Abstract
Background and objectives
High serum IL-6 is a major risk factor for cardiovascular disease (CVD) in the general population. This cytokine is substantially increased in patients with CKD, but it is still unknown whether the link between IL-6 and CVD in CKD is causal in nature.
Design, setting, participants, & measurements
In a cohort of 755 patients with stages 2–5 CKD, consecutively recruited from 22 nephrology units in southern Italy, this study assessed the relationship of serum IL-6 with history of CVD, as well as with incident cardiovascular (CV) events (mean follow up±SD, 31±10 months) and used the functional polymorphism (−174 G/C) in the promoter of the IL-6 gene to investigate whether the link between IL-6 and CV events is causal.
Results
In adjusted analyses, serum IL-6 above the median value was associated with history of CVD (P<0.001) and predicted the incidence rate of CV events (hazard ratio, 1.66; 95% confidence interval [95% CI], 1.11 to 2.49; P=0.01). Patients homozygous for the risk allele (C) of the −174 G/C polymorphism had higher levels of IL-6 than did those with other genotypes (P=0.04). Homozygous CC patients more frequently had a history of CVD (odds ratio, 2.15; 95% CI, 1.15 to 4.00; P=0.02) as well as a 87% higher rate of incident CV events (hazard ratio, 1.87; 95% CI, 1.02 to 3.44; P=0.04) compared with other genotypes.
Conclusions
In patients with stages 2–5 CKD, high serum IL-6 is associated with history of CVD and predicts incident CV events. The parallel relationship with history of CVD and incident CV events of the −174 G/C polymorphism in the IL-6 gene suggests that IL-6 may be causally involved in the high CV risk in this population.
Keywords: epidemiology and outcomes, chronic kidney disease, polymorphisms, cardiovascular, risk factors
Introduction
Classic experimental studies by Russel Ross et al. in the 1990s solidly established inflammation as a critical component of the atherosclerosis process (1). During the last two decades, large cohort studies in the general population have shown strong links between biomarkers of inflammation and cardiovascular (CV) outcomes in the general population (2–5) and in patients with CV disease (CVD) (6,7). Observational studies are methodologically vulnerable for testing causality because these studies are open to various sources of bias and confounding. Mendelian randomization—i.e., the random assortment of alleles at conception—offers an intriguing opportunity to limit problems inherent to observational studies because categorization of patients according to pertinent alleles is a sort of genetic randomization. Genetic variants may thus be used as indicators of environmental exposures in the observational context (8). Large-scale Mendelian randomization studies applying genetic polymorphisms of inflammatory cytokines (9) strongly support the hypothesis that, as seen in experimental animals (10,11), the link between inflammation and atherosclerosis complication is causal in nature.
CV risk is a multifactorial problem in patients with CKD (12). Systemic inflammation is common in these patients, particularly those with stage 5 CKD undergoing dialysis (13,14). In line with studies in the general population (5,15), IL-6, a major proinflammatory cytokine, is an established strong predictor of adverse clinical outcomes in patients with stage 5D CKD (16–20). However, the relationship between IL-6 and CVD at earlier CKD stages was investigated in just one relatively small study by Barreto et al. (21), which was based on a limited number of CV events (just 22 events). In addition, to date we lack specific proof that this relationship in the CKD population is causal.
Circulating levels of IL-6 are genetically regulated. Because transmission of genes is a random phenomenon, gene polymorphisms modulating IL-6 synthesis may represent an unbiased means for testing whether the link between IL-6 and CV outcomes in patients with CKD is causal (Mendelian randomization). The −174 G/C single-nucleotide polymorphism is a functional variant located in the promoter region of the IL-6 gene that regulates the rate of IL-6 gene transcription (22–28) and therefore represents a reliable research tool for testing the nature (causal versus noncausal) of the link between IL-6 and CV outcomes in CKD. With this background in mind, we set out to confirm findings by Barreto et al. (21) in a large observational study with a carefully characterized cohort of 755 patients with stages 2–5 CKD and to test whether this relationship may underlie a causal link by applying the Mendelian randomization approach (i.e., by stratifying the study population according to the functional −174 G/C polymorphism in the IL-6 gene).
Materials and Methods
Study Protocol
The study protocol conformed with the ethical guidelines of our institution and was approved by ethical committees of all participating units. Each participant provided written informed consent.
Patients with CKD
Our study population included a genetically homogenous series of 755 white patients from the same geographic area (southern Italy) (29), consecutively recruited from 22 nephrology units between October 2005 and September 2008. Eligible patients were age 18–75 years and in stable clinical condition. Exclusion criteria included acute or rapidly evolving renal disease, kidney transplant, acute intercurrent infections or acute inflammatory processes, pregnancy, cancer, or diseases in the terminal phase. This cohort was described in detail elsewhere (30).
Control Population
To compare the allelic frequencies of the −174 G/C polymorphism observed in patients with CKD, we studied a sample of 463 consecutive blood donors of the general population from the same geographical area of patients with CKD.
Follow-up and Study Outcome
After the initial assessment, patients were monitored for a mean±SD of 31±10 months (range, 0.3–48 months). The study endpoint was fatal and nonfatal CV events as described elsewhere (30). These events included myocardial infarction, documented by electrocardiography and biomarkers of myocardial injury; heart failure, defined as dyspnea in addition to two of the following conditions: raised jugular pressure, bi-basilar crackles, pulmonary venous hypertension, or interstitial edema on chest radiography requiring hospitalization; electrocardiography-documented arrhythmia; stroke; peripheral vascular disease; and major arterial or venous thrombotic episodes. These events were accurately recorded during the follow-up period.
The history of CVD was defined as the presence of at least one of the following comorbidities at enrollment: myocardial infarction, heart failure, peripheral vascular disease, stroke, transient ischemic attack, or coronary surgery/angioplasty.
Laboratory Measurements
In the whole study population, blood sampling was performed in the early morning after an overnight fast and plasma was stored at −80°C until analysis. Serum glucose, lipids, hemoglobin, albumin, creatinine, and C-reactive protein (CRP) were measured by standard methods in the routine clinical laboratory. Serum IL-6 was measured by ELISA (R&D Systems, Inc., Minneapolis, MN). The eGFR was calculated by using the four-variables Modification of Diet in Renal Disease study equation (31) and not by the CKD-Epidemiology Collaboration formula because the creatinine data were not traceable by isotope dilution mass spectrophotometry during the study period. All patients with CKD underwent a 24-hour urinary collection for the measurement of proteinuria.
Genotyping of −174G/C Polymorphism
Allelic discrimination of −174 G/C polymorphism was performed using a custom TaqMan SNP Genotyping Assay provided by Applied Biosystems (Foster City, CA). In this assay, primers were designed to amplify a region that included the mutation site specifically recognized by a few probes able to discriminate wild-type and mutated alleles. The sequences of primers and probes were: 5′-CGACCTAAGCTGCACTTTTCC-3′ (forward primer) and 5′-GGGCTGATTGGAAACCTTATTAAGATTG-3′ (reverse primer); 5′-CCTTTAGCAT[G]GCAAGAC-3′ (C allele-specific probe) and 5′- CCTTTAGCAT[C]GCAAGAC-3′ (G allele-specific probe). Allelic discrimination was performed on a 7900HT Fast Real-Time PCR platform and its accompanying Sequence Detection System software, version 2.4 (Applied Biosystems). Briefly, genomic DNA was extracted from peripheral blood mononuclear cells using standard salting out procedure (32). The reaction system contained 20 ng of genomic DNA, 12.5 μl of 2X TaqMan Universal PCR Master Mix No AmpErase UNG, 1,25 μl of 40X Assay Mix (including unlabeled PCR primers, FAM and VIC dye-labeled TaqMan MGB probes), and H2O for a total volume of 25 μl. A random 10% of samples were independently repeated to confirm genotyping results. The genotype results for these samples were completely consistent. All analyses were done blinded to clinical information.
Statistical Analyses
Data were expressed as mean±SD, median and interquartile range, or percentage frequency. Comparisons between two groups were made by t-test, Mann–Whitney test, or chi-square test, as appropriate. The comparison among more than two groups was performed by ANOVA for log-transformed variables, when appropriate. The deviation from Hardy–Weinberg equilibrium was assessed by the chi-square test comparing observed and expected genotype frequencies. The 95% confidence interval (95% CI) of the risk allele frequency was calculated as suggested by the standard method (33).
The functional form of serum IL-6 (as continuous, binary, quartile, or quintile data) was formally investigated by the analysis of Martingale residuals, and the binary form (below/above the median) provided the best data fitting.
The relationships between serum levels of IL-6 and CVD was evaluated by two approaches. First, we analyzed the baseline association between serum IL-6 and history of CVD. Second, we investigated the predictive power of serum IL-6 in the prospective cohort study. In both logistic and survival analyses, we considered variables that met criteria to be confounders (i.e., variables related [P≤0.10] to both the exposure under investigation [serum IL-6 levels below/above the median value] and history of CVD or incident fatal and nonfatal CV events, which are not an effect of the exposure and are not in the causal pathway between the exposure and outcome) (34). Tested covariates included traditional risk factors (age, sex, smoking, diabetes and glucose, cholesterol, and BP), factors specific to CKD (hemoglobin, albumin, eGFR, and urinary protein), antihypertensive treatment, body mass index, and CRP. In both logistic and Cox regression models, eGFR was always forced because of the strong and significant correlation between eGFR and serum IL-6 (Table 1). To further investigate the causal role of IL-6 in the pathway leading to CV events in patients with CKD, we applied a Mendelian randomization approach (i.e., we stratified the study population according to the −174 G/C polymorphism). These analyses were appropriately adjusted for variables that differed between CC and GC or GG patients and that appeared to be potential confounders (i.e., age, sex, cholesterol) for the interpretation of the link between the risk genotype (CC) and the study outcomes.
Table 1.
Main demographic and clinical characteristics of the study population according to serum IL-6 levels and −174 G/C polymorphism
| Characteristic | Whole Group (n=755) | Serum IL-6 Level | Genotype | ||||
|---|---|---|---|---|---|---|---|
| <2.5 pg/ml (n=380) | ≥2.5 pg/ml (n=375) | P Value | CC (n=52) | GC/GG (n=703) | P Value | ||
| Age (yr) | 62±11 | 59±12 | 64±9 | <0.001 | 59±13 | 62±10 | 0.09 |
| Men (%) | 453 (60) | 224 (59) | 229 (61) | 0.55 | 39 (75) | 414 (59) | 0.02 |
| Diabetes (%) | 263 (35) | 105 (28) | 158 (42) | <0.001 | 21 (40) | 242 (34) | 0.38 |
| Current smokers (%) | 98 (13) | 55 (14) | 43 (11) | 0.22 | 9 (17) | 89 (13) | 0.34 |
| CV comorbidities (%) | 221 (29) | 82 (22) | 139 (37) | <0.001 | 23 (44) | 198 (28) | 0.01 |
| Body mass index (kg/m2) | 28.2±4.7 | 27.3±4.3 | 29.0±4.8 | <0.001 | 27.9±3.8 | 28.2±4.7 | 0.54 |
| Systolic BP (mmHg) | 134±18 | 132±17 | 135±19 | 0.03 | 132±16 | 134±18 | 0.41 |
| Diastolic BP (mmHg) | 78±11 | 79±10 | 77±11 | 0.01 | 76±10 | 78±10 | 0.15 |
| Antihypertensive treatment (%) | 691 (92) | 341 (90) | 350 (93) | 0.89 | 44 (85) | 647 (92) | 0.69 |
| Glucose (mg/dl) | 116±49 | 109±41 | 122±56 | <0.001 | 98 (87–127) | 99 (88–120) | 0.65 |
| Total cholesterol (mg/dl) | 187±45 | 189±43 | 184±46 | 0.12 | 173±43 | 188±45 | 0.02 |
| Hemoglobin (g/dl) | 12.8±1.8 | 13.1±1.8 | 12.6±1.8 | <0.001 | 12±2.0 | 13±1.8 | 0.29 |
| Albumin (g/dl) | 4.2±0.5 | 4.2±0.5 | 4.1±0.5 | 0.01 | 4.1±0.4 | 4.2±0.5 | 0.53 |
| CRP (mg/L) | 2.4 (1.0–5.5) | 1.4 (0.7–2.8) | 4.3 (1.9–9.0) | <0.001 | 3.2 (1.2–10) | 2.3 (1.0–5.4) | 0.06 |
| Phosphate (mg/dl) | 3.7±0.8 | 3.6±0.7 | 3.8±0.8 | 0.02 | 3.71±0.68 | 3.72±0.78 | 0.92 |
| eGFR (ml/min per 1.73 m2) | 36±13 | 38±13 | 34±13 | <0.001 | 34±12 | 36±13 | 0.32 |
| Urinary protein (mg/24 hr) | 0.6 (0.2–1.5) | 0.5 (0.2–1 0.2) | 0.6 (0.2–1.7) | 0.01 | 0.7 (0.2–1.6) | 0.6 (0.2–1.5) | 0.53 |
Data are expressed as mean±SD, median and interquartile range, or percentage frequency, as appropriate. CV, cardiovascular; CRP, C-reactive protein.
In the prospective cohort study, the potential distortion on the study results due to the competing risks of death was assessed by comparing the incidence rate of death in exposed patients (high IL-6 levels or CC risk genotype) and unexposed patients (low IL-6 levels or GC/GG genotype). If a difference was found, the competing risk of death was accounted for by carrying out a survival analysis considering a combined outcome: death/CV events (35). Data were expressed as odds ratios (ORs; logistic regression analysis), hazard ratios (HRs; Cox regression model), 95% CIs, and P values. To internally validate the independent relationships of serum IL-6 and −174 G/C polymorphisms with history of CVD and incident CV events, a bootstrap resampling technique of 1000 samples (randomly extracted from the original sample) was performed (36). All potential effect modifications exerted by covariates on the relationship between the key exposures (serum IL-6 and −174 G/C polymorphism) and study outcomes were formally tested by introducing a multiplicative term into the models; no significant interaction was found.
All calculations were made by using a standard statistical package (IBM SPSS Statistics for Windows, version 21.0. 0.1, IBM, Armonk, NY).
Results
We studied 755 patients with stages 2–5 CKD (stage 2, 3%; stage 3a, 22%; stage 3b, 38%; stage 4, 34%; stage 5, 3%). Four hundred fifty-three patients were male (60%), 263 had type 2 diabetes (35%), and 98 were current smokers (13%). Two-hundred twenty-one patients (29%) had a history of CVD (Table 1). One hundred nine patients had only one previous CV event, and 112 of them had two or more previous CV events. The first CV event in this population was myocardial infarction in 19 patients, heart failure in 26 patients, peripheral vascular disease in 29 patients, stroke in 12 patients, transient ischemic attack in 12 patients, and coronary surgery/angioplasty in 11 patients. Six hundred ninety-one patients (92%) were receiving antihypertensive treatment: one medication in 19%, two medications in 29%, three medications in 28%, and four or more medications in 16%. The mean eGFR was 36±13 ml/min per 1.73 m2, and the median 24-hour urinary protein excretion was 0.6 mg/24 hours (interquartile range, 0.2–1.5 mg/24 hours). The median IL-6 level was 2.5 pg/ml (interquartile range, 1.6–4.0 pg/ml).
Analyses Based on Serum IL-6 Levels
Patients with IL-6 above the median value were significantly older, more frequently had diabetes, and had higher serum glucose and 24-hour urinary protein values than those with IL-6 below this threshold (Table 1). Values for eGFR, hemoglobin, and albumin were lower in patients with higher IL-6 than in those with IL-6 below the median value. Systolic BP was higher and diastolic BP was lower in patients with IL-6 levels above the median (Table 1). As expected, serum CRP levels were directly associated with IL-6 levels (r=0.57; P<0.001). On bivariate, multivariate, and bootstrapping validation analyses, patients with an IL-6 level above the median were more likely to have had a history of CVD (P≤0.01) than those with an IL-6 level below the median (Table 2).
Table 2.
Logistic regression models of history of cardiovascular disease
| Variable | Bivariate | Multivariate | Bootstrapping Validation | |||
|---|---|---|---|---|---|---|
| OR (95% CI) | P Value | OR (95% CI) | P Value | OR (95% CI) | P Value | |
| Serum IL-6 value (0 [<2.5 pg/ml]; 1 [≥2.5 pg/ml])a | 2.14 (1.55 to 2.95) | <0.001 | 1.58 (1.11 to 2.24) | 0.01 | 1.58 (1.12 to 2.24) | 0.01 |
| Age (in yr) | 1.05 (1.03 to 1.07) | <0.001 | 1.05 (1.03 to 1.07) | <0.001 | ||
| Diabetes (0=no; 1=yes) | 2.83 (1.99 to 4.03) | <0.001 | 2.83 (1.97 to 4.08) | <0.001 | ||
| Systolic BP (in mmHg) | 1.00 (0.99 to 1.01) | 0.93 | 1.00 (0.99 to 1.01) | 0.94 | ||
| Hemoglobin (in g/dl) | 1.04 (0.94 to 1.16) | 0.47 | 1.04 (0.93 to 1.16) | 0.49 | ||
| Albumin (in g/dl) | 0.74 (0.50 to 1.11) | 0.15 | 0.74 (0.50 to 1.11) | 0.14 | ||
| Phosphate (in mg/dl) | 1.02 (0.80 to 1.30) | 0.86 | 1.02 (0.78 to 1.34) | 0.87 | ||
| Urinary protein (in mg/24 hr) | 1.01 (0.89 to 1.13) | 0.93 | 1.01 (0.88 to 1.14) | 0.93 | ||
| eGFR (ml/min per 1.73 m2) | 1.00 (0.98 to 1.01) | 0.57 | 1.00 (0.98 to 1.01) | 0.57 | ||
OR, odds ratio; 95% CI, 95% confidence interval.
Below/above the median value (in parentheses). The description of the model building strategy is reported in the Materials and Methods section.
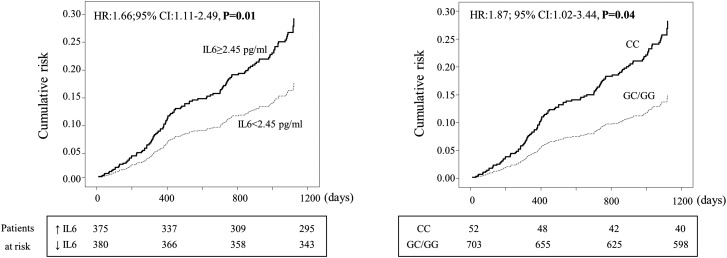
During the follow-up, 42 patients died. The incidence rate of mortality was three times higher (P=0.01) in patients with IL-6 above the median (3 deaths per 100 person-years; 95% CI, 2.0 to 4.1 deaths per 100 patient-years) than in those with IL-6 below this threshold (1 death per 100 person-years; 95% CI, 0.6 to 2.1 deaths per 100 patient-years). Overall, 117 patients had fatal and nonfatal CV events. As shown in Table 3, on bivariate, multivariate, and bootstrapping validation analyses, the incidence rate of fatal and nonfatal CV outcomes was significantly higher (P≤0.02) in patients with IL-6 levels above the median (8.6 CV events per 100 person-years; 95% CI, 2.5 to 5.0 CV events per 100 patient-years) than in those with IL-6 levels below this threshold (3.6 CV events per 100 person-years; 95% CI, 6.9 to 10.8 CV events per 100 patient-years) (Figure 1, left panel). CKD stages did not modify the relationship between IL-6 and CV risk (P for interaction=0.33). Further analyses investigating IL-6 as a continuous variable confirmed this biomarker as a strong and independent risk factor for study outcomes (history of CVD: bivariate analysis OR (1 pg/ml), 1.16 [95% CI, 1.10 to 1.26], P<0.001, and multivariate analysis OR, 1.10 [95% CI, 1.02 to 1.20], P=0.02; incident CV events, bivariate analysis HR (1 pg/ml), 1.14 [95% CI, 1.07 to 1.22], P<0.001, and multivariate analysis HR, 1.12 [95% CI, 1.03 to 1.22], P<0.001).
Table 3.
Cox regression models of incident cardiovascular events
| Variable | Bivariate | Multivariate | Bootstrapping Validation | |||
|---|---|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | |
| Serum IL-6 value (0 [<2.5 pg/ml]; 1 [≥2.5 pg/ml])a | 2.37 (1.61 to 3.51) | <0.001 | 1.66 (1.11 to 2.49) | 0.01 | 1.66 (1.10 to 2.52) | 0.02 |
| Age (in yr) | 1.07 (1.04 to 1.10) | <0.001 | 1.07 (1.03 to 1.10) | <0.001 | ||
| Diabetes (0=no; 1=yes) | 1.59 (1.09 to 2.32) | 0.02 | 1.59 (1.06 to 2.37) | 0.02 | ||
| Systolic BP (in mmHg) | 1.00 (1.00 to 1.02) | 0.31 | 1.00 (1.00 to 1.02) | 0.31 | ||
| Hemoglobin (in g/dl) | 0.91 (0.80 to 1.02) | 0.10 | 0.91 (0.81 to 1.02) | 0.09 | ||
| Albumin (in g/dl) | 0.82 (0.53 to 1.27) | 0.38 | 0.82 (0.52 to 1.29) | 0.39 | ||
| Phosphate (in mg/dl) | 1.13 (0.88 to 1.44) | 0.34 | 1.13 (0.88 to 1.45) | 0.35 | ||
| Urinary protein (in mg/24 hr) | 1.05 (0.94 to 1.17) | 0.38 | 1.05 (0.93 to 1.18) | 0.44 | ||
| eGFR (ml/min per 1.73 m2) | 1.01 (0.99 to 1.02) | 0.44 | 1.01 (0.99 to 1.02) | 0.49 | ||
HR, hazard ratio.
Below/above the median value (in parentheses). The description of the model building strategy is reported in the Materials and Methods section.
Figure 1.
IL-6 and risk of cardiovascular events. Left: Cumulative risk for incident fatal and nonfatal cardiovascular (CV) event–free survival in patients stratified according to serum IL-6 level. Data were adjusted for age, diabetes, systolic BP, hemoglobin, albumin, phosphate, urinary protein, and eGFR (see Table 3). The table under the x-axis indicates the number of patients at risk in each group (IL-6 above/below the median value) at relevant time points. Right: Cumulative risk for incident fatal and nonfatal CV event–free survival in patients stratified according to −174 G/C polymorphism in the IL-6 gene. Data were adjusted for age, sex, and cholesterol (see text). The table under the x-axis indicates the number of patients at risk in each group (CC and GC/GG genotype) at relevant time points. Data are expressed as hazard ratios (HRs), 95% confidence intervals (95% CIs), and P values.
To account for the potential effect of competing risks due to death on the relationship between IL-6 and CV outcomes, an additional adjusted analysis that considered a combined endpoint of death/CV events was performed. This multivariate analysis showed that the HR of the combined endpoint was about 1.5 times higher (HR, 1.54; 95% CI, 1.05 to 2.26; P=0.03) in patients with IL-6 levels above the median as compared with the remaining ones.
Analyses Based on the −174 G/C Polymorphism
In patients with CKD, the genotype distribution of −174 G/C polymorphism (GG, n=333 [44%]; GC, n=370 [49%]; CC, n=52 [7%]) significantly deviated from Hardy–Weinberg equilibrium (chi-square=14.3; P=0.001), whereas this was not true in healthy persons (GG, n=277 [60%]; GC, n=170 [37%]; CC, n=16 [3%]) (chi-square=2.70; P=0.10). Of note, the frequency of the C risk allele was significantly higher (P<0.02) in patients with CKD (31%; 95% CI, 28% to 34%) than in healthy study participants (22%; 95% CI, 18% to 26%).
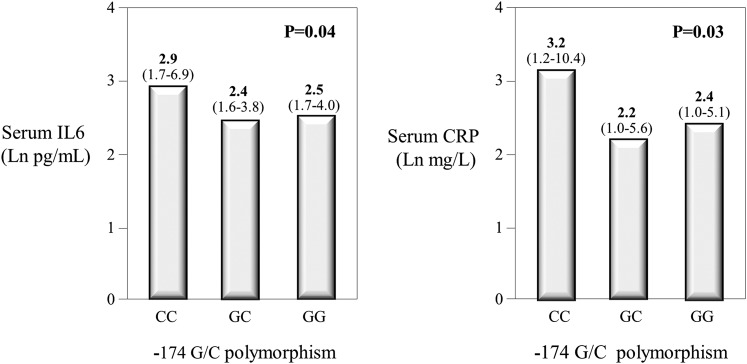
Patients with CKD and CC genotype had higher levels of IL-6 (median, 2.9 pg/ml [interquartile range, 1.7–6.9 pg/ml]) than in those with GC (2.4 pg/ml [interquartile range, 1.6–3.8 pg/ml]) and GG (2.5 pg/ml [interquartile range, 1.7–4.0 pg/ml]) (P=0.04) (Figure 2, left panel). This was also true for CRP (CC, median: 3.2 mg/L; interquartile range, 1.2–10.4 mg/L; GC, 2.2 mg/L [interquartile range, 1.0–5.6 mg/L]; GG, 2.4 mg/L [interquartile range, 1.0–5.1 mg/L]) (P=0.03) (Figure 2, right panel). These findings show that the relationship between −174 G/C polymorphism and inflammatory biomarkers is well described by a recessive model of inheritance (CC genotype versus GC/GG genotypes). For this reason, further data analysis was carried out using the recessive model. As shown in Table 1 and as expected from Mendelian randomization, CC patients and GC/GG patients did not differ for demographic and clinical characteristics except for a slight excess of men (75% versus 59%, respectively; P=0.02) in CC patients and slightly lower cholesterol levels (173±43 mg/dl versus 188±45 mg/dl, respectively; P=0.02) in patients with the same genotype.
Figure 2.
Inflammatory biomarkers and − 174 G/C polymorphism. Serum levels of IL-6 (left panel) and C-reactive protein (right panel) according to −174 G/C polymorphism in IL-6 gene. Data are expressed as medians. The interquartile ranges are indicated in parentheses. The comparison among groups was made by ANOVA.
The prevalence of a CVD history was higher in CC patients (44%) than in GC/GG patients (28%) (P=0.01). The OR for this outcome was more than twice as high in patients harboring the CC risk genotype (OR, 2.15; 95% CI, 1.15 to 4.00; P=0.02) as in those with other genotypes in a model adjusting for age, sex, and cholesterol (i.e., the covariates that differed [P≤0.10] between patients with CC and those with GC/GG genotypes; see Table 1). A bootstrapping validation model confirmed these results (OR, 2.14; 95% CI, 1.18 to 3.89; P=0.01).
In the cohort study, the incidence rate of mortality did not differ between patients with and those without the CC risk genotype (P=0.27), indicating that the competing risk of death on the link between −174 G/C polymorphism and CV events could be excluded. In close parallel with the strong association between serum IL-6 levels and CV events, the incidence rate of CV outcomes in patients with CC genotype was 87% higher (HR, 1.87; 95% CI, 1.02 to 3.44; P=0.04) than in those with the GC or GG genotypes (Figure 1, right panel) and this was also true in a bootstrapping validation model (HR, 1.87; 95% CI, 1.01 to 3.51; P=0.05).
Discussion
In this study, high serum IL-6 levels were associated with history of CVD and predicted the risk for incident CV events in patients with stages 2–5 CKD. Furthermore, the functional polymorphism −174 G/C in the promoter of the IL-6 gene is associated with history of CVD and predicts the risk for future CV events in this population. These results are compatible with the hypothesis that this inflammatory cytokine is causally implicated in the high CV risk in CKD.
Atherosclerosis is an inflammatory disease (1). Chronic inflammation is pervasive at all CKD stages, particularly stage 5D (13,14). IL-6 is considered an orchestrator of the inflammatory response (37) and a key player in atherosclerosis in humans (38). The IL-6 effect on the cardiovascular system might be mediated via downstream acute-phase proteins (39) or by IL-6 per se. IL-6 stimulates endothelial activation, vascular smooth muscle cell proliferation (40), and leukocyte recruitment (41), thereby contributing to the process of atherosclerotic plaque growth (42) and instability (43). IL-6 mRNA is overexpressed in atheromatous arteries, and IL-6 expression co-localizes with macrophages in areas of plaque rupture (43). In apoE-deficient mice, systemic administration of IL-6 accelerates atherosclerosis (10). Elevated levels of IL-6 are predictive of future CV events in healthy men (15) and women (44) and are markers of poor prognosis in patients with chronic angina (45) and acute coronary syndrome (46). IL-6 levels are markedly elevated in CKD, a phenomenon only in part explained by reduced renal clearance of this cytokine. Furthermore, the observation of a parallel increase in IL-6 and CRP in our study is in keeping with the notion that IL-6 drives the synthesis of CRP. High IL-6 has been solidly associated with mortality in patients with stage 5 CKD who are being maintained on long-term dialysis (16–20).
To the best of our knowledge, only one study has tested the relationship between IL-6 and CV mortality in CKD (21). This seminal study was carried out in a small cohort of 125 patients, was based on a limited number of CV events (n=22), and also included dialysis patients (34% of the whole cohort) (21). Our study, based on a large CKD cohort composed exclusively of predialysis patients and including a large number of CV events, confirmed pilot data by Barreto et al. (21) and showed that high IL-6 is coherently associated both with history of CVD as well as with incident CV events.
Being observational in nature, findings in studies discussed above, including our analysis based on circulating IL-6, remain hypothesis-generating and as such leave unresolved the critical question of whether this cytokine is causally implicated in CV complications in patients with predialysis CKD. This question can be resolved only by a full-fledged clinical trial.
The Mendelian randomization approach is useful step in the pathway to discovery in clinical research. Since genetic polymorphisms are distributed randomly at gamete formation and since genotypes precede phenotypes and do not change over time, comparing individuals harboring a given risk allele for the expression of a corresponding risk factor with those without the risk allele in question may allow unbiased assessment of the link between the attendant risk factor and relevant clinical outcomes. In this perspective, we used the functional polymorphism −174 G/C in the promoter of the IL-6 gene as a marker to further investigate the link between IL-6 and CV events in patients with CKD. The −174 G/C polymorphism is a common variant that regulates the serum concentration of IL-6 (22–28). In keeping with previous studies in patients with CVD (28), coronary artery bypass grafting surgery (23,25), carotid atherosclerosis (24), abdominal aortic aneurysm (22), and dialysis (26), we found that patients with CKD and CC genotype had higher circulating levels of IL-6 and CRP than those harboring GC or GG genotypes, specifically legitimating the use of this genetic marker as an unbiased means for assessing the causal nature of the link between the gene product (IL-6) of this polymorphism and CV complications in CKD. Interestingly, this analysis showed that this polymorphism is independently associated both with the history of CVD as well as with incident CV events. Such associations, which went along with the previously described relationships of serum IL-6 with the same outcomes, further strengthen the hypothesis that IL-6 is a direct player in atherosclerotic complications in CKD. Moreover, the observation that the distribution of genotypes frequency of the −174 G/C polymorphism in patients with CKD was not in Hardy–Weinberg equilibrium and that the frequency of the C allele was significantly higher in patients CKD compared with general population of the same geographic area, offers additional circumstantial evidence that IL-6 is a causal risk factor for CV events in this population (47).
Mendelian randomization studies support causal interpretations but do not constitute definitive proof for causality, which demands specific experimental evidence (i.e., a formal randomized clinical trial). In this respect, meta-analytic data from two genetic consortia exploring the effect of a polymorphism in the IL-6 receptor on the risk of coronary artery disease showed the allele that attenuated IL-6 signaling was significantly associated with reduced risk of coronary heart disease (48). Notably, the relevance of this genetic association for the control of inflammation is shown by a meta-analysis of clinical trials testing a monoclonal antibody against the IL-6 receptor (tocilizumab) in rheumatoid arthritis and documenting that lowering serum levels of IL-6 is an effective strategy to induce the remission of this chronic inflammatory disease (49).
Some limitations should be acknowledged. First, even though our cohort study registered a sizable number of cardiovascular events, the number of deaths (n=42) was limited, preventing adequately adjusted analyses focusing on this major outcome. However, the analysis of the primary outcome in this study, incident CV events, which was robustly based on 117 events, showed parallel links between serum IL-6 and the genetic marker of this cytokine with the same events.
Second, although Mendelian randomization is a powerful approach for inferring causality in observational studies, its application has potential limitations. Genetic variants can be indicators of environmental exposures on condition that there is no genetic admixture, pleiotropy or linkage disequilibrium. However, it is reasonable to believe that in our study all these assumptions are fulfilled. Our population is genetically homogeneous (29) and the −174G/C polymorphism is a functional variant directly responsible for serum levels of IL-6 (22–28). Furthermore, pleiotropy seems highly unlikely because of the location of −174 G/C polymorphism in the promoter region of the gene.
Third, although we demonstrated that our findings had high internal validity by bootstrap modeling, replication of the results in a second cohort is a required proof for the external generalizability of findings in observational studies. In this respect, our observational study in a large southern European cohort confirms findings in a small central European cohort (21). Furthermore, our study is the first applying a genetic marker of IL-6 to express the nature (causal versus not causal) of the link between IL-6 and CV events in patients with CKD.
In conclusion, high serum IL-6 is associated with both a history of CVD and future CV events in patients with CKD, and these associations are fully confirmed by the application of a functional polymorphism in the IL-6 gene. Overall, this study is compatible with the hypothesis that the IL-6 plays a causal role in the high CV risk of patients with CKD.
Disclosures
None.
Acknowledgments
We thank Cristina Politi and Maria Cristina Sanguedolce for their technical collaboration in genetic study.
This study is part of the Syskid project, which is supported through European Union’s FP7, grant agreement number HEALTH-F2-2009-241544.
Contributors: members of the Southern Italy CKD Cohort Working Group (in alphabetical order): Audino A, Bruzzese V, Caglioti A, Campo S, Caridi G, Catalano F, Chiarella S, Cicchetti T, D'Anello E, Enia G, Fabiano F, Fatuzzo P, Ferini S, Garozzo M, Grandinetti F, Gullo M, Mafrica A, Maimone I, Mancuso F, Mannino M, Marino F, Natale G, Palma L, Papalia T, Parlongo G, Pinciaroli A, Pinna M, Plutino D, Postorino M, Pugliese A, Rapisarda F, Santoro O, Tramontana D.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Ross R: Atherosclerosis—an inflammatory disease. N Engl J Med 340: 115–126, 1999 [DOI] [PubMed] [Google Scholar]
- 2.Ridker PM, Cushman M, Stampfer MJ, Tracy RP, Hennekens CH: Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N Engl J Med 336: 973–979, 1997 [DOI] [PubMed] [Google Scholar]
- 3.Ridker PM, Hennekens CH, Buring JE, Rifai N: C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med 342: 836–843, 2000 [DOI] [PubMed] [Google Scholar]
- 4.Pai JK, Pischon T, Ma J, Manson JE, Hankinson SE, Joshipura K, Curhan GC, Rifai N, Cannuscio CC, Stampfer MJ, Rimm EB: Inflammatory markers and the risk of coronary heart disease in men and women. N Engl J Med 351: 2599–2610, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Danesh J, Kaptoge S, Mann AG, Sarwar N, Wood A, Angleman SB, Wensley F, Higgins JP, Lennon L, Eiriksdottir G, Rumley A, Whincup PH, Lowe GD, Gudnason V: Long-term interleukin-6 levels and subsequent risk of coronary heart disease: two new prospective studies and a systematic review. PLoS Med 5: e78, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ridker PM, Rifai N, Pfeffer MA, Sacks FM, Moye LA, Goldman S, Flaker GC, Braunwald E, Cholesterol and Recurrent Events (CARE) Investigators : Inflammation, pravastatin, and the risk of coronary events after myocardial infarction in patients with average cholesterol levels. Circulation 98: 839–844, 1998 [DOI] [PubMed] [Google Scholar]
- 7.Libby P, Ridker PM, Maseri A: Inflammation and atherosclerosis. Circulation 105: 1135–1143, 2002 [DOI] [PubMed] [Google Scholar]
- 8.Davey Smith G, Hemani G: Mendelian randomization: genetic anchors for causal inference in epidemiological studies. Hum Mol Genet 23[R1]: R89–R98, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hingorani AD, Casas JP, Interleukin-6 Receptor Mendelian Randomisation Analysis (IL6R MR) Consortium : The interleukin-6 receptor as a target for prevention of coronary heart disease: a mendelian randomisation analysis. Lancet 379: 1214–1224, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huber SA, Sakkinen P, Conze D, Hardin N, Tracy R: Interleukin-6 exacerbates early atherosclerosis in mice. Arterioscler Thromb Vasc Biol 19: 2364–2367, 1999 [DOI] [PubMed] [Google Scholar]
- 11.Schuett H, Oestreich R, Waetzig GH, Annema W, Luchtefeld M, Hillmer A, Bavendiek U, von Felden J, Divchev D, Kempf T, Wollert KC, Seegert D, Rose-John S, Tietge UJ, Schieffer B, Grote K: Transsignaling of interleukin-6 crucially contributes to atherosclerosis in mice. Arterioscler Thromb Vasc Biol 32: 281–290, 2012 [DOI] [PubMed] [Google Scholar]
- 12.Zoccali C: Traditional and emerging cardiovascular and renal risk factors: An epidemiologic perspective. Kidney Int 70: 26–33, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Stenvinkel P, Ketteler M, Johnson RJ, Lindholm B, Pecoits-Filho R, Riella M, Heimbürger O, Cederholm T, Girndt M: IL-10, IL-6, and TNF-alpha: Central factors in the altered cytokine network of uremia—the good, the bad, and the ugly. Kidney Int 67: 1216–1233, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Zoccali C, Mallamaci F, Tripepi G: Inflammatory proteins as predictors of cardiovascular disease in patients with end-stage renal disease. Nephrol Dial Transplant 19[Suppl 5]: V67–V72, 2004 [DOI] [PubMed] [Google Scholar]
- 15.Ridker PM, Rifai N, Stampfer MJ, Hennekens CH: Plasma concentration of interleukin-6 and the risk of future myocardial infarction among apparently healthy men. Circulation 101: 1767–1772, 2000 [DOI] [PubMed] [Google Scholar]
- 16.Pecoits-Filho R, Bárány P, Lindholm B, Heimbürger O, Stenvinkel P: Interleukin-6 is an independent predictor of mortality in patients starting dialysis treatment. Nephrol Dial Transplant 17: 1684–1688, 2002 [DOI] [PubMed] [Google Scholar]
- 17.Zoccali C, Tripepi G, Mallamaci F: Dissecting inflammation in ESRD: Do cytokines and C-reactive protein have a complementary prognostic value for mortality in dialysis patients? J Am Soc Nephrol 17[Suppl 3]: S169–S173, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Honda H, Qureshi AR, Heimbürger O, Barany P, Wang K, Pecoits-Filho R, Stenvinkel P, Lindholm B: Serum albumin, C-reactive protein, interleukin 6, and fetuin a as predictors of malnutrition, cardiovascular disease, and mortality in patients with ESRD. Am J Kidney Dis 47: 139–148, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Meuwese CL, Snaedal S, Halbesma N, Stenvinkel P, Dekker FW, Qureshi AR, Barany P, Heimburger O, Lindholm B, Krediet RT, Boeschoten EW, Carrero JJ: Trimestral variations of C-reactive protein, interleukin-6 and tumour necrosis factor-α are similarly associated with survival in haemodialysis patients. Nephrol Dial Transplant 26: 1313–1318, 2011 [DOI] [PubMed] [Google Scholar]
- 20.Rao M, Guo D, Perianayagam MC, Tighiouart H, Jaber BL, Pereira BJ, Balakrishnan VS: Plasma interleukin-6 predicts cardiovascular mortality in hemodialysis patients. Am J Kidney Dis 45: 324–333, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Barreto DV, Barreto FC, Liabeuf S, Temmar M, Lemke HD, Tribouilloy C, Choukroun G, Vanholder R, Massy ZA, European Uremic Toxin Work Group (EUTox) : Plasma interleukin-6 is independently associated with mortality in both hemodialysis and pre-dialysis patients with chronic kidney disease. Kidney Int 77: 550–556, 2010 [DOI] [PubMed] [Google Scholar]
- 22.Jones KG, Brull DJ, Brown LC, Sian M, Greenhalgh RM, Humphries SE, Powell JT: Interleukin-6 (IL-6) and the prognosis of abdominal aortic aneurysms. Circulation 103: 2260–2265, 2001 [DOI] [PubMed] [Google Scholar]
- 23.Brull DJ, Montgomery HE, Sanders J, Dhamrait S, Luong L, Rumley A, Lowe GD, Humphries SE: Interleukin-6 gene -174g>c and -572g>c promoter polymorphisms are strong predictors of plasma interleukin-6 levels after coronary artery bypass surgery. Arterioscler Thromb Vasc Biol 21: 1458–1463, 2001 [DOI] [PubMed] [Google Scholar]
- 24.Jerrard-Dunne P, Sitzer M, Risley P, Steckel DA, Buehler A, von Kegler S, Markus HS, Carotid Atherosclerosis Progression Study : Interleukin-6 promoter polymorphism modulates the effects of heavy alcohol consumption on early carotid artery atherosclerosis: the Carotid Atherosclerosis Progression Study (CAPS). Stroke 34: 402–407, 2003 [DOI] [PubMed] [Google Scholar]
- 25.Wypasek E, Undas A, Sniezek-Maciejewska M, Kapelak B, Plicner D, Stepien E, Sadowski J: The increased plasma C-reactive protein and interleukin-6 levels in patients undergoing coronary artery bypass grafting surgery are associated with the interleukin-6-174G > C gene polymorphism. Ann Clin Biochem 47: 343–349, 2010 [DOI] [PubMed] [Google Scholar]
- 26.Liu Y, Berthier-Schaad Y, Fallin MD, Fink NE, Tracy RP, Klag MJ, Smith MW, Coresh J: IL-6 haplotypes, inflammation, and risk for cardiovascular disease in a multiethnic dialysis cohort. J Am Soc Nephrol 17: 863–870, 2006 [DOI] [PubMed] [Google Scholar]
- 27.Gillerot G, Goffin E, Michel C, Evenepoel P, Biesen WV, Tintillier M, Stenvinkel P, Heimbürger O, Lindholm B, Nordfors L, Robert A, Devuyst O: Genetic and clinical factors influence the baseline permeability of the peritoneal membrane. Kidney Int 67: 2477–2487, 2005 [DOI] [PubMed] [Google Scholar]
- 28.Jenny NS, Tracy RP, Ogg MS, Luong A, Kuller LH, Arnold AM, Sharrett AR, Humphries SE: In the elderly, interleukin-6 plasma levels and the -174G>C polymorphism are associated with the development of cardiovascular disease. Arterioscler Thromb Vasc Biol 22: 2066–2071, 2002 [DOI] [PubMed] [Google Scholar]
- 29.Di Gaetano C, Voglino F, Guarrera S, Fiorito G, Rosa F, Di Blasio AM, Manzini P, Dianzani I, Betti M, Cusi D, Frau F, Barlassina C, Mirabelli D, Magnani C, Glorioso N, Bonassi S, Piazza A, Matullo G: An overview of the genetic structure within the Italian population from genome-wide data. PLoS ONE 7: e43759, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zoccali C, Leonardis D, Enia G, Postorino M, Mallamaci F, MAURO study working group : The MAURO study: Multiple intervention and audit in renal diseases to optimize care. J Nephrol 21: 20–22, 2008 [PubMed] [Google Scholar]
- 31.Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, Kusek JW, Van Lente F, Chronic Kidney Disease Epidemiology Collaboration : Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med 145: 247–254, 2006 [DOI] [PubMed] [Google Scholar]
- 32.Miller SA, Dykes DD, Polesky HF: A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res 16: 1215, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Newcombe RG: Confidence Intervals for Proportion and Related Measures of Effect Size. Boca Raton, FL: CRC Press, 2013, pp 181–182 [Google Scholar]
- 34.Jager KJ, Zoccali C, Macleod A, Dekker FW: Confounding: What it is and how to deal with it. Kidney Int 73: 256–260, 2008 [DOI] [PubMed] [Google Scholar]
- 35.Varadhan R, Weiss CO, Segal JB, Wu AW, Scharfstein D, Boyd C: Evaluating health outcomes in the presence of competing risks: A review of statistical methods and clinical applications. Med Care 48[Suppl]: S96–S105, 2010 [DOI] [PubMed] [Google Scholar]
- 36.Steverberg EW: Clinical Prediction Models. A Practical Approach to Development,Validation, and Updating, New York, Springer, 2009. pp. 93–98 and 303–304 [Google Scholar]
- 37.Naka T, Nishimoto N, Kishimoto T: The paradigm of IL-6: From basic science to medicine. Arthritis Res 4[Suppl 3]: S233–S242, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hartman J, Frishman WH: Inflammation and atherosclerosis: A review of the role of interleukin-6 in the development of atherosclerosis and the potential for targeted drug therapy. Cardiol Rev 22: 147–151, 2014 [DOI] [PubMed] [Google Scholar]
- 39.Baumann H, Gauldie J: Regulation of hepatic acute phase plasma protein genes by hepatocyte stimulating factors and other mediators of inflammation. Mol Biol Med 7: 147–159, 1990 [PubMed] [Google Scholar]
- 40.Morimoto S, Nabata T, Koh E, Shiraishi T, Fukuo K, Imanaka S, Kitano S, Miyashita Y, Ogihara T: Interleukin-6 stimulates proliferation of cultured vascular smooth muscle cells independently of interleukin-1 beta. J Cardiovasc Pharmacol 17[Suppl 2]: S117–S118, 1991 [DOI] [PubMed] [Google Scholar]
- 41.Romano M, Sironi M, Toniatti C, Polentarutti N, Fruscella P, Ghezzi P, Faggioni R, Luini W, van Hinsbergh V, Sozzani S, Bussolino F, Poli V, Ciliberto G, Mantovani A: Role of IL-6 and its soluble receptor in induction of chemokines and leukocyte recruitment. Immunity 6: 315–325, 1997 [DOI] [PubMed] [Google Scholar]
- 42.Schieffer B, Selle T, Hilfiker A, Hilfiker-Kleiner D, Grote K, Tietge UJ, Trautwein C, Luchtefeld M, Schmittkamp C, Heeneman S, Daemen MJ, Drexler H: Impact of interleukin-6 on plaque development and morphology in experimental atherosclerosis. Circulation 110: 3493–3500, 2004 [DOI] [PubMed] [Google Scholar]
- 43.Schieffer B, Schieffer E, Hilfiker-Kleiner D, Hilfiker A, Kovanen PT, Kaartinen M, Nussberger J, Harringer W, Drexler H: Expression of angiotensin II and interleukin 6 in human coronary atherosclerotic plaques: Potential implications for inflammation and plaque instability. Circulation 101: 1372–1378, 2000 [DOI] [PubMed] [Google Scholar]
- 44.Volpato S, Guralnik JM, Ferrucci L, Balfour J, Chaves P, Fried LP, Harris TB: Cardiovascular disease, interleukin-6, and risk of mortality in older women: The Women’s Health and Aging Study. Circulation 103: 947–953, 2001 [DOI] [PubMed] [Google Scholar]
- 45.Ikonomidis I, Andreotti F, Economou E, Stefanadis C, Toutouzas P, Nihoyannopoulos P: Increased proinflammatory cytokines in patients with chronic stable angina and their reduction by aspirin. Circulation 100: 793–798, 1999 [DOI] [PubMed] [Google Scholar]
- 46.Ikeda U, Ito T, Shimada K: Interleukin-6 and acute coronary syndrome. Clin Cardiol 24: 701–704, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang J, Shete S: A test for genetic association that incorporates information about deviation from Hardy-Weinberg proportions in cases. Am J Hum Genet 83: 53–63, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sarwar N, Butterworth AS, Freitag DF, Gregson J, Willeit P, Gorman DN, Gao P, Saleheen D, Rendon A, Nelson CP, Braund PS, Hall AS, Chasman DI, Tybjærg-Hansen A, Chambers JC, Benjamin EJ, Franks PW, Clarke R, Wilde AA, Trip MD, Steri M, Witteman JC, Qi L, van der Schoot CE, de Faire U, Erdmann J, Stringham HM, Koenig W, Rader DJ, Melzer D, Reich D, Psaty BM, Kleber ME, Panagiotakos DB, Willeit J, Wennberg P, Woodward M, Adamovic S, Rimm EB, Meade TW, Gillum RF, Shaffer JA, Hofman A, Onat A, Sundström J, Wassertheil-Smoller S, Mellström D, Gallacher J, Cushman M, Tracy RP, Kauhanen J, Karlsson M, Salonen JT, Wilhelmsen L, Amouyel P, Cantin B, Best LG, Ben-Shlomo Y, Manson JE, Davey-Smith G, de Bakker PI, O’Donnell CJ, Wilson JF, Wilson AG, Assimes TL, Jansson JO, Ohlsson C, Tivesten Å, Ljunggren Ö, Reilly MP, Hamsten A, Ingelsson E, Cambien F, Hung J, Thomas GN, Boehnke M, Schunkert H, Asselbergs FW, Kastelein JJ, Gudnason V, Salomaa V, Harris TB, Kooner JS, Allin KH, Nordestgaard BG, Hopewell JC, Goodall AH, Ridker PM, Hólm H, Watkins H, Ouwehand WH, Samani NJ, Kaptoge S, Di Angelantonio E, Harari O, Danesh J, IL6R Genetics Consortium Emerging Risk Factors Collaboration : Interleukin-6 receptor pathways in coronary heart disease: A collaborative meta-analysis of 82 studies. Lancet 379: 1205–1213, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Navarro G, Taroumian S, Barroso N, Duan L, Furst D: Tocilizumab in rheumatoid arthritis: A meta-analysis of efficacy and selected clinical conundrums. Semin Arthritis Rheum 43: 458–469, 2014 [DOI] [PubMed] [Google Scholar]