Abstract
Metastasis is a challenging clinical problem and the primary cause of death in breast cancer patients. However, there is no therapeutic agent against metastasis of breast cancer cells. Here we report that phloroglucinol, a natural phlorotannin component of brown algae suppresses metastatic ability of breast cancer cells. Treatment with phloroglucinol effectively inhibited mesenchymal phenotypes of basal type breast cancer cells through downregulation of SLUG without causing a cytotoxic effect. Importantly, phloroglucinol decreased SLUG through inhibition of PI3K/AKT and RAS/RAF-1/ERK signaling. In agreement with in vitro data, phloroglucinol was also effective against in vivo metastasis of breast cancer cells, drastically suppressing their metastatic ability to lungs, and extending the survival time of mice. Collectively, our findings demonstrate a novel anticancer activity of phloroglucinol against metastasis of breast cancer cells, implicating its clinical relevance.
Keywords: basal type breast cancer cells, cancer metastasis, epithelial-mesenchymal transition, phloroglucinol, SLUG
Breast cancer is the second-most lethal cancer in women. The main cause of death in breast cancer is the metastatic spread of malignant neoplasm to distant sites.1 Metastasis is a multistep process in which cancer cells are disseminated from the primary tumor and locally invade the surrounding tissue, where they enter blood vessels (intravasation) and subsequently exit the bloodstream (extravasation), colonizing the new microenvironment and forming secondary tumors.2–4 For the molecular mechanisms on cancer metastasis, epithelial-mesenchymal cell transition (EMT) is well established.5,6 Previously, EMT is known as a normal embryonic process, during which polarized epithelial cells convert into motile mesenchymal ones for complex body patterning and morphogenesis.5,7 During cancer progression, similar but physiopathological transitions occur and endow cancer cells with increased motility and invasiveness. By an EMT program, cancer cells appear to acquire molecular alterations that cause dysfunctional cell–cell adhesive interactions, loss of cell–cell junctions, and restructuring of the cytoskeleton, which collectively result in the loss of apical polarity and the acquisition of a more spindle-shaped morphology.5 Beyond metastasis, many studies also suggested that EMT is associated with other malignant phenotypes of cancer cells such as resistance to cancer therapies and the maintenance of cancer stem cells, indicating that EMT is a complex cellular program driving to the multifaceted cancer progression. For these reasons, development of a new therapeutic agent against EMT is required for the treatment of metastatic cancer.
Phloroglucinol is a phenolic compound that chemical structure includes an aromatic phenyl ring, consisting of a hydroxyl group. Similar to other phenolic compounds, phloroglucinol shows a variety of biological activity and is commercially widely used in medicine, cosmetics, pesticides, paints, cements and dyeing,8 implicating its safety as a drug. The toxicity of phloroglucinol has not been reported yet. Rather, it has been shown to protect cells from H2O2-induced oxidative stress via catalase activation.9 In addition, phloroglucinol confers radioprotection against gamma radiation-induced cell damage by inhibiting oxidative stress.10 In contrast to the protective roles of phloroglucinol in living organisms, we report that phloroglucinol suppresses metastatic ability of breast cancer cells through inhibition of EMT. Importantly, phloroglucinol caused a decrease of EMT master regulator SLUG through RAS/RAF-1/ERK and PI3K/AKT signaling in breast cancer cells. Taken together, our findings suggest that phloroglucinol could be a novel therapeutic agent that suppresses cancer metastasis by inhibition of EMT.
Materials and Methods
Chemical reagents and antibodies
Polyclonal antibodies to phospho-AKT (Ser-473), phospho-AKT (Thr-308), phospho-ERK1/2 (Thr-202/Tyr-204), ERK1 and N-cadherin were obtained from Cell Signaling Technology (Beverly, MA, USA). Polyclonal antibodies to AKT, ZEB1, SNAIL, and SLUG were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). The polyclonal antibody vimentin (VIM) was obtained from Thermo Scientific (Seoul, Korea). 4,6-Diamidino-2-phenylindole (DAPI), and monoclonal antibodies to β-actin were obtained from Sigma (St Louis, MO, USA). Anti-mouse Alexa Fluor 488, and anti-rabbit Alexa Fluor 488 were purchased from Invitrogen (Carlsbad, CA, USA). The polyclonal antibody E-cadherin was obtained from BD Biosciences (San Jose, CA, USA). The polyclonal antibody KRAS was purchased from abcam (Seoul, Korea). Phloroglucinol (1, 3, 5-trihydroxybenzene) was purchased from Sigma.
Cell culture
Human breast cancer BT549 and MDA-MB-231 cell lines were obtained from the American Type Culture Collection (Manassas, VA, USA). MDA-MB-231 cells were grown in DMEM and BT549 cells in RPMI supplemented with 10% fetal bovine serum, penicillin (100 units/mL), and streptomycin (100 g/mL). Cells were incubated in a humidified 5% CO2 atmosphere at 37°C.
Western blot analysis
Cell lysates were prepared by extracting proteins with lysis buffer [40 mM Tris–HCl (pH 8.0), 120 mM NaCl, 0.1% Nonidet-P40] supplemented with protease inhibitors. Proteins were separated by SDS-PAGE, and transferred to a nitrocellulose membrane (Amersham, Arlington Heights, IL, USA). The membrane was blocked with 5% non-fat dry milk in Tris-buffered saline, and incubated with primary antibodies for overnight at 4°C. The Blots were developed with a peroxidase-conjugated secondary antibody, and proteins were visualized by enhanced chemiluminescence (ECL) procedures (Amersham), using the manufacturer's protocol.
Activated RAS affinity precipitation assay
Activated Ras affinity precipitation assay was performed according to the manufacturer's protocol. Briefly, cell lysates were incubated with 5 μg of Raf-1 RBD agarose beads (upstate, Charlotsville, VA, USA) for 30 min at 4°C. After extensive washing of the agarose beads three times with washing buffer (25 mM HEPES (pH 7.5), 10 mM MgCl2, 150 mM NaCl, 1 mM EDTA, 1% Nonidet P-40, 1 mM Na3VO4, 10% glycerol, 10 μg/mL leupeptin, 10 μg/mL aprotinin, and 25 mM NaF), the activated KRAS (GTP-Ras) bound to Raf-1 RBD argarose beads was released by addition of SDS-PAGE sample buffer. The amount of activated KRAS was determined by immunoblotting with a KRAS antibody.
Transfection with siRNA
siRNA duplexes were introduced into cells using Lipofectamine 2000 reagent (Invitrogen, California, USA) according to the procedure recommended by the manufacturer. Cells were harvested after 48 h for subsequent experiments. All siRNA were purchased from Samchully Pharmaceutical (Korea, Seoul).
Immunocytochemistry
Cells were fixed with 4% paraformaldehyde and permeabilized with 0.1% Triton X-100 in PBS. Following cell fixation, cells were incubated with the appropriate primary antibodies in a solution of PBS with 1% bovine serum albumin and 0.1% Triton X-100 at 4°C overnight. Antibodies used were as follows: human anti-E-cadherin (mouse polyclonal antibody, 1:200), -N-cadherin (mouse polyclonal antibody, 1:200), and -Fibronectin (FN1; mouse polyclonal antibody, 1:200). Staining was visualized using anti-rabbit or anti-mouse Alexa Flour 488 (Molecular Probes, Seoul, Korea). Nuclei were counterstained using 4, 6-diamidino-2-phenylindole (DAPI; Sigma). Stained cells were visualized with a fluorescence-microscope (Olympus IX71, Seoul, Korea).
Invasion and migration assays
Cells (2 × 104 cells/well) were suspended in 0.2 mL of growth medium for invasion and migration assays. For invasion assay, the cells were loaded in the upper well of the Transwell chamber (8-mm pore size; Corning Glass, Seoul, Korea) that was precoated with 10 mg/mL growth factor-reduced Matrigel (BD Biosciences) on an upper side of the chamber with the lower well filled with 0.8 mL of growth medium. After incubation for 48 h at 37°C, noninvaded cells on the upper surface of the filter were removed with a cotton swab, and migrated cells on the lower surface of the filter were fixed and stained with a Diff-Quick kit (Fisher, Pittsburgh, PA, USA) and photographed (magnification ×20). Invasiveness was determined by counting cells in five microscopic fields per well, and the extent of invasion was expressed as an average number of cells per microscopic field. Cells were imaged by phase contrast microscopy (Leica Microsystems, Bannockburn, IL, USA). For migration assay, we used the Transwell chambers with inserts that contained the same type of membrane but without the Matrigel coating. For 3D-culture system, cells were plated in growth medium that was solidified with mixture of common ECM components such as collagen I and matrigel. Their invasiveness was then visualized by H&E staining after perpendicular section of the gels.
Animal experiments
Green fluorescence protein (GFP)-labeled metastatic MDA-MB231 (5 × 105 cells) cells were injected into fourth mammary fat pad of NOD scid gamma (NSG) mice (n = 5/group; Orient, Gyeonggido, Korea). Phloroglucinol was then treated four times every other day by i.p injection (25 mg of phloroglucinol/kg of body). Tumor size was measured with a caliper (calculated volume = shortest diameter2 × longest diameter/2) at 3-day intervals. Lung metastasis was analyzed by counting the number of foci in the lung. Mice were monitored every day and the survival rate was calculated based on finding of dead mice. For tail vein injection, GFP-labeled metastatic MDA-MB231 cells (1 × 106 cells) were injected into athymic BALB/c nude mice (n = 4/group; Orient) by intravenous injection. This study was reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) of Center for Laboratory Animal Sciences, Medical Research Coordinating Center, HYU industry-University Cooperation Foundation.
Statistical analysis
All experimental data are reported as mean and the error bars represent the experimental standard error. Statistical analysis was performed by the non-parametric Student's t-test.
Results
Phloroglucinol suppresses the migratory and invasive properties of breast cancer cells
To investigate whether phloroglucinol, a natural phlorotannin component of brown algae, has an anticancer activity (Fig.1a), we first treated MDA-MB231 breast cancer cells with various concentrations of phloroglucinol (0–100 μM) and analyzed its toxicity, cell growth and the effect on migration and invasion of the breast cancer cells. When cell death was analyzed by FACS after propidium iodide staining, there was no significant cell death caused by phloroglucinol in all concentrations that are tested (Fig. S1A). Importantly, however, treatment with phloroglucinol effectively suppressed the migratory and invasive ability of MDA-MB231 breast cancer cells in a dose-dependent manner (Fig.1b,c), indicating that phloroglucinol suppresses malignant phenotypes of breast cancer cells without causing cell death. Since the 50% inhibitory concentration (IC50) of phloroglucinol on migration and invasion was about 50 μM, this concentration was used for further experiments, unless otherwise mentioned. In agreement, when other basal type BT549 cells was treated with the phloroglucinol (50 μM) and analyzed the migratory and invasiveness, phloroglucinol also suppressed effectively the migratory and invasive properties of the basal type breast cancer cells in Trans-wells (Fig.1d). To confirm the effect of phloroglucinol, the invasiveness of cancer cells were also analyzed in the presence or absence of phloroglucinol by 3D-culture system, where common extracellular matrix components such as collagen I and matrigel were mixed and solidified in growth medium, mimicking in vivo tissue environment. Consistently, phloroglucinol effectively suppressed the infiltration of MDA-MB231 cells in the 3D-culture system (Fig.1e). In cell growth, treatment with phloroglucinol also caused a decrease in cell proliferation at 72 h; however, no significant increase in cell numbers was observed at 48 h after the treatment, the time point at which migration and invasion assays were performed. To consolidate this issue, MDA-MB231 and BT549 cancer cells were also treated with various concentration of phloroglucinol and the increases of cell number were analyzed at 48 h. Importantly, either concentration of phloroglucinol had no effect on breast cancer cell growth at the time point, indicating that cell growth did not affect on the accuracy of migration and invasion assays (Fig. S1B). Taken together, these results suggest that phloroglucinol has no cellular toxicity; however, it suppresses the migratory and invasive properties of breast cancer cells.
Figure 1.
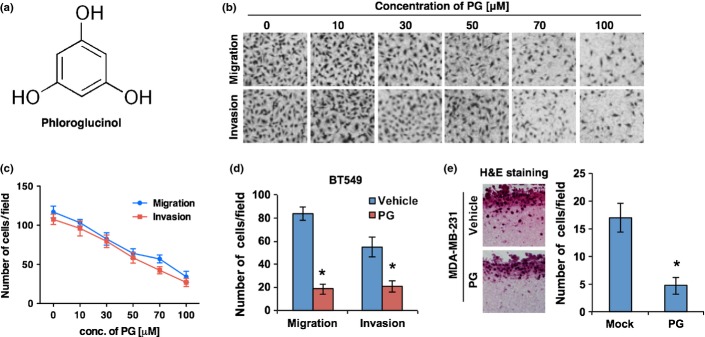
Phloroglucinol suppresses the migratory and invasive properties of breast cancer cells. (a) The chemical structure of phloroglucinol. (b, c) Dose-dependent effect of phloroglucinol on migration and invasion. Representative images are shown in (b) and migration and invasion are quantified in (c) after treatment of MDA-MB231 basal type breast cancer cells with various concentration of phloroglucinol. (d) Migration and invasion assay of BT549 basal type breast cancer cells after treatment with phloroglucinol (50 μM). (e) Effect of phloroglucinol on invasiveness of MDA-MB231 cells in 3D culture condition. Invasiveness was visualized and quantified after hematoxylin and eosin (H&E) staining. β-actin was used as a loading control. Error bars represent mean ± SD of triplicate samples. *P < 0.01 vs control.
Phloroglucinol suppresses mesenchymal traits of breast cancer cells
Tumor cell invasion is involved with the loss of cell–cell interaction together with acquisition of migratory properties, and is often associated with EMT.5 We next examined whether phloroglucinol suppresses the migratory and invasive properties of breast cancer cells through inhibition of EMT. To this end, we examined whether phloroglucinol decreases mesenchymal cell markers. Importantly, treatment with phloroglucinol decreased mesenchymal cell markers such as N-cadherin, FN1 and VIM, while increasing epithelial cell marker E-cadherin in basal type breast cancer cells (Fig.2a,b). Since these EMT markers are directly regulated by the EMT transcription factors such as SLUG, SNAIL, ZEB1 and TWIST, we analyzed whether phloroglucinol could inhibit the expression of these EMT regulators. Notably, SLUG expression was markedly decreased by treatment with phloroglucinol, whereas SNAIL, ZEB1 and TWIST expression were not altered (Fig.2c). Because phloroglucinol decreased only SLUG among four EMT transcription factors, we next examined whether downregulation of SLUG alone can suppress EMT. As expected, treatment with siRNA targeting SLUG caused a decrease of mesenchymal cell markers such as FN1, VIM and N-cadherin, while it increased E-cadherin in breast cancer cells (Fig.2d). In agreement, downregulation of SLUG effectively suppressed migratory and invasive properties of breast cancer cells (Fig.2e). Taken together, these results suggest that phloroglucinol suppresses mesenchymal phenotypes of breast cancer cells through downregulation of SLUG.
Figure 2.
Phloroglucinol suppresses mesenchymal traits of basal type breast cancer cells. (a, b) Western blot (a) and immunocytochemical analysis (b) for EMT markers in basal type breast cancer cells after treatment with vehicle or phloroglucinol (10, 30 or 50 μM). (c) Western blot analysis for EMT master regulators after treatment with vehicle or phloroglucinol (10, 30 or 50 μM). (d) Western blot analysis for EMT markers in basal type breast cancer cells after treatment with siRNA targeting SLUG. (e) Migration and invasion assay after treatment with siRNA targeting SLUG in basal type breast cancer cells. β-actin was used as a loading control.
Phloroglucinol inhibits PI3K/AKT and RAS/RAF-1/ERK signaling pathways
Because PI3K/AKT and RAS/RAF-1/ERK signaling pathways have been shown to regulate EMT in many cancer cells,11–13 we examined whether phloroglucinol shows its effect by inhibition of these pathways. To this end, we examined whether phloroglucinol could inhibit the activity of PI3K, AKT, KRAS, RAF-1 and ERK signaling components in basal type MDA-MB231 and BT549 breast cancer cells. Importantly, treatment with phloroglucinol inhibited the activity of PI3K and the phosphorylation of AKT in basal type breast cancer cells (Figs3a, S2A). Also, phloroglucinol effectively decreased the active form of KRAS that could interact with RAF-1 (Fig.3b). In parallel, treatment with phloroglucinol decreased the activity of RAF-1 and the phosphorylation of ERK (Figs3b, S2B).
Figure 3.
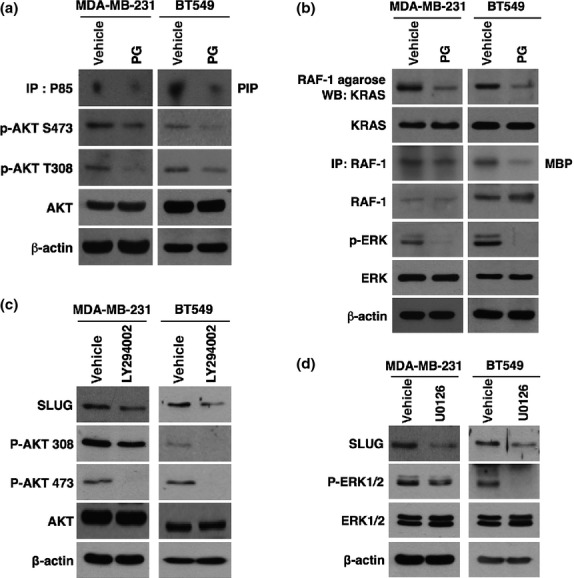
Phloroglucinol suppresses mesenchymal traits of basal type breast cancer cells through inhibition of PI3K/AKT and KRAS/RAF-1/ERK signaling pathways. (a) PI3 kinase assay and western blot analysis for phosphorylation status of AKT after treatment with phloroglucinol. (b) Activated KRAS affinity precipitation assay, RAF-1 kinase assay and western blot analysis for phosphorylation status of ERK after treatment with phloroglucinol. (c, d) Western blot analysis for SLUG after treatment with PI3K specific inhibitor LY294002 (c) or ERK inhibitor U0126 (d). β-actin was used as a loading control.
Since phloroglucinol inhibited PI3K/AKT and KRAS/RAF-1/ERK signaling pathways, we next examined whether phloroglucinol suppresses mesenchymal phenotypes of breast cancer cells through inhibition of these signaling pathways. Likely to the effect of phloroglucinol, treatment with PI3K specific inhibitor LY294002 or ERK inhibitor U0126 decreased the expression level of SLUG in basal type breast cancer cells (Fig.3c,d). Taken together, these results suggest that phloroglucinol blocks PI3K/AKT and KRAS/RAF-1/ERK signaling pathways, thereby suppressing mesenchymal phenotypes of basal type breast cancer cells.
Phloroglucinol inhibits in vivo metastatic ability of breast cancer cells
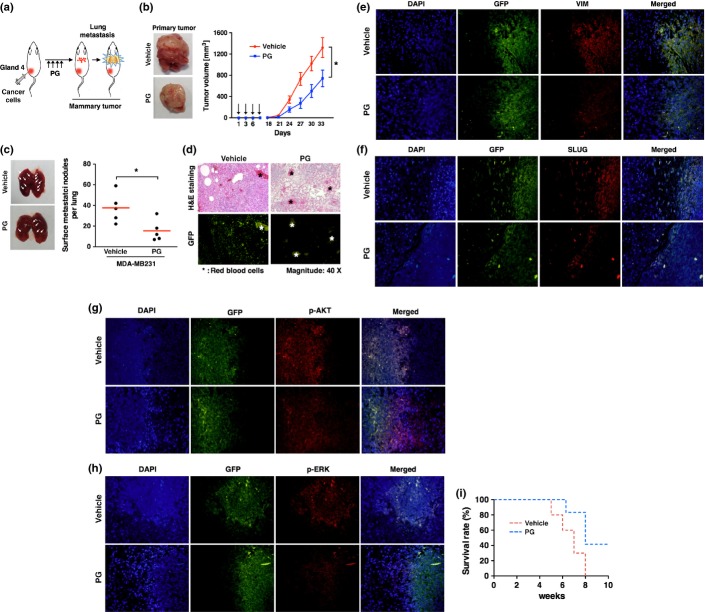
Because phloroglucinol inhibited the invasiveness of breast cancer cells in vitro, we next examined whether phloroglucinol could suppress metastasis of breast cancer in vivo. To this end, GFP-labeled metastatic MDA-MB231 cells were transplanted into mammary fat pads of NOD-scid gamma (NSG) mice and phloroglucinol were treated four times on alternate days as indicated in Figure4(a). In tumor volumes, we observed that treatment with phloroglucinol attenuated the primary tumor formation in mammary fat pads (Fig.4b). In parallel, lung metastasis was detected markedly less in phloroglucinol-treated mice than vehicle-treated one (Fig.4c). Not surprisingly, GFP was detected in the tumor tissues, indicating that the tumors had originated from GFP-labeled cancer cells (Fig.4d). When the primary tumors in fat pad were stained by mesenchymal cell marker VIM and regulator SLUG, the expression levels of those proteins were lower in mice that are treated with phloroglucinol, compared to vehicle-treated mice (Fig.4e,f). In a similar way with in vitro data, treatment with phloroglucinol decreased phosphorylation of AKT and ERK in the primary tumors of mice (Fig.4g,h). In agreement with these results, phloroglucinol-treated mice were survived longer than the control mice (Fig.4i).
Figure 4.
Phloroglucinol suppresses primary tumor formation and inhibits EMT in vivo. (a) Schematic experimental procedure for the mammary fat pad injection of cancer cells and treatment with vehicle or phloroglucinol. (b) Representative images of primary tumors (left) and tumor growth curves (right). Green fluorescent protein (GFP)-labeled MDA-MB231 cells were injected into mammary fat pad of NSG mice (n = 5) and then were treated four times with phloroglucinol or vehicle by i.p injection. (c) Representative images and quantification of lung metastases foci generated by GFP-labeled metastatic MDA-MB231 cells after mammary fat pad injection. (d) H&E staining and GFP florescence of lung metastases foci after mammary fat pad injection. (e–h) Immunohistochemistry for VIM (e), SLUG (f), p-AKT (g) and p-ERK (h) in fat pad primary tumor tissues. (i) Kaplan–Meier survival curves of mice that was treated with phloroglucinol or vehicle after mammary fat pad injection of cancer cells. Error bars represent mean ± SD. *P < 0.01 vs control.
Since phloroglucinol attenuated tumor formation as well as the metastasis, the less lung metastasis in phloroglucinol-treated mice could be caused by inhibition of primary tumor formation. Thus, to clarify the more direct effect of phloroglucinol on metastatic ability of breast cancer cells, we introduced GFP-labeled metastatic MDA-MB231 cells into athymic nude mice by intravenous injection. Mice were then treated with vehicle or phloroglucinol by i.p injection four times on alternate days (Fig.5a). By 5 weeks, we analyzed the frequency of lung metastasis in mice that were treated with phloroglucinol. Notably, treatment with phloroglucinol effectively attenuated lung metastasis of MDA-MB231 cells, compared to counterpart control in mice (Fig.5b). Again, GFP was detected in the tumor tissues, indicating that the tumors had originated from GFP-labeled MDA-MB231 cells (Fig.5c). By immunohistochemistry, we observed that AKT and ERK were highly activated in lung metastasized tumors, compared to counterpart normal tissues (Fig.5d,e). However, AKT and ERK were less phosphorylated in the tumors of mice that are treated with phloroglucinol (Fig.5d,e), indicating that phloroglucinol also inhibits AKT and ERK signaling in vivo. In agreement, the survival rate was higher in mice that are treated with phloroglucinol, compared to vehicle-treated mice (Fig.5f). Taken together, these results suggest that phloroglucinol inhibits in vivo metastatic ability of breast cancer cells.
Figure 5.
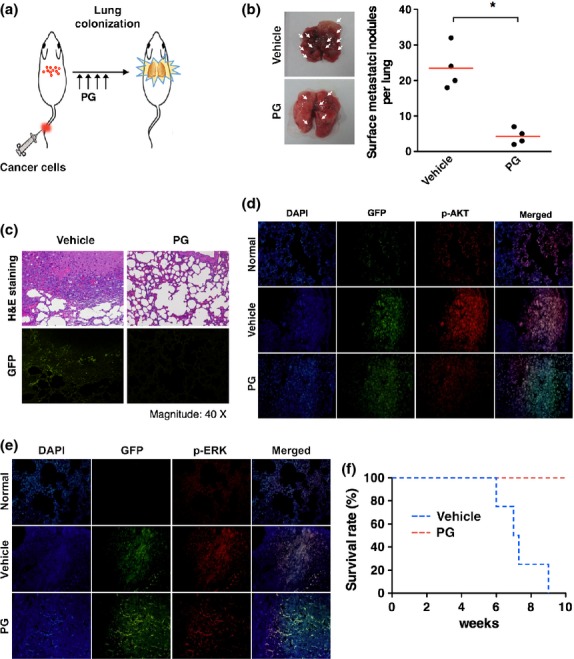
Phloroglucinol suppresses metastatic ability of breast cancer cells to lung. (a) Schematic experimental procedure for tail vein injection of cancer cells into athymic nude mice and treatment with vehicle or phloroglucinol. (b) Quantification of lung metastases foci generated by green fluorescent protein (GFP)-labeled metastatic MDA-MB231 cells after tail vein injection (n = 4). (c) H&E staining and GFP florescence of lung metastases foci after tail vein injection. (d, e) Immunohistochemistry for p-AKT (d) and p-ERK (e) in lung metastasized tumor tissues. (f) Kaplan–Meier survival curves of mice that were treated with phloroglucinol or vehicle after tail vein injection of cancer cells. Error bars represent mean ± SD. *P < 0.01 vs control.
Discussion
Metastasis to distant organs is a major reason behind cancer-associated deaths, and many studies have implicated a major mechanistic role of EMT in the metastasis of cancer.14 Thus, therapeutic strategies to suppress EMT-caused metastases might have a potential to impact on cancer mortality.
In this current study, we assessed the effect of phloroglucinol on metastatic ability of breast cancer cells. Phloroglucinol, a natural phlorotannin component of brown algae has previously been shown to exert a protective effect in normal cells, guarding against H2O2-induced oxidative stress via radical quenching and catalase activation.9,10 Since phloroglucinol prevents collagen destruction by inhibiting matrix metalloproteinase-1 (MMP-1) in human keratinocytes, phloroglucinol has been also considered as a preventive agent for skin aging.15 Although phloroglucinol has no toxicity but rather protective roles in cells of normal tissues, we found that phloroglucinol has an anticancer activity that suppresses the metastatic ability of malignant basal type breast cancer cells. Importantly, phloroglucinol attenuated the migratory and invasive properties of basal type breast cancer cells in Trans-wells. Also, it decreased the infiltration of breast cancer cells in 3D-culture system that mimics in vivo tissue environment. Moreover, treatment with phloroglucinol effectively attenuated the metastasis of breast cancer cells to lungs in mice. Of note, phloroglucinol suppressed the metastatic ability of breast cancer cells by inhibiting the mesenchymal phenotypes, as evidenced by decrease of mesenchymal cell markers such as N-cadherin, VIM and FN1.
Clinical outcome of luminal type breast cancer is greatly improved by endocrine therapy; however, there is no available effective clinical therapy for the treatment of basal type breast cancer.16 Moreover, basal type breast cancer cells have been known as the most aggressive breast cancer type.17–19 Very recently, several lines of evidence suggested that expression of EMT markers and regulators are closely associated with the basal-like subtype and cancer stem-like cell phenotypes.20,21 In particular, Storci et al.22 reported that SLUG is overexpressed in basal type breast cancer cells and contributes to the maintenance of basal type breast cancer phenotypes. They showed that downregulation of SLUG alone can inhibit in vitro and in vivo invasiveness of breast cancer cells, implicating SLUG as a critical regulator for EMT in breast cancer. In this study, we found that phloroglucinol causes a decrease in SLUG expression and thereby mitigates the mesenchymal phenotypes of basal type breast cancer cells. Phloroglucinol effectively decreased SLUG through inhibition of PI3K/AKT and Ras/Raf-1/ERK signaling pathways that previously have been shown to regulate EMT in many cancer cells.11–13 Consistent with our findings, very recent study suggested that phloroglucinol inhibit PI3K/AKT and RAS/RAF/ERK signaling in colorectal cancer.23 Also, Sun et al.24 reported that aspidin PB, a phloroglucinol derivative inhibits PI3K/AKT signaling in human hepatocarcinoma cells. All together, our findings and previous studies suggest that phloroglucinol has an inhibitory effect on PI3K/AKT and RAS/RAF/ERK signaling pathways, by which phloroglucinol suppressed mesenchymal phenotypes of breast cancer cells through inhibition of SLUG.
In summary, we demonstrated that phloroglucinol, a natural phlorotannin component of brown algae, inhibits metastatic ability of breast cancer cells. Of importance, phloroglucinol decreased the expression of SLUG, EMT master regulator through inhibition of PI3K/AKT and Ras/Raf-1/ERK signaling pathways. Since there is no appropriate therapeutic agent that blocks EMT and basal type breast cancer cells, our findings could have a clinical implication for the treatment of metastatic breast cancer.
Disclosure Statement
The authors declare no conflict of interest for this article.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Fig. S1. Effect of phloroglucinol on cell death and proliferation.
Fig. S2. Phloroglucinol inhibits AKT and ERK signaling pathways.
References
- Polyak K. Breast cancer: origins and evolution. J Clin Invest. 2007;117:3155–63. doi: 10.1172/JCI33295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaffer CL, Weinberg RA. A perspective on cancer cell metastasis. Science. 2011;331:1559–64. doi: 10.1126/science.1203543. [DOI] [PubMed] [Google Scholar]
- Liang X. EMT: new signals from the invasive front. Oral Oncol. 2011;47:686–7. doi: 10.1016/j.oraloncology.2011.04.016. [DOI] [PubMed] [Google Scholar]
- Christofori G. New signals from the invasive front. Nature. 2006;441:444–50. doi: 10.1038/nature04872. [DOI] [PubMed] [Google Scholar]
- Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002;2:442–54. doi: 10.1038/nrc822. [DOI] [PubMed] [Google Scholar]
- Valastyan S, Weinberg RA. Tumor metastasis: molecular insights and evolving paradigms. Cell. 2011;147:275–92. doi: 10.1016/j.cell.2011.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micalizzi DS, Farabaugh SM, Ford HL. Epithelial-mesenchymal transition in cancer: parallels between normal development and tumor progression. J Mammary Gland Biol Neoplasia. 2010;15:117–34. doi: 10.1007/s10911-010-9178-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh IP, Sidana J, Bharate SB, Foley WJ. Phloroglucinol compounds of natural origin: synthetic aspects. Nat Prod Rep. 2010;27:393–416. doi: 10.1039/b914364p. [DOI] [PubMed] [Google Scholar]
- Kang KA, Lee KH, Chae S, et al. Cytoprotective effect of phloroglucinol on oxidative stress induced cell damage via catalase activation. J Cell Biochem. 2006;97:609–20. doi: 10.1002/jcb.20668. [DOI] [PubMed] [Google Scholar]
- Kang KA, Zhang R, Chae S, et al. Phloroglucinol (1,3,5-trihydroxybenzene) protects against ionizing radiation-induced cell damage through inhibition of oxidative stress in vitro and in vivo. Chem Biol Interact. 2010;185:215–26. doi: 10.1016/j.cbi.2010.02.031. [DOI] [PubMed] [Google Scholar]
- Larue L, Bellacosa A. Epithelial-mesenchymal transition in development and cancer: role of phosphatidylinositol 3′ kinase/AKT pathways. Oncogene. 2005;24:7443–54. doi: 10.1038/sj.onc.1209091. [DOI] [PubMed] [Google Scholar]
- Yao K. Ye PP, Tan J, Tang XJ, Shen Tu XC. Involvement of PI3K/Akt pathway in TGF-beta2-mediated epithelial mesenchymal transition in human lens epithelial cells. Ophthalmic Res. 2008;40:69–76. doi: 10.1159/000113884. [DOI] [PubMed] [Google Scholar]
- Grille SJ, Bellacosa A, Upson J, et al. The protein kinase Akt induces epithelial mesenchymal transition and promotes enhanced motility and invasiveness of squamous cell carcinoma lines. Cancer Res. 2003;63:2172–8. [PubMed] [Google Scholar]
- Nguyen DX, Bos PD, Massague J. Metastasis: from dissemination to organ-specific colonization. Nat Rev Cancer. 2009;9:274–84. doi: 10.1038/nrc2622. [DOI] [PubMed] [Google Scholar]
- Piao MJ, Zhang R, Lee NH, Hyun JW. Phloroglucinol attenuates ultraviolet B radiation-induced matrix metalloproteinase-1 production in human keratinocytes via inhibitory actions against mitogen-activated protein kinases and activator protein-1. Photochem Photobiol. 2012;88:381–8. doi: 10.1111/j.1751-1097.2012.01074.x. [DOI] [PubMed] [Google Scholar]
- Turner NC, Jones AL. Management of breast cancer–Part II. BMJ. 2008;337:a540. doi: 10.1136/bmj.a540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haffty BG, Yang Q, Reiss M, et al. Locoregional relapse and distant metastasis in conservatively managed triple negative early-stage breast cancer. J Clin Oncol. 2006;24:5652–7. doi: 10.1200/JCO.2006.06.5664. [DOI] [PubMed] [Google Scholar]
- Carey LA, Dees EC, Sawyer L, et al. The triple negative paradox: primary tumor chemosensitivity of breast cancer subtypes. Clin Cancer Res. 2007;13:2329–34. doi: 10.1158/1078-0432.CCR-06-1109. [DOI] [PubMed] [Google Scholar]
- Perou CM, Sorlie T, Eisen MB, et al. Molecular portraits of human breast tumours. Nature. 2000;406:747–52. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- Sarrio D, Rodriguez-Pinilla SM, Hardisson D, Cano A, Moreno-Bueno G, Palacios J. Epithelial-mesenchymal transition in breast cancer relates to the basal-like phenotype. Cancer Res. 2008;68:989–97. doi: 10.1158/0008-5472.CAN-07-2017. [DOI] [PubMed] [Google Scholar]
- Choi Y, Lee HJ, Jang MH, et al. Epithelial-mesenchymal transition increases during the progression of in situ to invasive basal-like breast cancer. Hum Pathol. 2013;44:2581–9. doi: 10.1016/j.humpath.2013.07.003. [DOI] [PubMed] [Google Scholar]
- Storci G, Sansone P, Trere D, et al. The basal-like breast carcinoma phenotype is regulated by SLUG gene expression. J Pathol. 2008;214:25–37. doi: 10.1002/path.2254. [DOI] [PubMed] [Google Scholar]
- Kang MH, Kim IH, Nam TJ. Phloroglucinol induces apoptosis through the regulation of insulin-like growth factor 1 receptor signaling pathways in human colon cancer HT-29 cells. Int J Oncol. 2014;45:1036–42. doi: 10.3892/ijo.2014.2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Gao C, Luo M, et al. Aspidin PB, a phloroglucinol derivative, induces apoptosis in human hepatocarcinoma HepG2 cells by modulating PI3K/Akt/GSK3beta pathway. Chem Biol Interact. 2013;201:1–8. doi: 10.1016/j.cbi.2012.11.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Effect of phloroglucinol on cell death and proliferation.
Fig. S2. Phloroglucinol inhibits AKT and ERK signaling pathways.