Abstract
The presentation of an exogenous antigen in a major histocompatibility complex class-I-restricted fashion to CD8+ T cells is called cross-presentation. Heat shock proteins (HSPs) such as Hsp70, gp96, and Hsp90 have been shown to elicit efficient CTL responses by cross-presentation through an as-yet entirely unknown mechanism. Hsp90 is the most abundant cytosolic HSP and is known to act as a molecular chaperone. We have shown that a tumor antigen peptide complexed with Hsp90 could be cross-presented by dendritic cells (DCs) through an endosomal pathway in a murine system. However, it has not been determined whether human DCs also cross-present an Hsp90–peptide complex and induce peptide-specific CTLs. In this study, we found that an Hsp90–cancer antigen peptide complex was efficiently cross-presented by human monocyte-derived DCs and induced peptide-specific CTLs. Furthermore, we observed that the internalized Hsp90–peptide complex was strictly sorted to the Rab5+, EEA1+ static early endosome and the Hsp90-chaperoned peptide was processed and bound to MHC class I molecules through an endosome-recycling pathway. Our data indicate that targeting of the antigen to a “static” early endosome by Hsp90 is essential for efficient cross-presentation.
Keywords: Antigen presentation/processing, dendritic cells, heat shock protein, T cells, tumor immunity
The generation of specific CD8+ CTLs is thought to play a key role in the control of virus-infected cells and tumors. However, immunization with peptides or recombinant proteins generally fails to elicit CTLs because an immunized antigen (Ag) acts as an exogenous Ag. Generally, an exogenous Ag enters the MHC class II pathway and is presented to CD4+ T cells in the context of MHC class II molecules. However, professional Ag-presenting cells, particularly DCs, can take up exogenous Ag and present them on their MHC class I molecules. This process is called cross-presentation and plays an important role in the control of virus-infected cells and tumor growth.1 There are two pathways of cross-presentation: cytosolic (endoplasmic reticulum–Golgi-dependent) and vacuolar (endosomal) pathways.2,3 One of the reasons for inefficiency of a vaccine strategy is that the vaccine Ag is usually administered as an exogenous Ag, and it is therefore difficult to introduce the vaccine Ag into the cross-presentation pathway. To overcome this problem, various methods have been developed to target an exogenous Ag into the endogenous MHC class I-restricted pathway. In our previous studies, we showed that extracellular Hsp90–peptide complexes are efficiently cross-presented through the endosome-recycling pathway.4 In this Hsp90-mediated cross-presentation, the receptor-dependent endocytosed Hsp90–peptide complex was transferred to the early endosome in which a cysteine protease such as cathepsin S processed the precursor peptide. The resulting MHC class I epitope was transferred onto recycling MHC class I molecules, thereby resulting in the expression of an MHC class I–epitope complex on the cell surface. Furthermore, we have shown that immunization with Hsp90–tumor Ag peptide complexes induces Ag-specific CTL responses and strong antitumor immunity in vivo. However, how the Hsp90–peptide complex is sorted out after receptor-dependent endocytosis remains unclear. In the present work, we found that Hsp90 complexed with a human tumor Ag peptide derived from survivin-2B5,6 is cross-presented by human Mo-DCs resulting in the stimulation of peptide-specific CTLs. In addition, we found that Hsp90 targets a chaperoned Ag peptide into the “static” early endosome within Mo-DCs, resulting in cross-presentation of the antigenic peptide through the recycling pathway.
Materials and Methods
The study protocol was approved by the Clinic Institutional Ethical Review Board of the Medical Institute of Bioregulation, Sapporo Medical University (Sapporo, Japan). The patients and their families as well as healthy donors gave informed consent for the use of blood samples in our research.
Patient treatment
The patients were vaccinated with survivin-2B80-88 (1 mg) plus Montanide ISA 51 (1 mL; Seppic, Paris, France) s.c. four times at 14-day intervals. In addition, IFN-α (3 000 000 IU; Dainippon-Sumitomo Pharmaceutical Co., Osaka, Japan) was given s.c. twice a week close to the site of vaccination. Hematological examinations were carried out before and after each vaccination.
Induction of human monocyte-derived immature dendritic cells
Autologous monocytes were purified from PBMCs from each patient that were isolated using Lymphoprep (Nycomed, Oslo, Norway). Monocytes (1 × 104/well) in a 24-well plate were cultured in complete RPMI-1640 with 10% FCS and GM-CSF (1000 U/mL) and IL-4 (1000 U/mL) for 7 days. The medium with GM-CSF and IL-4 was gently replaced on day 2 and 4. Human recombinant GM-CSF was a kind gift from Kirin (Tokyo, Japan). Human recombinant IL-4 was purchased from Invitrogen (Carlsbad, CA, USA).
Peptides and proteins
The following peptides were used (underlined sequences representing the precise MHC class I-binding epitope): survivin-2B80-88 (AYACNTSTL); and survivin-2B75-93 (GPGTVAYACNTSTLGGRGG). All peptides were purchased from Sigma-Genosys (Ishikari, Japan). Human Hsp90 was purchased from StressGen (Ann Arbor, MI, USA). Human LDL was purchased from Sigma-Aldrich (St. Louis, MO, USA) and stored at 20 mg/mL in PBS at −80°C.
Antibodies
Confocal laser microscopy was used to detect organelles with specific antibodies: an anti-Rab5 pAb (MBL, Nagoya, Japan) and EEA1 (Abcam, Cambridge, MA, USA) for early endosomes, and anti-LAMP-1 pAb (Santa Cruz Biotechnology, Santa Cruz, CA, USA) for late endosomes/lysosomes. Alexa Fluor 594 (Molecular Probes, Eugene, OR, USA) was used for labeling Hsp90 and LDL.
Generation of Hsp90–peptide complex in vitro
As previously described,4 Hsp90 was mixed with survivin-2B75-93 (GPGTVAYACNTSTLGGRGG) in a 50:1 peptide:protein molar ratio in 0.7 M NaCl containing sodium-phosphate buffer and heated at 45°C for 30 min, then incubated for 30 min at room temperature.
Establishment of survivin-2B80-88-specific CTL clone
We generated survivin-2B80-88-specific CTL clones from a patient with colon cancer (patient 1 in Table1). After the fourth vaccination, PBMCs were isolated from blood samples by Ficoll–Conray density gradient centrifugation. Phytohemagglutinin blasts were derived from PBMCs by culturing in AIM V medium (Invitrogen) containing 10% human serum, IL-2 (100 U/mL; Takeda Pharmaceutical Co., Osaka, Japan), and PHA (1 mg/mL; Wako Chemicals, Osaka, Japan) for 3 days, followed by washing and cultivation in the presence of IL-2 (100 U/mL) for 4 days. HLA-A*2402-survivin-2B80-88 peptide tetramer-positive (MBL) CTLs were sorted and subsequently cloned to single cells using FACSAria (Becton Dickinson, San Jose, CA, USA). Survivin-2B80-88-specific CTL clones were restimulated with survivin-2B80-88 peptide-pulsed PHA blasts every 7 days in AIM V medium supplemented with 50 U/mL IL-2.
Table 1.
Quantitation of survivin-2B-specific CD8+ T cells by tetramer assay
| Patient no. | Tumor | Survivin-2B80-88-specific CD8+ T cell frequency (tetramer staining) |
Effect | |||||
|---|---|---|---|---|---|---|---|---|
| In vitro stimulation | (−) | Survivin-2B80-88 | Hsp90 | Survivin-2B75-93 | Hsp90–survivin-2B75-93 | |||
| 1 | Colon | 0.06 | 4.87 | 4.47 | 3.24 | 8.47 | †† | |
| 2 | Colon | 0.32 | 2.87 | 0.77 | 4.01 | 1.30 | No | |
| 3 | Pancreas | 0.70 | 6.27 | 1.70 | 1.88 | 6.64 | †† | |
| 4 | Pancreas | 0.48 | 4.56 | 0.84 | 1.28 | 3.02 | † | |
| 5 | Ampulla of Vater | 1.27 | 4.60 | 0.97 | 2.24 | 6.50 | †† | |
| 6 | Breast | 3.59 | 3.78 | 3.00 | 3.06 | 3.82 | †† | |
| 7 | Breast | 3.98 | 3.91 | 1.94 | 2.28 | 6.19 | †† | |
| 8 | Breast | 2.76 | 3.92 | 2.91 | 2.08 | 6.07 | †† | |
Frequency of survivin-2B-specific CD8+ T cells stimulated with heat shock protein 90 (Hsp90)–survivin-2B75-93 peptide complex was increased compared with stimulation with survivin-2B75-93 precursor peptide.
Frequency of survivin-2B-specific CD8+ T cells stimulated with Hsp90–survivin-2B75-93 peptide was increased compared with stimulation with both survivin-2B80-88 peptide and survivin-2B75-93 peptide. (−), negative control; No, no effect.
In vitro cross-presentation assay
Human Mo-DCs (1 × 105) were pulsed with Hsp90 (400 μg/mL), survivin-2B75-93 (400 μg/mL) alone, a complex of Hsp90 (100 or 400 μg/mL) and survivin-2B75-93 (100 or 400 μg/mL), a simple mixture of both or survivin-2B80-88 (400 μg/mL) for 2 h at 37°C in 100 μL Opti-MEM and then fixed for 1 min with 0.01% glutaraldehyde. Fixation was stopped by addition of 2 M L-lysine and the cells were washed twice with RPMI-1640 medium and cultured overnight with 1 × 105 survivin-2B peptide-specific CTL clone. Activation of CTLs was measured as IFN-γ production using ELISA. In a dose titration assay, Mo-DCs (1 × 105) were loaded with various doses of survivin-2B80-88 peptide or Hsp90–precursor peptide (survivin-2B75-93) complex for 2 h in 100 μL Opti-MEM and fixed with 0.01% glutaraldehyde. The cells were washed and cultured overnight with 1 × 105 survivin-2B80-88-peptide-specific CTL clone. Interferon-γ in the culture supernatant was measured using ELISA.
In vitro stimulation of PBMCs with Mo-DC loaded with Hsp90–precursor peptide complex
Peripheral blood mononuclear cells were isolated from eight patients suffering from various types of cancer who had been vaccinated with survivin-2B peptide in our clinical study.7,8 These patients' PBMCs were shown to contain survivin-2B-specific CD8+ T cells. The PBMCs were stimulated with Mo-DCs loaded with survivin-2B80-88 (400 μg/mL), Hsp90 (400 μg/mL), survivin-2B75-93 (400 μg/mL), and Hsp90 (100 or 400 μg/mL)–survivin-2B75-93 (100 or 400 μg/mL) complex in AIM V medium (Life Technologies, Grand Island, NY, USA) containing 10% human serum. Interleukin-2 was added at a final concentration of 50 U/mL on days 2, 4, and 6. On day 7 of culture, the PBMCs were analyzed by tetramer staining and ELISPOT assay.
Assessment of stimulation of Ag-specific CTLs using tetramer assay
The FITC-labeled HLA-A*2402-HIV peptide (RYLRDQQLL) and PE-labeled HLA-A*2402-survivin-2B80-88 peptide tetramers were purchased from MBL. For flow cytometric analysis, PBMCs, which were stimulated in vitro as described above, were stained with HIV tetramer or survivin-2B tetramer at 37°C for 20 min. Then a PE-Cy5-conjugated anti-CD8 antibody (eBioscience, San Diego, CA, USA) was added at 4°C for 30 min. Cells were washed twice with PBS. After washing, cells were fixed with 0.5% paraformaldehyde and analyzed by flow cytometry using FACSCalibur and CellQuest software (Becton Dickinson). CD8+ living cells were gated and cells labeled with survivin-2B tetramer were referred to as tetramer-positive cells. The frequency of CTL precursors was calculated as the number of tetramer-positive cells divided by the number of CD8+ T cells.
Assessment of stimulation of Ag-specific CTLs using ELISPOT assay
The ELISPOT plates were coated sterilely overnight with anti-IFN-γ capture antibody (BD Biosciences, San Jose, CA, USA) at 4°C. The plates were then washed once and blocked with AIM V medium containing 10% human serum for 2 h at room temperature. CD8-positive T cells separated from patients' PBMCs (5 × 103 cells/well), which were stimulated in vitro as described above, were then added to each well along with HLA-A24-transfected T2 (T2-A24) cells (5 × 104 cells/well) that had been preincubated with survivin-2B80-88 (10 μg/mL) or HIV with an HIV peptide as a negative control. After incubation in a 5% CO2 humidified chamber at 37°C for 24 h, the wells were washed vigorously five times with PBS and incubated with a biotinylated anti-human IFN-γ antibody (R&D Systems, Minneapolis, MN, USA) and HRP-conjugated avidin. Spots were visualized and analyzed using KS ELISPOT (Carl Zeiss, Jena, Germany).
Immunocytological localization of Hsp90–survivin-2B75-93 peptide complex
Heat shock protein 90 and LDL were conjugated with Alexa Fluor 594 (Molecular Probes) according to the manufacturer's instructions. Monocyte-derived DCs were incubated at 37°C with Alexa Fluor 594-labeled Hsp90 (20 μg) complexed with survivin-2B75-93 peptide (20 μg) for 1 h. Following incubation, cells were washed twice with ice-cold PBS and fixed with ice-cold acetone for 1 min. Organelles were stained with an anti-Rab5 pAb and EEA1 mAb for early endosomes and anti-LAMP-1 pAb for late endosomes followed by Alexa 488-conjugated goat anti-rabbit IgG (Molecular Probes) or anti-mouse IgG (Molecular Probes) and then visualized with a Bio-Rad MRC1024ES confocal scanning laser microscope system (Bio-Rad, Richmond, CA, USA). For evaluation of colocalization, a single z-plane of one cell was evaluated. For each protein and organelle combination, a total of 150 cells (50 cells from three independent experiments) were analyzed.
Inhibition studies
Monocyte-derived DCs were pre-incubated with chloroquine (Sigma-Aldrich) or primaquine (ICN Biomedicals, Irvine, CA, USA) at 37°C for 2 h, and then loaded with survivin-2B80-88 peptide alone or Hsp90–precursor peptide (survivin-2B75-93) complex for 2 h. The Mo-DCs were then fixed, washed, and cultured overnight with survivin-2B80-88-specific CTL clone. Activation of CTLs was measured as IFN-γ production using ELISA.
Statistical analysis
All experiments were independently carried out three times in triplicate. Results are shown as means + SEM. Comparisons between two groups were performed using Student's t-test, with a P-value < 0.05 considered to be statistically significant.
Results
Heat shock protein 90–survivin-2B75-93 peptide complex is cross-presented by Mo-DCs in vitro
We first examined whether human Hsp90 facilitated cross-presentation of the chaperoned precursor peptide by human Mo-DCs. The Mo-DCs were pulsed with Hsp90 alone, the survivin-2B75-93 precursor peptide alone, a simple mixture of both, a complex of them generated in vitro at double concentration, or survivin-2B80-88 peptide (for positive control) for 2 h at 37°C and then fixed, washed, and cultured with survivin-2B80-88-specific CTL clone. The Hsp90–survivin-2B75-93 precursor peptide complex elicited a significant amount of IFN-γ production both at 100 and 100 μg/mL, whereas Hsp90 alone, survivin-2B75-93 precursor peptide alone, or a simple mixture of both did not induce IFN-γ production by CTLs (Fig.1a). Strikingly, IFN-γ production induced by Hsp90–survivin-2B precursor peptide complex was much greater than that induced by survivin-2B peptide. These results indicated that cross-presentation of survivin-2B-derived peptide was enhanced when an exogenous precursor peptide was complexed to Hsp90. To confirm these observations, we compared the efficacy of CTL activation between survivin-2B80-88 peptide and Hsp90–survivin-2B75-93 precursor peptide complex in a dose titration assay (Fig.1b). We observed that stimulation of the survivin-2B80-88-specific CTL clone with Hsp90–survivin-2B75-93 precursor peptide complex was more effective than stimulation with survivin-2B80-88 peptide at any dose.
Figure 1.
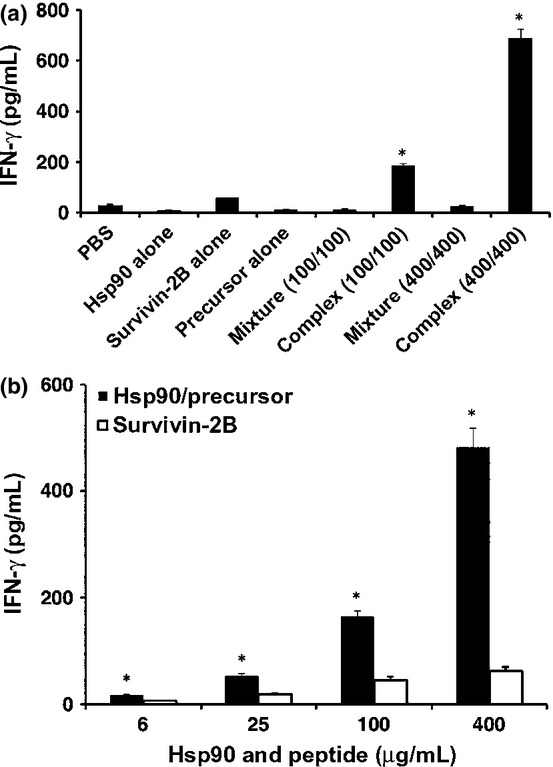
Cross-presentation of heat shock protein 90 (Hsp90)-chaperoned peptides by human monocyte-derived dendritic cells (Mo-DCs). (a) Human Mo-DCs (1 × 105) were pulsed with Hsp90 (400 μg/mL), precursor peptide survivin-2B75-93 (400 μg/mL) alone, a complex of Hsp90 (100 or 400 μg/mL) and survivin-2B75-93 (100 or 400 μg/mL), a simple mixture of both, or survivin-2B80-88 peptide (for positive control) for 2 h at 37°C and then fixed with 0.01% glutaraldehyde, washed, and cultured with survivin-2B80-88-specific CTL clone (1 × 105/well). Activation of CTLs was measured as γ-interferon (IFN-γ) production using ELISA. (b) Mo-DCs (1 × 105) were loaded with various doses of survivin-2B80-88 peptide (6, 25, 100, and 400 μg/mL) or Hsp90–survivin-2B75-93 precursor peptide complex (6/6, 25/25, 100/100, and 400/400 μg/mL) for 2 h in 100 μL Opti-MEM and fixed with 0.01% glutaraldehyde. The cells were washed and cultured overnight with 1 × 105 survivin-2B80-88-specific CTL clone. Activation of CTLs was measured as IFN-γ production using ELISA. Data are shown as means + SEM of three independent experiments. *P < 0.01.
Peptide-specific precursor CTLs are activated by cross-presentation of Hsp90–peptide complex
As we had shown that the Hsp90–survivin-2B75-93 precursor peptide complex was efficiently cross-presented, we next examined whether cross-presentation of Hsp90–peptide complex could activate and expand peptide-specific memory CD8+ T cells from patients who had been vaccinated with survivin-2B peptide with incomplete Freund's adjuvant. Activated and expanded survivin-2B-specific CD8+ T cells were detected by tetramer staining. As shown in Figure2, the survivin-2B75-93 precursor peptide chaperoned by Hsp90 was able to activate and expand survivin-2B-specific memory CD8+ T cells more vigorously than was the precursor peptide alone. Interestingly, peptide-specific T-cell frequency was higher when stimulated with Hsp90–survivin-2B75-93 precursor peptide complex than that with survivin-2B80-88 peptide, indicating that a long peptide chaperoned by Hsp90 was efficiently cross-presented and was able to stimulate peptide-specific CD8+ T cells. To confirm these observations, we compared the efficacy of activation of survivin-2B-specific memory CD8+ T cells by stimulation with survivin-2B80-88, survivin-2B75-93 precursor peptide, or Hsp90–survivin-2B75-93 precursor peptide complex in eight patients. As shown in Table1, stimulation with Hsp90–survivin-2B75-93 complex could expand survivin-2B-specific memory CD8+ T cells from seven out of eight patients compared with stimulation with survivin-2B75-93. More importantly, in six out of eight patients, stimulation with Hsp90–survivin-2B75-93 complex expanded survivin-2B-specific memory CD8+ T cells more efficiently compared with stimulation with survivin-2B80-88.
Figure 2.
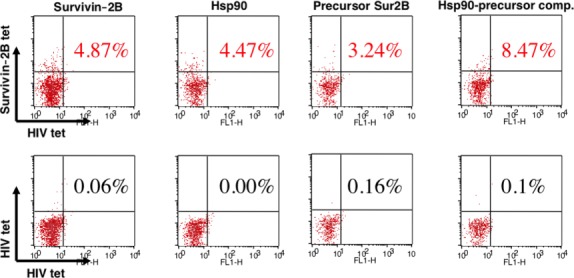
Peptide-specific precursor CTLs were activated by cross-presentation of heat shock protein 90 (Hsp90)–peptide complex. PBMCs were isolated from patient 1 suffering from colon cancer (Table1) who had been vaccinated with survivin-2B80-88 peptide in our clinical study. The patient's PBMCs were shown to contain the survivin-2B-specific CD8+ T cells. PBMCs were stimulated with human monocyte-derived dendritic cells loaded with survivin-2B80-88 (400 μg/mL), Hsp90 (400 μg/mL), survivin-2B75-93 precursor peptide (400 μg/mL), and Hsp90 (400 μg/mL)–survivin-2B75-93 precursor peptide (400 μg/mL) complex in AIM V medium containing 10% human serum and interleukin-2 (50 U/mL) for 7 days. The stimulated PBMCs were stained with HIV tetramer (tet) or survivin-2B tetramer at 37°C for 20 min. Then a phycoerythrin-Cy5-conjugated anti-CD8 antibody was added at 4°C for 30 min. Cells were washed twice with PBS. After washing, cells were fixed with 0.5% paraformaldehyde and analyzed by flow cytometry using FACSCalibur and CellQuest software. CD8+ living cells were gated, and cells labeled with survivin-2B tetramer were referred to as tetramer-positive cells. The frequency of CTL precursors was calculated as the number of tetramer-positive cells divided by the number of CD8+ cells. Data are shown as means + SEM of three independent experiments. *P < 0.01.
Memory CD8+ T cells activated by cross-presentation of Hsp90–peptide complex become functional peptide-specific CTLs
To further confirm whether survivin-2B-specific CD8+ T cells activated by Hsp90-mediated cross-presentation were functional or not, we carried out an ELSPOT assay using CD8+ T cells from a patient who had been vaccinated with survivin-2B peptide with incomplete Freund's adjuvant. Figure3 shows that stimulation of CD8+ T cells from the patient with Hsp90–survivin-2B75-93 precursor peptide complex clearly increased functionally positive survivin-2B-specific CD8+ T cells compared with stimulation with survivin-2B75-93 precursor peptide or survivin-2B80-88 peptide. When CD8+ T cells from the patient were stimulated with Hsp90 (400 μg/mL)–precursor peptide (400 μg/mL) complex, the number of IFN-γ-positive spots was less than that of CD8+ T cells stimulated with Hsp90 (100 μg/mL)–precursor peptide (100 μg/mL) complex. These results were due to the formation of fused large spots that were observed when stimulated with Hsp90 (400 μg/mL)–precursor peptide (400 μg/mL) complex and therefore the number of ELISPOT counted became smaller than that of Hsp90 (100 μg/mL)–precursor peptide (100 μg/mL) complex. These findings indicated that Hsp90–peptide complex is efficiently cross-presented by human Mo-DCs and is capable of stimulating peptide-specific CTLs.
Figure 3.
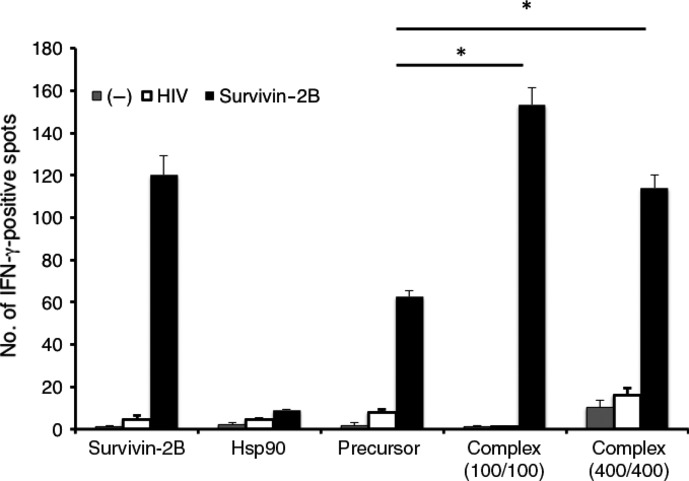
Memory CD8+ T cells activated by cross-presentation of heat shock protein 90 (Hsp90)–peptide complex became functional peptide-specific CTLs. CD8+ T cells separated from PBMCs (5 × 103 cells/well) from patient 1 (Table1) were stimulated with human monocyte-derived dendritic cells loaded with survivin-2B80-88 (400 μg/mL), Hsp90 (400 μg/mL), precursor peptide survivin-2B75-93 (400 μg/mL), and Hsp90 (100 or 400 μg/mL)–survivin-2B75-93 (100 or 400 μg/mL) complex, were added to each well along with HLA-A24-transfected T2 (T2-A24) cells (5 × 104 cells/well) that had been preincubated with survivin-2B80-88 (10 μg/mL) or HIV with an HIV peptide as a negative control (−). After incubation in a 5% CO2 humidified chamber at 37°C for 24 h, the wells were washed vigorously five times with PBS and incubated with a biotinylated anti-human γ-interferon (IFN-γ) antibody and HRP-conjugated avidin. Spots were visualized and analyzed using KS ELISPOT. Data are shown as means + SEM of three independent experiments. *P < 0.01.
Immunocytological localization of Hsp90–survivin2B75-93 peptide complex
For further support of the above-described results, we investigated the intracellular routing of Hsp90 after uptake of it in DCs, using confocal laser microscopy. The Mo-DCs were incubated with Alexa 594-labeled Hsp90–survivin2B75-93 peptide complex for 1 h. Following incubation, the cells were fixed and stained with antibodies against markers for organelle structures including EEA1, Rab5, and LAMP-1. Alexa 594-labeled Hsp90–peptide complex was detected in EEA1+ and Rab5+-early endosomes but not in lysosomes (Fig.4a). Quantitative analysis of the colocalization between the exogenous Hsp90–peptide complex and Rab5, EEA1, and LAMP1 revealed average colocalization incidences of 78.0%, 88.7%, and 7.3%, respectively, providing further evidence that the exogenous Hsp90–peptide complex was delivered to the endosome-recycling pathway (Fig.4b). We also examined the dynamics of Alexa 594-labeled LDL as a positive control protein for the dynamic early endosomal pathway (Fig.5). Alexa594-labeled soluble LDL localized to the Rab5+-early endosome as well as the LAMP-1+-late endosome/lysosome, but not to the EEA1+-compartment, thus indicating the dynamic endosomal pathway. These results indicated that the Hsp90–peptide complex was sorted into the static endosomal pathway, not the dynamic endosomal pathway, within human Mo-DCs. In contrast, the soluble LDL protein, which underwent degradation, was translocated to the dynamic endosomal pathway. These results suggested that targeting to the “static” early endosome was required for efficient cross-presentation by Mo-DCs.
Figure 4.
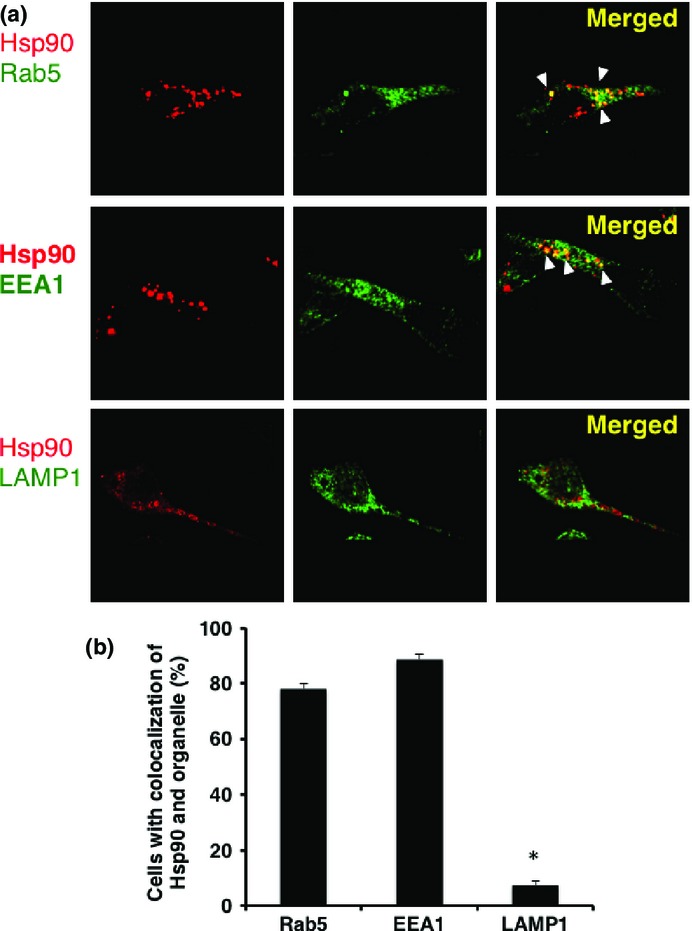
Heat shock protein 90 (Hsp90)–survivin-2B75-93 precursor peptide complex localized to static early endosomes within human monocyte-derived dendritic cells (Mo-DCs). (a) Human Mo-DCs were incubated at 37°C with Alexa 594-labeled Hsp90–survivin-2B75-93 peptide complex for 1 h and then washed and fixed. Organelles were stained with an anti-EEA1 mAb for early endosomes, anti-Rab5 polyclonal antibody for early endosomes, and anti-LAMP-1 polyclonal antibody for late endosomes/lysosomes followed by Alexa 488-conjugated goat anti-rabbit IgG or anti-mouse IgG and were visualized with confocal laser microscopy. Arrowheads indicate colocalization of the internalized Hsp90–survivin-2B75-93 peptide complex and each organelle. (b) To quantify the percentage of colocalization, a single z-plane of one cell was evaluated. For each protein and organelle combination, a total of 150 cells (50 cells from three independent experiments) were analyzed. Data are shown as means + SEM of three independent experiments. *P < 0.01.
Figure 5.
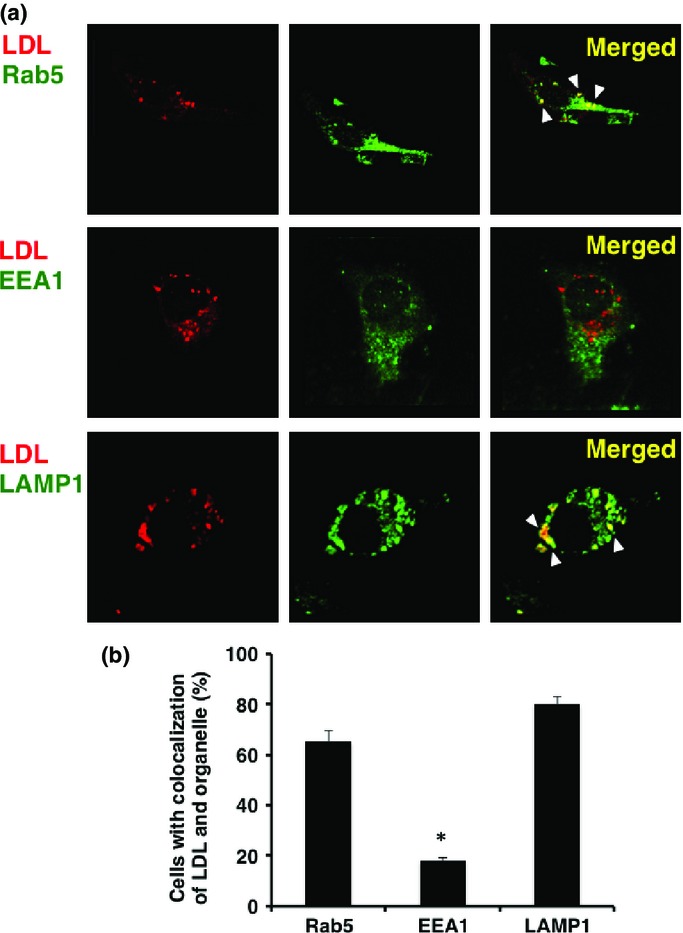
Low-density lipoprotein (LDL) was targeted to the dynamic early endosome followed by translocation to the late endosome/lysosome for degradation. (a) Human monocyte-derived dendritic cells were incubated at 37°C with Alexa 594-labeled LDL. Organelles were stained with an anti-EEA1 mAb, anti-Rab5 polyclonal antibody, and anti-LAMP-1 polyclonal antibody, followed by Alexa 488-conjugated goat anti-rabbit IgG or anti-mouse IgG. Arrowheads indicate colocalization of internalized LDL and each organelle. (b) To quantify the percentage of colocalization, a single z-plane of one cell was evaluated. For each protein and organelle combination, a total of 150 cells (50 cells from three independent experiments) were analyzed. Data are shown as means + SEM of three independent experiments. *P < 0.01.
Heat shock protein 90–peptide complex is cross-presented by human DCs through an endosome-recycling pathway
We then examined whether Hsp90–precursor peptide complex was cross-presented by human Mo-DCs through an endosomal pathway after targeting to the static early endosome. We used chloroquine for inhibition of endosomal acidification and primaquine for inhibition of the membrane recycling pathway. As shown in Figure6(a), Mo-DCs that were pre-incubated with increasing concentrations of chloroquine completely blocked cross-presentation of Hsp90–survivin-2B75-93 precursor peptide complex but had no substantial effect on survivin-2B80-88 peptide presentation. These results indicated that cross-presentation of Hsp90–precursor peptide complex depended on endosomal acidification, possibly including proteolysis by endosomal proteases. Moreover, Mo-DC incubated with primaquine could not present the Hsp90-chaperoned precursor peptide-derived survivin-2B80-88 peptide to CTL (Fig.6b). These results indicated that the Hsp90-chaperoned precursor peptide or processed peptide entered recycling endosomes and were transferred onto recycling MHC class I molecules.
Figure 6.
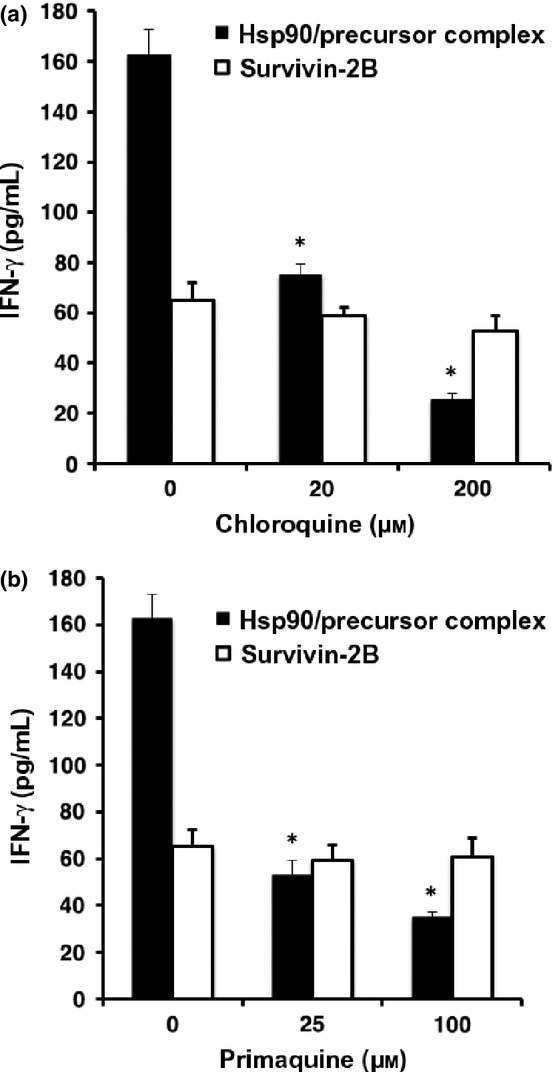
Heat shock protein 90 (Hsp90)–peptide complex is cross-presented through an endosome-recycling pathway. Human monocyte-derived dendritic cells (Mo-DCs) were pre-incubated with chloroquine (a) or primaquine (b) at 37°C for 2 h and then loaded with survivin-2B80-88 peptide alone or Hsp90–survivin-2B75-93 precursor peptide complex for 2 h. The Mo-DCs were then fixed, washed, and cultured overnight with survivin-2B80-88-specific CTL clone. Activation of CTL was measured as γ-interferon (IFN-γ) production using ELISA.
Discussion
It has been shown that immunization with tumor-derived HSPs or HSPs complexed with an Ag peptide/protein elicits tumor-or Ag-specific CD8+ T cell responses.1,9 Importantly, it has been shown that Hsp70–Ag and gp96–Ag complexes facilitate Ag presentation in association with MHC class I molecules.10–13 Recently, we3,4 and Calderwood's group14 have shown that Hsp90 also acted as an excellent navigator for associated antigens to enter the cross-presentation pathway in the murine system. We here showed that human Hsp90–cancer Ag peptide complex was efficiently cross-presented by human Mo-DCs. These results hold promise for the development of a safe and efficient immunomodulator for cancer immunotherapy. More importantly, we showed that translocation of the Hsp90–Ag complex into the static early endosome after endocytosis was crucial for efficient cross-presentation. It has been shown that the pathway for cross-presentation is comprised of two distinct intracellular routes, a proteasome–TAP-dependent pathway and an endosome-recycling pathway.2,3 Recent studies have revealed the pathway in which peptide exchange onto recycling MHC class I molecules occurs within early endosomal compartments.15 We have shown that Hsp90–peptide complex-mediated4 and ORP150–peptide complex-mediated16 cross-presentation was independent of TAP and was sensitive to primaquine, indicating that sorting of peptides onto MHC class I occurs through an endosome-recycling pathway. Lakadamyali et al.17 have shown that early endosomes are comprised of two distinct populations: a dynamic population that is highly mobile on microtubules and matures rapidly toward the late endosome, and a static population that matures much more slowly. Cargos destined for degradation, including LDL, epidermal growth factor, and influenza virus, are internalized and targeted to the Rab5+, EEA1−-dynamic population of early endosomes as we have observed using LDL, thereafter trafficking to Rab7+-late endosomes. In contrast, the recycling ligand transferrin is delivered to Rab5+, EEA1+-static early endosomes, followed by translocation to Rab11+-recycling endosomes. Furthermore, Burgdorf et al.18 clearly indicated that a mannose receptor introduced OVA specifically into an EEA-1+, Rab5+-stable early endosomal compartment for subsequent cross-presentation. In contrast, pinocytosis conveyed OVA to lysosomes for class II presentation. Of interest, OVA endocytosed by a scavenger receptor did not colocalize with EEA1 but colocalized with LAMP-1 in lysosomes, leading to presentation in the context of MHC class II molecules. We showed that the human Hsp90–peptide complex is targeted into Rab5+, EEA1+-early endosomes after internalization by Mo-DCs, suggesting that preferential sorting to the “static” endosome is necessary for cross-presentation of Hsp90–peptide complexes. In contrast, soluble LDL protein was targeted to the EEA1− and LAMP-1+-dynamic early endosome–late endosome/lysosome pathway, leading to degradation and presentation in the context of MHC class II molecules. These findings suggested that Hsp90 shuttled the chaperoned precursor peptide into the static endosome-recycling pathway, preventing further degradation, followed by transfer of the peptide onto recycling MHC class I molecules. Together, our findings indicate that the role of Hsp90 in cross-presentation is to navigate the associated Ag into static early endosomes within human Mo-DCs. Thus, Hsp90 appears to be a promising natural immunoactivator for use of cancer vaccine development due to its excellent ability to target human DCs and to induce specific CTLs.
Disclosure Statement
The authors have no conflict of interest.
Glossary
Abbreviations
- Ag
antigen
- DC
dendritic cell
- GM-CSF
granulocyte/macrophage colony-stimulating factor
- HSP
heat shock protein
- Hsp90
heat shock protein 90
- IFN
interferon
- IL
interleukin
- LDL
low-density lipoprotein
- Mo-DC
monocyte-derived dendritic cells
- OVA
ovalbumin
- pAb
polyclonal antibody
- PE
phycoerythrin
- PHA
phytohemagglutinin
- TAP
transporter associated with antigen processing
References
- Srivastava P. Interaction of heat shock proteins with peptides and antigen presenting cells: chaperoning of the innate and adaptive immune responses. Annu Rev Immunol. 2002;20:395–425. doi: 10.1146/annurev.immunol.20.100301.064801. [DOI] [PubMed] [Google Scholar]
- Shen L, Sigal LJ, Boes M, Rock KL. Important role of cathepsin S in generating peptides for TAP-independent MHC class I crosspresentation in vivo. Immunity. 2004;21:155–65. doi: 10.1016/j.immuni.2004.07.004. [DOI] [PubMed] [Google Scholar]
- Oura J, Tamura Y, Kamiguchi K, et al. Extracellular heat shock protein 90 plays a role in translocating chaperoned antigen from endosome to proteasome for generating antigenic peptide to be cross-presented by dendritic cells. Int Immunol. 2011;23:223–37. doi: 10.1093/intimm/dxq475. [DOI] [PubMed] [Google Scholar]
- Kurotaki T, Tamura Y, Ueda G, et al. Efficient cross-presentation by heat shock protein 90-peptide complex-loaded dendritic cells via an endosomal pathway. J Immunol. 2007;179:1803–13. doi: 10.4049/jimmunol.179.3.1803. [DOI] [PubMed] [Google Scholar]
- Hirohashi Y, Torigoe T, Maeda A, et al. An HLA-A24-restricted cytotoxic T lymphocyte epitope of a tumor-associated protein, survivin. Clin Cancer Res. 2002;8:1731–9. [PubMed] [Google Scholar]
- Kameshima H, Tsuruma T, Kutomi G, et al. Immunotherapeutic benefit of alpha-interferon (IFNalpha) in survivin2B-derived peptide vaccination for advanced pancreatic cancer patients. Cancer Sci. 2013;104:124–9. doi: 10.1111/cas.12046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuruma T, Iwayama Y, Ohmura T, et al. Clinical and immunological evaluation of anti-apoptosis protein, survivin-derived peptide vaccine in phase I clinical study for patients with advanced or recurrent breast cancer. J Transl Med. 2008;6:24. doi: 10.1186/1479-5876-6-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kameshima H, Tsuruma T, Torigoe T, et al. Immunogenic enhancement and clinical effect by type-I interferon of anti-apoptotic protein, survivin-derived peptide vaccine, in advanced colorectal cancer patients. Cancer Sci. 2011;102:1181–7. doi: 10.1111/j.1349-7006.2011.01918.x. [DOI] [PubMed] [Google Scholar]
- Tamura Y, Peng P, Liu K, Daou M, Srivastava PK. Immunotherapy of tumors with autologous tumor-derived heat shock protein preparations. Science. 1997;278:117–20. doi: 10.1126/science.278.5335.117. [DOI] [PubMed] [Google Scholar]
- Udono H, Levey DL, Srivastava PK. Cellular requirements for tumor-specific immunity elicited by heat shock proteins: tumor rejection antigen gp96 primes CD8+ T cells in vivo. Proc Natl Acad Sci U S A. 1994;91:3077–81. doi: 10.1073/pnas.91.8.3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato K, Torimoto Y, Tamura Y, et al. Immunotherapy using heat-shock protein preparations of leukemia cells after syngeneic bone marrow transplantation in mice. Blood. 2001;98:1852–7. doi: 10.1182/blood.v98.6.1852. [DOI] [PubMed] [Google Scholar]
- Noessner E, Gastpar R, Milani V, et al. Tumor-derived heat shock protein 70 peptide complexes are cross-presented by human dendritic cells. J Immunol. 2002;169:5424–32. doi: 10.4049/jimmunol.169.10.5424. [DOI] [PubMed] [Google Scholar]
- Berwin B, Rosser MF, Brinker KG, Nicchitta CV. Transfer of GRP94(Gp96)-associated peptides onto endosomal MHC class I molecules. Traffic. 2002;3:358–66. doi: 10.1034/j.1600-0854.2002.30505.x. [DOI] [PubMed] [Google Scholar]
- Murshid A, Gong J, Calderwood SK. Heat shock protein 90 mediates efficient antigen cross presentation through the scavenger receptor expressed by endothelial cells-I. J Immunol. 2010;185:2903–17. doi: 10.4049/jimmunol.0903635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleijmeer MJ, Escola JM, UytdeHaag FG, et al. Antigen loading of MHC class I molecules in the endocytic tract. Traffic. 2001;2:124–37. doi: 10.1034/j.1600-0854.2001.020207.x. [DOI] [PubMed] [Google Scholar]
- Kutomi G, Tamura Y, Okuya K, et al. Targeting to static endosome is required for efficient cross-presentation of endoplasmic reticulum-resident oxygen-regulated protein 150-peptide complexes. J Immunol. 2009;183:5861–9. doi: 10.4049/jimmunol.0803768. [DOI] [PubMed] [Google Scholar]
- Lakadamyali M, Rust MJ, Zhuang X. Ligands for clathrin-mediated endocytosis are differentially sorted into distinct populations of early endosomes. Cell. 2006;124:997–1009. doi: 10.1016/j.cell.2005.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgdorf S, Kautz A, Bohnert V, Knolle PA, Kurts C. Distinct pathways of antigen uptake and intracellular routing in CD4 and CD8 T cell activation. Science. 2007;316:612–6. doi: 10.1126/science.1137971. [DOI] [PubMed] [Google Scholar]


