 mg atorvastatin one time daily (n=619) or placebo (n=636). Participants were followed up at 4 weeks and every 6 months after randomization. At each follow-up visit, a blood sample was taken, and information about any suspected end point or serious adverse event was recorded.
Laboratory Procedures
We determined the concentrations of SAA(HDL) and SP-B(HDL) at baseline in available serum samples from the 4D Study with a newly developed ELISA. Briefly, apolipoprotein B–depleted serum was added to human HDL antibody–coated ELISA plates, and SAA(HDL) or SP-B(HDL) was detected by respective primary antibodies. The intra-assay values for variability for SAA(HDL) and SP-B(HDL) expressed by the correlation of variance were 10.9% and 21.6%, respectively. The correlation of variance values for the interassay variability were 15.9% for SAA(HDL) and 29.8% for SP-B(HDL). A detailed description and validation of our assay can be found in the Supplemental Material.
End Points
For the post hoc analysis presented here, we evaluated the following end points: (1) combined primary end point (composite of cardiac death, nonfatal myocardial infarction, or stroke), (2) cardiac death, (2a) sudden cardiac death, (3) nonfatal myocardial infarction, (4) fatal stroke, (5) nonfatal stroke, (6) all cardiac events combined (cardiac death and nonfatal myocardial infarction), (7) all cerebrovascular events combined, (8) stroke (fatal and nonfatal combined), (9) all-cause mortality, and (10) non-CVD mortality.
Statistical Analyses
For each marker investigated (i.e., SAA[HDL] and SP-B [HDL]), we examined characteristics of subgroups defined by quartiles of serum concentrations at baseline by calculating descriptive statistics (means and SDs for continuous variables and frequency tables for categorical variables). We compared the distribution of important cardiovascular parameters across quartiles by using ANOVA (for continuous variables) or chi-squared tests (for categorical variables). We used time-to-event analysis (extended Cox regression model allowing for multiple events) aimed to (1) evaluate the association of baseline SAA(HDL) and SP-B(HDL) concentrations on end point occurrence and (2) investigate the effect of baseline marker concentrations on the efficacy of atorvastatin intervention. First, for both markers SAA(HDL) and SP-B(HDL), we conducted a pooled analysis in both randomization groups including a dummy variable for randomization group and an interaction term. Second, because we found no evidence for interaction, we fitted a model without the interaction term. We used two different model parameterizations: one model included the marker quartile as a categorical variable, and one model included the marker quartile as a continuous variable. In the continuous model, the marker values were scaled in units of the SD of the population. Additionally, to further explore effect modification, we fitted efficacy models calculating hazard ratios (HRs) for atorvastatin versus placebo stratified by marker quartiles. All models were adjusted for all significant predictor variables selected for each end point separately by using a stepwise selection procedure (forward: P=0.05; backward: P=0.10) from a set of significant covariates (39). We conducted all statistical analyses using the statistical software package STATA (StataCorp 2011, Stata Statistical Software: Release 12; StataCorp LP, College Station, TX).
Results
Baseline Characteristics of the Study Population According to SAA(HDL) and SP-B(HDL) Quartiles
Characteristics of patient subgroups were determined by defined quartiles of HDL protein levels in serum at baseline. Characteristics of the study patients according to quartiles of SAA(HDL) are shown in Table 1. The mean values of SAA(HDL)±SDs in the quartiles were 1.82 (±0.65), 4.01 (±0.85), 8.35 (±1.82), and 20.29 (±6.00). Remarkably, patients in the fourth quartile showed a 10-fold higher SAA concentration compared with patients in the first quartile. Patients in the highest SAA(HDL) quartile had a higher prevalence of arrhythmia and congestive heart failure and increased C-reactive protein (CRP) levels. In contrast, total cholesterol and triglycerides were lower in higher SAA(HDL) quartiles. There were no differences in the distribution of body mass index, sex, or HDL-C across quartiles of SAA(HDL).
Table 1.
Baseline characteristics of the patients according to quartiles of serum amyloid A (HDL)
| Characteristic | Quartile 1 (n=288) | Quartile 2 (n=288) | Quartile 3 (n=288) | Quartile 4 (n=288) | P Value |
|---|---|---|---|---|---|
| SAA(HDL) | 1.82 (0.65) | 4.01 (0.85) | 8.35 (1.82) | 20.29 (6.00) | |
| SP-B(HDL) | 7.76 (8.86) | 8.71 (8.34) | 9.31 (8.38) | 12.96 (11.80) | <0.001 |
| Age, yr | 65.6 (9.2) | 65.9 (8.2) | 67.3 (8.1) | 66.5 (7.6) | 0.09 |
| Body mass index, kg/m2 | 27.5 (4.8) | 28.1 (4.8) | 27.7 (4.9) | 26.9 (4.6) | 0.02 |
| Sex (men), % | 173 (60) | 145 (50) | 143 (50) | 167 (58) | 0.02 |
| Nonsmoker, n (%) | 168 (58) | 183 (64) | 178 (62) | 159 (56) | 0.26 |
| History, n (%) | |||||
| Arrhythmia | 39 (14) | 50 (17) | 53 (18) | 70 (24) | 0.01 |
| Coronary artery disease | 91 (32) | 76 (26) | 80 (28) | 96 (33) | 0.23 |
| Congestive heart failure | 95 (33) | 81 (28) | 100 (35) | 136 (47) | <0.001 |
| Transitory ischemic attack | 55 (19) | 48 (17) | 54 (19) | 47 (16) | 0.76 |
| Systolic BP, mmHg | 147 (23) | 146 (22) | 145 (21) | 145 (21) | 0.49 |
| Diastolic BP, mmHg | 76 (11) | 77 (11) | 75 (11) | 76 (10) | 0.39 |
| Total cholesterol, mg/dl | 226.6 (45.3) | 223.1 (40.9) | 219.2 (40.3) | 208.7 (39.8) | <0.001 |
| LDL cholesterol, mg/dl | 125 (29) | 129 (30) | 128 (30) | 121 (28) | <0.01 |
| HDL cholesterol, mg/dl | 35 (13) | 37 (13) | 38 (14) | 35 (13) | 0.02 |
| Apolipoprotein A-I, mg/dl | 127.8 (25.6) | 129.1 (23.3) | 127.3 (23.5) | 120.8 (21.3) | <0.001 |
| Triglycerides, mg/dl | 263.5 [216.5] | 214.0 [161.5] | 196.5 [149.5] | 207.0 [173.5] | <0.001 |
| Phosphate, mg/L | 6.2 (1.5) | 6.0 (1.5) | 6.0 (1.6) | 6.0 (1.8) | 0.33 |
| C-reactive protein, mg/L | 2.9 [4.4] | 4.7 [9.1] | 8.0 [7.4] | 11.1 [15.8] | <0.001 |
| Albumin, g/dl | 3.9 (0.3) | 3.9 (0.3) | 3.8 (0.3) | 3.7 (0.3) | <0.001 |
| Hemoglobin, g/dl | 10.9 (1.2) | 11.1 (1.3) | 11.1 (1.4) | 10.6 (1.4) | <0.001 |
| HbA1c, % | 6.6 (1.2) | 6.8 (1.2) | 6.7 (1.2) | 6.8 (1.3) | 0.22 |
Data shown are means (SDs) or medians [interquartile ranges] if not otherwise mentioned. P values for comparison of groups were derived from ANOVA models (for continuous variables) or logistic regression models (for categorical variables).
Characteristics of the patients according to quartiles of baseline SP-B(HDL) are presented in Table 2. The mean values of SP-B(HDL)±SDs in the quartiles were 1.66 (±1.36), 4.92 (±0.91), 9.19 (±1.75), and 22.98 (±10.11). Similar to SAA, SP-B(HDL) was >10-fold higher in the fourth quartile compared with the first quartile. Individuals in higher SP-B(HDL) quartiles had an increased prevalence of congestive heart failure and lower total cholesterol and triglycerides. Patients in higher quartiles of SAA(HDL) and SP-B(HDL) had higher SP-B(HDL) and SAA(HDL), respectively (Tables 1 and 2), which were corroborated by a positive bivariate Spearman correlation coefficient (r=0.23, P<0.001).
Table 2.
Baseline characteristics of the patients according to quartiles of surfactant protein B (HDL)
| Characteristic | Quartile 1 (n=288) | Quartile 2 (n=288) | Quartile 3 (n=288) | Quartile 4 (n=288) | P Value |
|---|---|---|---|---|---|
| SP-B(HDL) | 1.66 (1.36) | 4.92 (0.91) | 9.19 (1.75) | 22.98 (10.11) | |
| SAA(HDL) | 6.25 (5.90) | 8.43 (7.83) | 9.11 (7.82) | 10.74 (8.76) | <0.001 |
| Age, yr | 63.8 (8.3) | 65.8 (8.2) | 67.3 (8.0) | 68.3 (7.9) | <0.001 |
| Body mass index, kg/m2 | 28.5 (5.2) | 28.0 (4.7) | 27.0 (4.5) | 26.7 (4.5) | <0.001 |
| Sex (men), % | 163 (57) | 160 (56) | 157 (55) | 148 (51) | 0.62 |
| Nonsmoker, n (%) | 176 (61) | 176 (61) | 176 (61) | 160 (56) | 0.49 |
| History, n (%) | |||||
| Arrhythmia | 50 (17) | 39 (14) | 59 (20) | 64 (22) | 0.04 |
| Coronary artery disease | 71 (25) | 79 (27) | 93 (32) | 100 (35) | 0.04 |
| Congestive heart failure | 82 (28) | 90 (31) | 121 (42) | 119 (41) | <0.001 |
| Transitory ischemic attack | 49 (17) | 59 (20) | 48 (17) | 48 (17) | 0.56 |
| Systolic BP, mmHg | 145 (22) | 146 (22) | 147 (22) | 145 (22) | 0.59 |
| Diastolic BP, mmHg | 75 (10) | 76 (11) | 77 (12) | 75 (11) | 0.62 |
| Total cholesterol, mg/dl | 228.9 (43.6) | 222.1 (42.0) | 214.0 (39.4) | 212.6 (41.5) | <0.001 |
| LDL cholesterol, mg/dl | 126 (30) | 127 (30) | 126 (27) | 124 (30) | 0.70 |
| HDL cholesterol, mg/dl | 36 (15) | 35 (11) | 36 (12) | 39 (15) | <0.01 |
| Apolipoprotein A-I, mg/dl | 127.8 (24.5) | 126.5 (22.3) | 124.6 (23.5) | 126.0 (24.4) | 0.45 |
| Triglycerides, mg/dl | 267.0 [239.0] | 238.5 [185.0] | 203.0 [150.0] | 185.0 [131.5] | <0.001 |
| Phosphate, mg/L | 6.3 (1.5) | 6.1 (1.5) | 5.8 (1.6) | 6.0 (1.8) | 0.002 |
| C-reactive protein, mg/L | 5.9 [8.8] | 5.9 [8.6] | 6.0 [8.3] | 7.0 [9.3] | 0.68 |
| Albumin, g/dl | 3.9 (0.3) | 3.8 (0.3) | 3.8 (0.3) | 3.8 (0.3) | <0.001 |
| Hemoglobin, g/dl | 10.9 (1.3) | 11.0 (1.4) | 10.9 (1.3) | 10.8 (1.3) | 0.21 |
| HbA1c, % | 6.9 (1.2) | 6.7 (1.2) | 6.7 (1.3) | 6.6 (1.2) | 0.06 |
Data shown are means (SDs) or medians [interquartile ranges] if not otherwise mentioned. P values for comparison of groups were derived from ANOVA models (for continuous variables) or logistic regression models (for categorical variables).
SAA(HDL) and Cardiac Events
In an adjusted multivariate Cox regression analysis examining potential associations of SAA(HDL) with the predefined study end points (described in Materials and Methods), the concentrations of SAA(HDL) at baseline were significantly associated with the end point of all cardiac events combined (HR per 1 SD higher in SAA[HDL] levels, 1.09; 95% confidence interval, 1.01 to 1.19; P=0.04) but not with the other end points (Table 3, Supplemental Table 1). The HRs for all cardiac events combined increased in parallel with the quartiles of SAA(HDL), and in about one half of the patients in the fourth quartile, cardiac events were observed (Figure 1, Table 3). Additional adjustment for HDL-C, apolipoprotein A-I, or CRP did not affect the association between SAA(HDL) and cardiac events (Supplemental Figure 9, Supplemental Table 1). Additional adjustment for SP-B(HDL) slightly decreased the association between SAA(HDL) and cardiac events indicated by the decreased P values (Supplemental Table 1). We found a potential effect modification of SAA(HDL) according to atorvastatin treatment on cardiac events described in detail in the Supplemental Material (Supplemental Material, Supplemental Figure 11), although the overall effect was not significant (P=0.27). For all other end points investigated, we did not observe any significant interaction.
Table 3.
End points according to quartiles of serum amyloid A (HDL)
| End Points | SAA(HDL) Quartile 1 | SAA(HDL) Quartile 2 | SAA(HDL) Quartile 3 | SAA(HDL) Quartile 4 | P value (chi square) | ||||
|---|---|---|---|---|---|---|---|---|---|
| No. | HR | No. | HR (95% CI) | No. | HR (95% CI) | No. | HR (95% CI) | ||
| Combined primary end point | 110 | 1.00 (reference) | 126 | 1.22 (0.94 to 1.58) | 129 | 1.31 (1.02 to 1.70) | 134 | 1.25 (0.96 to 1.63) | 0.18 |
| Death from cardiac causes | 59 | 1.00 (reference) | 57 | 1.03 (0.71 to 1.48) | 58 | 1.06 (0.73 to 1.53) | 72 | 1.28 (0.90 to 1.82) | 0.51 |
| Sudden cardiac death | 36 | 1.00 (reference) | 30 | 0.87 (0.53 to 1.42) | 35 | 1.06 (0.66 to 1.70) | 45 | 1.42 (0.90 to 2.23) | 0.20 |
| Nonfatal myocardial infarction | 33 | 1.00 (reference) | 41 | 1.42 (0.89 to 2.25) | 43 | 1.60 (1.01 to 2.53) | 34 | 1.27 (0.77 to 2.08) | 0.24 |
| Fatal stroke | 6 | 1.00 (reference) | 10 | 1.74 (0.62 to 4.85) | 7 | 1.17 (0.39 to 3.52) | 11 | 1.42 (0.66 to 3.06) | 0.72 |
| Nonfatal stroke | 12 | 1.00 (reference) | 18 | 1.47 (0.71 to 3.06) | 21 | 1.71 (0.84 to 3.49) | 17 | 1.42 (0.66 to 3.06) | 0.53 |
| All cardiac events combined | 122 | 1.00 (reference) | 134 | 1.22 (0.95 to 1.56) | 132 | 1.28 (0.10 to 1.64) | 146 | 1.43 (1.12 to 1.82) | 0.04 |
| All cerebrovascular events combined | 39 | 1.00 (reference) | 35 | 0.86 (0.54 to 1.36) | 43 | 1.10 (0.71 to 1.70) | 44 | 1.38 (0.89 to 2.13) | 0.21 |
| Stroke | 18 | 1.00 (reference) | 28 | 1.57 (0.87 to 2.85) | 28 | 1.55 (0.85 to 2.80) | 28 | 1.47 (0.80 to 2.71) | 0.44 |
| Death (all causes) | 130 | 1.00 (reference) | 118 | 0.97 (0.75 to 1.27) | 141 | 1.15 (0.90 to 1.47) | 175 | 1.22 (0.95 to 1.57) | 0.21 |
| Death (noncardiovascular disease causes) | 65 | 1.00 (reference) | 51 | 0.86 (0.60 to 1.24) | 76 | 1.24 (0.89 to 1.73) | 92 | 1.45 (1.04 to 2.03) | 0.02 |
P value of multivariate Cox regression model comparing the hazard ratios across the four groups. Risk factors included in the multivariate regression model were age, sex, phosphate, use of calcium antagonists, history of coronary artery disease, arrhythmia, congestive heart failure, peripheral vascular disease, and atorvastatin treatment. SAA, serum amyloid A; HR, hazard ratio; 95% CI, 95% confidence interval.
Figure 1.
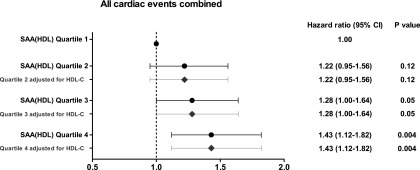
Hazard ratios for cardiac events according to SAA(HDL) quartiles. The dots and horizontal lines indicate HR points with 95% CI, respectively. The analysis was adjusted for following predictor variables: age, gender, phosphate, use of calcium antagonists, history of coronary artery disease, arrythmia, congestive heart failure, peripheral vascular disease. The squares and horizontal lines indicate HR points with 95% CI, respectively, adjusted for predictor variables and HDL-C.
SP-B(HDL) and All-Cause Mortality
Adjusted multivariate Cox regression analysis revealed that SP-B(HDL) was significantly associated with all-cause mortality and the single components death from cardiac causes, sudden cardiac death, and non-CVD death (Figure 2, Table 4). Notably, high SP-B(HDL) concentrations were associated with all-cause mortality (HR per 1 SD higher in SP-B [HDL] levels, 1.10; 95% confidence interval, 1.02 to 1.19; P=0.01), and about one half of the patients in the third quartile as well as approximately two thirds of the patients in the fourth quartile died (Table 4, Supplemental Table 2). This association remained significant after additional adjustment for HDL-C and SAA(HDL) (Supplemental Figure 10, Supplemental Table 2). Moreover, additional adjustment for apolipoprotein A-I or CRP did not alter the association between SP-B(HDL) and all-cause mortality (Supplemental Table 2). We did not observe any significant effect modification of atorvastatin treatment by SP-B(HDL) on any of the defined end points.
Figure 2.
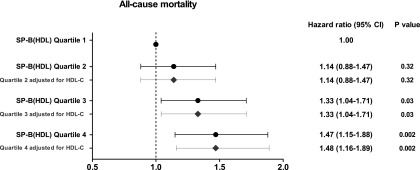
Hazard ratios for all-cause mortality according to SP-B(HDL) quartiles. The dots and horizontal lines indicate HR points with 95% CI, respectively. The analysis was adjusted for following predictor variables: age, gender, body mass index, total cholesterol, albumin, hemoglobin, glycated hemoglobin, phosphate, leukocytes, serum creatinine, diastolic blood pressure, duration of dialysis, duration of diabtets, use of statins, use of calcium antagonists, use of angiotension-converting enzyme inhibitors, history of coronary artery disease, ischemia, arrythmia, congestive heart failure, peripheral vascular disease. The squares and horizontal lines indicate HR points with 95% CI, respectively, adjusted for predictor variables and HDL-C.
Table 4.
End points according to quartiles of surfactant protein B (HDL)
| End Points | SP-B(HDL) Quartile 1 | SP-B(HDL) Quartile 2 | SP-B(HDL) Quartile 3 | SP-B(HDL) Quartile 4 | P value (chi square) | ||||
|---|---|---|---|---|---|---|---|---|---|
| No. | HR | No. | HR (95% CI) | No. | HR (95% CI) | No. | HR (95% CI) | ||
| Combined primary end point | 120 | 1.00 (reference) | 117 | 1.05 (0.81 to 1.35) | 118 | 1.15 (0.88 to 1.49) | 144 | 1.35 (1.05 to 1.73) | 0.08 |
| Death from cardiac causes | 56 | 1.00 (reference) | 52 | 1.01 (0.69 to 1.48) | 60 | 1.19 (0.82 to 1.72) | 78 | 1.58 (1.12 to 2.25) | 0.03 |
| Sudden cardiac death | 31 | 1.00 (reference) | 30 | 1.06 (0.64 to 1.76) | 36 | 1.29 (0.79 to 2.11) | 49 | 1.84 (1.16 to 2.91) | 0.03 |
| Nonfatal myocardial infarction | 47 | 1.00 (reference) | 35 | 0.81 (0.52 to 1.26) | 36 | 1.00 (0.64 to 1.56) | 33 | 0.91 (0.57 to 1.44) | 0.08 |
| Fatal stroke | 7 | 1.00 (reference) | 9 | 1.35 (0.50 to 3.66) | 4 | 0.63 (0.18 to 2.18) | 14 | 1.97 (0.76 to 5.09) | 0.19 |
| Nonfatal stroke | 10 | 1.00 (reference) | 21 | 2.24 (1.05 to 4.79) | 18 | 1.89 (0.86 to 4.13) | 19 | 1.91 (0.88 to 4.17) | 0.21 |
| All cardiac events combined | 143 | 1.00 (reference) | 117 | 0.87 (0.68 to 1.11) | 132 | 1.08 (0.85 to 1.38) | 142 | 1.17 (0.92 to 1.48) | 0.11 |
| All cerebrovascular events combined | 32 | 1.00 (reference) | 40 | 1.29 (0.81 to 2.07) | 34 | 1.18 (0.72 to 1.93) | 55 | 1.83 (1.17 to 2.85) | 0.04 |
| Stroke | 17 | 1.00 (reference) | 30 | 1.79 (0.98 to 3.26) | 22 | 1.47 (0.77 to 2.80) | 33 | 2.02 (1.11 to 3.68) | 0.12 |
| Death (all causes) | 117 | 1.00 (reference) | 128 | 1.14 (0.88 to 1.47) | 148 | 1.33 (1.04 to 1.71) | 171 | 1.47 (1.15 to 1.88) | 0.01 |
| Death (noncardiovascular disease causes) | 54 | 1.00 (reference) | 67 | 1.21 (0.84 to 1.75) | 84 | 1.63 (1.15 to 2.31) | 79 | 1.40 (0.98 to 2.00) | 0.05 |
P value of multivariate Cox regression model comparing the hazard ratios across the four groups. Risk factors included in the multivariate regression model were age, sex, body mass index, total cholesterol, albumin, hemoglobin, glycated hemoglobin, phosphate, leukocytes, serum creatinine, diastolic BP, duration of dialysis, duration of diabetes, use of statins, use of calcium antagonists, use of angiotension-coverting enzyme inhibitors, history of coronary artery disease, ischemia, arrhythmia, congestive heart failure, peripheral vascular disease, and atorvastatin treatment. SP-B, surfactant protein B; 95% CI, 95% confidence interval.
Discussion
In this post hoc analysis of the 4D Study, we found that the HDL-associated proteins SAA and SP-B were independently associated with cardiac events and overall mortality. The associations were robust against adjustment for potential confounding variables and HDL-C. This is the first study that evaluated and found an association of distinct disease-specific proteins directly on HDL particles with cardiovascular events and all-cause mortality. Moreover, these data suggest that the increased incorporation of SAA and SP-B into HDL particles might contribute to the high risk of cardiovascular events and mortality in these patients.
Patients with ESRD requiring dialysis are among the highest risk groups for CVD and mortality (17,18). Despite reduced serum HDL-C concentrations in ESRD, most studies in the Western population did not show a clear association of HDL-C with survival or cardiac events (40–42). In contrast, HDL-C was associated with a lower risk for myocardial infarction in the Japanese population (21). The association between higher HDL-C and reduced mortality risk and coronary artery disease severity is already abrogated in patients with only mildly reduced kidney function (22). This indicates that the HDL particles not only lose their atheroprotective properties in the setting of CKD but, also, are converted into deleterious molecules. In line, accumulating data suggests that HDLs are remodeled in patients with ESRD, acquire new proteins, such as SAA or SP-B, and thus, may become dysfunctional (25–27,33). A similar conversion of HDLs to presumably dysfunctional HDLs is observed in coronary artery disease and rheumatoid arthritis (31,34). The increased risk of cardiovascular events in ESRD results from pathophysiologic processes specific to CKD, making the prevention of CVD by interventions targeting traditional risk factors difficult (17). Individuals with diabetes constitute one of the largest groups of patients on dialysis and are at higher risk of cardiovascular events and mortality compared with the nondiabetic ESRD population, highlighting the importance of elucidating novel risk factors and the underlying pathophysiologic pathways (43,44).
The enrichment of distinct proteins in HDLs in various diseases states evolves as an important determinant for associations between HDL function and cardiovascular risk (16,30,45). Our improved understanding of the composition of HDLs can enable pharmacologic attempts in manipulating HDL composition and function to reduce cardiovascular risk. Moreover, quantification of specific HDL proteins as markers of impaired cardioprotective quality might be used to establish novel diagnostic biomarkers and assess the effectiveness of current and new therapeutic interventions (45). This concept is supported by our findings indicating that dysfunctional HDLs, measured by the incorporation of SAA and SP-B, occur in human chronic inflammatory syndromes and are associated with clinical events and mortality. Nevertheless, our results do not prove causality; remodeling of HDLs either has a direct pathophysiologic role or is secondary to other processes that contribute to disease etiology.
SAA is an apolipoprotein found predominantly in HDLs (46). SAA is a major acute-phase protein that is secreted in large amounts by the liver during infections or inflammatory processes. Increased incorporation of SAA into HDLs was documented in a number of chronic inflammatory diseases, most prominently in patients on dialysis, indicating that the presence of SAA in HDLs is a marker of ongoing inflammation (25,26,31,33,34). This hypothesis is supported by the correlation between SAA(HDL) and CRP in our study. However, only about 10% of the variance of CRP concentrations could be explained by SAA(HDL) quartiles (R2=0.10), and adjustment for CRP did not affect the association between SAA(HDL) and cardiac events.
SP-B has been detected in the HDLs of patients with ESRD and coronary artery disease (25,32). Moreover, SP-B accumulates in blood of individuals with acute respiratory distress syndrome and chronic heart failure (47,48). In addition, serum SP-B was associated with abdominal aortic plaques in current smokers (48). Pulmonary edema and pleural effusion are common in chronic heart failure and ESRD and may result in damage to the alveolar-capillary membrane. Consequently, SP-B is released into the circulation and may become incorporated into HDLs. SP-B(HDL) was not associated with CRP in our study, and adjustment for SAA(HDL) did not alleviate the association between SP-B(HDL) and mortality, suggesting that the pathogenetic mechanism that promotes incorporation of SP-B into HDLs is independent of inflammation.
There is increasing evidence that HDL-C does not affect cardiovascular events in different patient populations, because the amount of cholesterol within the HDL fraction does not necessarily correlate with the biologic functions of HDLs (6–8,11–13,49). Consequently, there is a strong need to develop new metrics for HDL functionality that are technically feasible and provide clinically applicable and relevant information in large and diverse populations. In such an effort, it was shown that the ability of HDLs to promote cholesterol efflux from macrophages correlates better and inversely with cardiovascular risk (10). However, this method of quantifying HDL function is technically challenging and therefore, unlikely to be widely applicable to clinical studies. In addition, HDL size and particle number assessed by nuclear magnetic resonance spectroscopy showed to be better predictors of cardiovascular risk than serum concentrations of HDL-C (50). Another approach that has been recently proposed to assess HDL quality is to monitor the protein cargo of HDLs (49). The findings of our study support this concept and provide evidence that quantification of individual HDL-associated proteins by a simple ELISA-based approach is useful to identify modified HDLs that are potentially relevant for clinical end points. Our results support the hypotheses that dysfunctional HDLs translate into clinical events and that quantification of specific HDL proteins may be used as surrogates for HDL functions.
To evaluate the full potential of SAA(HDL) and SP-B(HDL) for adverse clinical events in renal failure as well as other high-risk populations, additional studies are necessary to link HDL-associated proteins with cardiovascular risk association. Moreover, these markers might prove useful in earlier stages of CKD for assessing the risk of disease progression. Taking into account that HDL-C is associated with renal function (19,20,22), combined assessment of HDL proteins together with HDL-C could help to monitor disease progression and risk of cardiovascular outcome.
One limitation of our study is its post hoc design, and the results should be interpreted as explorative. For this reason, our data should be viewed as hypothesis-generating and thus, require additional evaluation in further studies.
In conclusion, our study provides evidence that SAA and SP-B measured directly on HDLs from patients with type 2 diabetes mellitus on hemodialysis are associated with cardiac events and mortality, respectively, independent of HDL-C. These findings suggest that remodeling of the HDL proteome contributes to the increased risk of cardiovascular events and mortality in patients with kidney disease.
Disclosures
None.
Supplementary Material
Acknowledgments
This study was supported by Else Kröner Fresenius-Stiftung Grant 010_A86 (to M.D.S. and T.W.).
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.06560714/-/DCSupplemental.
References
- 1.Gordon DJ, Probstfield JL, Garrison RJ, Neaton JD, Castelli WP, Knoke JD, Jacobs DR, Jr., Bangdiwala S, Tyroler HA: High-density lipoprotein cholesterol and cardiovascular disease. Four prospective American studies. Circulation 79: 8–15, 1989 [DOI] [PubMed] [Google Scholar]
- 2.Rye KA, Barter PJ: Cardioprotective functions of HDLs. J Lipid Res 55: 168–179, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Navab M, Reddy ST, Van Lenten BJ, Fogelman AM: HDL and cardiovascular disease: Atherogenic and atheroprotective mechanisms. Nat Rev Cardiol 8: 222–232, 2011 [DOI] [PubMed] [Google Scholar]
- 4.Säemann MD, Poglitsch M, Kopecky C, Haidinger M, Hörl WH, Weichhart T: The versatility of HDL: A crucial anti-inflammatory regulator. Eur J Clin Invest 40: 1131–1143, 2010 [DOI] [PubMed] [Google Scholar]
- 5.Rosenson RS, Brewer HB, Jr., Davidson WS, Fayad ZA, Fuster V, Goldstein J, Hellerstein M, Jiang XC, Phillips MC, Rader DJ, Remaley AT, Rothblat GH, Tall AR, Yvan-Charvet L: Cholesterol efflux and atheroprotection: Advancing the concept of reverse cholesterol transport. Circulation 125: 1905–1919, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schaefer EJ: Effects of cholesteryl ester transfer protein inhibitors on human lipoprotein metabolism: Why have they failed in lowering coronary heart disease risk? Curr Opin Lipidol 24: 259–264, 2013 [DOI] [PubMed] [Google Scholar]
- 7.Barter PJ, Caulfield M, Eriksson M, Grundy SM, Kastelein JJ, Komajda M, Lopez-Sendon J, Mosca L, Tardif JC, Waters DD, Shear CL, Revkin JH, Buhr KA, Fisher MR, Tall AR, Brewer B, ILLUMINATE Investigators : Effects of torcetrapib in patients at high risk for coronary events. N Engl J Med 357: 2109–2122, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Schwartz GG, Olsson AG, Barter PJ: Dalcetrapib in patients with an acute coronary syndrome. N Engl J Med 368: 869–870, 2013 [DOI] [PubMed] [Google Scholar]
- 9.Mahdy Ali K, Wonnerth A, Huber K, Wojta J: Cardiovascular disease risk reduction by raising HDL cholesterol—current therapies and future opportunities. Br J Pharmacol 167: 1177–1194, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khera AV, Cuchel M, de la Llera-Moya M, Rodrigues A, Burke MF, Jafri K, French BC, Phillips JA, Mucksavage ML, Wilensky RL, Mohler ER, Rothblat GH, Rader DJ: Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N Engl J Med 364: 127–135, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Briel M, Ferreira-Gonzalez I, You JJ, Karanicolas PJ, Akl EA, Wu P, Blechacz B, Bassler D, Wei X, Sharman A, Whitt I, Alves da Silva S, Khalid Z, Nordmann AJ, Zhou Q, Walter SD, Vale N, Bhatnagar N, O’Regan C, Mills EJ, Bucher HC, Montori VM, Guyatt GH: Association between change in high density lipoprotein cholesterol and cardiovascular disease morbidity and mortality: Systematic review and meta-regression analysis. BMJ 338: b92, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Voight BF, Peloso GM, Orho-Melander M, Frikke-Schmidt R, Barbalic M, Jensen MK, Hindy G, Hólm H, Ding EL, Johnson T, Schunkert H, Samani NJ, Clarke R, Hopewell JC, Thompson JF, Li M, Thorleifsson G, Newton-Cheh C, Musunuru K, Pirruccello JP, Saleheen D, Chen L, Stewart A, Schillert A, Thorsteinsdottir U, Thorgeirsson G, Anand S, Engert JC, Morgan T, Spertus J, Stoll M, Berger K, Martinelli N, Girelli D, McKeown PP, Patterson CC, Epstein SE, Devaney J, Burnett MS, Mooser V, Ripatti S, Surakka I, Nieminen MS, Sinisalo J, Lokki ML, Perola M, Havulinna A, de Faire U, Gigante B, Ingelsson E, Zeller T, Wild P, de Bakker PI, Klungel OH, Maitland-van der Zee AH, Peters BJ, de Boer A, Grobbee DE, Kamphuisen PW, Deneer VH, Elbers CC, Onland-Moret NC, Hofker MH, Wijmenga C, Verschuren WM, Boer JM, van der Schouw YT, Rasheed A, Frossard P, Demissie S, Willer C, Do R, Ordovas JM, Abecasis GR, Boehnke M, Mohlke KL, Daly MJ, Guiducci C, Burtt NP, Surti A, Gonzalez E, Purcell S, Gabriel S, Marrugat J, Peden J, Erdmann J, Diemert P, Willenborg C, König IR, Fischer M, Hengstenberg C, Ziegler A, Buysschaert I, Lambrechts D, Van de Werf F, Fox KA, El Mokhtari NE, Rubin D, Schrezenmeir J, Schreiber S, Schäfer A, Danesh J, Blankenberg S, Roberts R, McPherson R, Watkins H, Hall AS, Overvad K, Rimm E, Boerwinkle E, Tybjaerg-Hansen A, Cupples LA, Reilly MP, Melander O, Mannucci PM, Ardissino D, Siscovick D, Elosua R, Stefansson K, O’Donnell CJ, Salomaa V, Rader DJ, Peltonen L, Schwartz SM, Altshuler D, Kathiresan S: Plasma HDL cholesterol and risk of myocardial infarction: A mendelian randomisation study. Lancet 380: 572–580, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Capelleveen JC, Bochem AE, Motazacker MM, Hovingh GK, Kastelein JJ: Genetics of HDL-C: A causal link to atherosclerosis? Curr Atheroscler Rep 15: 326, 2013 [DOI] [PubMed] [Google Scholar]
- 14.Ansell BJ, Navab M, Hama S, Kamranpour N, Fonarow G, Hough G, Rahmani S, Mottahedeh R, Dave R, Reddy ST, Fogelman AM: Inflammatory/antiinflammatory properties of high-density lipoprotein distinguish patients from control subjects better than high-density lipoprotein cholesterol levels and are favorably affected by simvastatin treatment. Circulation 108: 2751–2756, 2003 [DOI] [PubMed] [Google Scholar]
- 15.Ray K, Wainwright NW, Visser L, Witteman J, Breteler M, Ambegaonkar B, Hofman A, Stricker B, Wareham N, Khaw KT, Sandhu M: Changes in HDL cholesterol and cardiovascular outcomes after lipid modification therapy. Heart 98: 780–785, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Toth PP, Barter PJ, Rosenson RS, Boden WE, Chapman MJ, Cuchel M, D’Agostino RB, Sr., Davidson MH, Davidson WS, Heinecke JW, Karas RH, Kontush A, Krauss RM, Miller M, Rader DJ: High-density lipoproteins: A consensus statement from the National Lipid Association. J Clin Lipidol 7: 484–525, 2013 [DOI] [PubMed] [Google Scholar]
- 17.Gansevoort RT, Correa-Rotter R, Hemmelgarn BR, Jafar TH, Heerspink HJ, Mann JF, Matsushita K, Wen CP: Chronic kidney disease and cardiovascular risk: Epidemiology, mechanisms, and prevention. Lancet 382: 339–352, 2013 [DOI] [PubMed] [Google Scholar]
- 18.Vaziri ND, Navab M, Fogelman AM: HDL metabolism and activity in chronic kidney disease. Nat Rev Nephrol 6: 287–296, 2010 [DOI] [PubMed] [Google Scholar]
- 19.Wang F, Zheng J, Ye P, Luo L, Bai Y, Xu R, Sheng L, Xiao T, Wu H: Association of high-density lipoprotein cholesterol with the estimated glomerular filtration rate in a community-based population. PLoS ONE 8: e79738, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baragetti A, Norata GD, Sarcina C, Rastelli F, Grigore L, Garlaschelli K, Uboldi P, Baragetti I, Pozzi C, Catapano AL: High density lipoprotein cholesterol levels are an independent predictor of the progression of chronic kidney disease. J Intern Med 274: 252–262, 2013 [DOI] [PubMed] [Google Scholar]
- 21.Shoji T, Masakane I, Watanabe Y, Iseki K, Tsubakihara Y, Committee of Renal Data Registry, Japanese Society for Dialysis Therapy : Elevated non-high-density lipoprotein cholesterol (non-HDL-C) predicts atherosclerotic cardiovascular events in hemodialysis patients. Clin J Am Soc Nephrol 6: 1112–1120, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zewinger S, Speer T, Kleber ME, Scharnagl H, Woitas R, Lepper PM, Pfahler K, Seiler S, Heine GH, März W, Silbernagel G, Fliser D: HDL cholesterol is not associated with lower mortality in patients with kidney dysfunction. J Am Soc Nephrol 25: 1073–1082, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Silbernagel G, Genser B, Drechsler C, Scharnagl H, Grammer TB, Stojakovic T, Krane V, Ritz E, Wanner C, März W: HDL cholesterol, apolipoproteins, and cardiovascular risk in hemodialysis patients [published online ahead of print July 10, 2014]. J Am Soc Nephrol doi:ASN.2013080816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kalantar-Zadeh K, Kopple JD, Kamranpour N, Fogelman AM, Navab M: HDL-inflammatory index correlates with poor outcome in hemodialysis patients. Kidney Int 72: 1149–1156, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Weichhart T, Kopecky C, Kubicek M, Haidinger M, Döller D, Katholnig K, Suarna C, Eller P, Tölle M, Gerner C, Zlabinger GJ, van der Giet M, Hörl WH, Stocker R, Säemann MD: Serum amyloid A in uremic HDL promotes inflammation. J Am Soc Nephrol 23: 934–947, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Holzer M, Birner-Gruenberger R, Stojakovic T, El-Gamal D, Binder V, Wadsack C, Heinemann A, Marsche G: Uremia alters HDL composition and function. J Am Soc Nephrol 22: 1631–1641, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yamamoto S, Yancey PG, Ikizler TA, Jerome WG, Kaseda R, Cox B, Bian A, Shintani A, Fogo AB, Linton MF, Fazio S, Kon V: Dysfunctional high-density lipoprotein in patients on chronic hemodialysis. J Am Coll Cardiol 60: 2372–2379, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rothblat GH, Phillips MC: High-density lipoprotein heterogeneity and function in reverse cholesterol transport. Curr Opin Lipidol 21: 229–238, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang R, Silva RA, Jerome WG, Kontush A, Chapman MJ, Curtiss LK, Hodges TJ, Davidson WS: Apolipoprotein A-I structural organization in high-density lipoproteins isolated from human plasma. Nat Struct Mol Biol 18: 416–422, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gordon S, Durairaj A, Lu JL, Davidson WS: High-density lipoprotein proteomics: Identifying new drug targets and biomarkers by understanding functionality. Curr Cardiovasc Risk Rep 4: 1–8, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vaisar T, Pennathur S, Green PS, Gharib SA, Hoofnagle AN, Cheung MC, Byun J, Vuletic S, Kassim S, Singh P, Chea H, Knopp RH, Brunzell J, Geary R, Chait A, Zhao XQ, Elkon K, Marcovina S, Ridker P, Oram JF, Heinecke JW: Shotgun proteomics implicates protease inhibition and complement activation in the antiinflammatory properties of HDL. J Clin Invest 117: 746–756, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Riwanto M, Rohrer L, Roschitzki B, Besler C, Mocharla P, Mueller M, Perisa D, Heinrich K, Altwegg L, von Eckardstein A, Lüscher TF, Landmesser U: Altered activation of endothelial anti- and proapoptotic pathways by high-density lipoprotein from patients with coronary artery disease: Role of high-density lipoprotein-proteome remodeling. Circulation 127: 891–904, 2013 [DOI] [PubMed] [Google Scholar]
- 33.Tölle M, Huang T, Schuchardt M, Jankowski V, Prüfer N, Jankowski J, Tietge UJ, Zidek W, van der Giet M: High-density lipoprotein loses its anti-inflammatory capacity by accumulation of pro-inflammatory-serum amyloid A. Cardiovasc Res 94: 154–162, 2012 [DOI] [PubMed] [Google Scholar]
- 34.Watanabe J, Charles-Schoeman C, Miao Y, Elashoff D, Lee YY, Katselis G, Lee TD, Reddy ST: Proteomic profiling following immunoaffinity capture of high-density lipoprotein: Association of acute-phase proteins and complement factors with proinflammatory high-density lipoprotein in rheumatoid arthritis. Arthritis Rheum 64: 1828–1837, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alwaili K, Bailey D, Awan Z, Bailey SD, Ruel I, Hafiane A, Krimbou L, Laboissiere S, Genest J: The HDL proteome in acute coronary syndromes shifts to an inflammatory profile. Biochim Biophys Acta 1821: 405–415, 2012 [DOI] [PubMed] [Google Scholar]
- 36.Wanner C, Krane V, März W, Olschewski M, Asmus HG, Krämer W, Kühn KW, Kütemeyer H, Mann JF, Ruf G, Ritz E, Deutsche Diabetes-Dialyse-Studie (4D) Study Group : Randomized controlled trial on the efficacy and safety of atorvastatin in patients with type 2 diabetes on hemodialysis (4D study): Demographic and baseline characteristics. Kidney Blood Press Res 27: 259–266, 2004 [DOI] [PubMed] [Google Scholar]
- 37.Wanner C, Krane V, März W, Olschewski M, Mann JF, Ruf G, Ritz E, German Diabetes and Dialysis Study Investigators : Atorvastatin in patients with type 2 diabetes mellitus undergoing hemodialysis. N Engl J Med 353: 238–248, 2005 [DOI] [PubMed] [Google Scholar]
- 38.Wanner C, Krane V, Ruf G, März W, Ritz E, Die Deutsche Diabetes Dialyse Studie Investigators : Rationale and design of a trial improving outcome of type 2 diabetics on hemodialysis. Kidney Int Suppl 71: S222–S226, 1999 [DOI] [PubMed] [Google Scholar]
- 39.März W, Genser B, Drechsler C, Krane V, Grammer TB, Ritz E, Stojakovic T, Scharnagl H, Winkler K, Holme I, Holdaas H, Wanner C, German Diabetes and Dialysis Study Investigators : Atorvastatin and low-density lipoprotein cholesterol in type 2 diabetes mellitus patients on hemodialysis. Clin J Am Soc Nephrol 6: 1316–1325, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kwan BC, Kronenberg F, Beddhu S, Cheung AK: Lipoprotein metabolism and lipid management in chronic kidney disease. J Am Soc Nephrol 18: 1246–1261, 2007 [DOI] [PubMed] [Google Scholar]
- 41.Moradi H, Vaziri ND, Kashyap ML, Said HM, Kalantar-Zadeh K: Role of HDL dysfunction in end-stage renal disease: A double-edged sword. J Ren Nutr 23: 203–206, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kilpatrick RD, McAllister CJ, Kovesdy CP, Derose SF, Kopple JD, Kalantar-Zadeh K: Association between serum lipids and survival in hemodialysis patients and impact of race. J Am Soc Nephrol 18: 293–303, 2007 [DOI] [PubMed] [Google Scholar]
- 43.Chang YT, Wu JL, Hsu CC, Wang JD, Sung JM: Diabetes and end-stage renal disease synergistically contribute to increased incidence of cardiovascular events: A nationwide follow-up study during 1998-2009. Diabetes Care 37: 277–285, 2014 [DOI] [PubMed] [Google Scholar]
- 44.Jardine MJ, Hata J, Woodward M, Perkovic V, Ninomiya T, Arima H, Zoungas S, Cass A, Patel A, Marre M, Mancia G, Mogensen CE, Poulter N, Chalmers J, ADVANCE Collaborative Group : Prediction of kidney-related outcomes in patients with type 2 diabetes. Am J Kidney Dis 60: 770–778, 2012 [DOI] [PubMed] [Google Scholar]
- 45.Shah AS, Tan L, Long JL, Davidson WS: Proteomic diversity of high density lipoproteins: Our emerging understanding of its importance in lipid transport and beyond. J Lipid Res 54: 2575–2585, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Whitehead AS, de Beer MC, Steel DM, Rits M, Lelias JM, Lane WS, de Beer FC: Identification of novel members of the serum amyloid A protein superfamily as constitutive apolipoproteins of high density lipoprotein. J Biol Chem 267: 3862–3867, 1992 [PubMed] [Google Scholar]
- 47.Doyle IR, Bersten AD, Nicholas TE: Surfactant proteins-A and -B are elevated in plasma of patients with acute respiratory failure. Am J Respir Crit Care Med 156: 1217–1229, 1997 [DOI] [PubMed] [Google Scholar]
- 48.Nguyen AB, Rohatgi A, Garcia CK, Ayers CR, Das SR, Lakoski SG, Berry JD, Khera A, McGuire DK, de Lemos JA: Interactions between smoking, pulmonary surfactant protein B, and atherosclerosis in the general population: The Dallas Heart Study. Arterioscler Thromb Vasc Biol 31: 2136–2143, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rosenson RS, Brewer HB, Jr., Ansell B, Barter P, Chapman MJ, Heinecke JW, Kontush A, Tall AR, Webb NR: Translation of high-density lipoprotein function into clinical practice: Current prospects and future challenges. Circulation 128: 1256–1267, 2013 [DOI] [PubMed] [Google Scholar]
- 50.Mora S, Glynn RJ, Ridker PM: High-density lipoprotein cholesterol, size, particle number, and residual vascular risk after potent statin therapy. Circulation 128: 1189–1197, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


