Abstract
Expression of the adenosine triphosphate-binding cassette B1 (ABCB1) transporter and P-glycoprotein are associated with resistance to anticancer drugs. The purpose of this study was to investigate the role of single nucleotide polymorphism in the ABCB1 and CYP3A genes in breast cancer patients who were treated with neoadjuvant chemotherapy. Stage II/III breast cancer patients were treated with three cycles of neoadjuvant, after which the patients received curative surgery and adjuvant chemotherapy. The polymorphisms of ABCB1 and CYP3A were genotyped. The correlation of polymorphism of ABCB1, CYP3A, and clinical outcomes was analyzed. Among the 216 patients, ABCB1 3435TT genotype had a longer overall survival (OS). than CC/CT. Multivariate analyses demonstrated that good PS, invasive ductal carcinoma, non-triple negative phenotype and initial operable stage were significantly associated with a lower death risk. ABCB1 3435TT genotype had a higher AUC than CC/CT for docetaxel. These higher AUCs in the C3435TT was associated with increased toxicities of neutropenia and diarrhea. This study showed that the genetic polymorphism of ABCB1 C3435T might be associated with a longer OS. Our results also suggest that the prediction of docetaxel toxicity might be possible for C3435T polymorphism. This study results provides valuable information on individualized therapy according to genotypes.
Keywords: ABCB1 Gene, breast cancer, C3435T, neoadjuvant chemotherapy, single nucleotide polymorphism
Randomized trials have proven the safety of neoadjuvant chemotherapy with comparable efficacy to adjuvant chemotherapy.1,2 These studies provide the theoretical roles supporting neoadjuvant chemotherapy. First of all, “downstaging” of the tumor from inoperable to operable stage in select patients may allow a substantial proportion of women to benefit from conservative surgery instead of having to resort to more radical approaches.3 Second, post-surgical treatment strategy could be optimized based on the previous tumor response during neoadjuvant chemotherapy. Last but not least, a subset of patients could achieve pathologic complete remission (pCR) from neoadjuvant therapy, which has been shown to be associated with a good prognosis.4,5
As neoadjuvant chemotherapy is becoming increasingly more important in clinical practice, a disputed issue is the development of resistance to the cytotoxic agents as well as cross-resistance to substances patients have never been exposed to before. Inter-individual variability of pharmacokinetics of anti-cancer drugs constitutes a major limitation to their clinical use. Since a variety of commonly used chemotherapeutic agents such as docetaxel and doxorubicin have narrow therapeutic indices and significant inter-individual variations in drug disposition, great efforts have been put in determining the mechanism of person-to-person pharmacokinetic diversities.
Single nucleotide polymorphisms (SNPs) in adenosine triphosphate-binding cassette B1 (ABCB1) and cytochrome P450 (CYP) genes are among potential candidates that explain the inter-individual variations in drug disposition. SNPs in ABCB1 gene are associated with phenotypic variation in P-glycoproteins (P-gp), a membrane-bound efflux pump, which removes chemotherapeutic drugs including docetaxel and doxorubicin from the cells. The intestinal P-gp plays a main role in the fecal elimination of drugs by modulating reabsorption of the drug after hepatobiliary secretion.6 The percentage of tumors expressing P-gp in breast cancer was 41.2% and the expression rate of P-gp increased after treatment which was three times more likely to fail in chemotherapy.7 Recently, SNPs of the ABCB1 gene have been reported to be associated with taxane clearance and the clinical outcome of patients who were treated with taxane.8,9 Doxorubicin was also shown to be functionally affected by SNPs of ABCB1 with regards to the pharmacokinetics.10,11 It has been shown that ABCB1 C3435T polymorphism in exon 26 is significantly related to clinical toxicity and high blood levels of docetaxel.12 ABCB1 G2677T/A polymorphism in exon 21 are associated with treatment outcomes after paclitaxel monotherapy.11 ABCB1 C1236T polymorphism in exon 12 is associated with docetaxel clearance.8,9
Most chemotherapeutic agents are eliminated via hepatic metabolism, which is mediated by cytochrome P450 (CYP), mainly CYP 3A4 and CYP3A5.13 Metabolism of docetaxel consists of CYP3A-mediated oxidation of the tert-butyl propionate side chain, which results in the formation of four metabolites with reduced cytotoxic activity.14 CYP3A4*1B and CYP3A5*3 are common polymorphisms, which have been shown to have clinical implications in some studies.14,15 CYP3A4*1B, an A-392G transition in the 5′-flanking region, is a promoter polymorphism. While the polymorphism is commonly found in the African-American population,14 its minor allele frequency is estimated 0% in Chinese, Taiwanese, Chinese Americans, and Japanese Americans.16,17 On the other hand, the CYP3A5*3 splice site variant that causes loss of hepatic expression of CYP3A5 is common, but does not account for inter-individual variability of docetaxel disposition in Asian populations.18,19 P-gp may affect the extent of CYP3A-mediated metabolism of drugs by limiting the intracellular substrate availability.20 This study hypothesized from the above findings that a genetic variant of ABCB1 and CYP3A5 contributes to the pharmacokinetic variability of docetaxel and doxorubicin.
This study investigated the distribution of SNPs in ABCB1 and CYP3A genes in breast cancer patients who were treated with neoadjuvant docetaxel and doxorubicin chemotherapy. The correlation between the genetic polymorphism, plasma concentration of chemotherapeutic agents and clinical outcomes was also explored.
Materials and Methods
Patients and study design
From September 2003 to September 2008, pathologically proven, previously untreated, clinical stage II or III breast cancer patients were enrolled in this prospective study. Patients had to have an Eastern Cooperative Oncology Group (ECOG) performance status (PS) of 0–2, objective measurable disease, and adequate bone marrow, hepatic, cardiac, and renal functions. Initial evaluations included physical examination, mammography, breast ultrasonography, computed tomography (CT) of the chest, bone scan, and breast magnetic resonance imaging (MRI). Initial nodal staging was evaluated by the physical examination and CT.
All patients were treated with three cycles of neoadjuvant chemotherapy which consisted of docetaxel (75 mg/m2) and doxorubicin (60 mg/m2) by intravenous infusion.21 Granulocyte colony-stimulating factor as primary prophylaxis was injected for 5 days every cycle. After completion of neoadjuvant treatment, the patients were re-evaluated for the response. After the primary surgery, patients were treated with three more cycles of docetaxel and doxorubicin as the adjuvant chemotherapy.21,22 Adequate radiation or hormonal therapy was performed as indicated.23,24 The radiological response was evaluated using breast MRI for the primary breast lesion and chest CT for the lymph node lesions with RECIST criteria 1.0.25 Pathologic complete response (pCR) was defined as complete disappearance of invasive carcinoma in both breast and axillary lymph nodes after three cycles of chemotherapy. For estrogen receptor (ER) and progesterone receptor (PR), cases with 10% or more positive staining were grouped as positive. Human epidermal growth factor receptor-2 (HER2) overexpression was demonstrated by immunohistochemistry scoring or fluorescence in situ hybridization (FISH) in paraffin sections of their primary carcinomas. Immunohistochemistry scores of only 3+ on a scale of 0 to 3+ or FISH positivity were defined as overexpression of HER2. Triple negative breast cancer was defined as the characteristics of the lack of expression of ER and PR and the absence of HER2 overexpression. Recurrence free survival (RFS) was calculated as the time from the first cycle of chemotherapy to the diagnosis of a recurrent disease in the ipsilateral breast, local, regional, or distant site, and the overall survival (OS) was the time from the first cycle of chemotherapy to death or last follow-up day. Adverse events were assessed in all patients with version 3.0 of the National Cancer Institute's Common Terminology Criteria for Adverse Events. The study protocol was approved by the Institutional Review Board of Seoul National University Hospital (IRB Registration No. H-0510-506-159, H-0610-020-186). This manuscript is a part of a clinical trial (ClinicalTrials.gov Identifier: NCT01396655).
Analysis of ABCB1 and CYP3A genomic polymorphism
Whole blood samples were obtained before the neoadjuvant chemotherapy. Genomic DNA was isolated from mononuclear cells of peripheral blood cells using QiaAmp blood mini kit (Qiagen, Hilden, Germany). ABCB1 C3435T,26,27 G2677T/A11,27 and C1236T28 polymorphisms were genotyped by polymerase chain reaction (PCR) and restriction fragment length polymorphism (RFLP) assays as previously described.29 CYP3A4*1B and CYP3A5*3 polymorphisms were done by the GoldenGate assay (Illumina, San Diego, CA, USA) that consisted of 96 custom selected SNPs within 34 genes. The dissolved biotinylated DNA pellet was extended and ligated. The supernatant was then used in PCR amplification. Double-stranded PCR products were immobilized onto paramagnetic particles, B Reagent (Illumina). The bound PCR products were denatured and the released ssDNAs were tralized with hybridization reagent (Illumina). Hybridization was done and then imaged using a BeadArray Reader (Illumina).
Pharmacokinetic studies
Seventy-two out of 216 patients participated in the prospective pharmacokinetic study of docetaxel and doxorubicin. These patients were grouped as the pharmacokinetic (PK) study group. Pharmacokinetic samplings were done at three time points. The plasma concentrations of docetaxel and doxorubicin at pre-concentration (just before infusion), post-concentration (just after infusion), and 24 h-concentration after infusion were determined by liquid chromatography tandem mass (LC/MS/MS) assays. Mass spectrometric analysis was done using an API 3000 MS system (Applied Biosystems, Foster City, CA, USA). All plasma samples were subjected to the sample preparation procedure described previously.30 With the above three blood samples, a graph was plotted of the sampling point as the x-axis against the drug concentration as the y-axis and was able to draw a diagram corresponding to the area under the plasma concentration (AUC) until 24 h after drug infusion. Based on this diagram, the drug concentration was calculated and was defined as the AUC in this study. The AUC of docetaxel and doxorubicin were dictated as AUCdoc, and AUCdox, respectively. The differences between the AUCs were examined until the 24 h-concentration after infusion, according to the genotypes of ABCB1 and CYP3A5.
Statistical analysis
This is a project of a phase II trial. The primary endpoint in this study was pCR. Secondary end points included rate of breast conserving operation, toxicity, relapse-free survival, overall survival and predictive/prognostic factors. The association between the allelic frequency of ABCB1 and clinical outcomes was assessed by the χ2 test or Fisher's exact test. The RFS and OS were estimated by the Kaplan–Meier method, and the difference in survival was compared using the log-rank test. Cox regression model was used to compare the clinical outcomes among the genotypes of the ABCB1 gene and CYP3A5. The crude hazard ratio (HR) of clinical events and the corresponding 95% confidence intervals (CI) were estimated. For genotype-pharmacokinetic study, Mann–Whitney test or Kruskal–Wallis test was done to compare the AUCs between post-concentration (just after infusion) and 24 h-concentration after infusion in the plasma concentrations according to the genotypes. Odds ratios and their respective 95% confidence intervals were used to show the relationship of the toxicities and covariates including the ABCB1 gene. SPSS program (version 12.0, Chicago, IL, USA) was used for all analyses, and two-sided P-value <0.05 was considered statistically significant.
Results
Patients and treatment outcomes
A total of 216 breast cancer patients were enrolled. The median follow-up duration was 85.4 months (range 26.2–117.8 months) and 75 patients (34.7%) experienced relapse and 43 patients (19.9%) died. Baseline characteristics of the study population are presented in Table1. The median age of the study population was 44 years (range 25–69 years). ABCB1 C3435T and ABCB1 C1236T were divided into two groups as the CC/CT vs. TT genotype and the ABCB1 G2677T/A was grouped into the GG vs. Non-GG group.
Table 1.
Baseline characteristics
| Characteristics | Total population (n = 216) (%) | Pharmacokinetic subgroup (n = 72) (%) |
|---|---|---|
| Age, (years) | ||
| Age <35 | 25 (11.6) | 4 (5.6) |
| Age ≥35 | 191 (88.4) | 68 (94.4) |
| Performance status | ||
| ECOG 0–1 | 213 (98.6) | 70 (97.2) |
| ECOG 2 | 3 (1.4) | 2 (2.8) |
| Pathologic characteristics | ||
| Invasive ductal carcinoma | 206 (95.4) | 69 (95.9) |
| Others* | 10 (4.6) | 3 (4.1) |
| Initial clinical stage | ||
| Operable(IIA, IIB, IIIA) | 155 (71.8) | 53 (73.6) |
| Inoperable (IIIB, IIIC) | 61 (28.2) | 19 (26.4) |
| Receptor status | ||
| ER | ||
| Positive | 99 (45.8) | 30 (41.7) |
| Negative | 117 (54.2) | 42 (58.3) |
| PR | ||
| Positive | 73 (33.8) | 23 (31.9) |
| Negative | 143 (66.2) | 49 (68.1) |
| HER2 | ||
| Positive | 67 (31.0) | 20 (27.8) |
| Negative | 149 (69.0) | 52 (72.2) |
| Triple negative phenotype | ||
| Non triple negative | 148 (68.5) | 49 (68.1) |
| Triple negative | 68 (31.5) | 23 (31.9) |
| ABCB1 C3435T | ||
| CC + CT | 185 (85.6) | 65 (90.3) |
| TT | 31 (14.4) | 7 (9.7) |
| ABCB1 G2677T/A | ||
| GG | 38 (17.6) | 13 (18.1) |
| Non-GG | 178 (82.4) | 59 (81.9) |
| ABCB1 C1236T | ||
| CC + CT | 136 (63.0) | 48 (66.7) |
| TT | 80 (37.0) | 24 (33.3) |
| CYP3A5*3 | ||
| AA | – | 3 (4.2) |
| AG | – | 29 (40.3) |
| GG | – | 40 (55.5) |
| Type of surgery | ||
| Breast conserving | 87 (40.3) | 25 (34.7) |
| Mastectomy | 129 (59.7) | 47 (65.3) |
| Menopausal status | ||
| Pre-menopause | 146 (67.6) | 50 (69.4) |
| Post-menopause | 70 (32.4) | 22 (30.6) |
ECOG, Eastern Cooperative Oncology Group; ER, estrogen receptor; PR, progesterone receptor; HER2, human epidermal growth factor receptor.
Among the 10 patients, 7 had invasive lobular carcinomas, 3 medullary carcinomas and 1 tubular carcinoma.
Pharmacokinetic analysis was done in 72 patients. Baseline characteristics of these patients did not differ greatly from those of the total patient group.
Association between genotypes and treatment outcomes
Frequency of ABCB1 and CYP3A genotypes
The frequency of ABCB1 3435TT genotype was 14.4% in total 216 patients and 9.7% in the PK group of 72 patients. In PK group, the AA genotype of CYP3A4 was 100% and the AA genotype of CYP3A5 had the lowest percentage, 4.2%. The proportion of each genotype was maintained similarly in 72 PK group patients. Each observed genotype distribution of ABCB1 genes conformed to Hardy–Weinberg equilibrium (each P > 0.050). Linkage disequilibrium for all pairs of exons 26, 21, and 12 were observed by use of the PL-EM algorithm (P < 0.001).
Analysis of clinical outcomes of the total patients
The overall clinical response rate (RR) was 76.8%. Twenty patients (9.3%) achieved clinical complete response (cCR) and 18 (8.3%) achieved pCR. Among the PK group, RR was 82.0% with 4.2% of pCR. ABCB1 C3435T, G2677T/A, and C1236T SNPs did not predict the responders. As shown in Figure1, ABCB1 3435TT genotype had a longer OS than the CT/TT genotype with statistical significance (P = 0.024). A similar trend was observed for the RFS, that is, ABCB1 3435TT genotype tended to have a longer RFS although it was not statistically significant (P = 0.234). ABCB1 G2677T/A and C1236T were analyzed, and there were no significant differences in the RFS and OS (Fig. S1).
Figure 1.
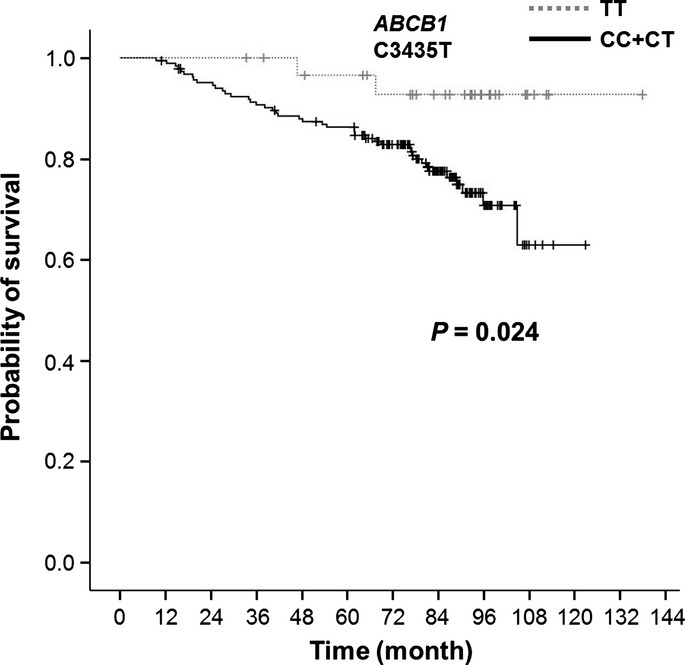
Overall survival (OS) according to ABCB1 C3435T polymorphism. ABCB1 C3435T TT genotype had longer OS compared to the others (P = 0.024).
With univariate analysis of the OS, good PS (ECOG 0-1), invasive ductal carcinoma, initial operable stage, ER-positivity, non-triple negative phenotype, breast conserving surgery and the TT genotype of ABCB1 C3435T were associated with a lower risk of death (Table2). Multivariate analyses of the OS demonstrated that poor PS (HR 9.526, 95% CI = 2.587–20.493; P = 0.003), triple negative phenotype (HR 2.106, 95% CI = 1.145–3.874; P = 0.017), other histologic type (HR 3.547, 95% CI = 1.205–10.444; P = 0.022), initial locally advanced clinical stage (HR 2.406, CI = 1.270–4.555; P = 0.003) and non-pathologic complete remission (HR 8.204, 95% CI = 1.810–22.112; P = 0.047) were significantly associated with the OS (Table3). The ABCB1 3435TT genotype was also associated with a lower risk of death with marginal significance not shown to be an independent prognostic factor (HR 0.245, 95% CI = 0.059–1.026; P = 0.054). The result was similar when breast cancer staging was adjusted instead of operable staging (HR 0.238; 95% CI, 0.057–0.999; P = 0.050).
Table 2.
Univariate analysis for overall survival (OS)
| Characteristics | Patient Number (n = 216) (%) | Median OS Hazard ratio† | 95% CI | P-value |
|---|---|---|---|---|
| Age, (years) | ||||
| Age <35 | 25 (11.6) | 1 | 0.898 | |
| Age ≥35 | 191 (88.4) | 1.063 | 0.419–2.696 | |
| Performance status | ||||
| ECOG 0–1 | 213 (98.6) | 1 | 0.048 | |
| ECOG 2 | 3 (1.4) | 3.754 | 0.904–12.588 | |
| Pathologic characteristics | ||||
| Invasive ductal carcinoma | 206 (95.4) | 1 | 0.008 | |
| Others* | 10 (4.6) | 3.684 | 1.312–10.341 | |
| Initial clinical stage | ||||
| Operable (IIA, IIB, IIIA) | 155 (71.8) | 1 | 0.030 | |
| Inoperable (IIIB, IIIC) | 61 (28.2) | 2.283 | 1.074–4.242 | |
| Receptor status | ||||
| ER | ||||
| Positive | 99 (45.8) | 1 | 0.049 | |
| Negative | 117 (54.2) | 2.081 | 0.996–3.189 | |
| PR | ||||
| Positive | 73 (33.8) | 1 | 0.258 | |
| Negative | 143 (66.2) | 1.542 | 0.754–2.832 | |
| HER2 | ||||
| Positive | 67 (31.0) | 1 | 0.730 | |
| Negative | 149 (69.0) | 1.117 | 0.697–2.177 | |
| Triple negative phenotype | ||||
| Non triple negative | 148 (68.5) | 1 | 0.033 | |
| Triple negative | 68 (31.5) | 1.995 | 1.004–3.447 | |
| ABCB1 C3435T | ||||
| CC + CT | 185 (85.6) | 1 | 0.024 | |
| TT | 31 (14.4) | 0.223 | 0.054–0.972 | |
| ABCB1 G2677T/A | ||||
| GG | 38 (17.6) | 1 | 0.566 | |
| Non-GG | 178 (82.4) | 1.168 | 0.543–2.510 | |
| ABCB1 C1236T | ||||
| CC + CT | 136 (63.0) | 1 | 0.126 | |
| TT | 80 (37.0) | 0.688 | 0.335–1.256 | |
| Type of surgery | ||||
| Breast conserving | 87 (40.3) | 1 | 0.036 | |
| Mastectomy | 129 (59.7) | 2.083 | 1.074–4.022 | |
| Menopausal status | ||||
| Pre-menopause | 146 (67.6) | 1 | 0.244 | |
| Post-menopause | 70 (32.4) | 1.323 | 0.724–2.419 | |
ECOG, Eastern Cooperative Oncology Group; ER, estrogen receptor; HER2, human epidermal growth factor receptor; PR, progesterone receptor.
Hazard ratio was calculated by Cox's proportional hazard model. If the hazard ratio is >1, the hazard ratio can be thought of as the average increased risk of death compared with the reference group (upper line).
Among the 10 patients, 7 had invasive lobular carcinomas, 3 medullary carcinomas and 1 tubular carcinoma.
Table 3.
Multivariate analysis for overall survival
| Characteristics | Category | Hazard ratio† | 95% CI | P-value |
|---|---|---|---|---|
| ECOG | 0–1 | 1 | ||
| 2 | 9.526 | 2.587–20.493 | 0.003 | |
| Histology | Invasive ductal carcinoma | 1 | ||
| Others* | 3.547 | 1.205–10.444 | 0.022 | |
| Triple negative | Non-triple negative | 1 | ||
| Phenotype | Triple negative | 2.106 | 1.145–3.874 | 0.017 |
| Initial stage‡ | Stage I-IIIA | 1 | ||
| Stage IIIB-IIIC | 2.406 | 1.270–4.555 | 0.007 | |
| Pathologic | pCR | 1 | ||
| Response | Non-pCR | 8.204 | 1.810–19.112 | 0.047 |
| ABCB1 C3435T | CC + CT | 1 | ||
| TT | 0.245 | 0.059–1.026 | 0.054 |
Eastern Cooperative Oncology Group.
Hazard ratio was calculated by Cox's proportional hazard model. If the hazard ratio is >1, the hazard ratio can be thought of as the average increased risk of death compared with the reference group (upper line).
According to the TNM classification of 2002 AJCC staging.
Among the 10 patients, 7 had invasive lobular carcinomas, 3 medullary carcinomas and 1 tubular carcinoma.
Toxicities
Toxicities according to the NCI-CTC version 3.0 are summarized in Table4. One hundred seventeen patients (54.2%) of the studied patients suffered one or more adverse event of any grade. Overall, Grade 3–4 toxicities were observed in 34 patients (15.7%). Grade 3–4 neutropenia developed in 21 patients (9.6%) with 9 (4.2%) febrile episodes, as grade 3–4 diarrhea did in 12 patients (5.6%). No treatment-related mortality was encountered in the study. TT genotype of ABCB1 C3435T was associated with higher frequency of grade 3–4 hematologic and non-hematologic toxicities including neutropenia (P = 0.037) and diarrhea (P = 0.017) compared with CC/CT genotype (Table5).
Table 4.
Toxicities in total patients (n = 216)
| Grade 1 (%) | Grade 2 (%) | Grade 3 (%) | Grade 4 (%) | |
|---|---|---|---|---|
| Neutropenia | 16 (7.4) | 13 (6.0) | 14 (6.4) | 7 (3.2) |
| Thrombocytopenia | 11 (5.1) | 5 (2.3) | – | – |
| Febrile neutropenia | – | – | 9 (4.2) | – |
| Nausea | 28 (13.0) | 13 (6.0) | 3 (1.4) | – |
| Vomiting | 20 (9.3) | 15 (6.9) | – | – |
| Stomatitis | 16 (7.4). | 6 (2.8) | 3 (1.4) | – |
| Diarrhea | 14 (6.5) | 9 (4.2) | 12 (5.6) | – |
| Neuropathy | 22 (10.1) | 7 (3.2) |
Toxicity was evaluated by CTCAE ver. 3. CI, confidence interval; HR, hazard ratio; IDC, invasive ductal carcinoma; OS, overall survival.
Table 5.
Toxicities according to the polymorphism of ABCB1 gene
| SNPs | Neutropenia† |
Febrile neutropenia† |
Nausea† |
Stomatitis† |
Diarrhea† |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % | RR‡ (95% CI) | P | % | RR (95% CI) | P | % | RR (95% CI) | P | % | RR (95% CI) | P | % | RR (95% CI) | P | |
| C3435T | |||||||||||||||
| CC + CT | 7.0 | 1 | 0.037 | 3.2 | 1 | 0.123 | 1.6 | 1 | 0.215 | 1.6 | – | 3.8 | 1 | 0.017 | |
| TT | 19.4 | 4.6 (1.3–11.6) | 9.7 | 3.2 (0.7–17.7) | 6.5 | 3.6 (0.5–21.0) | 0.0 | – | 16.1 | 3.3 (1.3–15.1) | |||||
| G2677T/A | |||||||||||||||
| GG | 6.7 | 1 | 0.201 | 3.2 | 1 | 0.209 | 2.8 | 1 | 0.121 | 1.6 | 1 | 0.114 | 5.1 | 1 | 0.115 |
| Non-GG | 11.2 | 2.1 (1.6–14.9) | 8.8 | 3.0 (1.2–14.5) | 5.5 | 1.8 (0.4–11.4) | 4.4 | 2.8 (1.3–17.4) | 8.6 | 3.3 (1.3–17.5) | |||||
| C1236T | |||||||||||||||
| CC + CT | 8.8 | 1 | 0.877 | 3.7 | 1 | 0.661 | 1.5 | 1 | 0.327 | 1.2 | – | 4.4 | 1 | 0.367 | |
| TT | 8.8 | 1.1 (0.3–3.7) | 5.0 | 1.4 (0.3–7.5) | 3.8 | 2.9 (0.3–18.6) | 0.0 | – | 7.5 | 3.2 (0.7–10.3) | |||||
Grade 3–4 Toxicities.
RR, relative risk; 95% CI, 95% confidence interval.
Pharmacokinetics and ABCB1and CYP3A5 genotypes
The ABCB1 3435TT genotype showed a higher AUCdoc than the CC/CT genotype with statistical significance (P = 0.031) (Fig.2). However, the ABCB1 G2677T/A or C1236T genotypes showed no association with AUCdoc or AUCdox. Among the genotypes of CYP3A5, AA (*1/*1)/AG (*1/*3) genotypes had a higher AUC of docetaxel than the GG (*3/*3) genotype with statistical significance (P = 0.024). The ABCB1 3435TT genotype was correlated with neutropenia (P = 0.039), febrile neutropenia (P = 0.218), and diarrhea (P = 0.057). CYP3A5 polymorphism did not affect survival and toxicities.
Figure 2.
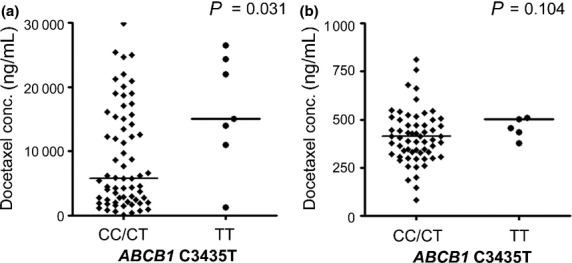
ABCB1 C3435T polymorphism against plasma drug level. (a) Docetaxel concentration in plasma (P = 0.031). (b) Doxorubicin concentration in plasma (P = 0.104).
Discussion
In recent years, various genetic variants in ABCB1 have been described to affect the transporter expression and function.31 Docetaxel and doxorubicin are subjected to transport by the ABCB1 transporter. ABCB1 3435C (glutamate) to T (glutamate) substitution does not result in an amino acid sequence change nor is this gene located in a regulatory region.8 This synonymous ABCB1 C3435T polymorphism has been associated with mRNA instability and a lower expression of P-gp in the duodenum.27 P-gp expression and ABCB1 C3435T polymorphism have been identified as an independent factor for RFS and RR for chemotherapy in breast cancer.11 Hoffmeyer et al. reported that the TT genotype of C3435T was correlated with reduced expression of P-gp and, therefore, reduced cellular elimination and maintained higher plasmaconcentrations of chemotherapeutic drugs.27,32 From these results, we hypothesize that patients who have the ABCB1 3435TT genotype might show a better treatment response to docetaxel and doxorubicin chemotherapy and get survival benefit compared to ABCB1 3435 CC/CT.
This study was designed to identify the clinical impact of ABCB1 SNPs in Korean patients with breast cancer who were treated with docetaxel and doxorubicin, a regimen widely accepted as neoadjuvant chemotherapeutic agents.21 In accordance with previous studies, this study showed that the TT genotype of ABCB1 3435 was associated with a longer OS and higher plasma concentration of docetaxel than CT/TT.33,34 It also suggests that a higher plasma level of docetaxel was maintained for a longer time in the TT genotype of ABCB1 translating into more intense exposure to docetaxel. As hematopoietic stem cells and intestinal epithelial cells are reported to express P-gp,35 higher drug concentrations in homozygote 3435TT leads to more frequent grade 3–4 neutropenia, febrile neutropenia, and diarrhea. Since the number of patients in the pharmacokinetic group was small, the OS tended to be longer in the ABCB1 3435TT genotype without statistical significance. There was a trend toward a higher percentage of grade 3–4 neutropenia in the ABCB1 3435TT subjects in the PK group (28.6% versus about 3.1% for the CC/CT subjects). It provides valuable information to clinicians that the ABCB1 homozygote carriers have increased efficacy and increased toxicity simultaneously. There were no significant differences in the OS at the analyzed data of G2677T/A and C1236T. To our knowledge, this is the first study demonstrating that ABCB1 C3435T polymorphism is significantly associated with plasma docetaxel concentration and OS after neoadjuvant docetaxel/doxorubicin combination chemotherapy in Asian patients with stage II or III breast cancer.
Since liver metabolism of docetaxel to inactive hydroxylated metabolites is mediated by CYP3A4 and CYP3A5,12,36 polymorphisms of such enzymes could affect the survival outcome of the treatment in breast cancer patients. Intuitively, it is reasonable to infer that the side effects of docetaxel treatment could be related to those metabolic enzymes. The most common variant, CYP3A4*1B allele founded in the African-American population, was not detected in the present study as expected based on previously published data.37 In contrast to CYP3A4*1B, the frequency of CYP3A5*3 allele was 65–85% in Asia18 and 76.8% in our study. The CYP3A5 is linked to a common transition in intron 3 of the CYP3A5 gene (CYP3A5*3) which introduces a frameshift during translation and results in a truncated, nonfunctional protein.38 At this point, homozygote (GG genotype,*3/*3) for CYP3A5*3 allele would be expected to be also strongly associated with low CYP3A5 protein content and AUC in a homozygote for the CYP3A5*3 allele which would be higher than CYP3A5*1. However, our findings show somewhat conflicting results: the homozygote of CYP3A5*3 tend to have a lower docetaxel concentration and a higher risk of grade 3 diarrhea than the CYP3A5*1/*1 genotype (P = 0.072). Concordantly, Goh et al. reported the same result that docetaxel clearance in patients with at least one CYP3A5*1 allele was significantly lower than CYP3A5*3 homozygotes.19 What causes this difference in predictions is as follows: the AA genotype (CYP3A5*1/*1) of CYP3A5 was only in three patients (4.2%) and was correlated with the TT genotype of C3435T with statistical significance. So a high docetaxel AUC revealed in the ABCB1 3435TT could be associated with the AA genotype of CYP3A5. Although CYP3A5 can account for >50% of the total CYP3A, we speculate that docetaxel metabolism was affected by the presence of the CYP3A5*3 allele to a small degree.
One of the merits of this study is the homogeneity of the population who received the same neoadjuvant chemotherapy followed by surgery for stage II/III breast cancer. Second, the study was designed prospectively; pharmacogenomics samples and plasma drug concentration samples were collected at specific time points according to the written prospective protocol in the chemotherapy-naïve patients. The clinical data were gained through the same therapeutic scheme. With all of these advantages, the major limitation of this study is the relatively small sample size of the pharmacokinetic study population. However, the distribution of genotype in this study was well matched to what has been reported previously in Koreans, and in a line with those with Asian population.39 In a sense, we believe the small sample size of this study did not led to selection bias, and thus does not undermine the significance of the findings.
In conclusion, this study shows that the genetic polymorphism of ABCB1 C3435T is associated with higher docetaxel concentration, a longer OS and more frequent toxicities. Our results suggest the future of guided drug selection by predicting drug toxicities according to the patient's genotype. Further prospective trials with large scale as well as in vitro and in vivo functional studies are warranted.
Acknowledgments
This research was supported by a grant from Basic Science Research Program (grant number : 2010-0022299) through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology and a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number : HI14C1277).
Disclosure Statement
The authors have no conflicts of interest.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Fig. S1. Kaplan–Meier survival analysis according to G2677TA and C1236T polymorphisms of the ABCB1 gene
References
- Fisher B, Brown A, Mamounas E, et al. Effect of preoperative chemotherapy on local-regional disease in women with operable breast cancer: findings from National Surgical Adjuvant Breast and Bowel Project B-18. J Clin Oncol. 1997;15:2483–93. doi: 10.1200/JCO.1997.15.7.2483. [DOI] [PubMed] [Google Scholar]
- Rastogi P, Anderson SJ, Bear HD, et al. Preoperative chemotherapy: updates of National Surgical Adjuvant Breast and Bowel Project Protocols B-18 and B-27. J Clin Oncol. 2008;26:778–85. doi: 10.1200/JCO.2007.15.0235. [DOI] [PubMed] [Google Scholar]
- Hortobagyi GN, Spanos W, Montague ED, Buzdar AU, Yap HY, Blumenschein GR. Treatment of locoregionally advanced breast cancer with surgery, radiotherapy, and combination chemoimmunotherapy. Int J Radiat Oncol Biol Phys. 1983;9:643–50. doi: 10.1016/0360-3016(83)90229-8. [DOI] [PubMed] [Google Scholar]
- Kuerer HM, Sahin AA, Hunt KK, et al. Incidence and impact of documented eradication of breast cancer axillary lymph node metastases before surgery in patients treated with neoadjuvant chemotherapy. Ann Surg. 1999;230:72–8. doi: 10.1097/00000658-199907000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher B, Mamounas EP. Preoperative chemotherapy: a model for studying the biology and therapy of primary breast cancer. J Clin Oncol. 1995;13:537–40. doi: 10.1200/JCO.1995.13.3.537. [DOI] [PubMed] [Google Scholar]
- van Zuylen L, Verweij J, Nooter K, Brouwer E, Stoter G, Sparreboom A. Role of intestinal P-glycoprotein in the plasma and fecal disposition of docetaxel in humans. Clin Cancer Res. 2000;6:2598–603. [PubMed] [Google Scholar]
- Trock BJ, Leonessa F, Clarke R. Multidrug resistance in breast cancer: a meta-analysis of MDR1/gp170 expression and its possible functional significance. J Natl Cancer Inst. 1997;89:917–31. doi: 10.1093/jnci/89.13.917. [DOI] [PubMed] [Google Scholar]
- Bosch TM, Huitema AD, Doodeman VD, et al. Pharmacogenetic screening of CYP3A and ABCB1 in relation to population pharmacokinetics of docetaxel. Clin Cancer Res. 2006;12:5786–93. doi: 10.1158/1078-0432.CCR-05-2649. [DOI] [PubMed] [Google Scholar]
- Green H, Soderkvist P, Rosenberg P, Horvath G, Peterson C. mdr-1 single nucleotide polymorphisms in ovarian cancer tissue: G2677T/A correlates with response to paclitaxel chemotherapy. Clin Cancer Res. 2006;12:854–9. doi: 10.1158/1078-0432.CCR-05-0950. [DOI] [PubMed] [Google Scholar]
- Kafka A, Sauer G, Jaeger C, et al. Polymorphism C3435T of the MDR-1 gene predicts response to preoperative chemotherapy in locally advanced breast cancer. Int J Oncol. 2003;22:1117–21. [PubMed] [Google Scholar]
- Tanabe M, Ieiri I, Nagata N, et al. Expression of P-glycoprotein in human placenta: relation to genetic polymorphism of the multidrug resistance (MDR)-1 gene. J Pharmacol Exp Ther. 2001;297:1137–43. [PubMed] [Google Scholar]
- Tran A, Jullien V, Alexandre J, et al. Pharmacokinetics and toxicity of docetaxel: role of CYP3A, MDR1, and GST polymorphisms. Clin Pharmacol Ther. 2006;79:570–80. doi: 10.1016/j.clpt.2006.02.003. [DOI] [PubMed] [Google Scholar]
- Shou M, Martinet M, Korzekwa KR, Krausz KW, Gonzalez FJ, Gelboin HV. Role of human cytochrome P450 3A4 and 3A5 in the metabolism of taxotere and its derivatives: enzyme specificity, interindividual distribution and metabolic contribution in human liver. Pharmacogenetics. 1998;8:391–401. doi: 10.1097/00008571-199810000-00004. [DOI] [PubMed] [Google Scholar]
- Ball SE, Scatina J, Kao J, et al. Population distribution and effects on drug metabolism of a genetic variant in the 5’ promoter region of CYP3A4. Clin Pharmacol Ther. 1999;66:288–94. doi: 10.1016/S0009-9236(99)70037-8. [DOI] [PubMed] [Google Scholar]
- Rebbeck TR, Jaffe JM, Walker AH, Wein AJ, Malkowicz SB. Modification of clinical presentation of prostate tumors by a novel genetic variant in CYP3A4. J Natl Cancer Inst. 1998;90:1225–9. doi: 10.1093/jnci/90.16.1225. [DOI] [PubMed] [Google Scholar]
- Hsieh KP, Lin YY, Cheng CL, et al. Novel mutations of CYP3A4 in Chinese. Drug Metab Dispos. 2001;29:268–73. [PubMed] [Google Scholar]
- Walker AH, Jaffe JM, Gunasegaram S, et al. Characterization of an allelic variant in the nifedipine-specific element of CYP3A4: ethnic distribution and implications for prostate cancer risk. Mutations in brief no. 191. Online. Hum Mutat. 1998;12:289. [PubMed] [Google Scholar]
- Kuehl P, Zhang J, Lin Y, et al. Sequence diversity in CYP3A promoters and characterization of the genetic basis of polymorphic CYP3A5 expression. Nat Genet. 2001;27:383–91. doi: 10.1038/86882. [DOI] [PubMed] [Google Scholar]
- Goh BC, Lee SC, Wang LZ, et al. Explaining interindividual variability of docetaxel pharmacokinetics and pharmacodynamics in Asians through phenotyping and genotyping strategies. J Clin Oncol. 2002;20:3683–90. doi: 10.1200/JCO.2002.01.025. [DOI] [PubMed] [Google Scholar]
- Lan LB, Dalton JT, Schuetz EG. Mdr1 limits CYP3A metabolism in vivo. Mol Pharmacol. 2000;58:863–9. doi: 10.1124/mol.58.4.863. [DOI] [PubMed] [Google Scholar]
- Han S, Kim SB, Kang SS, et al. A phase II study of neoadjuvant docetaxel plus doxorubicin (KBCS-01) in stage II, III breast cancer. Breast Cancer Res Treat. 2006;98:57–61. doi: 10.1007/s10549-005-9131-6. [DOI] [PubMed] [Google Scholar]
- Keam B, Im SA, Kim HJ, et al. Prognostic impact of clinicopathologic parameters in stage II/III breast cancer treated with neoadjuvant docetaxel and doxorubicin chemotherapy: paradoxical features of the triple negative breast cancer. BMC Cancer. 2007;7:203. doi: 10.1186/1471-2407-7-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recht A, Edge SB, Solin LJ, et al. Postmastectomy radiotherapy: clinical practice guidelines of the American Society of Clinical Oncology. J Clin Oncol. 2001;19:1539–69. doi: 10.1200/JCO.2001.19.5.1539. [DOI] [PubMed] [Google Scholar]
- Kong M, Hong SE. Which patients might benefit from postmastectomy radiotherapy in breast cancer patients with t1- tumor and 1-3 axillary lymph nodes metastasis? Cancer Res Treat. 2013;45:103–11. doi: 10.4143/crt.2013.45.2.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–16. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- Cascorbi I, Gerloff T, Johne A, et al. Frequency of single nucleotide polymorphisms in the P-glycoprotein drug transporter MDR1 gene in white subjects. Clin Pharmacol Ther. 2001;69:169–74. doi: 10.1067/mcp.2001.114164. [DOI] [PubMed] [Google Scholar]
- Hoffmeyer S, Burk O, von Richter O, et al. Functional polymorphisms of the human multidrug-resistance gene: multiple sequence variations and correlation of one allele with P-glycoprotein expression and activity in vivo. Proc Natl Acad Sci USA. 2000;97:3473–8. doi: 10.1073/pnas.050585397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang K, Ngoi SM, Gwee PC, et al. Distinct haplotype profiles and strong linkage disequilibrium at the MDR1 multidrug transporter gene locus in three ethnic Asian populations. Pharmacogenetics. 2002;12:437–50. doi: 10.1097/00008571-200208000-00004. [DOI] [PubMed] [Google Scholar]
- Yamaguchi H, Hishinuma T, Endo N, et al. Genetic variation in ABCB1 influences paclitaxel pharmacokinetics in Japanese patients with ovarian cancer. Int J Gynecol Cancer. 2006;16:979–85. doi: 10.1111/j.1525-1438.2006.00593.x. [DOI] [PubMed] [Google Scholar]
- Wang LZ, Goh BC, Grigg ME, Lee SC, Khoo YM, Lee HS. A rapid and sensitive liquid chromatography/tandem mass spectrometry method for determination of docetaxel in human plasma. Rapid Commun Mass Spectrom. 2003;17:1548–52. doi: 10.1002/rcm.1091. [DOI] [PubMed] [Google Scholar]
- Sparreboom A, Danesi R, Ando Y, Chan J, Figg WD. Pharmacogenomics of ABC transporters and its role in cancer chemotherapy. Drug Resist Updat. 2003;6:71–84. doi: 10.1016/s1368-7646(03)00005-0. [DOI] [PubMed] [Google Scholar]
- Kim RB, Leake BF, Choo EF, et al. Identification of functionally variant MDR1 alleles among European Americans and African Americans. Clin Pharmacol Ther. 2001;70:189–99. doi: 10.1067/mcp.2001.117412. [DOI] [PubMed] [Google Scholar]
- Cizmarikova M, Wagnerova M, Schonova L, et al. MDR1 (C3435T) polymorphism: relation to the risk of breast cancer and therapeutic outcome. Pharmacogenomics J. 2010;10:62–9. doi: 10.1038/tpj.2009.41. [DOI] [PubMed] [Google Scholar]
- Kurata Y, Ieiri I, Kimura M, et al. Role of human MDR1 gene polymorphism in bioavailability and interaction of digoxin, a substrate of P-glycoprotein. Clin Pharmacol Ther. 2002;72:209–19. doi: 10.1067/mcp.2002.126177. [DOI] [PubMed] [Google Scholar]
- Chaudhary PM, Roninson IB. Expression and activity of P-glycoprotein, a multidrug efflux pump, in human hematopoietic stem cells. Cell. 1991;66:85–94. doi: 10.1016/0092-8674(91)90141-k. [DOI] [PubMed] [Google Scholar]
- Puisset F, Chatelut E, Dalenc F, et al. Dexamethasone as a probe for docetaxel clearance. Cancer Chemother Pharmacol. 2004;54:265–72. doi: 10.1007/s00280-004-0823-0. [DOI] [PubMed] [Google Scholar]
- Spurdle AB, Goodwin B, Hodgson E, et al. The CYP3A4*1B polymorphism has no functional significance and is not associated with risk of breast or ovarian cancer. Pharmacogenetics. 2002;12:355–66. doi: 10.1097/00008571-200207000-00003. [DOI] [PubMed] [Google Scholar]
- Hustert E, Haberl M, Burk O, et al. The genetic determinants of the CYP3A5 polymorphism. Pharmacogenetics. 2001;11:773–9. doi: 10.1097/00008571-200112000-00005. [DOI] [PubMed] [Google Scholar]
- Kang RH, Jung SM, Kim KA, et al. Effects of CYP2D6 and CYP3A5 genotypes on the plasma concentrations of risperidone and 9-hydroxyrisperidone in Korean schizophrenic patients. J Clin Psychopharmacol. 2009;29:272–7. doi: 10.1097/JCP.0b013e3181a289e0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Kaplan–Meier survival analysis according to G2677TA and C1236T polymorphisms of the ABCB1 gene


