Abstract
A mimotope is an antibody-epitope-mimicking peptide retrieved from a phage display random peptide library. Immunization with antitumor antibody-derived mimotopes is promising for inducing antitumor immunity in hosts. In this study, we isolated linear and constrained mimotopes from HBJ127, a tumor-suppressing anti-CD98 heavy chain mAb, and determined their abilities for induction of antitumor activity equal to that of the parent antibody. We detected elevated levels of antipeptide responses, but failed to detect reactivity against native CD98-expressing HeLa cells in sera of immunized mice. Phage display panning and selection of mimotope-immunized mouse spleen-derived antibody Fab library showed that HeLa cell-reactive Fabs were successfully retrieved from the library. This finding indicates that native antigen-reactive Fab clones represented an undetectable minor population in mimotope-induced antibody repertoire. Functional and structural analysis of retrieved Fab clones revealed that they were almost identical to the parent antibody. From these results, we confirmed that mimotope immunization was promising for retrieving antitumor antibodies equivalent to the parent antibody, although the co-administration of adjuvant compounds such as T-cell epitope peptides and Toll-like receptor 4 agonist peptides is likely to be necessary for inducing stronger antitumor immunity than mimotope injection alone.
Keywords: CD98, mimotope, monoclonal antibody, phage display, recombinant Fab
Antibody medicines are widely used for treatment of tumors, autoimmune disorders, and infectious diseases. In tumor therapy, representatives are trastuzumab(1) and rituximab,(2) which are used for the treatment of c-erbB2-positive breast cancers and CD20-positive B-cell lymphoma, respectively. In rheumatoid arthritis therapy, an anti-tumor necrosis factor-α antibody, infliximab,(3) is used for treatment of methotrexate-unresponsive patients. Although antibody medicines show excellent efficacy in therapy of such diseases, they have some disadvantages, for example, repeated injection maybe necessary for maintaining efficacy, risk of anaphylaxis, and decreased effectiveness due to the anti-antibody response in the hosts. They are also very expensive medicines.
The development of new medicines having efficacy equivalent to antibody medicines without such drawbacks is in progress. One candidate is a peptide vaccine designed based on the epitope or epitope-mimicking (mimotope) sequence of antibody medicines or antibodies with biological activities. The goal is the induction of protective immunity by active immunization of the hosts with epitope or mimotope vaccines. Peptides identical to or mimicking epitope sequences obtained using phage display peptide libraries from cetuximab,(4) rituximab,(5) trastuzumab,(6) and anti-GD2 mAb(7) have successfully induced protective immunity in hosts. We have identified the epitope of the tumor-suppressive anti-CD98 mAb HBJ127(8) using a phage display peptide library(9) and evaluated HBJ127-derived epitope peptides for induction of antitumor immunity.(10)
CD98 was originally identified as a cell surface antigen associated with lymphocyte activation defined by 4F2 mAb;(11) it is expressed in proliferating normal tissues(12) and in almost all tumor cells.(8) CD98 heavy chain (hc) is a type II transmembrane glycoprotein(13) that is disulfide-linked to a non-glycosylated light chain of a member of the permease family.(14) It has been reported that CD98 was functionally involved in lymphocyte activation, cell proliferation, and malignant transformation. HBJ127, used in the present study, has been shown to inhibit tumor cell growth(15) and lymphocyte proliferation.(16) CD98 hc cDNA-transfected murine fibroblasts showed various malignant phenotypes.(17) In the present study, we evaluated the linear and cyclic peptides prepared from mimotope sequences of HBJ127 for induction of antitumor immunity. Linear mimotope sequences have been identified in our previous report.(9) A cyclic mimotope sequence was identified in this study by panning of phage display peptide libraries expressing disulfide-bond constrained cyclic heptamer amino acids against HBJ127. We first characterized the linear and cyclic peptide mimotope-immunized mice sera. We next constructed a Fab phage display library from peptide-immunized mice spleen cells and then retrieved and characterized recombinant Fab (rFab) clones reactive with native CD98 on tumor cells. Although both linear and cyclic peptides failed to induce sufficient antitumor activity in serum, the presence of HBJ127-like antibody repertoire was confirmed in the mimotope-immunized mice.
Materials and Methods
Cell lines
HeLa cells(18) were used for panning and screening of rFabs reactive with native CD98 expressed on the cell surface. HeLa cells were maintained in RPMI-1640 medium supplemented with 100 U/mL penicillin, 100 μg/mL streptomycin, and 10% heat-inactivated FBS at 37°C in a humidified 5% CO2 atmosphere.
Monoclonal antibody
HBJ127 (IgG1, κ)(8) selected from a hybridoma prepared by fusion between P3X63Ag.8653 mouse myeloma cells and spleen cells of a BALB/c mouse immunized with T24 human urinary bladder cancer cells was used for obtaining mimotope sequences using a phage display random peptide library.
Phage display random peptide library
The phage display random peptide library Ph.D.-C7C (New England BioLabs, Ipswich, MA, USA) was used for obtaining a cyclic heptapeptide mimotope of HBJ127. A disulfide bond-constrained cyclic heptapeptide with random sequence was expressed as a fusion protein with the N-terminal end of the minor coat protein of M13 phage. The library contained approximately 109 individual clones and the phage concentration was approximately 2 × 1013 pfu/mL according to the manufacturer's description.
Panning of cyclic peptide library against HBJ127
Panning of the Ph.D.-C7C library against HBJ127 was according to the procedure previously described.(9) Briefly, phage solution was added to the HBJ127-coated wells of Costar 3590 EIA/RIA plates (Corning Inc., Corning, NY, USA) and incubated for 1 h at room temperature. HBJ127-bound phages were then eluted and amplified by infection with Escherichia coli XL1-Blue cells (Stratagene, La Jolla, CA, USA). Amplified phages were precipitated with 20% PEG 8000 in 2.5 M sodium chloride solution (PEG/NaCl). The phages were finally suspended in 100 μL PBS and used for the next round of panning. Panning was repeated four times.
Screening of phage clones
Thirty blue plaques on the LB/isopropyl β-D-thiogalactopyranoside/X-Gal plate were randomly picked up and infected with 1:100 diluted XL1-Blue overnight cultures in 15 mL polypropylene tubes. After 4.5 h of shaking at 37°C, the amplified and cleared supernatants were used for testing the binding activity against HBJ127 (positive) and isotype-matched antibody with unrelated specificity (negative) by a phage ELISA. HBJ127-specific phage clones were selected, and then sequenced the ssDNA to identify the heptapeptide–mimotope sequence.
Preparation of peptide–carrier protein conjugates
Linear peptides with linker, LMP1 (HPMHFPS-GGGC) and LMP2 (YPRWQIP-GGGC), and a cyclic peptide with linker, CMP (cSWQIPGMc-GGGS: disulfide (cc)-constrained) were chemically synthesized (Torey Research Center, Tokyo, Japan). LMP1- and LMP2-carrier protein (KLH and BSA) conjugates were prepared by reaction of cysteine residue at the C-terminal end of peptide and maleimide-activated carrier proteins (Imject maleimide-activated carrier proteins; Pierce, Rockford, IL, USA). A CMP-carrier protein (KLH and BSA) conjugate was also prepared by the activated ester method using N-hydroxysuccinimide and water-soluble carbodiimide (e.g., 1-Ethyl-3-(3-dimethylaminopropyl)carbodiimide, EDC). Protein content of peptide–carrier protein conjugates was determined by BCA Protein Assay (Pierce), and used for immunization (KLH conjugates) and ELISA (BSA conjugates).
Immunization of mice
Immunization with mimotope–KLH conjugates was carried out as previously described.(10) Briefly, female BALB/c mice (Japan SLC, Hamamatsu, Japan) were immunized three times with mimotope–KLH at 14-day intervals. Two weeks after the third immunization, the mice received a booster i.v. injection of mimotope–KLH in saline. Three days after the final immunization, the mice were killed by cutting of the jugular vein and venous blood was collected. Spleens were also excised and used as antibody sources for library construction.
RNA isolation and library construction
Total RNA was isolated from spleens of immunized mice using an RNeasy Mini Kit (Qiagen, Tokyo, Japan) according to the manufacturer's instructions. After reverse transcription, the γ1 Fd region and κ chain were amplified by PCR and phage display libraries were constructed in the phage display vector pComb3, as previously described.(10)
Antibody library selection
Antibody libraries were selected using CD98-positive HeLa cells. Panning was carried out for four consecutive rounds, and phagemid DNA, isolated after the final round of panning, was reconstructed for production of soluble Fab fragments as previously described.(10)
Nucleic acid sequencing
Nucleic acid sequencing was carried out on a PRISM 310 Genetic Analyzer (Applied Biosystems, Foster City, CA, USA) using a BigDye Terminator v1.1 Cycle Sequencing Kit (Applied Biosystems). Sequencing primers were as previously reported.(10) Comparison to reported immunoglobulin germline sequences from Genbank/EMBL/DDBJ was carried out using NCBI blast against Ig sequences (Ig blast) (http://www.ncbi.nlm.nih.gov/igblast/) and IMGT/V-QUEST (http://www.imgt.org/IMGT_vquest/share/textes/) searches.
Indirect immunofluorescence
The reactivity of rFab fragments was determined by an indirect immunofluorescence (IIF) as previously described.(19) HeLa cells were detached from the culture flask with trypsin–EDTA (Invitrogen, Tokyo, Japan), washed once with PBS, and suspended in 100 μL PBS supplemented with 1% (w/v) BSA (BSA–PBS). The cells were successively treated with each rFab fragment (10 μg/mL in 1% BSA–PBS), and 1:200 diluted FITC-labeled rabbit anti-mouse IgG F(ab')2 (Jackson ImmunoResearch Laboratories, West Grove, PA, USA). The preparations were observed using a confocal laser microscope (LSM510; Carl Zeiss Japan, Tokyo, Japan).
In vitro tumor growth inhibition assay
Effect of rFabs on growth inhibition of HeLa cells was assessed according to the procedure reported previously.(10) Briefly, HeLa cells were seeded in triplicate into flat-bottomed 96-well culture plates at 1 × 104 cells per well. The rFab fragment cross-linked with the rabbit anti-mouse IgG F(ab')2 was incubated with cells for 72 h at 37°C. The rFab fragment was used at a final concentration of 50 μg/mL, and the equimolar concentration of HBJ127 was used as a positive control. The cell growth inhibitory effect of the rFab fragment was evaluated by the alamarBlue (Cosmo Bio, Tokyo, Japan) assay according to the manufacturer's instructions.
Results
Identification of cyclic peptide mimotopes reactive with HBJ127
To identify the mimotope sequence with constrained structure, four rounds of panning of the Ph.D.-C7C phage display peptide library were carried out against HBJ127. The eluted phage titer decreased at the second round but increased at the third and fourth rounds of panning, indicating the presence of phage clones bearing a peptide sequence reactive with HBJ127. The HBJ127-bound phage titer increased from 3.0 × 105 pfu/mL (second round) to 6.1 × 107 pfu/mL (fourth round). The library was finally concentrated to approximately 200-fold after four rounds of panning. Thirty blue color plaques were randomly picked from the plate of eluted phages after the final round. Each clone was amplified by infection with XL1-Blue, and then tested for reactivity against HBJ127 by a phage ELISA. As shown in Table 1, all positive clones (20/30) possessed the motif of WQIPGM in the constrained heptapeptide sequence, whereas none of the negative clones carried this sequence.
Table 1.
Sequences of cyclic peptide mimotopes after panning against HBJ127
| Sequence | Frequency | Reactivity |
|---|---|---|
| cSWQIPGMc | 19 | + |
| cTWQIPGMc | 1 | + |
| cNAPSSRSc | 2 | − |
| cSQYNRLVc | 2 | − |
| cPSRHGQVc | 2 | − |
| cSQWAQAFc | 2 | − |
| cRQSQYPTc | 1 | − |
| cLYAKGPHc | 1 | − |
Characterization of antisera obtained from mimotope–KLH immunized mice
Linear and cyclic mimotopes as shown in Table 2 were used for the haptens of immunogens. Serum obtained from hyperimmunized mice were determined by direct ELISA, and elevated reactivity against the hapten of the immunogen was confirmed (Table 3). Next, the reactivity of sera against CD98-positive HeLa cells was determined. None of the sera reacted at all with HeLa cells, as determined by either IIF or flow cytometry (data not shown).
Table 2.
Sequences of cyclic and linear peptide mimotopes using for immunization
| No. | Name | Sequence | Carrier protein |
|---|---|---|---|
| 1 | LMP1 | HPMHFPS-GGGC | KLH/BSA |
| 2 | LMP2 | YPRWQIP-GGGC | KLH/BSA |
| 3 | CMP | cSWQIPGMc-GGGS | KLH/BSA |
Table 3.
Serum titer against corresponding peptide–KLH and peptide–BSA conjugates
| Conjugate | LMP1 | LMP2 | CMP |
|---|---|---|---|
| Peptide–KLH | 1:105 | 1:105 | 1:103 |
| Peptide–BSA | 1:5 × 104 | 1:5 × 104 | 1:5 × 102 |
Serum titer that gives 50% of maximum reactivity against immunogen or antigen is shown.
Retrieval of rFab clones reactive with native human CD98 from linear and cyclic peptide immunized mice spleen cells
These results suggested that antibodies reactive with native CD98 were not elicited or were elicited but represented an undetectable population in immune sera. To confirm this hypothesis, we constructed a Fab phage display library from LMP1, LMP2, and CMP–KLH hyperimmunized mice spleen cells (libraries are abbreviated as LMP1-Lib, LMP2-Lib, and CMP-Lib, respectively). The resulting sizes of LMP1-Lib, LMP2-Lib, and CMP-Lib were 3.8 × 106, 6.1 × 106, and 4.7 × 106 c.f.u., respectively. The phage display libraries were panned against live HeLa cells. Four rounds of panning produced up to 50-, 35-, and 95-fold enrichment of antigen-bound phage populations in LMP1-Lib, LMP2-Lib, and CMP-Lib, respectively. Twenty clones of rFab from each library were tested for reactivity against live HeLa cells and 15, 18, and 20 clones from LMP1-Lib, LMP2-Lib, and CMP-Lib, respectively, showed intense staining with the surface of live HeLa cells. Because all positive clones in each library were found to be identical by BstNI fingerprinting,(20) we selected the clones A18, B3, and C17 from each library, and used them for following experiments.
Functional characterization of rFab clones
We first determined the reactivity of A18, B3, and C17 against live HeLa cells by IIF. All rFabs strongly stained the surfaces of HeLa cells (Fig. 1). We then determined the reactivity against HeLa cell lysates using these rFabs for antigen capture and human CD98-specific mAb for detection in sandwich ELISA. A18, B3, and C17 reacted with CD98 in HeLa cell lysates (Fig. 2). Next, we determined in vitro tumor growth inhibitory activity of A18, B3, and C17 using CD98-expressing HeLa cells. HeLa cells were treated with rFabs then cross-linked with rabbit anti-mouse IgG F(ab')2. After 72 h, the viability of cells was determined by alamarBlue assay. As shown in Figure 3, all rFab clones showed cell growth inhibition activity comparable to HBJ127. These results implied that mimotope-induced rFabs reacted with human CD98 antigen and showed the biological activities as comparable to HBJ127.
Fig. 1.
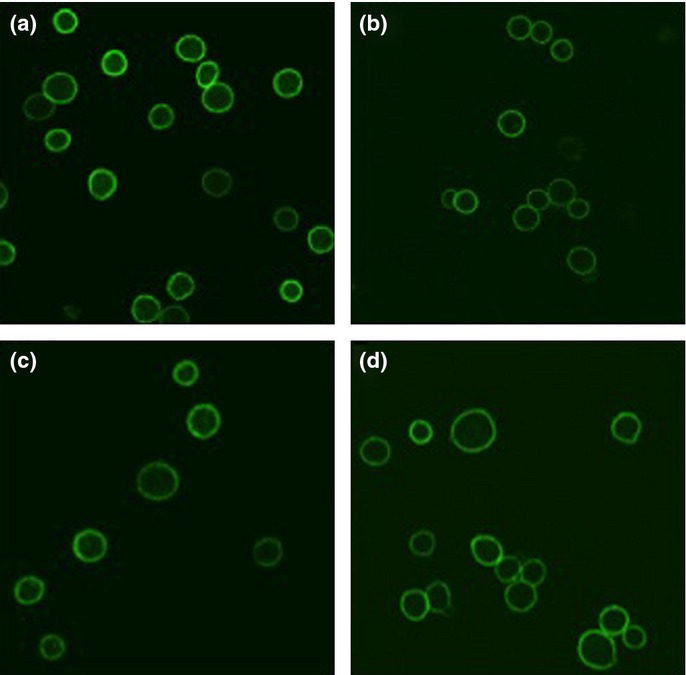
Reactivity of recombinant Fab clones against live HeLa cells by indirect immunofluorescence. Recombinant Fab (a) A18, (b) B3, and (c) C17 retrieved from peptide–KLH immunized mice were examined. The parental antibody (d) HBJ127 was used as a positive control.
Fig. 2.
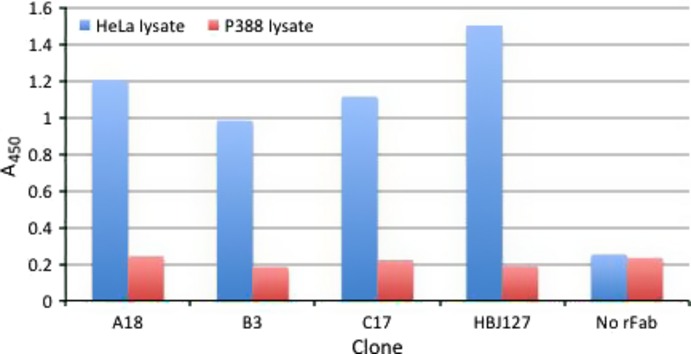
Reactivity of recombinant Fab (rFab) clones against HeLa lysates by capture sandwich ELISA. HeLa lysates captured by purified rFabs (A18, B3, C17) or parental antibody HBJ127 (positive control) were detected by biotinylated anti-CD98 mAb 1-10. Murine leukemia P388 cell lysates were used as a negative control.
Fig. 3.
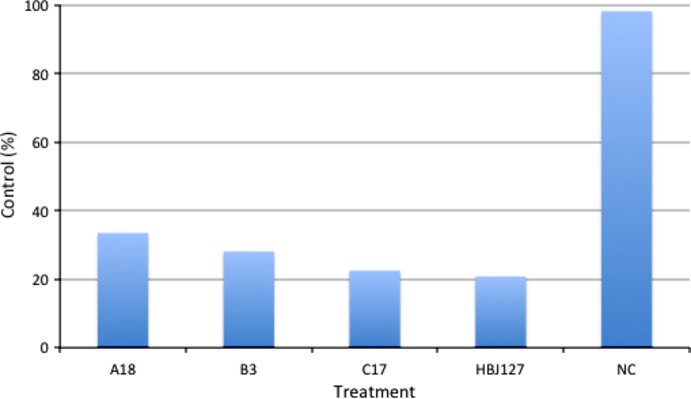
Effects of recombinant Fab clones on in vitro growth of HeLa cells. Cells (1 × 104) were cultured with recombinant Fabs (A18, B3, C17) plus anti-mouse IgG F(ab')2 or with mAbs (HBJ127 and isotype-matched unrelated antibody [NC]) for 72 h at 37°C. Cell growth activity was evaluated by alamarBlue assay.
Molecular structural analysis of rFab clones
To elucidate the structural similarity between the mimotope-induced rFabs and parental HBJ127, we sequenced and compared the variable heavy (VH) and variable light (VL) domains of A18, B3, and C17, respectively. These sequence data have been submitted to the DDBJ/EMBL/GenBank databases under accession numbers AB773873-4 (A18), AB773877-8 (B3), AB773881-2 (C17), respectively. The deduced amino acid sequences of the three clones are shown in Figure 4. In each clone, only a single mutation was found in the FR1 region of the VH domain (99% identical to HBJ127 VH). In contrast, multiple mutations were found mainly in FR regions of VL domain (90–95% identical to HBJ127 VL). As for the complementarity determining region (CDR) sequences, HCDR1-3 and LCDR1-2 were identical to those of HBJ127. Only a single amino acid difference (Thr to Ser) was found in LCDR3. This mutation may not influence the function of rFabs because of their comparable bioactivities to HBJ127. The gene usage and structural homologies between three clones and the parent antibody is shown in Table 4. The VH of the three clones were derived from VHQ52.a5.13, and the VL from IgVk8-30. VH (AB056115) and VL (AB056116) of HBJ127 are also derived from the same germline. From these results, the VH and VL of selected clones were considered to be almost identical to those of HBJ127. We confirmed that linear and cyclic mimotope peptides could induce antibodies structurally equivalent to parental HBJ127.
Fig. 4.

Deduced amino acid sequences of recombinant Fab clones (A18, B3, C17) compared with HBJ127 and the closest known germline. (a) Variable heavy domain (VH) sequences and (b) variable light domain (VL) sequences.
Table 4.
Comparison of gene usage and structural homologies of the variable heavy (VH) and variable light (VL) domains of recombinant Fab clones
| Fab | VH gene usage |
VL gene usage |
||||
|---|---|---|---|---|---|---|
| Nearest germline | Homology to germline % |
Nearest germline | Homology to germline % |
|||
| DNA | Protein | DNA | Protein | |||
| HBJ127 | VHQ52.a5.13 | 94.2 | 89.7 | IgVk8-30 | 96.7 | 93.1 |
| A18 | VHQ52.a5.13 | 92.8 | 88.7 | IgVk8-30 | 98.7 | 97.0 |
| B3 | VHQ52.a5.13 | 94.2 | 87.6 | IgVk8-30 | 99.7 | 99.0 |
| C17 | VHQ52.a5.13 | 93.2 | 88.7 | IgVk8-30 | 98.3 | 95.0 |
Discussion
In our previous reports, we identified the epitope sequence of tumor-suppressive anti-CD98 mAb, HBJ127 using a phage display random peptide library.(9) We next tried to induce 1antitumor immunity by immunization with peptide–KLH conjugates prepared based on the HBJ127 epitope sequence, but we failed to detect reactivity against CD98-expressing tumor cells in immunized serum.(10) In epitope analysis experiments, HBJ127-reactive but epitope-unrelated peptides were obtained dominantly.(9) These peptides, named mimotopes, may be more promising than epitope peptides in induction of antitumor immunity. Moreover, peptides with constrained sequences may be more promising than free peptides. Until now, isolation and characterization of mimotopes from antibody medicines, such as trastuzumab,(6,21–23) and rituximab(5,24–26) has been reported. Mimotopes conjugated with KLH, then emulsified with Freund's adjuvants or tetanus toxoids were given to mice. Elevated levels of antibody titer against mimotopes were confirmed and antitumor immunity equivalent to the parent antibody were successfully induced in the immunized mice sera.
In this study, we determined the efficacy of HBJ127-reactive mimotopes with free and constrained structures for induction of antitumor immunity, however, we failed to detect significant elevation in serum antitumor activity despite the successful induction of an antibody repertoire functionally and structurally equivalent to HBJ127. The possible reasons for failure of induction of antitumor immunity in this study are as follows.
Immune tolerance. CD98 hc has over 70% conserved sequence between human, rat, and mouse.(9) Mimotopes used in this study were derived from a conserved region in CD98 hc.(9) Thus, immune tolerance(27) may be the reason why antibody repertoire failed to be induced. Selection of rabbit or guinea pig as immunized hosts instead of mice may be used to counter this failure.
T-cell immunity. It is known that sufficient induction of antibody production from activated B cells requires helper T cell activation.(28) Immunization with only a B-cell mimotope may be insufficient for induction of antitumor immunity. Preparation of peptides following an analysis of the antigen-specific T-cell epitope and co-administration of both peptides may induce antitumor immunity in immunized hosts.
Toll-like receptor 4 (TLR4) activation. It has been recently reported that activation of natural immunity is required for induction of antibody production from B cells. Activation of natural immunity through TLR is known.(29) Among TLR, activation of TLR4 is related to infectious and inflammatory diseases and cancer.(30) The representative TLR4 ligand is LPS. Identification of a mimotope of anti-LPS antibody and application of mimotope-derived peptides as an adjuvant for antibody production has been recently reported.(31) We may be able to induce antitumor immunity by administration of our mimotopes concomitant with adjuvant peptides as described above. We plan to test the above possibilities for successful induction of tumor immunity in serum levels by human CD98 hc-oriented mimotopes.
Although we did not achieve our primary goal, we showed that phage display panning and selection against native antigen-expressing cells could obtain conformation-specific antibodies, which are, in general, difficult to select by peptide immunization and hybridoma technology. Our method may be applicable to prepare conformation-specific antibodies against proteins that are difficult to purify.
Acknowledgments
This work was supported in part by Grants-in-Aid for Scientific Research (C) from The Japan Society for Promotion of Science (20590063). The authors thank Dr. Dennis R. Burton of The Scripps Research Institute (La Jolla, CA, USA) for providing pComb3 vector.
Disclosure Statement
The authors have no conflict of interest.
References
- 1.Goldenberg MM. Trastuzumab, a recombinant DNA-derived humanized monoclonal antibody, a novel agent for the treatment of metastatic breast cancer. Clin Ther. 1999;21:309–18. doi: 10.1016/S0149-2918(00)88288-0. [DOI] [PubMed] [Google Scholar]
- 2.Anderson DR, Grillo-López A, Varns C, Chambers KS, Hanna N. Targeted anti-cancer therapy using rituximab, a chimaeric anti-CD20 antibody (IDEC-C2B8) in the treatment of non-Hodgkin's B-cell lymphoma. Biochem Soc Trans. 1997;25:705–8. doi: 10.1042/bst0250705. [DOI] [PubMed] [Google Scholar]
- 3.Maini RN, Breedveld FC, Kalden JR, et al. Therapeutic efficacy of multiple intravenous infusions of anti-tumor necrosis factor alpha monoclonal antibody combined with low-dose weekly methotrexate in rheumatoid arthritis. Arthritis Rheum. 1998;41:1552–63. doi: 10.1002/1529-0131(199809)41:9<1552::AID-ART5>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 4.Riemer AB, Kurz H, Klinger M, Scheiner O, Zielinski CC, Jensen-Jarolim E. Vaccination with cetuximab mimotopes and biological properties of induced anti-epidermal growth factor receptor antibodies. J Natl Cancer Inst. 2005;97:1663–70. doi: 10.1093/jnci/dji373. [DOI] [PubMed] [Google Scholar]
- 5.Li M, Yan Z, Han W, Zhang Y. Mimotope vaccination for epitope-specific induction of anti-CD20 antibodies. Cell Immunol. 2006;239:136–43. doi: 10.1016/j.cellimm.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 6.Riemer AB, Klinger M, Wagner S, et al. Generation of Peptide mimics of the epitope recognized by trastuzumab on the oncogenic protein Her-2/neu. J Immunol. 2004;173:394–401. doi: 10.4049/jimmunol.173.1.394. [DOI] [PubMed] [Google Scholar]
- 7.Riemer AB, Forster-Waldl E, Bramswig KH, et al. Induction of IgG antibodies against the GD2 carbohydrate tumor antigen by vaccination with peptide mimotopes. Eur J Immunol. 2006;36:1267–74. doi: 10.1002/eji.200535279. [DOI] [PubMed] [Google Scholar]
- 8.Masuko T, Abe J, Yagita H, Hashimoto Y. Human bladder cancer cell-surface antigens recognized by murine monoclonal antibodies raised against T24 bladder cancer cells. Jpn J Cancer Res. 1985;76:386–94. [PubMed] [Google Scholar]
- 9.Itoh K, Inoue K, Hayashi H, Suzuki T, Masuko T. Identification of cell proliferation-associated epitope on CD98 oncoprotein using phage display random peptide library. Cancer Sci. 2007;98:1696–700. doi: 10.1111/j.1349-7006.2007.00587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Itoh K, Ohshima M, Sonobe M, et al. Antibody epitope peptides as potential inducers of IgG antibodies against CD98 oncoprotein. Cancer Sci. 2009;100:126–31. doi: 10.1111/j.1349-7006.2008.00998.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haynes BF, Hemler ME, Mann DL, et al. Characterization of a monoclonal antibody (4F2) that binds to human monocytes and to a subset of activated lymphocytes. J Immunol. 1981;126:1409–14. [PubMed] [Google Scholar]
- 12.Hashimoto Y, Masuko T, Yagita H, Endo N, Kanazawa J, Tazawa J. A proliferation-associated rat cell surface antigen recognized by a murine monoclonal antibody. Gann. 1983;74:819–21. [PubMed] [Google Scholar]
- 13.Broer S, Broer A, Hamprecht B. The 4F2hc surface antigen is necessary for expression of system L-like neutral amino acid-transport activity in C6-BU-1 rat glioma cells: evidence from expression studies in Xenopus laevis oocytes. Biochem J. 1995;312:863–70. doi: 10.1042/bj3120863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kanai Y, Segawa H, Miyamoto K, Uchino H, Takeda E, Endou H. Expression cloning and characterization of a transporter for large neutral amino acids activated by the heavy chain of 4F2 antigen (CD98) J Biol Chem. 1998;273:23629–32. doi: 10.1074/jbc.273.37.23629. [DOI] [PubMed] [Google Scholar]
- 15.Yagita H, Masuko T, Hashimoto Y. Inhibition of tumor cell growth in vitro by murine monoclonal antibodies that recognize a proliferation-associated cell surface antigen system in rats and humans. Cancer Res. 1986;46:1478–84. [PubMed] [Google Scholar]
- 16.Yagita H, Masuko T, Takahashi N, Hashimoto Y. Monoclonal antibodies that inhibit activation and proliferation of lymphocytes. I. Expression of the antigen on monocytes and activated lymphocytes. J Immunol. 1986;136:2055–61. [PubMed] [Google Scholar]
- 17.Hara K, Kudoh H, Enomoto T, Hashimoto Y, Masuko T. Malignant transformation of NIH3T3 cells by overexpression of early lymphocyte activation antigen CD98. Biochem Biophys Res Commun. 1999;262:720–5. doi: 10.1006/bbrc.1999.1051. [DOI] [PubMed] [Google Scholar]
- 18.Scherer WF, Syverton JT, Gey GO. Studies on the propagation in vitro of poliomyelitis viruses. IV. Viral multiplication in a stable strain of human malignant epithelial cells (strain HeLa) derived from an epidermoid carcinoma of the cervix. J Exp Med. 1953;97:695–710. doi: 10.1084/jem.97.5.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Itoh K, Inoue K, Hirooka K, et al. Phage display cloning and characterization of monoclonal antibody genes and recombinant Fab fragment against the CD98 oncoprotein. Jpn J Cancer Res. 2001;92:1313–21. doi: 10.1111/j.1349-7006.2001.tb02155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Itoh K, Nakagomi O, Suzuki K, Inoue K, Tada H, Suzuki T. Recombinant human monoclonal Fab fragments against rotavirus from phage display combinatorial libraries. J Biochem. 1999;125:123–9. doi: 10.1093/oxfordjournals.jbchem.a022248. [DOI] [PubMed] [Google Scholar]
- 21.Jiang B, Liu W, Qu H, et al. A novel peptide isolated from a phage display peptide library with trastuzumab can mimic antigen epitope of HER-2. J Biol Chem. 2005;280:4656–62. doi: 10.1074/jbc.M411047200. [DOI] [PubMed] [Google Scholar]
- 22.Riemer AB, Kraml G, Scheiner O, Zielinski CC, Jensen-Jarolim E. Matching of trastuzumab (Herceptin) epitope mimics onto the surface of Her-2/neu–a new method of epitope definition. Mol Immunol. 2005;42:1121–4. doi: 10.1016/j.molimm.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 23.Riemer AB, Untersmayr E, Knittelfelder R, et al. Active induction of tumor-specific IgE antibodies by oral mimotope vaccination. Cancer Res. 2007;67:3406–11. doi: 10.1158/0008-5472.CAN-06-3758. [DOI] [PubMed] [Google Scholar]
- 24.Perosa F, Favoino E, Caragnano MA, Dammacco F. CD20 mimicry by a mAb rituximab-specific linear peptide: a potential tool for active immunotherapy of autoimmune diseases. Ann N Y Acad Sci. 2005;1051:672–83. doi: 10.1196/annals.1361.112. [DOI] [PubMed] [Google Scholar]
- 25.Perosa F, Favoino E, Vicenti C, Merchionne F, Dammacco F. Identification of an antigenic and immunogenic motif expressed by two 7-mer rituximab-specific cyclic peptide mimotopes: implication for peptide-based active immunotherapy. J Immunol. 2007;179:7967–74. doi: 10.4049/jimmunol.179.11.7967. [DOI] [PubMed] [Google Scholar]
- 26.Perosa F, Favoino E, Vicenti C, et al. Two structurally different rituximab-specific CD20 mimotope peptides reveal that rituximab recognizes two different CD20-associated epitopes. J Immunol. 2009;182:416–23. doi: 10.4049/jimmunol.182.1.416. [DOI] [PubMed] [Google Scholar]
- 27.Jerne NK. The somatic generation of immune recognition. Eur J Immunol. 1971;1:1–9. doi: 10.1002/eji.1830010102. [DOI] [PubMed] [Google Scholar]
- 28.Kennedy R, Celis E. Multiple roles for CD4+ T cells in anti-tumor immune responses. Immunol Rev. 2008;222:129–44. doi: 10.1111/j.1600-065X.2008.00616.x. [DOI] [PubMed] [Google Scholar]
- 29.Kumar H, Kawai T, Akira S. Toll-like receptors and innate immunity. Biochem Biophys Res Commun. 2009;388:621–5. doi: 10.1016/j.bbrc.2009.08.062. [DOI] [PubMed] [Google Scholar]
- 30.O'Neill LA, Bryant CE, Doyle SL. Therapeutic targeting of Toll-like receptors for infectious and inflammatory diseases and cancer. Pharmacol Rev. 2009;61:177–97. doi: 10.1124/pr.109.001073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shanmugam A, Rajoria S, George AL, Mittelman A, Suriano R, Tiwari RK. Synthetic Toll like receptor-4 (TLR-4) agonist peptides as a novel class of adjuvants. PLoS ONE. 2012;7:e30839. doi: 10.1371/journal.pone.0030839. [DOI] [PMC free article] [PubMed] [Google Scholar]


