Abstract
The endogenous chemotaxis of cells toward sites of tissue injury and/or biomaterial implantation is an important component of the host response. Implanted biomaterials capable of recruiting host stem/progenitor cells to a site of interest may obviate challenges associated with cell transplantation. An assay for the identification and quantification of chemotaxis induced by surgically placed biologic scaffolds composed of extracellular matrix is described herein.
Keywords: Chemotaxis, Stem cells, Cryptic peptides, Extracellular matrix
1 Introduction
One of the purported methods by which biologic scaffold materials composed of extracellular matrix (ECM) support constructive tissue remodeling involves the endogenous recruitment of stem and progenitor cells to the site of interest (1–10). This cell recruitment process has been attributed, in part, to chemotactic cryptic peptides generated during the process of in vivo scaffold degradation (11, 12). Specific peptides have been described with such chemotactic activity (13–15).
The directed movement of cells toward sites of tissue injury, inflammation, and infection plays a crucial role in homeostasis and response to injury (16–19). Identification of the bioactive factors that mediate such processes (i.e., specific stimuli) is important for the understanding of disease processes, understanding host physiologic and pathologic responses, and for the development of innovative and effective therapeutic strategies. This manuscript describes an in vitro assay to evaluate the chemotactic response of perivascular stem cells (PVSC) (3–6). These cells were originally isolated from human skeletal muscle and have been suggested to be a critical component of the mammalian response to injury in several anatomic locations (3, 20–24). The in vitro chemotactic assay described herein can be used for any cell type provided that the individual parameters of the assay are optimized for the cell type of interest.
Considering the current interest in various forms of cell therapy, the challenges in maintaining the presence and viability of cells at tissue sites of interest, and the potential role of paracrine factors in recruiting various cells to specific anatomic locations, this in vitro assay can be a valuable laboratory tool.
2 Materials
Cell type of interest (See Note 1).
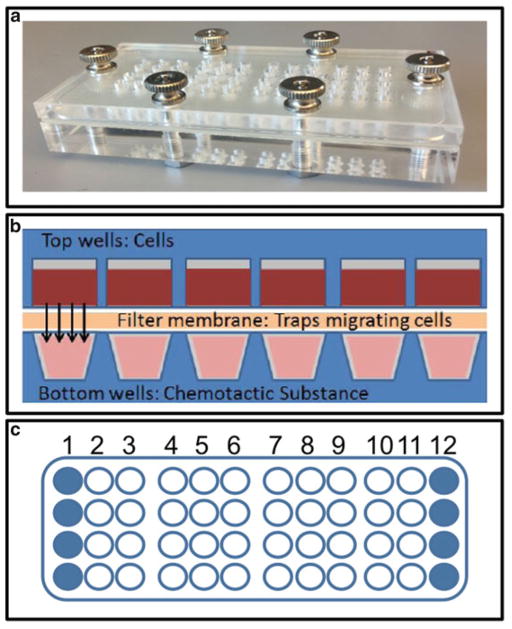
48 Well Micro Chemotaxis Chamber (Neuro Probe, Gaithersburg, MD, USA) (Fig. 1a).
Polycarbonate membrane filter (Neuro Probe, Gaithersburg, MD, USA) with 8 μm pore size (See Note 2).
Accessory pack for polycarbonate filters (Neuro Probe, Gaithersburg, MD, USA).
Collagen I from rat tail (BD Biosciences, Franklin Lakes, NJ, USA) (See Note 3).
95 % methanol.
Diff-Quik Stain Set (Source: Dade Behring, Deerfield, IL, USA, or equivalent) or a fluorescent nuclear stain such as Draq5 (Cell Signaling, Danvers, MA, USA) or 4′,6-diami-dino-2-phenylindole (DAPI; BD Biosciences, Franklin Lakes, NJ, USA).
2″ × 3″ Glass slides (Source: Kimble Scientific, Vineland, NJ, USA, or equivalent).
Cover slip.
Fig. 1.
An established assay for the identification of chemotaxis. Chemotaxis chamber used for the cell migration assay (a). Schematic representation of the chemotaxis assay (b). Outermost wells (solid circles) of the chamber are not used due to artifacts from edge evaporation (c). In a 48-well chamber up to ten substances can be tested (columns 2 through 11) in quadruplicate
3 Methods
3.1 Overview
Potential chemotactic substances to be evaluated are placed in the lower wells of the chemotaxis chamber. Migrating cells are placed in the upper wells. A porous filter membrane separates the upper and lower wells. As the substances diffuse cells begin to migrate and are trapped in the membrane where they can subsequently be stained and quantified (Fig. 1b).
3.2 Filter Preparation
Choose a polycarbonate filter with appropriate pore size (see Note 2). The appropriate pore size for PVSCs is 8 μm.
-
Coat filter with appropriate adhesion-promoting molecule (see Note 3). Collagen I is used as a substrate for PVSC chemotaxis.
Pour 0.05 mg/ml collagen I solution (prepared according to the manufacturer’s protocol) into a large 100 mm cell culture dish.
Immerse filters in collagen I solution, matte side down, making sure that both sides are coated.
Incubate filters in collagen solution for 30–45 min at room temperature.
Float filters over PBS, first with one side down in the PBS and then with the other side down in the PBS.
Clip large filter clamp (from Neuro Probe accessory pack) on the edge of one end of the filter, and hang at room temperature for 20 min to dry. Use filter for assay within 4 h of removal from the collagen I solution.
3.3 Preparation of Responding Cells
Established cell lines or primary cells being expanded in culture are serum starved for 18–24 h prior to the chemotaxis assay in basal growth media with 0.5 % serum. On the day of the assay, the cultured cells are trypsinized, counted, and resuspended in basal growth media without serum (migration media) to achieve desired cell density for the assay (see Note 4). Freshly isolated cells are counted and resuspended in migration media at desired cell density (see Note 4). PVSCs are resuspended at a concentration of 6 × 105 cells/mL, to allow easy pipetting of 30,000 cells in 50 μl per well.
Place cell suspension in a conical tube with loosened cap, and incubate at 5 % CO2/37 °C for 1 h. The chemoattractants and chemotaxis chamber are prepared during this incubation period.
3.4 Preparation of Chemoattractants
The chemoattractants and control chemoattractants are prepared and diluted in migration media at desired concentrations (see Note 5). Migration media alone and 20 % serum represent the negative and positive controls, respectively, for PVSC chemotaxis.
The chemoattractants are loaded into the bottom plate of the chemotaxis chamber on a flat bench top outside of the cell culture hood (see Note 6). Set up assay with quadruplicates when possible, and exclude edge wells due to potential artifacts caused by media evaporation (Fig. 1c).
Adjust micropipette so that filled wells on the bottom plate of the chemotaxis chamber form a slight positive meniscus. This volume is 27.5 μl for most liquids and 28 μl for more viscous samples.
3.5 Preparation of Chemotaxis Chamber
Carefully place filter membrane (matte side down) evenly on top of the filled wells of the bottom plate of the chemotaxis chamber without leaving any bubbles underneath the filter membrane (see Note 7).
Carefully add silicone gasket.
Push top plate down against bottom plate, and hold it firmly while applying thumbnuts (see Note 8).
Remove cells from incubator, and gently pipet cells to resuspend evenly.
Pipet 50 μl of resuspended cells into each upper well of the chemotaxis chamber (see Note 9).
Incubate at 5 % CO2/37 °C for 1 to 18 h, depending on cell type (see Note 10). Three hours is the optimal incubation period for PVSCs.
3.6 Disassembly of Chemotaxis Chamber
-
While firmly holding down the top plate, remove thumbnuts. Invert the entire chamber onto a paper towel while grasping the corners of the top plate (now on the bottom), and slowly lower the top plate until it rests on the bench top, making sure that the filter stays stuck to the gasket and top plate. The filter is now on top of the gasket, and migrated cells are facing up on the filter. The side of the filter now facing up is referred to as the migrated cell side (see Note 11).
Carefully (see Note 12) and quickly lift just the end of the filter, and clamp 1 mm of the edge with the large plastic clamp (from Neuro Probe accessory pack). Take care to place the clamp evenly on the filter, with the filter centered in the jaws of the clamp and the clamp parallel to the ends of the filter. Lift the filter with the plastic clamp, and while lifting the filter immediately attach the small metal clamp (from Neuro Probe accessory pack) to the other end in an even fashion so that the filter is centered in the jaws of the small clamp. Take care so that the filter does not fold over on itself.
Holding both clamps, with the migrated cell side up, wet the underside of the filter in a dish containing PBS. Take care not to allow the PBS to wash over the cell side of the filter.
While holding the filter by the large clamp, with the small clamp attached to the other end and hanging free, wipe the cells off the non-migrated side of the filter by drawing the filter up over the wiper blade (from Neuro Probe accessory pack). The blade should first contact the filter just below the jaws of the wide clamp. Apply light pressure evenly against the blade, and maintain an angle of about 30° from vertical for the portion of the filter above the wiper. It is important to complete the wiping carefully and quickly so that the cells do not dry on the filter; drying occurs in 10–20 s and will prevent complete removal of the non-migrated cells.
Clean the wiper with a kimwipe.
Repeat steps (b)–(d) two to three times.
3.7 Filter Fixation and Analysis
To fix the filter, carefully float on 95 % methanol in a 100 mm petri dish, migrated cell side down. Then carefully and evenly submerge the filter for 2 min.
Allow the filter to dry with migrated cell side up.
-
Using the small metal clamps placed evenly on both sides of the filter, stain as follows (see Note 13):
Dip the filter into a 50 ml tube of Diff-Quick-1, 5×, 1 s each time.
Dip the filter into a 50 ml tube of Diff-Quick-2, 5×, 1 s each time.
Dip the filter into a 50 ml tube of Diff-Quick-3, 5×, 1 s each time.
-
Dip the filter carefully into deionized filtered water several times to rinse off excess stain.
Dry well by touching the edges of the filter to a paper towel.
Remove the bottom clamp carefully, and lay the filter on a glass slide to partially dry.
Lay the filter on a second glass slide. Gently pull out any wrinkles with the small forceps (from Neuro Probe accessory pack).
The filter may be viewed and photographed as is or a drop of immersion oil may be added to cover the filter. Gently spread the oil on the filter with a smooth, blunt instrument to remove all bubbles and wrinkles.
View the slide using bright field microscopy (see Note 14).
Photograph three to five representative 20× fields of each well.
Count total cells in each field of view by hand or by using an image analysis software package such as ImageJ or Cell Profiler.
Graph results for analysis.
Footnotes
Cells used in this assay can be either freshly isolated, primary cultured cells or transformed cell lines. Cells of interest may include stem/progenitor cells, endothelial cells, and epithelial cells among others. The protocol described herein details an assay for the examination of PVSC chemotaxis.
Pore size is critical to the success of the assay and must be determined for each cell type.
The appropriate adhesion-promoting substrate (e.g., gelatin, fibronectin, laminin, collagen I, collagen IV) may be critical to the success of the assay and is cell type specific.
Density range varies for each cell type and is critical for the success of the assay. Most often, optimal density will be in the range of 10,000–250,000 cells per well.
Chemoattractants are substances with biologic activity, specifically, chemotactic potential. Common chemoattractant substances include pharmacologics, growth factors, secreted effector molecules, and degradation products of biologic scaffolds among others.
Include a negative control consisting of migration media alone.
When possible, include a positive control. The positive control may be a known specific chemoattractant (e.g., VEGF) at an appropriate concentration. For many cell types, basal media with 10–20 % serum is a good positive control.
The assay no longer requires sterility due to short incubation of migration periods (1–18 h).
Do not move filter once it is applied to prevent cross-contamination of wells and to minimize air bubble formation.
Maintain an even firm pressure on the top of the chamber while tightening the thumbnuts to prevent air bubbles from being drawn into the bottom wells. Thumbnuts should be finger-tight; do not use any tools to tighten.
The placement of cells into the chamber is performed on the bench top outside of the cell culture hood. Carefully add cells into each well. Eject the fluid with a rapid motion to dislodge air from the bottom of the well. Periodically resuspend the cells in the tube with a pipet to assure a single-cell suspension and even seeding densities.
Incubation time is critical to the success of the assay and must be determined for each cell type. Optimal incubation time for most cell types is between 2 and 4 h and is rarely longer than 6 h.
As the membrane filter is extremely thin, extreme care must be taken at each step from this point onward to assure that the filter remains intact and does not fold over on itself.
Care must be taken at each step from this point onward to assure that the filter remains intact and does not fold over on itself.
Alternatively, a fluorescent nuclear dye, such as Draq5 or Dapi, may be used to stain the filter for migrated cells.
Fluorescent microscopy must be used to image fluorescent nuclear dyes.
References
- 1.Reing JE, Zhang L, Myers-Irvin J, Cordero KE, Freytes DO, Heber-Katz E, Bedelbaeva K, McIntosh D, Dewilde A, Braunhut SJ, Badylak SF. Degradation products of extracellular matrix affect cell migration and proliferation. Tissue Eng Part A. 2009;15(3):605–614. doi: 10.1089/ten.tea.2007.0425. [DOI] [PubMed] [Google Scholar]
- 2.Agrawal V, Siu BF, Chao H, Hirschi KK, Raborn E, Johnson SA, Tottey S, Hurley KB, Medberry CJ, Badylak SF. Partial Characterization of the Sox2+ Cell Population in an Adult Murine Model of Digit Amputation. Tissue Eng Part A. 2012;18(13):1454–1463. doi: 10.1089/ten.tea.2011.0550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Crisan M, Yap S, Casteilla L, Chen CW, Corselli M, Park TS, Andriolo G, Sun B, Zheng B, Zhang L, Norotte C, Teng PN, Traas J, Schugar R, Deasy BM, Badylak S, Buhring HJ, Giacobino JP, Lazzari L, Huard J, Peault B. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3(3):301–313. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 4.Agrawal V, Johnson SA, Reing J, Zhang L, Tottey S, Wang G, Hirschi KK, Braunhut S, Gudas LJ, Badylak SF. Epimorphic regeneration approach to tissue replacement in adult mammals. Proc Natl Acad Sci U S A. 2010;107(8):3351–3355. doi: 10.1073/pnas.0905851106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tottey S, Corselli M, Jeffries EM, Londono R, Peault B, Badylak SF. Extracellular matrix degradation products and low-oxygen conditions enhance the regenerative potential of perivascular stem cells. Tissue Eng Part A. 2011;17 (1–2):37–44. doi: 10.1089/ten.tea.2010.0188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tottey S, Johnson SA, Crapo PM, Reing JE, Zhang L, Jiang H, Medberry CJ, Reines B, Badylak SF. The effect of source animal age upon extracellular matrix scaffold properties. Biomaterials. 2011;32(1):128–136. doi: 10.1016/j.biomaterials.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Badylak SF, Park K, Peppas N, McCabe G, Yoder M. Marrow-derived cells populate scaffolds composed of xenogeneic extracellular matrix. Exp Hematol. 2001;29(11):1310–1318. doi: 10.1016/s0301-472x(01)00729-9. [DOI] [PubMed] [Google Scholar]
- 8.Zantop T, Gilbert TW, Yoder MC, Badylak SF. Extracellular matrix scaffolds are repopulated by bone marrow-derived cells in a mouse model of Achilles tendon reconstruction. J Orthop Res. 2006;24(6):1299–1309. doi: 10.1002/jor.20071. [DOI] [PubMed] [Google Scholar]
- 9.Nieponice A, Gilbert TW, Johnson SA, Turner NJ, Badylak SF. Bone marrow-derived cells participate in the long-term remodeling in a mouse model of esophageal reconstruction. J Surg Res. 2012;182(1):e1–e7. doi: 10.1016/j.jss.2012.09.029. [DOI] [PubMed] [Google Scholar]
- 10.Brennan EP, Tang XH, Stewart-Akers AM, Gudas LJ, Badylak SF. Chemoattractant activity of degradation products of fetal and adult skin extracellular matrix for keratinocyte progenitor cells. J Tissue Eng Regen Med. 2008;2 (8):491–498. doi: 10.1002/term.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beattie AJ, Gilbert TW, Guyot JP, Yates AJ, Badylak SF. Chemoattraction of progenitor cells by remodeling extracellular matrix scaffolds. Tissue Eng Part A. 2009;15(5):1119–1125. doi: 10.1089/ten.tea.2008.0162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Valentin JE, Stewart-Akers AM, Gilbert TW, Badylak SF. Macrophage participation in the degradation and remodeling of extracellular matrix scaffolds. Tissue Eng Part A. 2009;15 (7):1687–1694. doi: 10.1089/ten.tea.2008.0419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Agrawal V, Kelly J, Tottey S, Daly KA, Johnson SA, Siu BF, Reing J, Badylak SF. An isolated cryptic Peptide influences osteogenesis and bone remodeling in an adult Mammalian model of digit amputation. Tissue Eng Part A. 2011;17(23–24):3033–3044. doi: 10.1089/ten.tea.2011.0257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Agrawal V, Tottey S, Johnson SA, Freund JM, Siu BF, Badylak SF. Recruitment of progenitor cells by an extracellular matrix cryptic peptide in a mouse model of digit amputation. Tissue Eng Part A. 2011;17(19–20):2435–2443. doi: 10.1089/ten.tea.2011.0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gilbert TW, Stewart-Akers AM, Simmons-Byrd A, Badylak SF. Degradation and remodeling of small intestinal submucosa in canine Achilles tendon repair. J Bone Joint Surg Am. 2007;89(3):621–630. doi: 10.2106/JBJS.E.00742. [DOI] [PubMed] [Google Scholar]
- 16.Schober A, Weber C. Mechanisms of monocyte recruitment in vascular repair after injury. Antioxid Redox Signal. 2005;7(9–10):1249–1257. doi: 10.1089/ars.2005.7.1249. [DOI] [PubMed] [Google Scholar]
- 17.Yates CC, Bodnar R, Wells A. Matrix control of scarring. Cell Mol Life Sci. 2011;68 (11):1871–1881. doi: 10.1007/s00018-011-0663-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Janis JE, Kwon RK, Lalonde DH. A practical guide to wound healing. Plast Reconstr Surg. 2010;125(6):230e–244e. doi: 10.1097/PRS.0b013e3181d9a0d1. [DOI] [PubMed] [Google Scholar]
- 19.Arnold L, Henry A, Poron F, Baba-Amer Y, van Rooijen N, Plonquet A, Gherardi RK, Chazaud B. Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis. J Exp Med. 2007;204(5):1057–1069. doi: 10.1084/jem.20070075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.LaBarge MA, Blau HM. Biological progression from adult bone marrow to mononucleate muscle stem cell to multinucleate muscle fiber in response to injury. Cell. 2002;111 (4):589–601. doi: 10.1016/s0092-8674(02)01078-4. [DOI] [PubMed] [Google Scholar]
- 21.Palermo AT, Labarge MA, Doyonnas R, Pomerantz J, Blau HM. Bone marrow contribution to skeletal muscle: a physiological response to stress. Dev Biol. 2005;279(2):336–344. doi: 10.1016/j.ydbio.2004.12.024. [DOI] [PubMed] [Google Scholar]
- 22.Lee JY, Qu-Petersen Z, Cao B, Kimura S, Jankowski R, Cummins J, Usas A, Gates C, Robbins P, Wernig A, Huard J. Clonal isolation of muscle-derived cells capable of enhancing muscle regeneration and bone healing. J Cell Biol. 2000;150(5):1085–1100. doi: 10.1083/jcb.150.5.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Relaix F, Rocancourt D, Mansouri A, Buckingham M. A Pax3/Pax7-dependent population of skeletal muscle progenitor cells. Nature. 2005;435(7044):948–953. doi: 10.1038/nature03594. [DOI] [PubMed] [Google Scholar]
- 24.Ten Broek RW, Grefte S, Von den Hoff JW. Regulatory factors and cell populations involved in skeletal muscle regeneration. J Cell Physiol. 2010;224(1):7–16. doi: 10.1002/jcp.22127. [DOI] [PubMed] [Google Scholar]