Abstract
Malignant pleural mesothelioma (MPM) is a rare and highly aggressive neoplasm that arises from the pleural, pericardial, or peritoneal lining. Although surgery, chemotherapy, radiotherapy, and combinations of these therapies are used to treat MPM, the median survival of such patients is dismal. Therefore, there is a compelling need to develop novel therapeutics with different modes of action. Ganglioside GM2 is a glycolipid that has been shown to be overexpressed in various types of cancer. However, there are no published reports regarding the use of GM2 as a potential therapeutic target in cases of MPM. In this study, we evaluated the efficacy of the anti-GM2 antibody BIW-8962 as an anti-MPM therapeutic using in vitro and in vivo assays. Consequently, the GM2 expression in the MPM cell lines was confirmed using flow cytometry. In addition, eight of 11 cell lines were GM2-positive (73%), although the GM2 expression was variable. BIW-8962 showed a significant antibody-dependent cellular cytotoxicity activity against the GM2-expressing MPM cell line MSTO-211H, the effect of which depended on the antibody concentration and effector/target ratio. In an in vivo orthotropic mouse model using MSTO-211H cells, BIW-8962 significantly decreased the incidence and size of tumors. Additionally, the GM2 expression was confirmed in the MPM clinical specimens. Fifty-eight percent of the MPM tumors were positive for GM2, with individual variation in the intensity and frequency of staining. These data suggest that anti-GM2 antibodies may become a therapeutic option for MPM patients.
Keywords: Antibodies, antibody-dependent cell cytotoxicity, ganglioside GM2, mesothelioma, therapeutics
Malignant pleural mesothelioma (MPM) is a rare and highly aggressive neoplasm that arises from the pleural, pericardial, or peritoneal lining. Malignant pleural mesothelioma was previously considered to be very rare; however, the worldwide incidence is expected to increase substantially in the next decades, as MPM is usually associated with chronic asbestos exposure and there is a long latency period between the time of exposure and tumor development.1,2 Although surgery, chemotherapy, radiotherapy, and combinations of these therapies are used to treat MPM, the median survival of such patients is dismal, at only 6–18 months.3–5 Despite the use of a novel systemic chemotherapy regimen using the combination of pemetrexed and cisplatin, the long-term survival of patients with MPM remains limited.6 Therefore, further specific, effective, and less toxic therapies are needed.7
The importance of antibody therapeutics is increasing due to the high efficacy and low toxicity of these agents. The mode of action of antibody therapeutics can be divided into two main types: antigen neutralization, and the killing of antigen-expressing cells due to antibody effector functions. The major effector functions of therapeutic antibodies include antibody-dependent cellular cytotoxicity (ADCC) and complement-dependent cytotoxicity (CDC), both of which are mediated by the recruitment of immune cells, including natural killer (NK) cells and complement, respectively.8 The ADCC activity, in particular, is considered to play an important role in the efficacy of antibody therapeutics for hematological and solid tumors. For example, trastuzumab has been shown to have clinical benefits in the setting of breast cancer. The beneficial effects of this drug depend on the patient's gene polymorphism of FcγRIIIA, the receptor for IgG expressed in NK cells.9,10 These data strongly suggest that ADCC activity is involved in the therapeutic activity of trastuzumab.
Ganglioside GM2, a glycolipid consisting of ceramide and oligosaccharide, is a component of the cell membrane. The activity of GM2 is suggested to be associated with neuronal cell survival.11 In addition, GM2 has been reported to be overexpressed in tissues of lung cancer, neuroblastoma, and glioma, although it is rarely expressed in normal cells.12,13 Therefore, GM2 is considered to be an attractive target for therapeutic antibodies against cancer. Jones et al.14 reported that melanoma patients with an elevated anti-GM2 antibody titer show prolonged survival. This finding indicates the potential importance of antibodies that recognize GM2 in the immunological response against cancer. Although vaccination using GM2-conjugated proteins has been attempted clinically, sufficient efficacy has not yet been achieved.15 Against this background, humanized anti-GM2 antibodies with potent ADCC and CDC activities have been generated.16 Recently, the ADCC-enhancing modification of fucose removal from core Fc-linked oligosaccharides was applied to this antibody, and a non-fucosylated humanized anti-GM2 antibody, BIW-8962, was successfully developed. BIW-8962 was subsequently shown to have an in vivo therapeutic activity in a SCID mouse model of multiple organ metastasis induced by GM2-positive small-cell lung cancer (SCLC) cell lines, and overexpression of GM2 was detected in SCLC clinical specimens.17 In order to further investigate the therapeutic potential of the non-fucosylated, humanized anti-GM2 antibody BIW-8962 as a novel anti-MPM agent, we evaluated the efficacy of BIW-8962 against MPM cell lines using in vitro ADCC and in vivo orthotropic mouse models. In addition, we analyzed GM2 expression levels in clinical samples of MPM.
Materials and Methods
Cell lines
Eleven human MPM cell lines were used in this study. ACC-MESO-1, Y-MESO-8A, Y-MESO-12, and Y-MESO-14 were established at the Aichi Cancer Research Center Institute (Nagoya, Japan).18 NCI-H290 and NCI-H513 were provided by Dr. Adi F. Gazdar (University of Texas Southwestern Medical Center, Dallas, TX, USA). MSTO-211H, NCI-H28, NCI-H226, NCI-H2052, and NCI-H2452 were purchased from ATCC (Rockville, MD, USA). These cells were cultured in RPMI-1640 medium supplemented with 10% FBS (Life Technologies, Grand Island, NY, USA), 100 U/mL penicillin, and 100 mg/mL streptomycin (Meiji Seika Kaisha, Tokyo, Japan).
Animals
Male SCID mice, 5–6 weeks of age, were obtained from CLEA Japan (Osaka, Japan) and maintained under specific pathogen-free conditions throughout this study. All animals were acclimatized for at least 1 week before the experiments. All animal experiments complied with the Guidelines for the Institute for Experimental Animals, Kanazawa University Advanced Science Research Center (Kanazawa, Japan).
Reagents
The anti-GM2 antibody BIW-8962 and isotype control anti-dinitrophenol (DNP) antibody (fucose-removed human IgG1) were prepared by Kyowa Hakko Kirin Co., Ltd.
Flow cytometry
The GM2 expression in the MPM cells was examined using flow cytometry.19 Briefly, cells (5 × 105) were resuspended in PBS, supplemented with 10% pooled AB serum to prevent non-specific binding to the Fc receptor, washed with cold PBS, and incubated on ice for 30 min with BIW-8962 or the isotype control. The cells were washed with cold PBS and incubated on ice for an additional 30 min with FITC-conjugated anti-human IgG antibodies (Beckman Coulter, Fullerton, CA, USA) then washed and resuspended in cold PBS. The cells were subsequently analyzed on a FACSCalibur flow cytometer using the CellQuest software program (Becton Dickinson, San Jose, CA, USA). The relative fluorescence intensity was calculated as the ratio of the mean fluorescence intensity of BIW-8962 to that of the isotype control.
Antibody-dependent cellular cytotoxicity activity
The in vitro ADCC activity was measured using the lactate dehydrogenase (LDH) release assay method. Human peripheral blood mononuclear cells (MNCs) prepared from healthy donors using Lymphoprep (Axis Shield, Dundee, UK) were used as effector cells, and the human MPM cell line MSTO-211H was used for the target cells. Detached MSTO-211H cells were plated at a density of 1 × 104 cells/well into round-bottom 96-well microplates, and freshly isolated MNCs were added to the same plates in order to achieve an appropriate effector/target (E/T) ratio (E/T = 25/1, 50/1, and 100/1). Serial dilutions of BIW-8962 were then added to the plates to start the reaction. Following incubation at 37°C for 4 h, the supernatants from each well were recovered by centrifugation at 50 g for 5 min. The LDH activity in each supernatant was measured using a non-radioactive cytotoxicity assay kit (Promega, Madison, WI, USA). The absorbance at 490 nm was determined using an ELISA reader. The specific cytotoxicity level was calculated according to the following formula:
where Exp is the amount of LDH experimentally released from the target cells incubated with the effector cells and antibodies, Espo is the amount of LDH spontaneously released from the effector cells, Tspo is the amount of LDH spontaneously released from the target cells, and Total is the maximum amount of LDH released from the target cells incubated with 9% Triton X.
Orthotropic in vivo assay
Cultured MSTO-211H cells were harvested using trypsin, washed twice, and resuspended in PBS, and 1 × 106 cells in 100 μL PBS were subsequently injected into the thoracic cavity of each SCID mouse, which was pretreated with TM-β1 antibodies (previously established mouse IL-2Rβ antibodies)20 2 days before cancer cell inoculation.21 The mice were then i.v. given BIW-8962 (10 μg/animal) and/or 1 × 106 cells/animal MNC prepared according to the above method on days 7 and 14. At 3 weeks after tumor cell inoculation, the mice were sacrificed, their thoracic tumors were carefully removed and weighed, and the volume of pleural effusion was measured.
Immunofluorescent staining
Twenty-six MPM clinical tumor specimens were purchased from Origene Technologies (Rockville, MD, USA). Tumor specimens obtained by orthotopic inoculation of H290 cells in SCID mice were also used as a positive control. Frozen sections of these samples were fixed with acetone, washed with PBS, and incubated with 1 μg/mL of DAPI (Vector Laboratories, Burlingame, CA, USA) at room temperature for 20 min. The sections were then washed with PBS, blocked with 5% FBS in PBS for 10 min and incubated with 30 μg/mL of Alexa Fluor488 (Invitrogen, Carlsbad, CA, USA) conjugated BIW-8962 or isotype control at 4°C for 6 h. After being washed with PBS, the sections were analyzed using fluorescence microscopy. The percentages of cells with positive cytoplasmic and/or membrane GM2 immunoreactivity were evaluated as 0–100% and the modal intensity of the positively staining cells were determined on a scale from 0 to 3+: 0, complete absence of staining; 1+, weaker staining than H290 cells; 2+, similar staining to H290 cells; 3+, clearly more intense staining than H290 cells (Fig. S1).
Statistical analysis
Differences in the results of the in vitro experiments were evaluated using Student's two-tailed t-test, and differences in the results of the in vivo experiments were analyzed according to Dunnett's multiple comparison test. In all analyses, differences were considered to be significant at a P-value of <0.05.
Results
GM2 expression in MPM cell lines
First, we carried out a flow cytometric analysis to determine the GM2 expression levels in the MPM cell lines. In this assay, 11 histologically different MPM cell lines were used, as follows: ACC-MESO-1, Y-MESO-12, NCI-H290, NCI-H513, NCI-H226, and NCI-H2452 as epithelioid type cells; NCI-H28 and NCI-H2052 as sarcomatoid type cells; and Y-meso-8A, Y-meso-14, and MSTO-211H as biphasic type cells. Membrane-bound GM2 antigens were detected using the anti-GM2 antibody BIW-8962. The GM2 expression levels in these cell lines were categorized into three groups based on the relative fluorescence intensity: high (>10) in four cell lines (36%); low (2–10) in four cell lines (36%); and negative (<2) in three cell lines (28%) (Fig.1). We found no cell type-dependent high expression of GM2, and no GM2 expression was detected in the sarcomatoid MPM cell lines.
Figure 1.
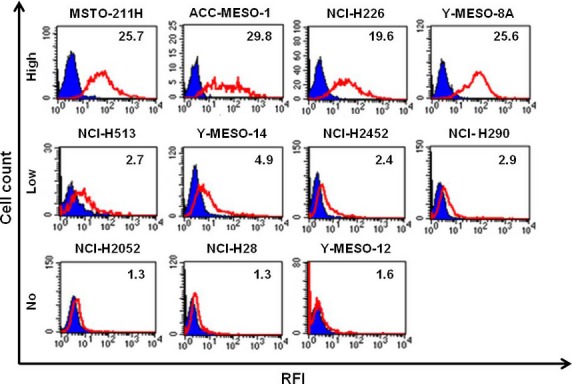
Expression levels of ganglioside GM2 were evaluated in malignant pleural mesothelioma cell lines. Cells were detached and incubated with BIW-8962 or anti-dinitrophenol (DNP) antibodies on ice for 30 min. Bound Abs were detected with FITC-conjugated goat anti-human IgG Abs. The fluorescence intensity of the stained cells was measured using flow cytometry, and the mean fluorescence intensity was calculated. The open red histograms represent BIW-8962-stained samples and the filled blue histograms represent anti-DNP antibody-stained samples. The relative fluorescent intensity (RFI) versus anti-DNP is indicated.
Antibody-dependent cellular cytotoxicity activity against MPM cell line
The in vitro ADCC activity of BIW-8962 against the MPM cell line was determined using MNCs obtained from four healthy volunteers as effector cells and MSTO-211H, as target cells, which highly express GM2. Consequently, BIW-8962 showed significant ADCC activity at antibody concentrations of 1 and 10 μg/mL (Fig.2), the efficacy of which increased in correlation with the E/T ratio (25, 50, and 100). The potent ADCC activity of BIW-8962 was consistently observed in MNCs obtained from the four donors.
Figure 2.
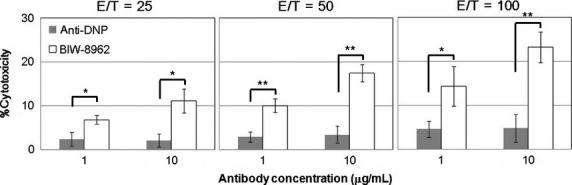
Anti-GM2 antibody BIW-8962 exerted antibody-dependent cellular cytotoxicity activity against MSTO-211H malignant pleural mesothelioma cells. Human peripheral blood mononuclear cells were purified from healthy donors and used as effector cells. MSTO-211H cells (target cells) were incubated with effector cells (effector/target = 25/1, 50/1, and 100/1) and antibodies (BIW-8962 or anti-dinitrophenol antibodies) at 37°C for 4 h. The released lactate dehydrogenase activity was measured and the % cytotoxicity was calculated. The experiments were carried out in triplicate, and the values are expressed as the mean of the values for four donors ± SD. *P < 0.01, **P < 0.001 BIW-8962 treatment versus anti-dinitrophenol antibody treatment.
In vivo therapeutic activity of BIW-8962 in orthotropic mouse model
The therapeutic efficacy of BIW-8962 was evaluated using an in vivo SCID mouse orthotropic model in which the animals were inoculated in the thoracic cavity with GM2-positive MSTO-211H cells and treated with BIW-8962 and/or human MNCs. BIW-8962 significantly decreased both the incidence and size of tumors (Fig.3, Table1). Notably, the concomitant administration of BIW-8962 and MNC had a greater effect on tumor incidence and size, whereas MNC injection alone showed weak antitumor activity. In addition, the incidence and volume of pleural effusion tended to be decreased by the co-administration of BIW-8962 and MNC.
Figure 3.
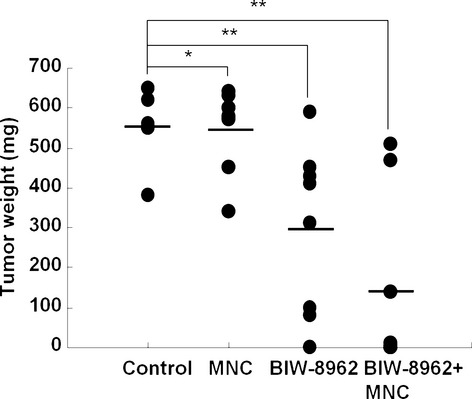
Anti-GM2 antibody BIW-8962 showed therapeutic activity in an MSTO-211H orthotropic mouse model. MSTO-211H malignant pleural mesothelioma cells were inoculated into the thoracic cavity in SCID mice, and the animals were treated with BIW-8962 and/or human peripheral blood mononuclear cells (MNC). The mice were then i.v. administered BIW-8962 and/or MNC on days 7 and 14. At 3 weeks after tumor cell inoculation, the mice were sacrificed and their tumor weights were measured. The bars represent the mean of the group data. *P < 0.05 and **P < 0.01 between each treatment and the control groups.
Table 1.
Therapeutic evaluation of anti-GM2 antibody BIW-8962 in an in vivo orthotropic malignant pleural mesothelioma model
| Treatment | Dose | Thoracic tumor |
Pleural effusion |
||||
|---|---|---|---|---|---|---|---|
| Incidence | Weight, mg |
Incidence | Volume, μL |
||||
| Median | Range | Median | Range | ||||
| Control | DW | 8/8 | 550 | 380–650 | 3/8 | 0 | 0–300 |
| MNC | 1 × 106 cells | 8/8 | 590 | 340–640 | 2/8 | 0 | 0–200 |
| BIW-8962 | 10 μg | 7/8 | 360 | 0–590 | 1/8 | 0 | 0–250 |
| BIW-8962 + MNC | 10 μg, 1 × 106 cells | 4/8 | <10 | 0–510 | 1/8 | 0 | 0–150 |
DW, distilled water; MNC, human peripheral blood mononuclear cells.
Expression of GM2 in MPM clinical specimens
In order to determine GM2 expression in clinical MPM specimens, immunofluorescent staining of frozen MPM tissue samples obtained from 26 patients was carried out. The GM2 expression was subsequently confirmed in 58% of the donors, although the intensity and frequency of staining varied greatly from donor to donor (Fig.4, Table2). In contrast, we found no correlation between GM2 expression pattern and MPM tissue type or stage. GM2 positivity was detected in four of eight biphasic type samples (50%), three of five desmoplastic type samples (60%), and one of two epithelial type samples (50%). Similarly, GM2-positive cells were detected in two of three stage I tumors (67%), three of four stage II tumors (75%), five of seven stage III tumors (71%), and two of five stage IV tumors (40%).
Figure 4.
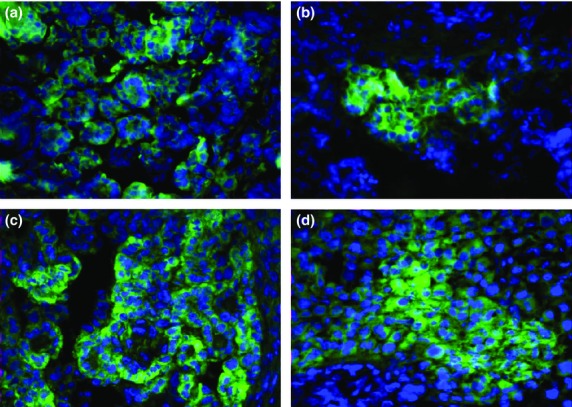
Ganglioside GM2 expression in clinical malignant pleural mesothelioma specimens. Frozen sections obtained from 26 mesothelioma patients were incubated in DAPI at room temperature for 20 min. After washing, the sections were incubated in 5% FBS–PBS for 10 min for blocking and subsequently incubated in 30 μg/mL BIW-8962 or anti-dinitrophenol antibodies conjugated with Alexa Fluor488 at 4°C for 6 h. Representative images are shown. Samples of patients #12 (a), 19 (b), 20 (c), and 23 (d) are shown.
Table 2.
Expression of ganglioside GM2 in mesothelioma patient samples
| Patient ID | Staining frequency, % | Staining intensity | Tissue type | Age, years | Sex | Stage |
|---|---|---|---|---|---|---|
| #1 | 0 | 0 | Biphasic | 58 | M | II |
| #2 | 30 | 2 | Biphasic | 69 | M | I |
| #3 | 0 | 0 | Meso | 53 | M | IV |
| #4 | 0 | 0 | Meso | 57 | F | III |
| #5 | 30 | 3 | Meso | 73 | M | NR |
| #6 | 0 | 0 | Biphasic | 52 | M | III |
| #7 | 0 | 0 | Biphasic | 71 | M | IV |
| #8 | 80 | 2 | Biphasic | 50 | M | IV |
| #9 | 30 | 1 | Meso | 65 | M | III |
| #10 | 0 | 0 | Meso | 71 | M | IIA |
| #11 | 0 | 0 | Desmoplastic | 81 | M | NR |
| #12 | 50 | 3 | Meso | 66 | M | II |
| #13 | 0 | 0 | Meso | 86 | M | NR |
| #14 | 100 | 2 | Desmoplastic | 55 | M | I |
| #15 | 0 | 0 | Desmoplastic | 63 | NR | I |
| #16 | 90 | 1 | Epithelial | 61 | M | IV |
| #17 | 10 | 1 | Meso | 68 | M | III |
| #18 | 40 | 3 | Biphasic | 62 | M | III |
| #19 | 80 | 3 | Desmoplastic | 57 | M | NR |
| #20 | 100 | 3 | Meso | 69 | M | II |
| #21 | 100 | 1 | Biphasic + desmoplastic | 68 | M | III |
| #22 | 100 | 3 | NR | 65 | M | NR |
| #23 | 50 | 3 | Meso | 69 | M | II |
| #24 | 20 | 2 | Meso | 57 | F | III |
| #25 | 0 | 0 | Biphasic | 50 | M | IV |
| #26 | 0 | 0 | Epithelial | 81 | M | NR |
Criteria for staining intensity: 0, negative; 1, faint; 2, moderate; 3, strong. F, female; M, male; Meso, unclassified mesothelioma; NR, not reported.
Discussion
Ganglioside GM2 is recognized to be a cancer-associated antigen. A previous histological analysis showed that GM2 is widely expressed in human lung cancer cells, including SCLC and Non-small-cell Lung cancer (NSCLC).22 However, GM2 expression in MPM has not yet been characterized. To the best of our knowledge, this study is the first experimental report to show GM2 expression in MPM cell lines and clinical MPM tumors. Furthermore, in this study, the non-fucosylated humanized anti-GM2 antibody, BIW-8962, showed an antitumor effect and a trend toward decreasing pleural effusion in the in vivo mouse orthotropic model. These results suggest the possibility that the anti-GM2 antibody BIW-8962 may be applied therapeutically in MPM patients.
Expression of GM2 was confirmed in eight of 11 MPM cell lines, four of which showed high levels of GM2 expression. A previous study showed that the main mode of actions of BIW-8962 are ADCC and CDC.17 Actually, BIW-8962 was shown to exert ADCC activity against the highly GM2-positive MPM cell line MSTO-211H in a dose-dependent and E/T ratio-dependent manner. In contrast, CDC activity against MPM cell lines was not observed when BIW-8962 was used at the high concentration of 100 μg/mL (data not shown). In addition, BIW-8962 treatment resulted in a significant reduction in the incidence and size of tumors in the in vivo orthotropic mouse model. As the concomitant administration of human MNC augmented the therapeutic activity of BIW-8962 in this study, the main mechanism of therapeutic action is thought to involve ADCC. One of the primary subsets of lymphocytes exerting an ADCC activity is NK cells, which are believed to be present in the tumors of MPM patients.23 These data suggest that the ADCC activity is stimulated in MPM tumors by treatment with BIW-8962, thus eliciting an antitumor effect. The therapeutic effect of BIW-8962 was observed at a dose of 10 μg/animal (roughly equal to 0.5 mg/kg) given i.v., and the efficacy of the therapy appeared to be higher than that of other antibody therapeutics reported previously.24,25 The antibody distribution in human MPM has not been defined. In an MPM mouse xenograft model, it was reported that biodistribution of 86Y labeled cetuximab and panitumumab in tumor was approximately 30% ID/g.26 Extrapolating from this report, antibody concentration in tumor when 0.5 mg/kg dose of antibody is given i.v. is calculated to be approximately 3 μg/mL. This is the sufficient concentration for BIW-8962 to exert ADCC activity, based on the in vitro experiment result. However, a previous study reported that antibody distribution in tumor was approximately 0.001% ID/g in colorectal cancer patients, lower than the mouse xenograft model.27 If we extrapolate the biodistribution ratio to MPM, a dose of 10 mg/kg can achieve an antibody concentration of more than 5 μg/mL in tumor, which is required for ADCC exertion.
In this study, GM2 expression was also confirmed in the clinical specimens of MPM patients, with individual differences in the expression levels and a rate of GM2 positivity of 58%. Although no correlations were observed between the GM2 expression profile and the histological findings or stage, all of the tissue types were GM2-positive. In the setting of MPM, differences in the histological type are known to affect the patient's prognosis, with cases of biphasic and sarcomatoid MPM having an especially poor prognosis.28,29 In this study, the antitumor activities of BIW-8962 were observed in the in vitro and in vivo models using the biphasic MPM cell line MSTO-211H. If this antibody possesses therapeutic activity in biphasic and sarcomatoid MPM tissues in the clinical setting, it would be a valuable treatment option for MPM patients.
In the in vivo orthotropic mouse model using the human MPM cell line, the mice given BIW-8962 had a tendency to develop a smaller amount of pleural effusion. As NK cells have been shown to be present in the fluid of pleural effusion,30 it is possible that ADCC reactions may occur in the pleural effusion of MPM patients. More than 60% of MPM patients present with pleural effusion associated with breathlessness, often accompanied by chest wall pain, which subsequently compromises their quality of life.31 BIW-8962 treatment may help to improve the prognosis of these patients as well as increase their quality of life by inhibiting the accumulation of pleural effusion.
Cytotoxic agents such as pemetrexed and cisplatin are primarily used in MPM therapy. Hence, the development of a novel therapeutic agent with a different mode of action is eagerly anticipated. In this study, we showed that GM2 is overexpressed in MPM clinical specimens and that the non-fucosylated anti-GM2 antibody BIW-8962 has therapeutic activity in an in vivo orthotropic MPM model. As the ADCC activity of antibody therapeutics plays an important role in the efficacy of oncologic treatment, anti-GM2 antibodies may become an effective therapy for MPM. A clinical study of BIW-8962 as monotherapy in subjects with NSCLC, SCLC, and mesothelioma is currently being carried out (NCT01898156).
Acknowledgments
We thank Dr. Hideki Murakami, Aichi Cancer Center Research Institute, for providing assistance with the immunofluorescent GM2 staining.
Disclosure Statement
Yusuke Machino, Mami Tsuchiya, Yui Suzuki, Ken-ichiro Nan-ya, Shigeru Iida, and Kazuyasu Nakamura are employees of Kyowa Hakko Kirin Co., Ltd. Seiji Yano received a research grant from Kyowa Hakko Kirin Co., Ltd.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Fig. S1. Representative staining of ganglioside GM2 in H290 cells in SCID mice.
References
- Connelly RR, Spirtas R, Myers MH, Percy CL, Fraumeni JF., Jr Demographic patterns for mesothelioma in the United States. J Natl Cancer Inst. 1987;78:1053–60. [PubMed] [Google Scholar]
- Ismail-Khan R, Robinson LA, Williams CC, Jr, Garrett CR, Bepler G, Simon GR. Malignant pleural mesothelioma: a comprehensive review. Cancer Control. 2006;13:255–63. doi: 10.1177/107327480601300402. [DOI] [PubMed] [Google Scholar]
- Ruffie P, Feld R, Minkin S, et al. Diffuse malignant mesothelioma of the pleura in Ontario and Quebec: a retrospective study of 332 patients. J Clin Oncol. 1989;7:1157–68. doi: 10.1200/JCO.1989.7.8.1157. [DOI] [PubMed] [Google Scholar]
- Pass HI, Kranda K, Temeck BK, Feuerstein I, Steinberg SM. Surgically debulked malignant pleural mesothelioma: results and prognostic factors. Ann Surg Oncol. 1997;4:215–22. doi: 10.1007/BF02306613. [DOI] [PubMed] [Google Scholar]
- Rusch VW, Piantadosi S, Holmes EC. The role of extrapleural pneumonectomy in malignant pleural mesothelioma. A Lung Cancer Study Group trial. J Thorac Cardiovasc Surg. 1991;102:1–9. [PubMed] [Google Scholar]
- Vogelzang NJ, Rusthoven JJ, Symanowski J, et al. Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol. 2003;21:2636–44. doi: 10.1200/JCO.2003.11.136. [DOI] [PubMed] [Google Scholar]
- Kurai J, Chikumi H, Hashimoto K, et al. Therapeutic antitumor efficacy of anti-epidermal growth factor receptor antibody, cetuximab, against malignant pleural mesothelioma. Int J Oncol. 2012;41:1610–8. doi: 10.3892/ijo.2012.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota T, Niwa R, Satoh M, Akinaga S, Shitara K, Hanai N. Engineered therapeutic antibodies with improved effector functions. Cancer Sci. 2009;100:1566–72. doi: 10.1111/j.1349-7006.2009.01222.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musolino A, Naldi N, Bortesi B, et al. Immunoglobulin G fragment C receptor polymorphisms and clinical efficacy of trastuzumab-based therapy in patients with HER-2/neu-positive metastatic breast cancer. J Clin Oncol. 2008;26:1789–96. doi: 10.1200/JCO.2007.14.8957. [DOI] [PubMed] [Google Scholar]
- Tamura K, Shimizu C, Hojo T, et al. FcgammaR2A and 3A polymorphisms predict clinical outcome of trastuzumab in both neoadjuvant and metastatic settings in patients with HER2-positive breast cancer. Ann Oncol. 2011;22:1302–7. doi: 10.1093/annonc/mdq585. [DOI] [PubMed] [Google Scholar]
- Usuki S, Ren J, Utsunomiya I, Cashman NR, Inokuchi J, Miyatake T. GM2 ganglioside regulates the function of ciliary neurotrophic factor receptor in murine immortalized motor neuron-like cells (NSC-34) Neurochem Res. 2001;26:375–82. doi: 10.1023/a:1010999014657. [DOI] [PubMed] [Google Scholar]
- Jennemann R, Rodden A, Bauer BL, Mennel HD, Wiegandt H. Glycosphingolipids of human gliomas. Cancer Res. 1990;50:7444–9. [PubMed] [Google Scholar]
- Hakomori S. Aberrant glycosylation in cancer cell membranes as focused on glycolipids: overview and perspectives. Cancer Res. 1985;45:2405–14. [PubMed] [Google Scholar]
- Jones PC, Sze LL, Liu PY, Morton DL, Irie RF. Prolonged survival for melanoma patients with elevated IgM antibody to oncofetal antigen. J Natl Cancer Inst. 1981;66:249–54. [PubMed] [Google Scholar]
- Eggermont AM, Suciu S, Rutkowski P, et al. Adjuvant ganglioside GM2-KLH/QS-21 vaccination versus observation after resection of primary tumor >1.5 mm in patients with stage II melanoma: results of the EORTC 18961 randomized phase III trial. J Clin Oncol. 2013;31:3831–7. doi: 10.1200/JCO.2012.47.9303. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Tanaka Y, Shitara K, Hanai N. Construction of humanized anti-ganglioside monoclonal antibodies with potent immune effector functions. Cancer Immunol Immunother. 2001;50:275–84. doi: 10.1007/PL00006689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada T, Bando H, Takeuchi S, et al. Genetically engineered humanized anti-ganglioside GM2 antibody against multiple organ metastasis produced by GM2-expressing small-cell lung cancer cells. Cancer Sci. 2011;102:2157–63. doi: 10.1111/j.1349-7006.2011.02093.x. [DOI] [PubMed] [Google Scholar]
- Kawaguchi K, Murakami H, Taniguchi T, et al. Combined inhibition of MET and EGFR suppresses proliferation of malignant mesothelioma cells. Carcinogenesis. 2009;30:1097–105. doi: 10.1093/carcin/bgp097. [DOI] [PubMed] [Google Scholar]
- Nishioka Y, Yano S, Fujiki F, et al. Combined therapy of multidrug-resistant human lung cancer with anti-P-glycoprotein antibody and monocyte chemoattractant protein-1 gene transduction: the possibility of immunological overcoming of multidrug resistance. Int J Cancer. 1997;71:170–7. doi: 10.1002/(sici)1097-0215(19970410)71:2<170::aid-ijc8>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Tanaka T, Tsudo M, Karasuyama H, et al. A novel monoclonal antibody against murine IL-2 receptor beta-chain. Characterization of receptor expression in normal lymphoid cells and EL-4 cells. J Immunol. 1991;147:2222–8. [PubMed] [Google Scholar]
- Nakataki E, Yano S, Matsumori Y, et al. Novel orthotopic implantation model of human malignant pleural mesothelioma (EHMES-10 cells) highly expressing vascular endothelial growth factor and its receptor. Cancer Sci. 2006;97:183–91. doi: 10.1111/j.1349-7006.2006.00163.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K, Koike M, Shitara K, et al. Chimeric anti-ganglioside GM2 antibody with antitumor activity. Cancer Res. 1994;54:1511–6. [PubMed] [Google Scholar]
- Yamada N, Oizumi S, Kikuchi E, et al. CD8+ tumor-infiltrating lymphocytes predict favorable prognosis in malignant pleural mesothelioma after resection. Cancer Immunol Immunother. 2010;59:1543–9. doi: 10.1007/s00262-010-0881-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inami K, Abe M, Takeda K, et al. Antitumor activity of anti-C-ERC/mesothelin monoclonal antibody in vivo. Cancer Sci. 2010;101:969–74. doi: 10.1111/j.1349-7006.2009.01463.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abe S, Morita Y, Kaneko MK, et al. A novel targeting therapy of malignant mesothelioma using anti-podoplanin antibody. J Immunol. 2013;190:6239–49. doi: 10.4049/jimmunol.1300448. [DOI] [PubMed] [Google Scholar]
- Nayak TK, Garmestani K, Milenic DE, et al. HER1-targeted 86Y- panitumumab possesses superior targeting characteristics than 86Y-cetuximab for PET imaging of human malignant mesothelioma tumors xenografts. PLoS ONE. 2011;6:e18198. doi: 10.1371/journal.pone.0018198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallinger S, Reilly RM, Kirsh JC, et al. Comparative dual label study of first and second generation antitumor-associated glycoprotein-72 monoclonal antibodies in colorectal cancer patients. Cancer Res. 1993;53:271–8. [PubMed] [Google Scholar]
- Musk AW, Olsen N, Alfonso H, et al. Predicting survival in malignant mesothelioma. Eur Respir J. 2011;38:1420–4. doi: 10.1183/09031936.00000811. [DOI] [PubMed] [Google Scholar]
- Flores RM, Pass HI, Seshan VE, et al. Extrapleural pneumonectomy versus pleurectomy/decortication in the surgical management of malignant pleural mesothelioma: results in 663 patients. J Thorac Cardiovasc Surg. 2008;135:620–6. doi: 10.1016/j.jtcvs.2007.10.054. [DOI] [PubMed] [Google Scholar]
- Vacca P, Martini S, Garelli V, Passalacqua G, Moretta L, Mingari MC. NK cells from malignant pleural effusions are not anergic but produce cytokines and display strong antitumor activity on short-term IL-2 activation. Eur J Immunol. 2013;43:550–61. doi: 10.1002/eji.201242783. [DOI] [PubMed] [Google Scholar]
- Robinson BW, Lake RA. Advances in malignant mesothelioma. N Engl J Med. 2005;353:1591–603. doi: 10.1056/NEJMra050152. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Representative staining of ganglioside GM2 in H290 cells in SCID mice.


