Abstract
Background
Sexually transmitted co-infections increase HIV infectiousness through local inflammatory processes. The prevalence of STI among people living with HIV/AIDS has implications for containing the spread of HIV in general and the effectiveness of HIV treatments for prevention in particular.
Methods
A systematic review of studies examining STI co-infections in people living with HIV/AIDS. The review focuses on STI contracted after becoming HIV infected. Electronic database and manual searches located clinical and epidemiological studies of STI that increase HIV infectiousness.
Results
Thirty seven studies of STI-HIV co-infection prevalence were located. Studies of adults living with HIV/AIDS from developed and developing countries reported STI rates for 46 different samples (33 samples had clinical/laboratory confirmed STI). The overall mean point-prevalence for confirmed STI was16.3% (SD = 16.4), and median 12.4% STI prevalence in people living with HIV/AIDS. The most common STI studied were Syphilis with median 9.5% prevalence, gonorrhea 9.5%, Chlamydia 5%, and Trichamoniasis 18.8% prevalence. STI prevalence was greatest at the time of HIV diagnosis, reflecting the role of STI in HIV transmission. Prevalence of STI among individuals receiving HIV treatment was not appreciably different from untreated persons.
Conclusions
The prevalence of STI in people infected with HIV suggests that STI co-infections could undermine efforts to use HIV treatments for prevention by increasing genital secretion infectiousness.
Keywords: HIV STI co-infection, test and treat, HIV treatment for prevention, sexual health
Introduction
Sexually transmitted infections (STI) are among the most well established risk factors for HIV infection. STI facilitate HIV transmission by breaching protective mucosal barriers and recruiting susceptible immune cells (e.g., CD4+ T-helper cells, macrophages) to the site of infection. (1) Ulcerative and non-ulcerative STI also create portals of entry for HIV to access susceptible cells. The association between ulcerative STI and HIV transmission is well established with as many as half of newly HIV infected people demonstrating herpes simplex virus-2 (HSV-2) infection. (2) STI can also cause genital bleeding, further increasing the risk for exposure to HIV during sexual activity. (3–4) Trichomoniasis and bacterial vaginosis, for example, can increase the risks for vaginal bleeding more than twelve fold.(5)
Recent research has examined STI in people already infected with HIV. The effects of HIV infection on immunity can increase susceptibility to other STI as individuals who are immune compromised are less able to mount a protective response against sexually transmitted pathogens. (6–7) For example, HIV and HSV-2 co-infections are prevalent and both infections can facilitate acquisition of the other. In a study that illustrates the reciprocal relationship between HIV and HSV-2, Tobian et al. (8) followed 6,396 men in Uganda for two years and found a 1.09% HIV seroconversion rate in the cohort. However, HIV seroconversion was closely associated with HSV-2 seroconversion; more than half (56%) of HIV and HSV-2 infections occurred in the same time-frame. In 25% of cases, HIV infection preceded HSV-2 infection, and in 19% of cases HSV-2 infection preceded HIV transmission.
Sexually transmitted co-infections pose considerable health threats to people living with HIV/AIDS. Syphilis, for example, is related to both increased concentrations of HIV RNA in blood plasma and decreased CD4 cells. (9) Viral STI and genital ulcer diseases, particularly HSV-2, are also linked to increased concentrations of HIV in blood plasma and genital fluids. (10) In addition, viral STI appear to have a bidirectional pathogenic relationship with HIV; HIV can accelerate disease progression of other viral infections and vice versa. (11) When individuals are immune compromised, co-occurring STI are more difficult to treat and symptomatic periods may linger. Finally, local inflammation of the genital tract caused by viral and non-viral STI promotes HIV shedding, therefore increasing HIV infectiousness. (12) Unfortunately, multiple sexually transmitted co-infections are common because the pathogens share transmission routes. (13)
There is growing evidence that antiretroviral therapies (ART) can reduce HIV infectiousness and prevent HIV transmission.(14) Studies have reported that under optimal conditions HIV infected men and women who initiate ART are significantly less likely to infect their HIV negative sex partners. For example, Vernazza (15) found that men treated with a suppressive ART regimen and cleared of other STI had a 98% concordance between HIV RNA in blood plasma and semen; men who had undetectable virus in their blood plasma almost always had undetectable virus in their semen. A study by Donnell et al. (16) reported only one HIV infection among 349 HIV serodiscordant couples in which the infected partner had initiated treatment. In contrast, there were 102 HIV infections observed among the 3,032 couples with untreated HIV positive partners. These findings encourage the use of ART for HIV prevention. However, all participants in studies examining HIV treatment for prevention receive regular and routine STI screening and treatment services. Similarly, mathematical models that forecast the near elimination of HIV epidemics with universal coverage of HIV testing and treatment do not include population or individual level STI prevalence in their models.(17) Given the substantial role that STI co-infection plays in genital tract HIV viral shedding, projections reported by most models of ART for HIV prevention are likely unrealistically optimistic. (18) Thus, the prevalence of sexually transmitted co-infections in HIV infected populations has significant implications for expected outcomes of HIV test and treat programs. (19)
The purpose of this paper is to systematically review the research on prevalence of HIV-STI co-infection. We examined the point and time interval prevalence estimates of HIV-STI co-infection in people living with HIV/AIDS to provide a more realistic context for interpreting studies of the test and treat strategy and mathematical models that forecast the effects of ART on HIV transmission. Our review is based on the premise that increased genital infectiousness resulting from co-occurring STI will diminish the positive effects of reducing community level blood plasma viral load. Our aim is therefore to examine the prevalence of HIV-STI co-infections to better inform efforts to scale-up HIV treatment for prevention programs.
Co-occurring STI and HIV infectiousness
On a population level, concentrations of HIV RNA tend to be lower in genital secretions than blood plasma. However, the relative concentrations of HIV RNA in peripheral blood and genital compartments are reversed when there are co-occurring STI. A review of studies reporting the association between semen and blood plasma concentrations of HIV RNA found an average correlation of .44, with associations ranging between no relationship (r = 0.07) and nearly perfect (98%) concordance. Studies with the highest correspondence between blood plasma viral load and seminal plasma viral load were those which screened and treated men for co-occurring STI. (15) The prevalence of STI and HIV co-infection is therefore likely a significant factor in accounting for the overall low-correspondence between HIV in the genital tract and blood plasma. (20)
HIV concentrations in semen and vaginal fluids are directly associated with number of leukocytes migrating to the genital tract. A dose-relationship exists between leukocyte concentrations, a marker for inflammatory processes, and HIV viral shedding. (21) Various STI differ in their impact on HIV shedding and the same STI can have different effects on HIV concentrations in men and women. Overall the greater the inflammatory response the greater the impact on HIV infectiousness.(21) Gonorrhea and Chlamydia, for example, are associated with high concentrations of leukocytes in the genital tract, and therefore greater HIV shedding. (21) In addition, bacterial vaginosis increases HIV viral shedding by as much as six-fold. (22) Syphilis is associated with HIV shedding in blood plasma as well as the genital tract; blood plasma concentrations of HIV can increase as much as .22 log values prior to Syphilis treatment. (23) Syphilis is also related to decrements in CD4 cell counts. In addition, treating Syphilis improves both blood plasma viral loads and CD4 cells. (24) In contrast, human papillomavirus (HPV) does not significantly impact inflammatory responses in the genital compartment and is not associated with HIV shedding. (25–26) Similarly, Candida infections are not associated with increased HIV shedding. (22) Thus, the STI with greatest impact on HIV shedding are those that produce genital ulcers and urethral/vaginal discharge, specifically Syphilis, Chancroid, gonorrhea, Chlamydia, HSV-2, trichomoniasis, bacterial vaginosis, urethritis, cervicitis, and cervical mucopus. Our review of studies reporting sexually transmitted co-infections therefore focuses on these STI.
Literature Review
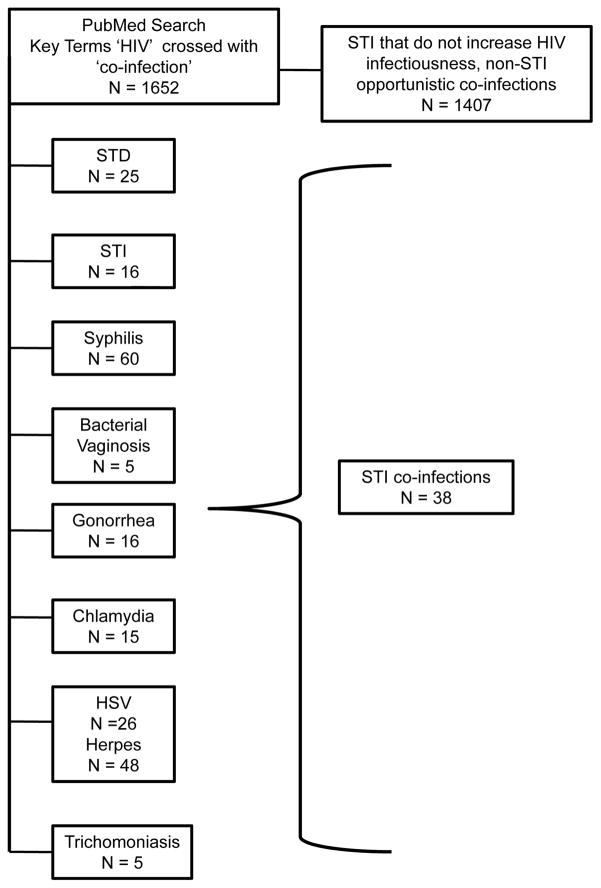
We conducted electronic and manual searches for studies that reported STI co-infections among people diagnosed with HIV. Details on review search strategies are available from the authors. We did not include HPV, Candida and other infections that have not demonstrated increased HIV infectiousness. Studies of antibodies to STI, particularly HSV-2 and Syphilis, do not necessarily reflect recent infection but are included because chronic infections will have a longer lasting effect on community viral load. Our search was restricted to studies reported since 2000 to produce STI prevalence rates relevant to contemporary models that forecast use of HIV treatments for prevention. The literature search was completed August 2010 and identified 1652 studies of HIV co-infections, with most concerning AIDS-related opportunistic co-infections unassociated with HIV infectiousness (e.g., TB, HPV, Hepatitis, etc.) The initial search for HIV co-infection studies was followed by searches within results for key terms, specifically STI diagnoses. We also conducted manual searches of leading relevant journals (e.g., AIDS, JAIDS, Sexually Transmitted Infections, Sexually Transmitted Diseases, AIDS and Behavior, and AIDS Patient Care and STDs). Figure 1 shows the derivation of the 37 studies included in the review.
Figure 1.
Results of automated literature search using key terms ‘HIV’ and ‘co-infection’ followed by manual searches within findings in key journals.
Prevalence of co-occurring STI and HIV
Table 1 shows the summary of 37 studies that report prevalence of STI in people living with HIV/AIDS. Studies that reported separate prevalence rates by gender were examined separately when possible; 21 samples of men, 12 samples of women, and 11 mixed genders. Studies were conducted in Africa (N= 3), Asia (N = 4), Australia (N = 1), Europe (N = 7), North America (N = 19), South America (N = 2), and the Caribbean (N = 1). A total of 708,296 people living with HIV/AIDS were included in the studies, with one study, Manning et al.(27), accounting for 90% of cases (N = 632,264) as it reported time interval prevalence data from STI records of all cases in New York City.
Table 1.
Summary of findings from studies of sexually transmitted co-infections in people living with HIV/AIDS.
| Study Authors, Date | Sample - all HIV+ | % Receiving ART | STI diagnosis/detection | STI point prevalence | STI time-interval prevalence |
|---|---|---|---|---|---|
| Africa and Middle East | |||||
| Machekano et al., 2000 (28) | 666 men factory workers, Zimbabwe | NR | Presentation with urethral discharge, genital ulcer, genital warts, or other | 11% Genital ulcers 5% Urethral discharge |
|
| Aboud et al., 2008 (5) | 2292 pregnant women enrolled in an HIV prevention trial, Malawi, Tanzania, Zambia | NR | External examination, serology, gram-stain | 47.8% bacterial vaginosis 2.6% Chlamydia 2.2% genital ulcer 1.7% gonorrhea 18.8% Trichomoniasis |
|
| Agmon-Levin et al., 2010 (47) | 1060 patients receiving HIV-related care, Israel | NR | Clinical records and serology | 14% syphilis | |
| Asia | |||||
| Zhang et al., 2007(48) | 16 men, VCT center, China | None, initial test | Serology | 31% Chlamydia 50% syphilis |
|
| Park et al., 2008(49) | 539 patients at HIV clinics, Republic of Korea | 100% taking HAART | Serology | 10% early syphilis 3% primary syphilis 7% secondary syphilis |
|
| Lee et al., 2009(50) | 116 men and 7 women attending HIV clinic, China | NR | Self-reported history, medical records, serology and urine screening | 8.9% had active STDs at enrollment | 19.5% new STI since HIV diagnosis |
| Sirivongrangson et al., in press | 131 MSM STI clinic patients, Thailand | 16% | Clinical examination, PCR, serology | 10% Chlamydia 13% Gonorrhea 9% Syphilis reactive 10% Genital ulcers |
|
| Australia | |||||
| Jin et al., 2007 (51) | 235 men in the Positive Health Cohort Australia | NR | Serology, urine and anal screening | 5.9% anal Chlamydia 2.2% urethral Chlamydia 3.2% anal gonorrhea 18.6% Syphilis reactive |
|
| Europe | |||||
| Stolte et al., 2006(52) | 222 men, STD clinic patients, The Netherlands | None | Serology and culture | 15.8% rectal gonorrhea 9.9% infectious Syphilis |
|
| Kofoed et al., 2006(24) | 2200 infectious disease patients, Denmark | 83% of Syphilis patients taking ART | Serology | 2% Syphilis cases diagnosed in 1 year | |
| Dodds et al., 2007(53) | 257 men, venue surveys, 3 cities in UK London n=176 Brighton n= 51 Manchester n=30 |
NR | Self-report STI diagnosis in past year | STI in the past year London 38% Brighton 43% Manchester 35% |
|
| Landes et al. 2007(54) | 1050 pregnant women, seven countries in Europe | 80% antenatal ART | Clinical and laboratory | Diagnosed during pregnancy 1% gonorrhea/Chlamydia 2% syphilis 12% Trichomoniasis |
|
| Diaz et al., 2009 (55) | 1,462 MSM diagnosed with HIV between 2003 and 2007 in Spain | NA | Clinical diagnosis | 31% diagnosed with Chlamydia, gonorrhea or Syphilis at time of testing HIV positive | |
| Branger et al., 2009(32) | 1105 HIV clinic patients with history of syphilis testing, The Netherlands | NR | Serology | 6.2% syphilis (33% asymptomatic) | |
| Dang et al., 2009(56) | 147 men, Swiss HIV Cohort Study, Switzerland | 71% combination ART | Anal swab specimens screened for Chlamydia trachomatis by PCR; Self-reported STI history | 10.9% Chlamydia trachomatis 2.7% gonorrhea 43% Syphilis reactive |
|
| North America | |||||
| Niccolai et al., 2000(57) | 1407 women at public HIV outpatient clinic, USA | NR | Clinical diagnosis Trichomoniasis gonorrhea, chlamydia, and primary or secondary syphilis | 27% a concurrent STI 29% Trichomoniasis 36% re-infected with Trichomoniasis |
|
| Kalichman et al., 2000(58) | 223 men, 112 women, 5 transgender recruited from community services, USA | NR | Self-reported | 3-month retrospective Men 3% Chlamydia 4% Gonorrhea 3% incident HSV 3% syphilis 1% Trichomoniasis Women 4% Chlamydia 6% Gonorrhea 6% incident HSV 4% syphilis 9% Trichomoniasis |
|
| Cu-Uvin et al., 2001(22) | 108 women, research site, USA | 30% untreated 25% Non-HAART 45% HAART |
Clinical diagnosis and culture | 11% bacterial vaginosis 4% trichomoniasis |
|
| Scheer et al., 2001(59) | 11, 516 people living with AIDS in San Francisco data extracted from central STI registry, USA | 79% received HIV therapy | Clinical reports to central registry of Chlamydia, gonorhrhea, non-gonococcal urethritis, Syphilis | 2% diagnosed with STI after AIDS diagnosis Of those with STI 5% Chlamydia 84% gonorhrhea 7% non-gonococcal urethritis 3% syphilis |
|
| Do et al.., 2001(30) | 36,102 patients attending over 100 health clinics, USA between 1991 and 1998 | 62% received HIV therapy | Culture, gram-stain | 1.6% urogenital gonorrhea infection | |
| Cu-Uvin et al., 2002(60) | 871 women in HERS Cohort - patients in health clinics in 4 cities, USA | NR | Microscopic evaluation, culture | 47% Bacterial vaginosis 29% Trichomoniasis estimated |
|
| Whittington et al., 2002(61) | 337 men receiving public health clinic services and recruited from other sites, USA | NR | Serology and urine screening | STD clinics 15% gonorrhea or Chlamydia Other recruitment sites 8% gonorrhea or Chlamydia 2% syphilis |
|
| Erbelding et al., 2003(31) | 796 men and 354 women public STI clinic patients, USA | NA | Clinical records from routine testing services | Co-infection at time of testing Men 14% gonorrhea 19% non-gonococcal urethritis 21% syphilis Women 9% gonorrhea 18% syphilis 20% Trichomoniasis |
|
| Bachmann et al., 2005(62) | 338 men, HIV clinic, USA | 76% HAART | Pharynx and rectal swabs; urine samples, cultures and PCR | 3% Chlamydia 1.5% gonorrhea |
|
| Phipps et al., 2005(63) | 814 primary care HIV patients, USA | NR | Serology, urine screening, pharyngeal and anal swabs | 10% gonorrhea/Chlamydia 1.8% syphilis |
|
| Kahle et al., 2007(64) | 4711 patients at HIV clinics and hospitals, USA | 74% history of HIV treatment | Serology, gram stain, culture, self-report, and referral | 1 year interval 2% Chlamydia 4% gonorrhea 1% syphilis |
|
| Manning et al., 2007(27) | 632,264 people living with HIV/AIDS in New York City data extracted from central STI registry, USA | NR | Clinical reports to central registry | People living with HIV 5% of men 2.5% women People living with AIDS 1.8% men 1.4% women Frequency of diagnoses among people living with HIV/AIDS with two year cumulative STI 20% Chlamydia 42% gonorrhea 31% syphilis 5% multiple STI 16% repeated STI |
|
| Sena et al., 2008(65) | 1460 newly diagnosed men and 2142 newly diagnosed women from public testing sites, USA | NA | Serology and clinical records | 7% Syphilis, men 13% Syphilis, women |
|
| Rieg et al., 2008(37) | 212 men receiving medical care at 2 HIV clinics, USA | NR | Serology, urine screening, self-report | 6% gonorrhea 5% Chlamydia |
|
| Mayer et al., 2009(66) | 398 men screened at a community research site, USA | 66% ART | Medical records of clinical examination, serology, PCR, | STI in past year >1% Chlamydia 3.1% gonorrhea 6.4% Syphilis |
|
| Horberg et al., 2009(67) | 622 patients in an integrated health care system, USA | 51% treated | Laboratory database searched for serological test results | 12.4% syphilis | |
| Kalichman et al., 2009(68) | 320 men, 137 women, 33 transgender recruited from community services, USA | 71% treated | Self-reported STI | STI diagnosis in past 6-month 14% new Chlamydia, gonorrhea, HSV, or syphilis STI diagnosis since testing HIV+ 20% Chlamydia 13% gonorrhea 37% HSV 36% syphilis |
|
| Romanowski et al., 2009(69) | 455 men, 174 women infectious disease clinic patients, Canada | 77% men, 60% women HAART | HSV-1 and HSV-2 serology | 78% HSV-1 positive 54% HSV-2 positive 58% of HSV-2positive without genital herpes symptoms |
|
| McCoy et al., 2009(6) | 56 men and 19 women with acute HIV infection, USA | NA | Clinical confirmed diagnosis | 30.6% Co-infected with STI Men > 1% Chlamydia 12% gonorrhea 7% syphilis Women 15% Bacterial vaginosis 10% Chlamydia 10% gonorrhea 26% Trichomoniasis |
|
| South America | |||||
| Griemberg et al., 2006 (70) | 87 HIV positive patients detected in clinics/hospitals, Argentina | NA | Clinical diagnosis | 58% Syphilis reactive at time of testing | |
| Grinsztejn et al., 2006 (71) | 458 women receiving care, Brazil | 67% | Clinical diagnosis | 7.0% Trichomoniasis 0.9% Neisseria gonorrhoeae 3.0% Chlamydia trachomatis 3.1% Herpes Simplex Virus 13.8% Syphilis 22.8% Bacterial vaginosis |
|
| Caribbean | |||||
| Hutton-Rose et al., 2008 (72) | 138 men and 132 women referral clinic, Jamaica | NR | History, clinical examination, and lab diagnosis | Men 4% chancroid 19% gonorrhea 19% non-gonococcal urethritis 8% syphilis 9% Trichomoniasis 14% genital ulcer Women 10% Bacterial vaginosis 2% chancroid 15% gonorrhea 19% non-gonococcal urethritis 6% syphilis 15% Trichomoniasis 7% genital ulcer |
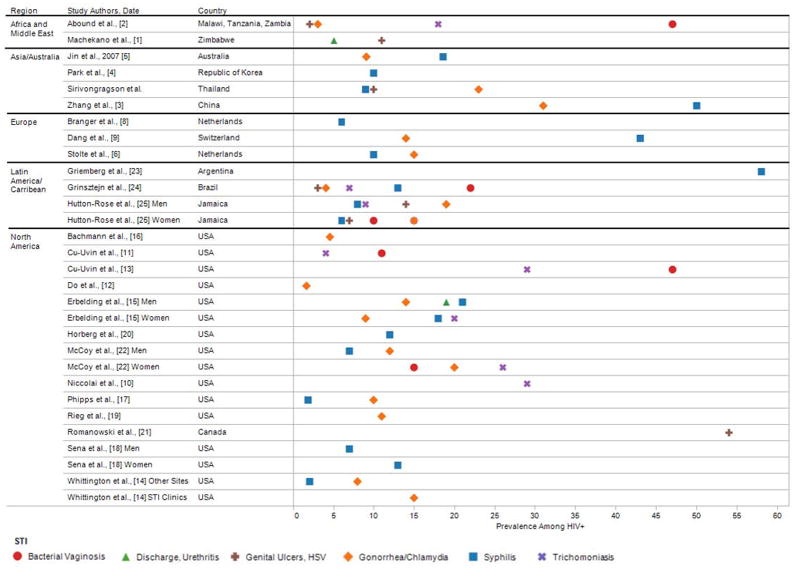
Overall, the studies demonstrated a mean point prevalence STI co-infection of 16.3% (SD = 16.4, median = 12.4%). Figure 2 shows the point prevalence of individually diagnosed STI segmented by geographical region. The most common STI studied were Syphilis with median 9.5% prevalence, gonorrhea 9.5%, Chlamydia 5%, and Trichamoniasis 18.8%. The figure shows that the highest point prevalence rates were for syphilis, genital ulcers - which included Herpes Simplex virus, and bacterial vaginosis. For studies reporting point prevalence, the mean STI in Africa was 11.3 (SD = 4.6), Asia 17.4 (SD = 15.3), Europe 14.7 (SD = 10.6), and North America 16.1 (SD = 18.4). STI prevalence was similar for men (13.6%, SD = 10.3), women (15.8%, SD=9.9), t(23) = 0.5, n.s., and somewhat higher in mixed gender samples (24.0%, SD=30.6). Reports of time interval STI had a mean prevalence of 16.9 (SD = 13.88, median = 14.0).
Figure 2.
Prevalence of specific STI that have significant effects on HIV genital shedding reported in studies of people living with HIV/AIDS.
Sexually transmitted co-infections over the course of HIV disease
The greatest prevalence of HIV/STI co-infections occur among individuals newly diagnosed with HIV. (28) Studies of people who tested HIV positive at the time of STI testing found an average STI prevalence of 19.6%. These studies reflect the importance of STI as reliable risk factors for HIV transmission. (29–30) New STI are also often contracted relatively soon after HIV transmission. Erbelding et al. (31) reported that men contracted a new STI within 415 days and women within 176 days of their HIV diagnosis. However, studies show that STI co-infections occur throughout the years of HIV infection, not just at the time of, or soon after, HIV seroconversion. The average STI prevalence among HIV clinic patients, most of which have been living with HIV for some time, was 14%.
People receiving HIV treatment also demonstrated significant prevalence of STI co-infections. A total of 14 samples reported ART exposure, with a mean proportion of samples taking ART 67.4% (SD = 19.9, median 71%). The overall STI point prevalence in the studies that reported participants receiving ART was 16.2% (SD = 23.7) compared to 16.5% (SD = 13.3) among the studies that did not report ART use, a non-significant difference, t(31) = 0.9, n.s. Among the samples that reported ART use, there was no association between percent of the sample using ART and prevalence of STI co-infections, r = .013, n.s. Studies also showed relatively stable rates of gonorrhea infections among people infected with HIV in the US during the late 1990s when ART was being prescribed early in the course of HIV infection, with diagnoses ranging from 7.6 cases per 1000 person years to 14.3 per 1000 person years.(30)
Methodological limitations of studies of STI among people living with HIV/AIDS
The literature on STI and HIV co-infection over-represents countries in North America and Europe, regions with relatively low HIV prevalence. The few studies conducted in Africa and Asia therefore limits the usefulness of the literature in informing models that estimate the impact of test and treat programs in developing countries. Several studies reported aggregate STI that were not the focus of the current review. Rather, we focused our review on studies of STI that are known to increase HIV infectiousness. The literature is also limited by mixed gender samples that do not report gender separate estimates. In addition, STI rates that rely on self-reported symptoms over time intervals are non-specific and were not included. Also of concern are asymptomatic STI that can lead to underestimates of HIV-STI co-infections; one third of Syphilis infections in people living with HIV/AIDS are asymptomatic. (32) Asymptomatic STI are especially concerning because these infections worsen and increase HIV infectiousness when untreated, and do not alert the person to reduce risk practices.
Estimates of time interval STI were difficult to interpret because studies used a range of periods. It is also unknown if point prevalence estimates stemming from different data sources, such as chart abstraction, clinical diagnosis, and laboratory confirmation yield varying prevalence estimates. The literature is also limited by incomplete reporting of HIV disease stage and exposure to ART. The literature is therefore hampered by non-standardized procedures and allow only for a partial picture of the prevalence of STI and HIV co-infection.
Implications of STI co-infections for using treatment for HIV prevention
While HIV treatments reduce blood plasma viral load and may reduce infectiousness, ART does not eliminate risks for HIV transmission. HIV shedding in the genital tract is well documented in men and women who have undetectable blood plasma viral load.(33) STI among people receiving ART further increase viral shedding and infectiousness. Studies show that the number of people living with HIV who are STI co-infected has increased over the years since access to ART has improved. (34) In addition, evidence that ART reduces infectiousness in persons who are HIV-STI co-infected is mixed. Some studies show that ART does not attenuate the association between STI and HIV shedding. (22) In contrast, Sadiq et al. (35) found that men with urethritis who were not treated with ART had a five-fold increase in semen concentrations of HIV RNA relative to men receiving ART.
Assuring that ART reduces HIV infectiousness will require aggressive behavioral interventions that include STI screening and treatment for all sexually active HIV infected persons. Treating STI has repeatedly demonstrated reductions in genital tract HIV RNA. Wang et al. (36) for example reported a 3.2 fold reduction in HIV concentrations following treatment of vaginal infections. However, infrequent screening for STI in sexually active people living with HIV will likely prove insufficient because of the amount of time that people with undetected STI remain infectious. It is also insufficient to rely on sexually active persons to self-detect STI symptoms because as many as one in four STI in people with HIV/AIDS are asymptomatic. (37–39)
Sporadic patterns of sexual behavior demand a broader definition of patients for STI screening. Clinical visits for monitoring blood plasma viral load in response to HIV treatment offer an opportunity for frequent routine STI screening. Although treatment guidelines are regularly revised, the standard of care is to routinely monitor viral load for people on ART every three or four months in order to detect viral rebound prior to the onset of drug resistance.(40) All patients who receive ART for HIV prevention should therefore receive routine STI screening in the same monitoring time-frame, every three to four months. In addition, patients receiving HIV treatment for prevention should receive repeated counseling to address erroneous beliefs regarding infectiousness. Risk compensation beliefs are common in response to viral suppression and must be addressed to avoid increases in risk behaviors.(41) Patients should also routinely be offered condoms when receiving HIV treatment for prevention. Clinical guidelines should therefore be enhanced to recommend frequent and routine STI screening in the context of using HIV treatment for prevention.
The findings from this review also have implications for mathematical models that forecast the effects of universal HIV testing and treatment for HIV prevention. Models that demonstrate the potential for ART to change the trajectory of HIV epidemics have not included the impact of STI co-infections on infectiousness (42) and are therefore overly optimistic in their projections.(43–45) Overall, the potential for ART to reduce genital tract infectiousness will be impeded for at least 15% of persons receiving treatment who contract a new STI. In addition, estimates of treatment effects on HIV transmission vary for vaginal and anal intercourse suggesting that the differential impact of STI will also affect estimates of infectiousness by route of HIV transmission.(46) In addition, economic analyses of the cost-effectiveness of HIV treatment for prevention have not considered the costs for STI screening and treatment. Failure to address ongoing STI-HIV co-epidemics will therefore undermine the potential benefits of using HIV treatment for prevention.
Key Messages.
Sexually transmitted co-infections are prevalent among people living with HIV/AIDS.
High-rates of co-occurring sexually transmitted infections in people living with HIV/AIDS will impede efforts to prevent HIV transmission by using HIV treatments to reduce infectiousness.
Sexual risk reduction interventions are needed for people living with HIV/AIDS to control sexually transmitted co-infections and reduce infectiousness.
Acknowledgments
This project was supported by grants from the National Institute of Mental Health (NIMH) grants R01-MH71164 and R01-MH82633 and National Institute of Alcohol Abuse and Alcoholism RC1AA018983.
Footnotes
Competing Interest: None declared.
License for Publication
The Corresponding Author has the right to grant on behalf of all authors and does grant on behalf of all authors, an exclusive license (or non exclusive for government employees) on a worldwide basis to the BMJ Publishing Group Ltd to permit this article (if accepted) to be published in STI and any other BMJPGL products and sublicenses such use and exploit all subsidiary rights, as set out in our license http://group.bmj.com/products/journals/instructions-for-authors/licence-forms.”
References
- 1.Ward H, Ronn M. Contribution of sexually transmitted infections to the sexual transmission of HIV. Curr Opin HIV AIDS. 2010 Jul;5(4):305–10. doi: 10.1097/COH.0b013e32833a8844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barbour JD, Sauer MM, Sharp ER, et al. HIV-1/HSV-2 co-infected adults in early HIV-1 infection have elevated CD4+ T cell counts. PLoS ONE. 2007;2(10):e1080. doi: 10.1371/journal.pone.0001080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Royce R, Sena A, Cates W, et al. Sexual transmission of HIV. N Engl J Med. 1997;336:1072–8. doi: 10.1056/NEJM199704103361507. [DOI] [PubMed] [Google Scholar]
- 4.Boily MC, Baggley R, Wang L, et al. Heterosexual risk of HIV-1 infection per sexual act: systematic review and meta-analysis of observational studies. Lancet. 2009;9(2):118–29. doi: 10.1016/S1473-3099(09)70021-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aboud S, Msamanga G, Read JS, et al. Genital tract infections among HIV-infected pregnant women in Malawi, Tanzania and Zambia. Int J STD AIDS. 2008 Dec;19(12):824–32. doi: 10.1258/ijsa.2008.008067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McCoy SI, Eron JJ, Kuruc JD, et al. Sexually transmitted infections among patients with acute HIV in North Carolina. Sex Transm Dis. 2009 Jun;36(6):372–4. doi: 10.1097/OLQ.0b013e3181997252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cohen MS. When people with HIV get syphilis: triple jeopardy. Sex Transm Dis. 2006 Mar;33(3):149–50. doi: 10.1097/01.olq.0000204530.19762.e4. [DOI] [PubMed] [Google Scholar]
- 8.Tobian AA, Serwadda D, Quinn TC, et al. Male circumcision for the prevention of HSV-2 and HPV infections and syphilis. N Engl J Med. 2009 Mar 26;360(13):1298–309. doi: 10.1056/NEJMoa0802556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buchacz K, Patel P, Taylor M, et al. Syphilis increases HIV viral load and decreases CD4 cell counts in HIV-infected patients with new syphilis infections. AIDS. 2004 Oct 21;18(15):2075–9. doi: 10.1097/00002030-200410210-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duffus WA, Mermin J, Bunnell R, et al. Chronic herpes simplex virus type-2 infection and HIV viral load. Int J STD AIDS. 2005 Nov;16(11):733–5. doi: 10.1258/095646205774763298. [DOI] [PubMed] [Google Scholar]
- 11.White MK, Gorrill TS, Khalili K. Reciprocal transactivation between HIV-1 and other human viruses. Virology. 2006 Aug 15;352(1):1–13. doi: 10.1016/j.virol.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 12.Cohen MS, Hoffman IF, Royce RA, et al. Reduction of concentration of HIV-1 in semen after treatment of urethritis: implications for prevention of sexual transmission of HIV-1. Lancet. 1997;349:1868–73. doi: 10.1016/s0140-6736(97)02190-9. [DOI] [PubMed] [Google Scholar]
- 13.Mehta SD, Ghanem KG, Rompalo AM, et al. HIV seroconversion among public sexually transmitted disease clinic patients: analysis of risks to facilitate early identification. J Acquir Immune Defic Syndr. 2006 May;42(1):116–22. doi: 10.1097/01.qai.0000200662.40215.34. [DOI] [PubMed] [Google Scholar]
- 14.Dieffenbach CW, Fauci AS. Universal voluntary testing and treatment for prevention of HIV transmission. JAMA. 2009 Jun 10;301(22):2380–2. doi: 10.1001/jama.2009.828. [DOI] [PubMed] [Google Scholar]
- 15.Vernazza PL, Troiani L, Flepp MJ, et al. Potent antiretroviral treatment of HIV infection results in suppression of the seminal shedding of HIV. AIDS. 2000;14(2):117–21. doi: 10.1097/00002030-200001280-00006. [DOI] [PubMed] [Google Scholar]
- 16.Donnell DBJ, Kiarie K, et al. Heterosexual HIV-1 transmission after initiation of antiretroviral therapy: A prospective cohort analysis. Lancet. 2010 doi: 10.1016/S0140-6736(10)60705-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lima VD, Hogg RS, Montaner JS. Expanding HAART treatment to all currently eligible individuals under the 2008 IAS-USA Guidelines in British Columbia, Canada. PLoS ONE. 2010;5(6):e10991. doi: 10.1371/journal.pone.0010991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cohen MS, Gay CL. Treatment to prevent transmission of HIV-1. Clin Infect Dis. 2010 May 15;50( Suppl 3):S85–95. doi: 10.1086/651478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kalichman S, Eaton L. Strategies for Preventing HIV Transmission. JAMA. 2009 Oct 14;302(14):1531. doi: 10.1001/jama.2009.1443. author reply -2. [DOI] [PubMed] [Google Scholar]
- 20.Chakraborty H, Sen PK, Helms RW, et al. Viral burden in genital secretions determines male-to-female sexual transmission of HIV-1: a probabilistic empiric model. AIDS. 2001 Mar 30;15(5):621–7. doi: 10.1097/00002030-200103300-00012. [DOI] [PubMed] [Google Scholar]
- 21.Johnson LF, Lewis DA. The effect of genital tract infections on HIV-1 shedding in the genital tract: a systematic review and meta-analysis. Sex Transm Dis. 2008 Nov;35(11):946–59. doi: 10.1097/OLQ.0b013e3181812d15. [DOI] [PubMed] [Google Scholar]
- 22.Cu-Uvin S, Hogan JW, Caliendo AM, et al. Association between bacterial vaginosis and expression of human immunodeficiency virus type 1 RNA in the female genital tract. Clin Infect Dis. 2001 Sep 15;33(6):894–6. doi: 10.1086/322613. [DOI] [PubMed] [Google Scholar]
- 23.Buchacz K, Klausner JD, Kerndt PR, et al. HIV incidence among men diagnosed with early syphilis in Atlanta, San Francisco, and Los Angeles, 2004 to 2005. J Acquir Immune Defic Syndr. 2008 Feb 1;47(2):234–40. [PubMed] [Google Scholar]
- 24.Kofoed K, Gerstoft J, Mathiesen LR, et al. Syphilis and human immunodeficiency virus (HIV)-1 coinfection: influence on CD4 T-cell count, HIV-1 viral load, and treatment response. Sex Transm Dis. 2006 Mar;33(3):143–8. doi: 10.1097/01.olq.0000187262.56820.c0. [DOI] [PubMed] [Google Scholar]
- 25.Muller EE, Chirwa TF, Lewis DA. Human papillomavirus (HPV) infection in heterosexual South African men attending sexual health services: associations between HPV and HIV serostatus. Sex Transm Infect. 2010 Jun;86(3):175–80. doi: 10.1136/sti.2009.037598. [DOI] [PubMed] [Google Scholar]
- 26.Smith JS, Moses S, Hudgens MG, et al. Increased risk of HIV acquisition among Kenyan men with human papillomavirus infection. J Infect Dis. 2010 Jun 1;201(11):1677–85. doi: 10.1086/652408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Manning SE, Pfeiffer MR, Nash D, et al. Incident sexually transmitted infections among persons living with diagnosed HIV/AIDS in New York City, 2001–2002: a population-based assessment. Sex Transm Dis. 2007 Dec;34(12):1008–15. doi: 10.1097/OLQ.0b013e3180eaa243. [DOI] [PubMed] [Google Scholar]
- 28.Machekano RN, Bassett MT, Zhou PS, et al. Report of sexually transmitted diseases by HIV infected men during follow up: time to target the HIV infected? Sex Transm Infect. 2000 Jun;76(3):188–92. doi: 10.1136/sti.76.3.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Torian LV, Makki HA, Menzies IB, et al. High HIV seroprevalence associated with gonorrhea: New York City Department of Health, sexually transmitted disease clinics, 1990–1997. AIDS. 2000 Jan 28;14(2):189–95. doi: 10.1097/00002030-200001280-00015. [DOI] [PubMed] [Google Scholar]
- 30.Do AN, Hanson DL, Dworkin MS, et al. Risk factors for and trends in gonorrhea incidence among persons infected with HIV in the United States. AIDS. 2001 Jun 15;15(9):1149–55. doi: 10.1097/00002030-200106150-00010. [DOI] [PubMed] [Google Scholar]
- 31.Erbelding EJ, Chung SE, Kamb ML, et al. New sexually transmitted diseases in HIV-infected patients: markers for ongoing HIV transmission behavior. J Acquir Immune Defic Syndr. 2003 Jun 1;33(2):247–52. doi: 10.1097/00126334-200306010-00021. [DOI] [PubMed] [Google Scholar]
- 32.Branger J, van der Meer JT, van Ketel RJ, et al. High incidence of asymptomatic syphilis in HIV-infected MSM justifies routine screening. Sex Transm Dis. 2009 Feb;36(2):84–5. doi: 10.1097/OLQ.0b013e318186debb. [DOI] [PubMed] [Google Scholar]
- 33.Cu-Uvin S, Delong AK, Venkatesh KK, et al. Genital tract HIV-1 RNA shedding among women with below detectable plasma viral load. AIDS. 2010 Aug 25; doi: 10.1097/QAD.0b013e32833e5043. [DOI] [PubMed] [Google Scholar]
- 34.Scheer S, Chu PL, Klausner JD, et al. Effect of highly active antiretroviral therapy on diagnoses of sexually transmitted diseases in people with AIDS. Lancet. 2001 Feb 10;357(9254):432–5. doi: 10.1016/S0140-6736(00)04007-1. [DOI] [PubMed] [Google Scholar]
- 35.Sadiq ST, Taylor S, Copas AJ, et al. The effects of urethritis on seminal plasma HIV-1 RNA loads in homosexual men not receiving antiretroviral therapy. Sexually Transmitted Diseases. 2005;81:120–3. doi: 10.1136/sti.2004.010249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang CC, McClelland RS, Reilly M, et al. The effect of treatment of vaginal infections on shedding of human immunodeficiency virus type 1. J Infect Dis. 2001 Apr 1;183(7):1017–22. doi: 10.1086/319287. [DOI] [PubMed] [Google Scholar]
- 37.Rieg G, Lewis RJ, Miller LG, et al. Asymptomatic sexually transmitted infections in HIV-infected men who have sex with men: prevalence, incidence, predictors, and screening strategies. AIDS Patient Care STDs. 2008 Dec;22(12):947–54. doi: 10.1089/apc.2007.0240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Winter AJ, Taylor S, Workman J, et al. Asymptomatic urethritis and detection of HIV-1 RNA in seminal plasma. Sex Transm Dis. 1999;75:261–3. doi: 10.1136/sti.75.4.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moreira C, Venkatesh KK, DeLong A, et al. Effect of treatment of asymptomatic bacterial vaginosis on HIV-1 shedding in the genital tract among women on antiretroviral therapy: a pilot study. Clin Infect Dis. 2009 Sep 15;49(6):991–2. doi: 10.1086/605540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Volberding PA, Deeks SG. Antiretroviral therapy and management of HIV infection. Lancet. 2010 Jul 3;376(9734):49–62. doi: 10.1016/S0140-6736(10)60676-9. [DOI] [PubMed] [Google Scholar]
- 41.Eaton LA, Kalichman S. Risk compensation in HIV prevention: implications for vaccines, microbicides, and other biomedical HIV prevention technologies. Curr HIV/AIDS Rep. 2007 Dec;4(4):165–72. doi: 10.1007/s11904-007-0024-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Boily MC, Bastos FI, Desai K, et al. Changes in the transmission dynamics of the HIV epidemic after wide-scale use of antiretroviral therapy could explain increases in sexually transmitted infections. Sexually Transmitted Diseases. 2004;31:100–13. doi: 10.1097/01.OLQ.0000112721.21285.A2. [DOI] [PubMed] [Google Scholar]
- 43.Granich RM, Gilks CF, Dye C, et al. Universal voluntary HIV testing with immediate antiretroviral therapy as a strategy for elimination of HIV transmission: a mathematical model. Lancet. 2009;373(9657):48–57. doi: 10.1016/S0140-6736(08)61697-9. [DOI] [PubMed] [Google Scholar]
- 44.Wilson DP, Law MG, Grulich AE, et al. Relation between HIV viral load and infectiousness: a model-based analysis. Lancet. 2008;372(9635):314–20. doi: 10.1016/S0140-6736(08)61115-0. [DOI] [PubMed] [Google Scholar]
- 45.Blower SM, Gershengorn HB, Grant RM. A tale of two futures: HIV and antiretroviral therapy in San Francisco. Science. 2000;287:650–654. doi: 10.1126/science.287.5453.650. [DOI] [PubMed] [Google Scholar]
- 46.Baggaley RF, White RG, Boily MC. HIV transmission risk through anal intercourse: systematic review, meta-analysis and implications for HIV prevention. Int J Epidemiol. 2010 Aug;39(4):1048–63. doi: 10.1093/ije/dyq057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Agmon-Levin N, Elbirt D, Asher I, et al. Syphilis and HIV co-infection in an Israeli HIV clinic: incidence and outcome. Int J STD AIDS. 2010 Apr;21(4):249–52. doi: 10.1258/ijsa.2009.009011. [DOI] [PubMed] [Google Scholar]
- 48.Zhang X, Wang C, Hengwei W, et al. Risk factors of HIV infection and prevalence of co-infections among men who have sex with men in Beijing, China. AIDS. 2007 Dec;21( Suppl 8):S53–7. doi: 10.1097/01.aids.0000304697.39637.4c. [DOI] [PubMed] [Google Scholar]
- 49.Park WB, Jang HC, Kim SH, et al. Effect of highly active antiretroviral therapy on incidence of early syphilis in HIV-infected patients. Sex Transm Dis. 2008 Mar;35(3):304–6. doi: 10.1097/OLQ.0b013e31815b0148. [DOI] [PubMed] [Google Scholar]
- 50.Lee HC, Lee NY, et al. Trends in sexually transmitted diseases and risky behaviors among HIV-infected patients at an outpatient clinic in souther Taiwan. Sex Transm Dis. 2009;36(12) doi: 10.1097/OLQ.0b013e3181bd8301. [DOI] [PubMed] [Google Scholar]
- 51.Jin F, Prestage GP, Zablotska I, et al. High rates of sexually transmitted infections in HIV positive homosexual men: data from two community based cohorts. Sex Transm Infect. 2007 Aug;83(5):397–9. doi: 10.1136/sti.2007.025684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stolte IG, de Wit JB, Kolader M, et al. Association between ‘safer sex fatigue’ and rectal gonorrhea is mediated by unsafe sex with casual partners among HIV-positive homosexual men. Sex Transm Dis. 2006 Apr;33(4):201–8. doi: 10.1097/01.olq.0000194596.78637.8e. [DOI] [PubMed] [Google Scholar]
- 53.Dodds JP, Johnson AM, Parry JV, et al. A tale of three cities: persisting high HIV prevalence, risk behaviour and undiagnosed infection in community samples of men who have sex with men. Sex Transm Infect. 2007 Aug;83(5):392–6. doi: 10.1136/sti.2006.021782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Landes M, Thorne C, Barlow P, et al. Prevalence of sexually transmitted infections in HIV-1 infected pregnant women in Europe. Eur J Epidemiol. 2007;22(12):925–36. doi: 10.1007/s10654-007-9188-0. [DOI] [PubMed] [Google Scholar]
- 55.Diaz A, Junquera ML, Esteban V, et al. HIV/STI co-infection among men who have sex with men in Spain. Euro Surveill. 2009;14(48) doi: 10.2807/ese.14.48.19426-en. [DOI] [PubMed] [Google Scholar]
- 56.Dang T, Jaton-Ogay K, Flepp M, et al. High prevalence of anorectal chlamydial infection in HIV-infected men who have sex with men in Switzerland. Clin Infect Dis. 2009;49(10):1532–5. doi: 10.1086/644740. [DOI] [PubMed] [Google Scholar]
- 57.Niccolai LM, Kopicko JJ, Kassie A, et al. Incidence and predictors of reinfection with Trichomonas vaginalis in HIV-infected women. Sex Transm Dis. 2000 May;27(5):284–8. doi: 10.1097/00007435-200005000-00009. [DOI] [PubMed] [Google Scholar]
- 58.Kalichman SC, Rompa D, Cage M. Sexually transmitted infections among HIV seropositive men and women. Sex Transm Infect. 2000 Oct;76(5):350–4. doi: 10.1136/sti.76.5.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Scheer S, McQuitty M, Denning P, et al. Undiagnosed and unreported AIDS deaths: results from the San Francisco Medical Examiner. J Acquir Immune Defic Syndr. 2001 Aug 15;27(5):467–71. doi: 10.1097/00126334-200108150-00008. [DOI] [PubMed] [Google Scholar]
- 60.Cu-Uvin S, Ko H, Jamieson DJ, et al. Prevalence, incidence, and persistence or recurrence of trichomoniasis among human immunodeficiency virus (HIV)-positive women and among HIV-negative women at high risk for HIV infection. Clin Infect Dis. 2002 May 15;34(10):1406–11. doi: 10.1086/340264. [DOI] [PubMed] [Google Scholar]
- 61.Whittington WL, Collis T, Dithmer-Schreck D, et al. Sexually transmitted diseases and human immunodeficiency virus-discordant partnerships among men who have sex with men. Clin Infect Dis. 2002 Oct 15;35(8):1010–7. doi: 10.1086/342693. [DOI] [PubMed] [Google Scholar]
- 62.Bachmann LH, Grimley DM, Waithaka Y, et al. Sexually transmitted disease/HIV transmission risk behaviors and sexually transmitted disease prevalence among HIV-positive men receiving continuing care. Sex Transm Dis. 2005 Jan;32(1):20–6. doi: 10.1097/01.olq.0000148293.81774.e4. [DOI] [PubMed] [Google Scholar]
- 63.Phipps W, Stanley H, Kohn R, et al. Syphilis, chlamydia, and gonorrhea screening in HIV-infected patients in primary care, San Francisco, California, 2003. AIDS Patient Care STDs. 2005 Aug;19(8):495–8. doi: 10.1089/apc.2005.19.495. [DOI] [PubMed] [Google Scholar]
- 64.Kahle E, Zhang Q, Golden M, et al. Trends in evaluation for sexually transmitted infections among HIV-infected people, King County, Washington. Sex Transm Dis. 2007 Dec;34(12):940–6. doi: 10.1097/olq.0b013e31813e0a48. [DOI] [PubMed] [Google Scholar]
- 65.Sena AC, Torrone EA, Leone PA, et al. Endemic early syphilis among young newly diagnosed HIV-positive men in a southeastern U.S state. AIDS Patient Care STDs. 2008 Dec;22(12):955–63. doi: 10.1089/apc.2008.0077. [DOI] [PubMed] [Google Scholar]
- 66.Mayer KH, O’Cleirigh C, Skeer M, et al. Which HIV-infected men who have sex with men in care are engaging in risky sex and acquiring sexually transmitted infections: findings from a Boston community health centre. Sex Transm Infect. 2010 Feb;86(1):66–70. doi: 10.1136/sti.2009.036608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hoerberg MARD, Quesenberry CP. Syphilis epidemiology and clinical outcomes in HIV-infected and HIV-uninfected patients in Kaiser Permanente Northern California. Sex Transm Dis. 2009:36. doi: 10.1097/OLQ.0b013e3181b6f0cc. [DOI] [PubMed] [Google Scholar]
- 68.Kalichman SC, Eaton L, Cherry C. Sexually transmitted infections and infectiousness beliefs among people living with HIV/AIDS: implications for HIV treatment as prevention. HIV Med. 2010 Mar 1; doi: 10.1111/j.1468-1293.2009.00818.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Romanowski B, Myziuk LN, Walmsley SL, et al. Seroprevalence and Risk Factors for Herpes Simplex Virus Infection in a Population of HIV-Infected Patients in Canada. Sex Transm Dis. 2008 Dec 18; doi: 10.1097/OLQ.0b013e31818d3fb6. [DOI] [PubMed] [Google Scholar]
- 70.Griemberg G, Bautista CT, Pizzimenti MC, et al. High prevalence of syphilis-HIV co-infection at four hospitals of the city of Buenos Aires, Argentina. Rev Argent Microbiol. 2006 Jul-Sep;38(3):134–6. [PubMed] [Google Scholar]
- 71.Grinsztejn B, Bastos FI, Veloso VG, et al. Assessing sexually transmitted infections in a cohort of women living with HIV/AIDS, in Rio de Janeiro, Brazil. Int J STD AIDS. 2006 Jul;17(7):473–8. doi: 10.1258/095646206777689071. [DOI] [PubMed] [Google Scholar]
- 72.Hutton-Rose N, Blythe C, Ogbonna C, et al. The prevalence of other sexually transmitted infections in confirmed HIV cases at a referral clinic in Jamaica. J R Soc Promot Health. 2008 Sep;128(5):242–7. doi: 10.1177/1466424008092799. [DOI] [PubMed] [Google Scholar]