Abstract
Background and objectives
Both valganciclovir and high-dose valacyclovir are recommended for cytomegalovirus prophylaxis after renal transplantation. A head-to-head comparison of both regimens is lacking. The objective of the study was to compare valacyclovir prophylaxis with valganciclovir, which constituted the control group.
Design, settings, participants, & measurements
In a randomized, open-label, single-center trial, recipients of renal transplants (recipient or donor cytomegalovirus-seropositive) were randomly allocated (1:1) to 3-month prophylaxis with valacyclovir (2 g four times daily) or valganciclovir (900 mg daily). Enrollment occurred from November of 2007 to April of 2012. The primary end points were cytomegalovirus DNAemia and biopsy-proven acute rejection at 12 months. Analysis was by intention to treat.
Results
In total, 119 patients were assigned to valacyclovir (n=59) or valganciclovir prophylaxis (n=60). Cytomegalovirus DNAemia developed in 24 (43%) of 59 patients in the valacyclovir group and 18 (31%) of 60 patients in the valganciclovir group (adjusted hazard ratio, 1.35; 95% confidence interval, 0.71 to 2.54; P=0.36). The incidence of cytomegalovirus disease was 2% with valacyclovir and 5% with valganciclovir prophylaxis (adjusted hazard ratio, 0.21; 95% confidence interval, 0.01 to 5.90; P=0.36). Significantly more patients with valacyclovir prophylaxis developed biopsy-proven acute rejection (18 of 59 [31%] versus 10 of 60 [17%]; adjusted hazard ratio, 2.49; 95% confidence interval, 1.09 to 5.65; P=0.03). The incidence of polyomavirus viremia was higher in the valganciclovir group (18% versus 36%; adjusted hazard ratio, 0.43; 95% confidence interval, 0.19 to 0.96; P=0.04).
Conclusions
Valganciclovir shows no superior efficacy in cytomegalovirus DNAemia prevention compared with valacyclovir prophylaxis. However, the risk of biopsy-proven acute rejection is higher with valacyclovir.
Keywords: cytomegalovirus, renal transplantation, prevention, valganciclovir, valacyclovir
Introduction
Despite major advances in diagnosis and prevention, cytomegalovirus (CMV) continues to be one of the most common opportunistic pathogens in solid organ transplant recipients (1). Transplantation outcomes are complicated primarily by indirect effects of CMV. CMV has been shown to enhance the immune response to alloantigens and increase the incidence of acute rejection episodes and chronic graft injury, such as interstitial fibrosis and tubular atrophy (IF/TA), after renal transplantation (2–4). Additional indirect effects include increased risk of other opportunistic infections, post-transplant lymphoproliferative disorder, cardiovascular events, and new-onset diabetes (1,5,6). CMV viremia and disease have been shown to be independent risk factors of mortality and/or graft failure in the late renal post-transplant period (7). This association has been confirmed by studies using modern preventive strategies (8–10).
Prevention of CMV makes up a critical part of post-transplant management. With regards to renal transplant recipients, the recent international guidelines recommend universal prophylaxis and a preemptive approach showing comparable efficacy (1). Both approaches have drawbacks; the main limitations are development of late-onset CMV disease after prophylaxis has been discontinued as well as the failure of the preemptive approach if not meeting the stringent logistic requirements (11–13). Valganciclovir is currently the drug most commonly used in prophylaxis (12,14,15). An alternative for patients with renal transplants is high-dose valacyclovir, and its efficacy has been documented in randomized studies (10,16,17). Some centers could find valacyclovir to be an attractive option for economic reasons or because of less bone marrow suppression (18). In addition, valacyclovir has been associated with a lower incidence of acute rejection episodes in several studies (16,17,19), with the efficacy of valacyclovir comparable with that of oral ganciclovir (19). However, there has been no randomized study designed to make a head-to-head comparison of valacyclovir with valganciclovir to date.
This randomized study Two Valine Esters Study (2VAL) is the first to compare the efficacy and safety of 3-month prophylaxis with valacyclovir and valganciclovir in renal transplant recipients while also assessing the incidence of CMV indirect effects.
Materials and Methods
Study Design and Patients
This was an open-label, single-center, randomized study. From November of 2007 to April to 2012, all adult renal transplant recipients with recipient and/or donor positive for CMV serology were eligible for inclusion. Exclusion criteria included donor-negative/recipient-negative serostatus, allergy to (val)ganciclovir or (val)acyclovir, severe leukopenia or thrombocytopenia, participation in another clinical trial, and inability to provide informed consent. The study was approved by the local ethics committee and conducted in compliance with the Declaration of Helsinki and the Declaration of Istanbul on Organ Trafficking and Transplant Tourism. Written informed consent was obtained before enrollment. Patients were randomized by the transplant physician using a random number table at a 1:1 ratio to valganciclovir or valacyclovir prophylaxis. Randomization was stratified by donor/recipient CMV serostatus. Sequentially numbered sealed envelopes were used for allocation concealment. (The trial is registered at Australian New Zealand Clinical Trials Registry: ACTRN1260000016033.)
Interventions
Patients received valganciclovir (Valcyte; Hoffman-La Roche, Grenzach-Wyhlen, Germany) at a dose of 900 mg daily or valacyclovir (Valtrex; Glaxo Wellcome, Dartford, UK) at a dose of 2 g four times daily for 3 months beginning day 7 post-transplant at the latest. The doses of antiviral drugs were tapered on the basis of renal function according to the manufacturers’ instructions. PCR for CMV DNA from whole blood was performed at 2-week intervals for the first 3 months and at 4, 5, 6, 9, and 12 months thereafter. PCR was likewise performed if clinically required. Asymptomatic CMV DNAemia occurring during or after prophylaxis was not treated, regardless of the viral load.
The standard immunosuppressive protocol included cyclosporin, mycophenolate mofetil, and corticosteroids. Immunologic high-risk patients received induction by antithymocyte globulin (Thymoglobulin; Genzyme, Marcy I’Eoile, France) and tacrolimus. Recipients of grafts from highly marginal donors were treated with anti–IL-2R mAb (basiliximab) and low-dose tacrolimus. Patients received prophylaxis with trimethoprim-sulfamethoxazole for 4 months and oral amphotericin solution for 1 month. Plasma was tested for polyoma virus DNAemia every 1 month for the first 6 months and at 9 and 12 months with preemptive immunosuppression reduction at a significant viral load (≥10,000 copies/ml).
Study Outcomes and Follow-Up
The primary end points were the incidences of CMV DNAemia and biopsy-proven acute rejection (BPAR) at 12 months post-transplant. Secondary end points included CMV disease, patient and graft survival (not censored for death), subclinical rejection and IF/TA assessed by protocol biopsy at month 3, renal function, other infections, and safety evaluated by recording adverse events and routine laboratory parameters. In addition, other potential indirect effects of CMV, such as cardiovascular events or new-onset diabetes mellitus, malignancy, and economic data, were recorded prospectively. All patients were followed for a minimum of 12 months post-transplant or until death.
CMV DNAemia was defined by detection of CMV DNA. CMV disease was defined as symptomatic CMV DNAemia and included both CMV syndrome and tissue-invasive disease (20). Suspected acute rejection was confirmed by core biopsy using the updated Banff classification (21). BPAR was defined as grade ≥IA or antibody-mediated rejection.
Sample Size and Statistical Analyses
The anticipated CMV DNAemia and BPAR rates in the valacyclovir group were 60% and 12%, respectively (16,19). To detect a reduction in the incidence of CMV DNAemia to 35% and an increase in the incidence of BPAR to 30% in the valganciclovir group, it was necessary to enroll at least 60 and 72 patients, respectively, to ensure 80% power for detection of a treatment difference with type 1 error of 0.05. Given the anticipated number of patients lost to follow-up, 80 patients were planned to enrollment. Because of a clinically important trend to a lower BPAR rate in the valganciclovir group (12.5% versus 25%; P=0.14 by not-adjusted log-rank test) after analysis of a planned study population, it was decided to increase sample size. With the assumption of the same difference in BPAR, at least 114 patients were required. Finally, 124 patients were planned for enrollment to anticipate patients being lost to follow-up.
Quantitative parametric data were compared using the t test and the Mann–Whitney U test in nonparametric distribution. Qualitative data were analyzed using the chi-squared test with Yates correction or the Fisher exact test. Incidence of CMV DNAemia and disease, BPAR, patient and graft survival, polyoma BK virus (BKV) viremia, and polyomavirus-associated nephropathy were calculated using Kaplan–Meier curves, with the log-rank test used for comparison. The Cox proportional hazard model adjusting for age, previous transplantation, peak panel reactive antibodies, HLA mismatches, calcineurin inhibitor, induction therapy, donor age, donor type, expanded criteria donor, and delayed graft function was used to calculate adjusted hazard ratios (aHRs) and 95% confidence intervals (95% CIs) for selected variables. Data were analyzed according to the intention-to-treat principle. Statistical calculations were made using SigmaStat 3.1 and Statistica 9.0 software. Values of P<0.05 were considered statistically significant. Detailed methods are in the Supplemental Material.
Results
Participants
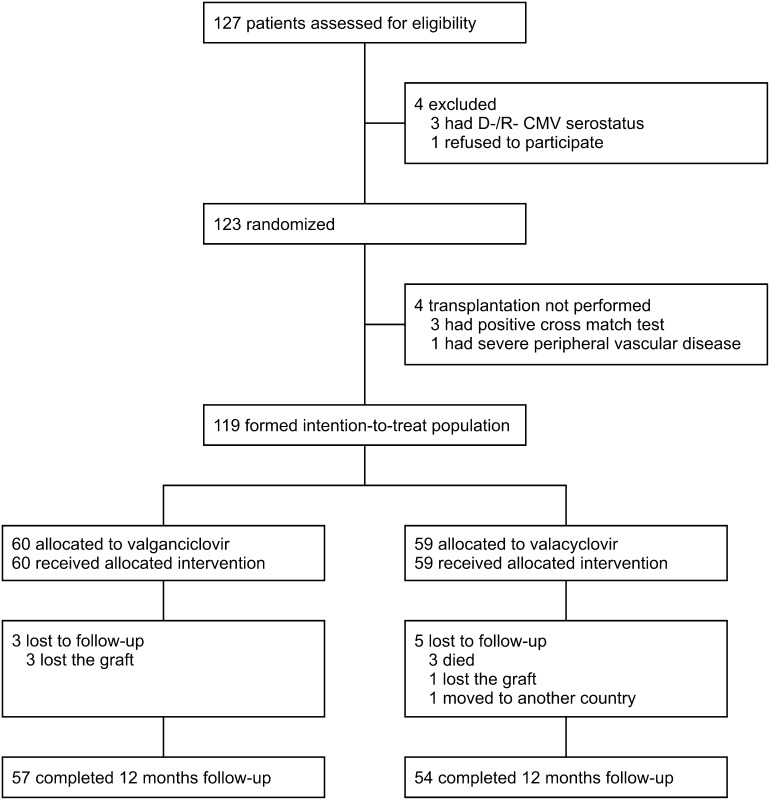
The intention-to-treat population included a total of 119 patients (Figure 1); 60 and 59 patients were randomized to valacyclovir and valganciclovir prophylaxis, respectively. Mean daily doses of valacyclovir and valganciclovir were 6.0±1.4 g and 636±198 mg, respectively. The groups did not differ in prophylaxis duration (90±11 versus 93±12 days, respectively; P=0.33). No major differences were seen in demographic and immunologic parameters. Except for more frequent induction therapy with basiliximab in the valganciclovir group, initial immunosuppressive therapy was comparable in both groups (Table 1). The immunosuppressive protocol as well as immunosuppressive drug levels and doses did not differ during the first 1 year (Supplemental Table 1).
Figure 1.
Flow of patients through the study. CMV, cytomegalovirus; D, donor; R, recipient.
Table 1.
Characteristics of the intention-to-treat population
| Variables | Valganciclovir (n=60) | Valacyclovir (n=59) |
|---|---|---|
| Recipient | ||
| Age (yr), mean±SD | 48±13 | 50±11 |
| Sex (men) | 47 (78) | 37 (63) |
| Cause of renal disease | ||
| Chronic GN | 30 (50) | 21 (36) |
| Polycystic kidney disease | 14 (23) | 10 (17) |
| Hypertensive nephrosclerosis | 6 (10) | 11 (19) |
| Diabetic nephropathy | 2 (3) | 6 (10) |
| Chronic interstitial nephritis | 2 (3) | 4 (7) |
| Other | 6 (10) | 7 (12) |
| Preemptive transplantation | 6 (10) | 5 (8) |
| Previous transplantation | 9 (15) | 7 (12) |
| HLA mismatches, mean ± SD | 3.5±1.2 | 3.6±1.5 |
| Pretransplant PRA≥20% | 11 (18) | 9 (15) |
| CMV serostatus | ||
| D+/R− | 7 (12) | 4 (7) |
| D+/R+ | 44 (73) | 49 (83) |
| D−/R+ | 9 (15) | 6 (10) |
| Donor | ||
| Age (yr), mean±SD | 50±16 | 49±16 |
| Donor type (deceased) | 57 (95) | 54 (92) |
| Expanded criteria donora | 34 (57) | 32 (54) |
| Donation after cardiac death | 3 (5) | 3 (6) |
| Dual kidney transplantation | 5 (9) | 3 (6) |
| Primary immunosuppression | ||
| Cyclosporin + mycophenolate mofetil | 25 (42) | 35 (59) |
| Tacrolimus + mycophenolate mofetil | 35 (58) | 24 (41) |
| No induction therapy | 25 (42) | 34 (58) |
| Basiliximabb | 26 (43) | 14 (24) |
| Thymoglobulin | 9 (15) | 11 (19) |
Data are n (%) unless otherwise indicated. PRA, panel reactive antibody; CMV, cytomegalovirus; D, donor; R, recipient.
According to the United Network for Organ Sharing criteria.
P=0.04.
Primary End Points: CMV DNAemia and Allograft Rejection
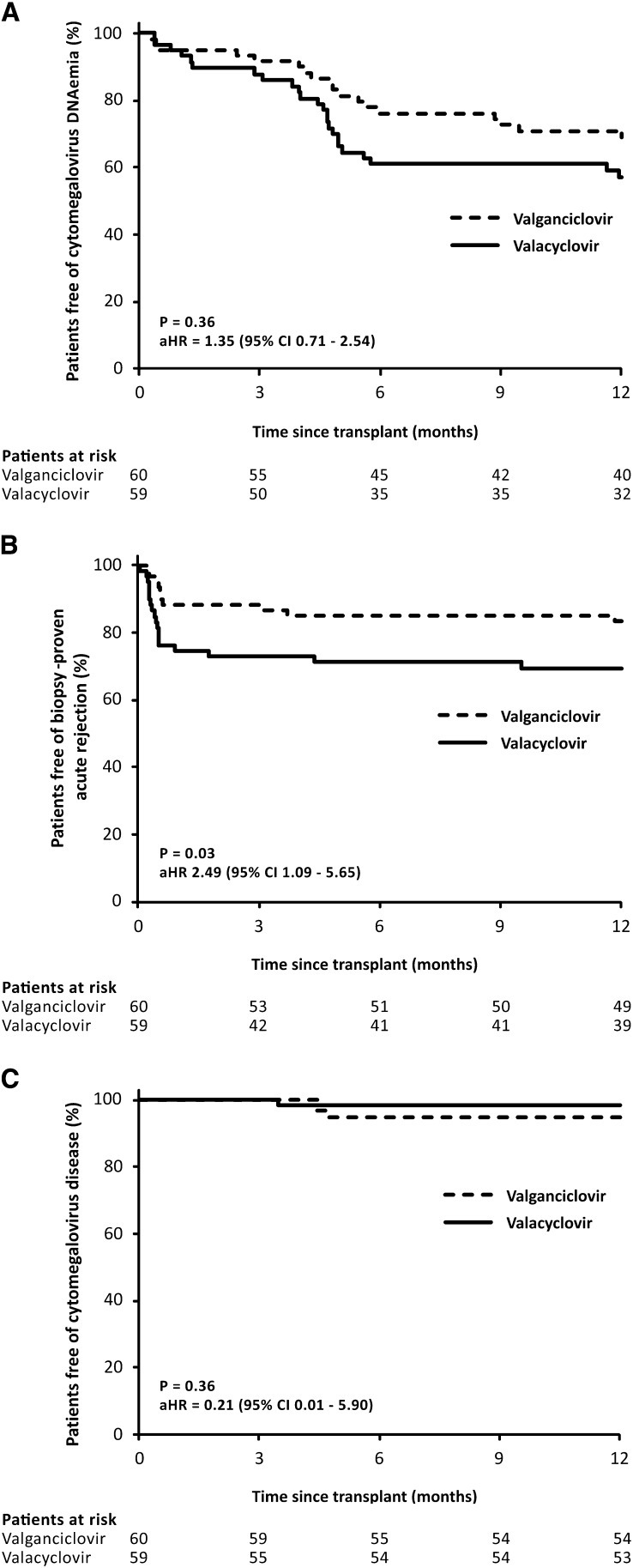
The incidence of CMV DNAemia in valacyclovir prophylaxis was comparable with that seen in the valganciclovir group (24 of 59 [43%] versus 18 of 60 [31%]; aHR, 1.35; 95% CI, 0.71 to 2.54; P=0.36) (Figure 2A). The median time to CMV DNAemia was likewise similar (137 versus 145 days; P=0.37). During prophylaxis, CMV DNAemia developed in eight (14%) and five (8%; P=0.53) patients in the valacyclovir and valganciclovir groups, respectively. Likewise, no major differences were found in the other parameters of CMV DNAemia (Table 2).
Figure 2.
Kaplan–Meier curves for the cumulative probability of freedom from (A) cytomegalovirus DNAemia, (B) biopsy-proven acute rejection, and (C) cytomegalovirus disease. Valacyclovir and valganciclovir prophylaxis showed similar efficacy in prevention of cytomegalovirus DNAemia or disease however, the incidence of biopsy-proven acute rejection was significantly higher with valacyclovir. The Cox proportional hazard model adjusting for age, previous transplantation, peak panel reactive antibodies, HLA mismatches, calcineurin inhibitor, induction therapy, donor age, donor type, expanded criteria donor, and delayed graft function was used for comparison. aHR, adjusted hazard ratio; 95% CI, 95% confidence interval.
Table 2.
Characteristics of cytomegalovirus DNAemia during 12 months
| Variables | Valganciclovir (n=60) | Valacyclovir (n=59) | P Value |
|---|---|---|---|
| CMV DNAemia | 18 (31) | 24 (43) | 0.36a |
| CMV DNAemia during prophylaxisb | 5 (8) | 8 (14) | 0.54 |
| CMV DNAemia by D/R status | |||
| D+/R− | 3 (46) | 2 (50) | 0.66 |
| D+/R+ | 12 (26) | 21 (46) | 0.08 |
| D−/R+ | 3 (37) | 1 (17) | 0.49 |
| CMV DNAemia≥2000 copies/ml | 6 (10) | 5 (9) | 0.86 |
| Peak viral load (copies/ml), median (25th–75th percentiles) | 300 (50–2800) | 750 (100–1650) | 0.66 |
| Time to CMV DNAemia (d), median (25th–75th percentiles) | 145 (87–181) | 137 (64–150) | 0.37 |
| Duration of CMV DNAemia (d), median (25th–75th percentiles) | 39 (22–85) | 31 (14–69) | 0.39 |
Data are n (%) unless otherwise indicated. D, donor; R, recipient.
Hazard ratio, 1.35; 95% confidence interval, 0.71 to 2.54 after adjustment for age, previous transplantation, peak panel reactive antibodies, HLA mismatches, calcineurin inhibitor, induction therapy, donor age, donor type, expanded criteria donor, and delayed graft function.
During prophylaxis, all episodes of cytomegalovirus viremia were with viral load not in excess of 1000 copies/ml. The only exception was a patient with valacyclovir prophylaxis whose viral load reached 1600 copies/ml shortly after pulse methylprednisolone antirejection therapy. Cytomegalovirus viremia remained asymptomatic, with spontaneous clearance within 2 weeks.
Biopsy for cause was performed in 38 (64%) and 32 (53%; P=0.29) patients in the valacyclovir and valganciclovir groups, respectively. On the basis of biopsies for cause, the incidence of BPAR was significantly higher in patients randomized to valacyclovir compared with the valganciclovir prophylaxis (18 of 59 [31%] versus 10 of 60 [17%]; aHR, 2.49; 95% CI, 1.09 to 5.65; P=0.03) (Figure 2B, Table 3, Supplemental Table 2). There were only two patients developing BPAR after CMV DNAemia, whereas in 11 patients, BPAR preceded the development of CMV DNAemia; the remaining 15 patients were not diagnosed with CMV DNAemia.
Table 3.
Characteristics of acute rejection and histologic findings on protocol biopsy at 3 months post-transplant
| Variables | Valganciclovir (n=60) | Valacyclovir (n=59) | P Value |
|---|---|---|---|
| Biopsy-proven acute rejection | 10 (17) | 18 (31) | 0.03a |
| Grade IA | 5 (8) | 8 (14) | |
| Grade IB | 1 (2) | 2 (3) | |
| Grade IIA | 3 (5) | 3 (5) | |
| Grade IIB | 1 (2) | 2 (3) | |
| Grade III | 0 (0) | 0 (0) | |
| Antibody-mediated rejectionb | 2 (3) | 3 (5) | |
| Depleting ALA for rejection | 5 (8) | 7 (12) | 0.74 |
| Protocol biopsy | |||
| No. of patients | 59 | 54 | |
| Glomeruli per biopsy, mean±SD | 10.1±4.9 | 10.9±6.3 | 0.88 |
| Arteries per biopsy, mean±SD | 1.3±1.0 | 1.2±1.1 | 0.52 |
| Subclinical rejection | 2 (3) | 2 (4) | 0.68 |
| Borderline changes | 7 (12) | 11 (20) | 0.33 |
| IF/TA (all grades) | 15 (25) | 9 (17) | 0.37 |
| Moderate-to-severe IF/TAc | 3 (5) | 3 (6) | 0.76 |
| Chronic ci+ct score, mean±SD | 0.81±1.11 | 0.70±1.10 | 0.62 |
| Chronic antibody-mediated rejection | 1 (2) | 0 (0) | 0.97 |
| Chronic T cell-mediated rejection | 0 (0) | 1 (2) | 0.97 |
| Calcineurin inhibitor toxicity | 6 (10) | 4 (7) | 0.85 |
| Vascular nephrosclerosis | 21 (36) | 15 (28) | 0.49 |
| Polyomavirus-associated nephropathy | 6 (10) | 0 (0) | 0.05 |
Data are n (%) unless otherwise indicated. ALA, antilymphocyte antibody; IF/TA, interstitial fibrosis and tubular atrophy; ci, interstitial fibrosis; ct, tubular atrophy.
Hazard ratio, 2.49; 95% confidence interval, 1.09 to 5.65 after adjustment for age, previous transplantation, peak panel reactive antibodies, HLA mismatches, calcineurin inhibitor, induction therapy, donor age, donor type, expanded criteria donor, and delayed graft function.
Both patients with antibody-mediated rejection in the valganciclovir group suffered from concomitant acute T cell-mediated rejection.
Grade II or III according to the Banff 09 classification.
CMV Disease
CMV disease was diagnosed in one (2%) patient of the valacyclovir group and three (5%) patients of the valganciclovir group (aHR, 0.21; 95% CI, 0.01 to 5.90; P=0.36) (Figure 2C). They were all patients with late-onset CMV disease on completion of prophylaxis with a good response to (val)ganciclovir therapy without recurrence (Supplemental Table 3).
Protocol Biopsy Findings
Protocol biopsy at 3 months was performed in all patients with a functioning graft; the only exception was one patient in the valacyclovir group who was lost to follow-up. One patient (valacyclovir group) could not be included into the analysis, because not enough material was available. Although there were no differences in the incidence of subclinical rejection, borderline changes, or IF/TA, the incidence of polyomavirus-associated nephropathy was higher in the valganciclovir group (Table 3).
Survival and Renal Function
The cumulative patient and graft survival rates at 12 months did not differ between the groups. There were three deaths (valacyclovir group) not related to CMV. In the valganciclovir group, graft loss was documented in three patients. Other than the three deaths with a functioning graft, there was one patients with graft failure in the valacyclovir group. Renal function was comparable in both groups throughout the study (Supplemental Table 4).
Polyomavirus and Other Infections
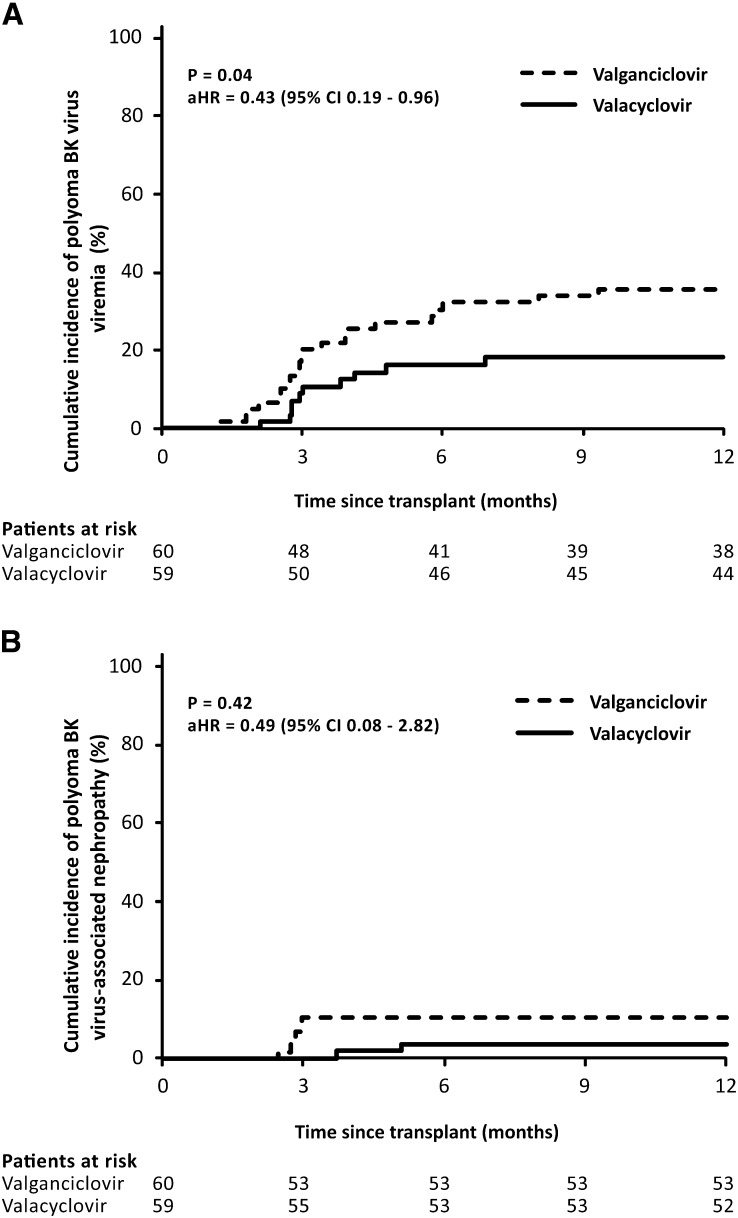
The groups did not differ in the incidence of viral, bacterial, and fungal infections, with a major exception being polyoma BKV infection. The incidence of polyoma BKV viremia was significantly lower in patients receiving valacyclovir prophylaxis (10 of 59 [18%] versus 21 of 60 [36%]; aHR, 0.43; 95% CI, 0.19 to 0.96; P=0.04) (Figure 3A, Supplemental Table 2). However, no differences were observed in peak viral load and the incidence of BKV viremia with a viral load ≥10,000 copies/ml (Table 4). Polyomavirus-associated nephropathy was diagnosed in two (4%) patients in the valacyclovir group compared with six (10%) patients in the valganciclovir group (P=0.42) (Figure 3B).
Figure 3.
Kaplan–Meier curves for the cumulative probability of (A) polyoma BK virus viremia and (B) polyoma BK virus-associated nephropathy. Significantly lower incidence of polyoma BK virus viremia was observed in patients treated with valacyclovir prophylaxis. The Cox proportional hazard model adjusting for age, previous transplantation, peak panel reactive antibodies, HLA mismatches, calcineurin inhibitor, induction therapy, donor age, donor type, expanded criteria donor, and delayed graft function was used for comparison. aHR, adjusted hazard ratio.
Table 4.
Incidence of infections except for cytomegalovirus at 12 months
| Variables | Valganciclovir (n=60) | Valacyclovir (n=59) | P Value |
|---|---|---|---|
| Herpes simplex virus | 9 (15) | 8 (14) | 0.97 |
| Varicella zoster virus | 2 (3) | 0 (0) | 0.48 |
| Epstein–Barr virus disease | 1 (2) | 1 (2) | 0.48 |
| Polyoma BK virus | |||
| Viremia | 21 (36) | 10 (18) | 0.04a |
| Viremia≥10,000 copies/ml | 5 (8) | 3 (5) | 0.73 |
| Nephropathy | 6 (10) | 2 (4) | 0.42 |
| Peak viral load (copies/ml), mean±SD | 7400±15,000 | 8950±15,800 | 0.50 |
| Other viral infection | 15 (25) | 18 (31) | 0.64 |
| Fungal infection | 6 (10) | 10 (17) | 0.40 |
| Pneumocystis | 0 (0) | 0 (0) | — |
| Any bacterial infection | 30 (50) | 39 (66) | 0.11 |
| Urinary tract infection | 11 (18) | 19 (32) | 0.13 |
| Pneumonia | 5 (8) | 4 (7) | 0.98 |
| Sepsis | 11 (18) | 10 (17) | 0.97 |
Data are n (%) unless otherwise indicated.
Hazard ratio, 0.43; 95% confidence interval, 0.19 to 0.96 after adjustment for age, previous transplantation, peak panel reactive antibodies, HLA mismatches, calcineurin inhibitor, induction therapy, donor age, donor type, expanded criteria donor, and delayed graft function.
Safety
Although the incidence of leukopenia and neutropenia was higher in patients treated with valganciclovir, the differences were not significant (Table 5). Granulocyte colony–stimulating factor was used in three (5%) patients of the valacyclovir group and 10 (17%) patients of the valganciclovir group (P=0.08). By contrast, no major differences were noted in psychiatric side effects. A summary of side effects is presented in Table 5. Treatment was discontinued because of undesirable effects of valganciclovir in 13 (22%) patients, whereas valacyclovir was discontinued because of undesirable effects in six (10%) patients.
Table 5.
Summary of adverse events
| Variables | Valganciclovir (n=60) | Valacyclovir (n=59) | P Value |
|---|---|---|---|
| Leukopenia | 20 (33) | 13 (22) | 0.24 |
| Neutropenia | 21 (35) | 13 (22) | 0.17 |
| G-CSF treatment | 10 (17) | 3 (5) | 0.08 |
| Thrombocytopenia | 9 (15) | 11 (19) | 0.76 |
| Anemia | 8 (13) | 11 (19) | 0.56 |
| Hallucinations/confusion | 9 (15) | 13 (22) | 0.45 |
| Moderate to severe | 7 (12) | 6 (10) | 0.97 |
| Headache | 6 (10) | 2 (3) | 0.29 |
| Insomnia | 3 (5) | 2 (3) | 0.99 |
| Nausea | 15 (25) | 14 (24) | 0.96 |
| Diarrhea | 22 (37) | 26 (44) | 0.53 |
| New-onset diabetes or IGTa | 23 (41) | 18 (35) | 0.62 |
| Hypercholesterolemia | 45 (75) | 50 (85) | 0.27 |
| Elevated liver enzymes | 15 (25) | 25 (42) | 0.07 |
| Cardiovascular events | 14 (23) | 11 (19) | 0.69 |
| Malignancy | 1 (2) | 0 (0) | 0.99 |
| Study drug discontinuationb | 13 (22) | 6 (10) | 0.14 |
Data are n (%) unless otherwise indicated. G-CSF, granulocyte colony–stimulating factor; IGT, impaired glucose tolerance.
Patients without diabetes before transplantation were analyzed.
Reasons for discontinuation in valganciclovir prophylaxis were leukopenia and neutropenia (13), and reasons for discontinuation in valacyclovir prophylaxis were leukopenia and neutropenia (3), hallucinations/confusion (1), and oral drugs intolerance (1).
Discussion
Our randomized study has shown that valganciclovir prophylaxis in renal transplant recipients is not superior to valacyclovir in preventing CMV DNAemia. The safety profile was acceptable with both protocols. Quite unexpectedly, valganciclovir prophylaxis was associated with a major reduction in the risk of BPAR development. Another remarkable finding was the increased incidence of polyomavirus viremia in patients treated with valganciclovir. This study is the first to compare valganciclovir with valacyclovir using a randomized design. Both drugs are currently recommended for CMV prophylaxis in renal transplant recipients (1).
Just like valganciclovir, valacyclovir was compared with oral ganciclovir after solid organ transplantation; both agents were found to be comparably effective in the prevention of CMV viremia and disease (19,22,23). Consistent with this finding, our data did not document a major reduction in the incidence of CMV DNAemia in a head-to-head comparison of valganciclovir and valacyclovir. No differences were documented in the time of onset and duration of the DNAemia or peak viral load. The incidence of CMV DNAemia in the range of 30%–40%, like in our group, and its delayed development after completion of prophylaxis were reported in earlier studies involving populations of renal transplant recipients with a low proportion of patients at risk of primary CMV infection (15,16). Nonetheless, the reported incidence is not negligible from the clinical perspective given the recently published association between late-onset CMV viremia and IF/TA or even impaired graft survival (4,10). Both protocols were effective in reducing the incidence of CMV disease to 5%, an effect to be considered in the context of the composition of our patients. In studies involving only patients who were donor positive/recipient negative, the incidence of late-onset CMV disease continues to be unsatisfactory and poses the main limitation to prophylaxis (12,14,23,24). Extending prophylaxis to 6 months would only partly eliminate the problem (12,14); hence, there are efforts to improve prophylactic regimens by determining CMV-specific T cell–mediated immunity (24). Another option is to use the preemptive therapy approach, where late-onset CMV disease does not pose a problem (10,25). Some meta-analyses have shown an increased risk of late-onset CMV disease with valganciclovir compared with nonganciclovir therapies (26). The sample size of our study and the low incidence of CMV disease do not allow us to conclusively evaluate possible differences.
CMV infection is an established risk factor for allograft rejection (2,27,28). Intragraft CMV infection has been implicated in chronic allograft dysfunction and graft loss (29,30). The reduced incidence of acute rejection in patients receiving CMV prophylaxis has been primarily explained by preventing the development of CMV disease and viremia (31). Interestingly, the beneficial effect on the incidence of acute rejection in renal transplant recipients has been related to valacyclovir prophylaxis (16,17,19). However, our study has shown a significantly lower incidence of BPAR in patients with valganciclovir prophylaxis, with a 60% relative risk reduction after adjustment. Given the negative effect of acute rejection on graft outcome, this finding is most important for clinical practice. The difference is unlikely to be because of differences in CMV suppression. In addition to the fact no major differences were observed in the incidence of CMV DNAemia or disease, the overwhelming majority of BPAR developed before the onset of CMV DNAemia or in patients without CMV DNAemia. Despite randomization, the valganciclovir group included more patients with basiliximab induction. Nonetheless, the differences in BPAR persisted even after adjustment for induction therapy and the type of calcineurin inhibitor used. Moreover, a detailed analysis of the immunosuppressive therapy showed a similar distribution of immunosuppressive agents, their doses, and their levels throughout the 12-month study. A plausible explanation for the reduced incidence of acute rejection could be the additive immunosuppressive effect of valganciclovir. Unlike acyclovir, ganciclovir inhibits lymphocyte functions in healthy volunteers (32). In healthy volunteers, ganciclovir has been shown to suppress lymphocyte proliferation and activation by inhibiting DNA synthesis (33). Although in an analysis of lymphocyte function in a subgroup of the 2VAL Study, valganciclovir was shown to reduce lymphocyte proliferation and activated T-cell count, this effect was not documented with valacyclovir (34). It should be noted that an earlier head-to-head comparison of valacyclovir with oral ganciclovir did not show a difference in the incidence of acute rejection (22); our earlier data even showed a lower incidence of acute rejection with valacyclovir. However, the increase in the incidence of acute rejection with oral ganciclovir was only because of the higher proportion of patients with delayed graft function (19). Systemic exposure to ganciclovir at standard valganciclovir doses is significantly higher compared with oral ganciclovir (23); hence, the effect on lymphocyte function need not necessarily be identical.
The significantly higher incidence of polyoma BKV viremia in the group with valganciclovir prophylaxis undergoing systematic screening for BKV replication is a unique finding to date, which may provide additional support to the hypothesis of the clinically relevant immunosuppressive potential of valganciclovir. Polyoma BKV infection is a serious complication in renal transplant recipients that can result in polyomavirus-associated nephropathy, a condition that is associated with a risk of graft loss as high as 90% (35,36). Just as in CMV, the key factor for polyomavirus control is the BKV-specific T-cell immune response (37,38). However, caution is to be exercised when interpreting the results of our study. The difference was caused by the higher incidence of BKV viremia with low viral load, and its clinical effect is poorly understood. However, the finding of polyomavirus-associated nephropathy in protocol biopsy and graft failure in two patients in the valganciclovir group warrants caution and additional research. Deceased donor transplantation as well as routine ureteral stent placement during transplant surgery result in higher risk for polyomavirus-associated nephropathy in our population (35,39).
The tolerability of both protocols was acceptable. As expected, the incidence of leukopenia and neutropenia was higher in the valganciclovir group. Likewise, management of neutropenia was more challenging with valganciclovir, requiring more frequent therapy with granulocyte colony–stimulating factor and discontinuation of valganciclovir. Inconsistent with earlier reports, the incidence of psychiatric adverse events was not increased with valacyclovir (16,17,19). This may have been because of deferred valacyclovir prophylaxis in patients with delayed or slow graft function until the end of the first post-transplant week. A high incidence of hallucinations or confusion in the early post-transplant period was reported in patients with impaired renal function (16,17).
This study has several limitations. The sample size is limited, and the study population comprised mostly patients who were donor positive/recipient positive. Smaller differences in CMV DNAemia rate could not be detected. The low proportion of patients who were donor positive/recipient negative precludes a conclusive comparison of both regimens in this group of patients. The single-center design does not rule out the possibility of different results with another immunosuppressive protocol or composition of donors. The reduced risk of acute rejection in the valganciclovir group may not be demonstrable in a patient population with a low incidence of acute rejection. However, the wide 95% CI allows for even more substantial harm with risk of acute rejection in patients receiving valacyclovir. It should be noted, however, that the composition of our group, which included immunologic high-risk patients and >50% of patients receiving grafts from expanded criteria donors, is consistent with the common transplant population in most European nations.
In conclusion, our data have shown that, in patients undergoing renal transplant, valganciclovir prophylaxis is not superior to high-dose valacyclovir in preventing CMV DNAemia or disease. However, despite comparable data of CMV prevention, choice of the prophylactic antiviral agent may result in differences in not only the spectrum of adverse events but also, major clinical parameters. The significant reduction in BPAR at the expense of increased incidence of low-grade polyoma BKV viremia in patients receiving valganciclovir prophylaxis supports current concepts on the effect of valganciclovir on lymphocyte functions. Long-term follow-up is needed to ascertain whether these differences may influence transplant outcomes, such as renal function or graft survival.
Disclosures
None.
Supplementary Material
Acknowledgments
The authors thank Lenka Karlikova for assistance in data collection.
This work was supported by European Regional Development Fund Grant ED2.1.00/03.0076 and the Charles University Research Fund (P36).
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.07020714/-/DCSupplemental.
References
- 1.Kotton CN, Kumar D, Caliendo AM, Asberg A, Chou S, Danziger-Isakov L, Humar A, Transplantation Society International CMV Consensus Group : Updated international consensus guidelines on the management of cytomegalovirus in solid-organ transplantation. Transplantation 96: 333–360, 2013 [DOI] [PubMed] [Google Scholar]
- 2.Reischig T, Jindra P, Svecová M, Kormunda S, Opatrný K, Jr., Treska V: The impact of cytomegalovirus disease and asymptomatic infection on acute renal allograft rejection. J Clin Virol 36: 146–151, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Reischig T, Jindra P, Hes O, Bouda M, Kormunda S, Treska V: Effect of cytomegalovirus viremia on subclinical rejection or interstitial fibrosis and tubular atrophy in protocol biopsy at 3 months in renal allograft recipients managed by preemptive therapy or antiviral prophylaxis. Transplantation 87: 436–444, 2009 [DOI] [PubMed] [Google Scholar]
- 4.Smith JM, Corey L, Bittner R, Finn LS, Healey PJ, Davis CL, McDonald RA: Subclinical viremia increases risk for chronic allograft injury in pediatric renal transplantation. J Am Soc Nephrol 21: 1579–1586, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Humar A, Gillingham K, Payne WD, Sutherland DE, Matas AJ: Increased incidence of cardiac complications in kidney transplant recipients with cytomegalovirus disease. Transplantation 70: 310–313, 2000 [DOI] [PubMed] [Google Scholar]
- 6.Hjelmesaeth J, Sagedal S, Hartmann A, Rollag H, Egeland T, Hagen M, Nordal KP, Jenssen T: Asymptomatic cytomegalovirus infection is associated with increased risk of new-onset diabetes mellitus and impaired insulin release after renal transplantation. Diabetologia 47: 1550–1556, 2004 [DOI] [PubMed] [Google Scholar]
- 7.Sagedal S, Hartmann A, Nordal KP, Osnes K, Leivestad T, Foss A, Degré M, Fauchald P, Rollag H: Impact of early cytomegalovirus infection and disease on long-term recipient and kidney graft survival. Kidney Int 66: 329–337, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Arthurs SK, Eid AJ, Pedersen RA, Kremers WK, Cosio FG, Patel R, Razonable RR: Delayed-onset primary cytomegalovirus disease and the risk of allograft failure and mortality after kidney transplantation. Clin Infect Dis 46: 840–846, 2008 [DOI] [PubMed] [Google Scholar]
- 9.Luan FL, Kommareddi M, Ojo AO: Impact of cytomegalovirus disease in D+/R- kidney transplant patients receiving 6 months low-dose valganciclovir prophylaxis. Am J Transplant 11: 1936–1942, 2011 [DOI] [PubMed] [Google Scholar]
- 10.Reischig T, Hribova P, Jindra P, Hes O, Bouda M, Treska V, Viklicky O: Long-term outcomes of pre-emptive valganciclovir compared with valacyclovir prophylaxis for prevention of cytomegalovirus in renal transplantation. J Am Soc Nephrol 23: 1588–1597, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reischig T: Advances in cytomegalovirus-preventive strategies in solid organ transplantation: Defending pre-emptive therapy. Expert Rev Anti Infect Ther 10: 51–61, 2012 [DOI] [PubMed] [Google Scholar]
- 12.Humar A, Lebranchu Y, Vincenti F, Blumberg EA, Punch JD, Limaye AP, Abramowicz D, Jardine AG, Voulgari AT, Ives J, Hauser IA, Peeters P: The efficacy and safety of 200 days valganciclovir cytomegalovirus prophylaxis in high-risk kidney transplant recipients. Am J Transplant 10: 1228–1237, 2010 [DOI] [PubMed] [Google Scholar]
- 13.Kliem V, Fricke L, Wollbrink T, Burg M, Radermacher J, Rohde F: Improvement in long-term renal graft survival due to CMV prophylaxis with oral ganciclovir: Results of a randomized clinical trial. Am J Transplant 8: 975–983, 2008 [DOI] [PubMed] [Google Scholar]
- 14.Humar A, Limaye AP, Blumberg EA, Hauser IA, Vincenti F, Jardine AG, Abramowicz D, Ives JA, Farhan M, Peeters P: Extended valganciclovir prophylaxis in D+/R- kidney transplant recipients is associated with long-term reduction in cytomegalovirus disease: Two-year results of the IMPACT study. Transplantation 90: 1427–1431, 2010 [DOI] [PubMed] [Google Scholar]
- 15.Khoury JA, Storch GA, Bohl DL, Schuessler RM, Torrence SM, Lockwood M, Gaudreault-Keener M, Koch MJ, Miller BW, Hardinger KL, Schnitzler MA, Brennan DC: Prophylactic versus preemptive oral valganciclovir for the management of cytomegalovirus infection in adult renal transplant recipients. Am J Transplant 6: 2134–2143, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Reischig T, Jindra P, Hes O, Svecová M, Klaboch J, Treska V: Valacyclovir prophylaxis versus preemptive valganciclovir therapy to prevent cytomegalovirus disease after renal transplantation. Am J Transplant 8: 69–77, 2008 [DOI] [PubMed] [Google Scholar]
- 17.Lowance D, Neumayer HH, Legendre CM, Squifflet JP, Kovarik J, Brennan PJ, Norman D, Mendez R, Keating MR, Coggon GL, Crisp A, Lee IC, International Valacyclovir Cytomegalovirus Prophylaxis Transplantation Study Group : Valacyclovir for the prevention of cytomegalovirus disease after renal transplantation. N Engl J Med 340: 1462–1470, 1999 [DOI] [PubMed] [Google Scholar]
- 18.Kielberger L, Bouda M, Jindra P, Reischig T: Pharmacoeconomic impact of different regimens to prevent cytomegalovirus infection in renal transplant recipients. Kidney Blood Press Res 35: 407–416, 2012 [DOI] [PubMed] [Google Scholar]
- 19.Reischig T, Jindra P, Mares J, Cechura M, Svecová M, Hes O, Opatrný K, Jr., Treska V: Valacyclovir for cytomegalovirus prophylaxis reduces the risk of acute renal allograft rejection. Transplantation 79: 317–324, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Humar A, Michaels M, AST ID Working Group on Infectious Disease Monitoring : American Society of Transplantation recommendations for screening, monitoring and reporting of infectious complications in immunosuppression trials in recipients of organ transplantation. Am J Transplant 6: 262–274, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Sis B, Mengel M, Haas M, Colvin RB, Halloran PF, Racusen LC, Solez K, Baldwin WM, 3rd, Bracamonte ER, Broecker V, Cosio F, Demetris AJ, Drachenberg C, Einecke G, Gloor J, Glotz D, Kraus E, Legendre C, Liapis H, Mannon RB, Nankivell BJ, Nickeleit V, Papadimitriou JC, Randhawa P, Regele H, Renaudin K, Rodriguez ER, Seron D, Seshan S, Suthanthiran M, Wasowska BA, Zachary A, Zeevi A: Banff ’09 meeting report: Antibody mediated graft deterioration and implementation of Banff working groups. Am J Transplant 10: 464–471, 2010 [DOI] [PubMed] [Google Scholar]
- 22.Pavlopoulou ID, Syriopoulou VP, Chelioti H, Daikos GL, Stamatiades D, Kostakis A, Boletis JN: A comparative randomised study of valacyclovir vs. oral ganciclovir for cytomegalovirus prophylaxis in renal transplant recipients. Clin Microbiol Infect 11: 736–743, 2005 [DOI] [PubMed] [Google Scholar]
- 23.Paya C, Humar A, Dominguez E, Washburn K, Blumberg E, Alexander B, Freeman R, Heaton N, Pescovitz MD, Valganciclovir Solid Organ Transplant Study Group : Efficacy and safety of valganciclovir vs. oral ganciclovir for prevention of cytomegalovirus disease in solid organ transplant recipients. Am J Transplant 4: 611–620, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Manuel O, Husain S, Kumar D, Zayas C, Mawhorter S, Levi ME, Kalpoe J, Lisboa L, Ely L, Kaul DR, Schwartz BS, Morris MI, Ison MG, Yen-Lieberman B, Sebastian A, Assi M, Humar A: Assessment of cytomegalovirus-specific cell-mediated immunity for the prediction of cytomegalovirus disease in high-risk solid-organ transplant recipients: A multicenter cohort study. Clin Infect Dis 56: 817–824, 2013 [DOI] [PubMed] [Google Scholar]
- 25.Spinner ML, Saab G, Casabar E, Bowman LJ, Storch GA, Brennan DC: Impact of prophylactic versus preemptive valganciclovir on long-term renal allograft outcomes. Transplantation 90: 412–418, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kalil AC, Freifeld AG, Lyden ER, Stoner JA: Valganciclovir for cytomegalovirus prevention in solid organ transplant patients: An evidence-based reassessment of safety and efficacy. PLoS ONE 4: e5512, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reischig T: Cytomegalovirus-associated renal allograft rejection: New challenges for antiviral preventive strategies. Expert Rev Anti Infect Ther 8: 903–910, 2010 [DOI] [PubMed] [Google Scholar]
- 28.Freeman RB, Jr.: The ‘indirect’ effects of cytomegalovirus infection. Am J Transplant 9: 2453–2458, 2009 [DOI] [PubMed] [Google Scholar]
- 29.Dzabic M, Rahbar A, Yaiw KC, Naghibi M, Religa P, Fellström B, Larsson E, Söderberg-Nauclér C: Intragraft cytomegalovirus protein expression is associated with reduced renal allograft survival. Clin Infect Dis 53: 969–976, 2011 [DOI] [PubMed] [Google Scholar]
- 30.Reischig T, Nemcová J, Vanecek T, Jindra P, Hes O, Bouda M, Treska V: Intragraft cytomegalovirus infection: A randomized trial of valacyclovir prophylaxis versus pre-emptive therapy in renal transplant recipients. Antivir Ther 15: 23–30, 2010 [DOI] [PubMed] [Google Scholar]
- 31.Kalil AC, Levitsky J, Lyden E, Stoner J, Freifeld AG: Meta-analysis: The efficacy of strategies to prevent organ disease by cytomegalovirus in solid organ transplant recipients. Ann Intern Med 143: 870–880, 2005 [DOI] [PubMed] [Google Scholar]
- 32.Heagy W, Crumpacker C, Lopez PA, Finberg RW: Inhibition of immune functions by antiviral drugs. J Clin Invest 87: 1916–1924, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Battiwalla M, Wu Y, Bajwa RP, Radovic M, Almyroudis NG, Segal BH, Wallace PK, Nakamura R, Padmanabhan S, Hahn T, McCarthy PL, Jr.: Ganciclovir inhibits lymphocyte proliferation by impairing DNA synthesis. Biol Blood Marrow Transplant 13: 765–770, 2007 [DOI] [PubMed] [Google Scholar]
- 34.Reischig T, Prucha M, Sedlackova L, Lysak D, Jindra P, Bouda M, Matejovic M: Valganciclovir prophylaxis against cytomegalovirus impairs lymphocyte proliferation and activation in renal transplant recipients. Antivir Ther 16: 1227–1235, 2011 [DOI] [PubMed] [Google Scholar]
- 35.Hirsch HH, Randhawa P, AST Infectious Diseases Community of Practice : BK polyomavirus in solid organ transplantation. Am J Transplant 13[Suppl 4]: 179–188, 2013 [DOI] [PubMed] [Google Scholar]
- 36.Schaub S, Hirsch HH, Dickenmann M, Steiger J, Mihatsch MJ, Hopfer H, Mayr M: Reducing immunosuppression preserves allograft function in presumptive and definitive polyomavirus-associated nephropathy. Am J Transplant 10: 2615–2623, 2010 [DOI] [PubMed] [Google Scholar]
- 37.Binggeli S, Egli A, Schaub S, Binet I, Mayr M, Steiger J, Hirsch HH: Polyomavirus BK-specific cellular immune response to VP1 and large T-antigen in kidney transplant recipients. Am J Transplant 7: 1131–1139, 2007 [DOI] [PubMed] [Google Scholar]
- 38.Bestard O, Lucia M, Crespo E, Van Liempt B, Palacio D, Melilli E, Torras J, Llaudó I, Cerezo G, Taco O, Gil-Vernet S, Grinyó JM, Cruzado JM: Pretransplant immediately early-1-specific T cell responses provide protection for CMV infection after kidney transplantation. Am J Transplant 13: 1793–1805, 2013 [DOI] [PubMed] [Google Scholar]
- 39.Kayler L, Zendejas I, Schain D, Magliocca J: Ureteral stent placement and BK viremia in kidney transplant recipients. Transpl Infect Dis 15: 202–207, 2013 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.