Abstract
Early-onset cataracts are associated with insufficient antioxidative activity, and, therefore, a potential risk of cancer. This study investigated the risk of cancer after being diagnosed with early-onset cataracts. Retrospective claims data from the Taiwan National Health Insurance Research Database were analyzed. Study subjects were comprised of patients with early-onset cataracts, aged 20–55 years (International Classification of Diseases, 9th Revision, Clinical Modification [ICD-9-CM] code 366.00, 366.01, 366.02, 366.03, 366.04, 366.09, 366.17 and 366.18) and newly diagnosed between 1997 and 2010 (n = 1281), and a comparison cohort without the disease (n = 5124). Both cohorts were followed up until 2010 to estimate the incidences of cancer. We used the Poisson regression model to compare incidence rate ratios and the 95% confidence interval (CI). Cox proportional hazards regression was used to assess the hazard ratio (HR) of cancer associated with early-onset cataracts. The overall incidence rate of all cancers was 2.19-fold higher in the early-onset cataract cohort than in the comparison cohort (8.06 vs 3.68 per 1000 person-years) with an adjusted HR of 2.13 (95% CI = 1.48, 3.07). The site-specific analysis also showed a strong relationship, with adjusted HR of 3.24 ((95% CI = 1.30, 8.10) for head and neck cancer, 3.29 (95% CI 1.16, 9.31) for hepatoma and 3.19 (95% CI 1.34, 7.58) for breast cancer. The present study suggests that patients with early-onset cataracts are at an increased risk of being diagnosed with cancer in subsequent years.
Keywords: Cancer, cohort study, early-onset cataracts, incidence, retrospective
Cataracts are a common cause of reduced visual transparency in elderly patients, and are associated with decreased metabolic transport of antioxidants in the aging lens.(1) In normal aging, the oxidation of nuclear components increases in the lens and the lens continues to grow lens fibers, reducing transparency. The activities of antioxidant enzymes, such as glutathione peroxidase, may be reduced in older adults for metabolizing oxidants, such as hydrogen peroxide.(2) The oxidative damage may start in the nucleus of the lens because of the low metabolic activity; the amount of modified proteins susceptible to oxidation accumulate with age.(3) Changes in other parts of the lens may occur to varying degrees, including the cortical and posterior subcapsular parts.(1)
Age-related cataracts usually occur in patients older than 60 years,(4) but some patients experience cataracts before the age of 55. One of the causes of early-onset cataracts might be insufficient antioxidative function. Cancer is another disease associated with oxidative stress. The production of reactive oxygen species (ROS) plays important roles in genomic instability and uncontrolled cell proliferation.(5) The overproduction of ROS and faulty antioxidant and DNA repair lead to oxidative damage to cellular macromolecules and contribute to carcinogenesis.
Because the two diseases have a similar mechanism, using a nationwide population-based dataset from Taiwan, the present study investigates whether patients with early-onset cataracts are at risk of subsequent cancer diagnosis in the years following the formation of cataracts.
Materials and Methods
Data sources
This study was designed as a population-based retrospective cohort study to investigate the relationship between having been diagnosed with early-onset cataracts and the occurrence of cancer. All datasets were obtained from the reimbursement database of Taiwan National Health Insurance (NHI), a single-payer universal insurance system. The insurance system covers more than 99% of the approximate 23 million citizens in Taiwan (Bureau of National Health Insurance, Department of Health, Executive Yuan, Taiwan [http://www.nhi.gov.tw/english/index.aspx]) In this study, we used the claims data of the Longitudinal Health Insurance Database 2000 (LHID2000) established by the National Health Research Institutes (NHRI), Department of Health, Taiwan. There were no statistically significant differences in the distribution of sex, age or health-care costs between cohorts in the LHID2000 and all insurance enrollees, as reported by the NHRI in Taiwan. Data files were linked with the identifications of patients that had been anonymized, with identification numbers encrypted, and maintained in the NHI reimbursement data, to protect the privacy of the individuals. The comprehensive claim files provided information on the registry of medical facilities, details of inpatient orders, types of ambulatory care, socio-demographic status of patients and health-care services received by each patient, including all payments for outpatient visits, hospitalizations and prescriptions. The data for each outpatient visit or hospitalization contained up to five diagnoses that were coded according to the International Classification of Diseases, 9th Revision Clinical Modification (ICD-9-CM) classification.
Ethics statement
Using a retrospective cohort study design, this study was approved by the ethics review committee at the China Medical University and Hospital. Consent was not obtained from patients, which was approved by the ethical review committee.
Study participants
We identified 1281 patients aged 20 to 55 years with newly diagnosed early-onset cataracts (ICD-9-CM codes 366.00, 366.01, 366.02, 366.03, 366.04, 366.09, 366.17 and 366.18) diagnosed from 1997 to 2010 with at least three claims for outpatient and/or hospitalization visits as the study cohort by using the diagnosed date as the index date. Juvenile infantile cataracts and traumatic cataracts were excluded. The index date for the patient was the date of the first outpatient visit for early-onset cataracts. We used a systematic random sampling method to select a comparison cohort from the rest of the insured population that was free from early-onset cataracts and cancer; the frequency was randomly matched by age (every 5 years), sex and the year of the index date.
Outcome definition
For this study, to identify subjects later diagnosed with cancer, we obtained the Registry of Catastrophic Illness Patient Database (RCIPD) for all patients who were diagnosed with cancer from 1 January 1997 to 31 December 2010. Any of the diagnoses of cancer except metastatic cancer (ICD-9-CM codes 140–195 and 200–208) made by doctors and officials of the NHI system was considered an acceptable code. We excluded patients aged under 20 years and those who were diagnosed with any type of cancer (ICD-9-CM codes 140–208) before the index date. Nine groups of cancer were evaluated: head and neck cancer, colorectal cancer, hepatoma, breast cancer, uterine cancer, bladder cancer, kidney cancer, thyroid cancer, and others.
Variables of interest (exposure)
In addition, we measured patients with at least three claims for outpatient visits or hospitalization visits at the baseline by the principle and secondary diagnoses for diseases considered possible comorbidities associated with cancer. Based on ICD-9-CM codes, diseases included were hypertension (codes 401 to 405), diabetes mellitus (code 250) hyperlipidemia (code 272), stroke (codes 430 to 438), ischemic heart disease (codes 410 to 414), asthma (code 493), chronic obstructive pulmonary disease (COPD) (codes 490 to 496), alcohol-related illness (including alcoholic psychoses (code 291), alcohol dependence syndrome (code 303), alcohol abuse (code 305), alcoholic fatty liver (571.0), acute alcoholic hepatitis (code 571.1), alcoholic cirrhosis (code 571.2) and alcoholic liver damage (code 571.3).
Statistical analysis
The distributions of the categorical socio-demographic characteristics and comorbidities were compared between the study cohort and the comparison cohort, and the differences were examined using the χ2-test. Subjects with stratified age groups of 20–35 years, 36–45 years and 46–55 years at the index date of early-onset cataracts were analyzed. The person-years of follow-up time were calculated for each patient until cancer was diagnosed or censored. The follow-up person-years were calculated to assess the incidence density rates. We used the Poisson regression model to assess the study cohort to compare cohort incidence rate ratios (IRR) and the 95% confidence interval (CI). Cox proportional hazards regression analysis was used to assess the cancer risk associated with early-onset cataracts by adjusting for cofactors significantly related to early-onset cataracts. All analyses were performed using the sas statistical package (version 9.1 for Windows; SAS institute, Cary, NC, USA). A two-tailed P-value of <0.05 indicated the statistical significance level.
Results
Socio-demographic characteristics are shown in Table 1. No significant differences in distributions of age and sex were found between the early-onset cataract cohort and the comparison cohort. There were more women than men and near two-thirds of the patients aged 46 to 55 years in both cohorts (mean age approximately 46.2 ± 7.4 years). The early-onset cataract cohort had higher prevalence of hypertension, diabetes mellitus, hyperlipidemia, stroke, ischemic heart disease, asthma, chronic obstructive pulmonary disease and alcohol-related illnesses (P < 0.05).
Table 1.
Comparisons in demographic characteristics and comorbidities between with and without early onset cataracts
| Early onset cataract |
|||
|---|---|---|---|
| No (n = 5124) | Yes (n = 1281) | P-value | |
| Gender | |||
| Women | 2508 (48.9) | 627 (48.9) | 0.99 |
| Men | 2616 (51.1) | 654 (51.0) | |
| Age stratified | |||
| 20–35 | 456 (8.90) | 114 (8.90) | 0.99 |
| 36–45 | 1216 (23.7) | 304 (23.7) | |
| 46–55 | 3452 (67.4) | 863 (67.4) | |
| Age, mean ± SD* | 46.2 ± 7.48 | 46.3 ± 7.37 | 0.53 |
| Comorbidity | |||
| Diabetes | 390 (7.61) | 309 (24.1) | <0.001 |
| Hypertension | 876 (17.1) | 363 (28.3) | <0.001 |
| Hyperlipidemia | 706 (13.8) | 354 (27.6) | <0.001 |
| Stroke | 60 (1.17) | 24 (1.87) | 0.048 |
| Ischemic heart disease | 317 (6.19) | 139 (10.9) | <0.0001 |
| Asthma | 179 (3.49) | 72 (5.62) | 0.0004 |
| Chronic obstructive pulmonary disease | 900 (17.6) | 348 (27.2) | <0.0001 |
| Alcohol-related illness | 121 (2.36) | 48 (3.75) | 0.006 |
χ2-test;
t-test.
The incidence rate and adjusted HR among the early-onset cataract cohort and the comparison cohort is shown in Table 2. The overall incidence rate of all cancer was 2.19-fold higher in the early-onset cataract cohort than in the comparison cohort (8.06 vs 3.68 per 1000 person-years), with an adjusted HR of 2.13 (95% CI = 1.48–3.07). In the early-onset cataract cohort, the incidence of cancer was higher in women than in men. Compared with the comparison cohort, the adjusted HR of cancer in the early-onset cataract cohort was also higher for women than men. The incidence increased with age, with the age-specific adjusted HR of cancer significant only for the early-onset cataract cohort aged 46–55 years (adjusted HR = 2.14, 95% CI = 1.41–3.25). Patients with comorbidities of diabetes, hyperlipidemia, stroke, ischemic heart disease, COPD or alcohol-related illnesses had an increased cancer incidence. However, the early-onset cataract patients without comorbidities were more likely to have significant adjusted HR of cancer.
Table 2.
Incidence, incidence rate ratio and adjusted hazard ratio of cancer by sex, age and comorbidities compared between cohorts with and without early onset cataracts
| Early onset cataract |
||||||||
|---|---|---|---|---|---|---|---|---|
| No |
Yes |
Compared to without early onset cataract |
||||||
| Event | PY | Rate‡ | Event | PY | Rate‡ | IRR§ (95% CI) | Adjusted HR† (95% CI) | |
| All | 89 | 24 207 | 3.68 | 48 | 5953 | 8.06 | 2.19 (1.88, 2.56)*** | 2.13 (1.48, 3.07)*** |
| Sex | ||||||||
| F | 43 | 12 316 | 3.49 | 27 | 3051 | 8.85 | 2.54 (2.04, 3.15)*** | 2.73 (1.67, 4.47)*** |
| M | 46 | 11 891 | 3.87 | 21 | 2903 | 7.23 | 1.87 (1.50, 2.33)*** | 1.64 (0.95, 2.83) |
| Age | ||||||||
| 20–35 | 2 | 2243 | 0.89 | 1 | 583 | 1.72 | 1.92 (1.09, 3.40)* | 1.54 (0.13, 18.3) |
| 36–45 | 18 | 5713 | 3.15 | 10 | 1384 | 7.23 | 2.29 (1.68, 3.14)*** | 2.00 (0.88, 4.53) |
| 46–55 | 69 | 16 251 | 4.25 | 37 | 3986 | 9.28 | 2.19 (1.81, 2.63)*** | 2.14 (1.41, 3.25)*** |
| Comorbidity | ||||||||
| Diabetes | ||||||||
| No | 82 | 22 356 | 3.67 | 34 | 4570 | 7.44 | 2.03 (1.71, 2.41)*** | 2.02 (1.35, 3.03)*** |
| Yes | 7 | 1851 | 3.78 | 14 | 1383 | 10.12 | 2.68 (1.69, 4.24)*** | 2.71 (1.08, 6.82)* |
| Hypertension | ||||||||
| No | 63 | 20 171 | 3.12 | 35 | 4253 | 8.23 | 2.64 (2.22, 3.13)*** | 2.68 (1.76, 4.08)*** |
| Yes | 26 | 4036 | 6.44 | 13 | 1701 | 7.64 | 1.19 (0.84, 1.67) | 1.19 (0.59, 2.38) |
| Hyperlipidemia | ||||||||
| No | 78 | 20 976 | 3.72 | 34 | 4387 | 7.75 | 2.08 (1.75, 2.48)*** | 2.06 (1.37, 3.12)*** |
| Yes | 11 | 3231 | 3.40 | 14 | 1567 | 8.93 | 2.62 (1.82, 3.79)*** | 2.56 (1.13, 5.78)* |
| Stroke | ||||||||
| No | 87 | 23 978 | 3.63 | 47 | 5853 | 8.03 | 2.21 (1.89, 2.59)*** | 2.14 (1.48, 3.10)*** |
| Yes | 2 | 229 | 8.74 | 1 | 100 | 9.97 | 1.14 (0.36, 3.64) | 0.89 |
| Ischemic heart disease | ||||||||
| No | 79 | 22 740 | 3.47 | 39 | 5342 | 7.30 | 2.10 (1.78, 2.47)*** | 2.16 (1.46, 3.20)*** |
| Yes | 10 | 1466 | 6.82 | 9 | 611 | 14.7 | 2.16 (1.29, 3.63)** | 2.08 (0.79, 5.49) |
| COPD | ||||||||
| No | 75 | 19 972 | 3.76 | 33 | 4319 | 7.64 | 2.03 (1.70, 2.43)*** | 1.95 (1.27, 2.98)** |
| Yes | 15 | 4234 | 3.31 | 15 | 1635 | 9.18 | 2.78 (1.98, 3.89)*** | 2.73 (1.29, 5.77)** |
| Asthma | ||||||||
| No | 87 | 23 461 | 3.71 | 47 | 5683 | 8.27 | 2.23 (1.91, 2.61)*** | 2.15 (1.49, 3.10)*** |
| Yes | 2 | 746 | 2.68 | 1 | 270 | 3.70 | 1.38 (0.56, 3.41) | 2.24 (0.12, 40.5) |
| Alcohol-related illness | ||||||||
| No | 84 | 23 823 | 3.53 | 46 | 5797 | 7.93 | 2.25 (1.93, 2.63)*** | 2.24 (1.54, 3.26)*** |
| Yes | 5 | 383 | 13.0 | 2 | 156 | 12.8 | 0.98 (0.39, 2.48) | 0.63 (0.10, 4.11) |
P < 0.05
P < 0.01
P < 0.001.
Adjusted HR: multivariable analysis including sex, age and comorbidities of diabetes, hypertension, hyperlipidemia, stroke, ischemic heart disease, asthma, chronic obstructive pulmonary disease and alcohol-related illness.
Rate, incidence rate, per 1000 person-years.
IRR, incidence rate ratio, per 1000 person-years. –, not available; COPD, chronic obstructive pulmonary disease; PY, person-years.
Table 3 presents a site-specific analysis of cancer risks between the study and comparison cohorts. Compared to the comparison group, half (25 cases) of all cancer occurrences were higher for head and neck cancer, hepatoma and breast cancer, with adjusted HR of 3.24 (95% CI = 1.30–8.10), 3.29 (95% CI = 1.16–9.31) and 3.19 (95% CI = 1.34–7.58), respectively, for patients with early-onset cataracts.
Table 3.
Incidence, incidence rate ratio and adjusted hazard ratio of sub-division cancer between with and without early onset cataracts
| Early onset cataract |
||||||
|---|---|---|---|---|---|---|
| Cancer (ICD-9-CM) | No |
Yes |
Compared to without early onset cataract |
|||
| Event | Rate‡ | Event | Rate‡ | IRR*(95% CI) | Adjusted HR† (95% CI) | |
| Head and neck (140–149) | 12 | 0.50 | 8 | 1.34 | 2.71 (2.27, 3.24)*** | 3.24 (1.30, 8.10)* |
| Colon (153, 154) | 8 | 0.33 | 5 | 0.84 | 2.54 (2.10, 3.07)*** | 2.25 (0.70, 7.28) |
| Hepatoma (155) | 8 | 0.33 | 8 | 1.34 | 4.07 (3.41, 4.85)*** | 3.29 (1.16, 9.31)* |
| Breast (174) | 13 | 0.54 | 9 | 1.51 | 2.82 (2.35, 3.37)*** | 3.19 (1.34, 7.58)** |
| Uterus (180–183) | 7 | 0.29 | 2 | 0.34 | 1.16 (0.93, 1.45) | 1.03 (0.21, 5.22) |
| Bladder (188) | 3 | 0.12 | 2 | 0.34 | 2.71 (2.23, 3.30)*** | 3.26 (0.53, 20.10) |
| Kidney (189) | 3 | 0.12 | 4 | 0.67 | 5.42 (4.51, 6.52)*** | 3.78 (0.76, 18.70) |
| Thyroid (193) | 1 | 0.04 | 2 | 0.34 | 8.13 (6.64, 9.96)*** | 9.54 (0.86, 106.40) |
| Others | 34 | 1.40 | 8 | 1.34 | 1.36 (1.10, 1.67)*** | 0.85 (0.38, 1.89) |
P < 0.05
P < 0.01
P < 0.001.
Adjusted HR: multivariable analysis including sex, age and comorbidities of diabetes, hypertension, hyperlipidemia, stroke, ischemic heart disease, asthma, chronic obstructive pulmonary disease and alcohol-related illness.
Rate, incidence rate, per 1000 person-years. IRR, incidence rate ratio, per 1000 person-years.
Kaplan–Meier analysis showed that patients with early-onset cataracts had significantly higher cumulative incidence rates for all cancers than the comparison group (8.13 vs 3.90) by the end of the 12-year follow up, especially for head and neck cancer (1.07 vs 0.39%), hepatoma (1.25 vs 0.36%) and breast cancer (1.59 vs 0.39%) (Fig. 1).
Fig. 1.
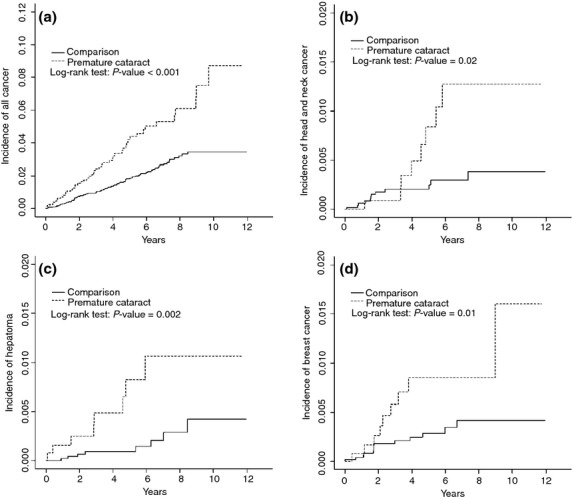
Cumulative incidences of compared between early-onset cataract cohort and comparison cohort for (a) all cancers, (b) head and neck cancer, (c) hepatoma and (d) breast cancer.
Discussion
Cataracts are common in the older population, while the early-onset cataract is rare for the younger population. No previous study has reported the relationship between early-onset cataracts and the risk of cancer. The present retrospective cohort study found that the cancer risk was more than twofold greater for patients with early-onset cataracts than for the general population, particularly for neck cancer, hepatoma and breast cancer. This finding is important for patients with early-onset cataracts, although the mechanism of developing cancer deserves further exploration.
The association with comorbidities revealed that subjects with diabetes and hyperlipidemia had a higher risk of developing cancer compared to the comparison group, with adjusted HR of 2.71 (95% CI = 1.09–6.75) and 2.51 (95% CI = 1.12–5.64), respectively. This finding is consistent with other epidemiological study findings.(6,7) Forte et al. found that diabetes and obesity are inextricably linked, which is associated with an increased incidence of solid tissue cancers.(6) Chen et al. found a twofold to threefold increase in the risk of hepatoma in patients with diabetes. Diabetes and obesity have synergistic effects with hepatitis B or C, increasing the risk more than 100-fold.(7)
Patients with metabolic syndrome have a constellation of problems, including obesity, dyslipidemia, diabetes and insulin resistance.(8) Among developed world studies, the Australian Blue Mountain Eye Study has shown that the metabolic syndrome is associated with all three types of cataracts (nuclear, cortical, and posterior subcapsular cataracts) in a cohort of the elderly.(9) A European study also reports increased odds of cataracts for middle-aged women with arterial pressure, central obesity and higher triglycerides.(10) Diabetes and hyperglycemia may advance the formation of glycation of lens proteins and the hyperosmotic effects of sorbitol on lens fibers through the aldose reductase pathway.(11) Although the mechanism linking hyperlipidemia and cataracts is unclear, certain pathophysiological mechanisms have been proposed. Leptin, secreted mainly by adipocytes, is a cytokine that has also been involved in the cataract formation.(12) People with hyperlipidemia are likely to have hyperleptinemia and leptin resistance.(13) Thus, hyperlipidemia may promote cataract formation. Furthermore, a Swedish study for women aged <65 years also demonstrates that metabolic syndrome components and their combination could increase the risk of cataract extraction.(14) Consequently, controlling metabolic syndrome and its components is considered vital for preventing early-onset cataracts.
In addition to metabolic syndrome, several possible mechanisms exist for the association between early-onset cataracts and cancer, including inflammation, ROS and genetic factors. ROS alters gene expression patterns and contributes to the carcinogenesis process through oxidative stress in cancer-associated fibroblasts.(5,15–19) The ROS effect on the oxidative damage to lens proteins may result in lens opacification.(3) Oxidative stress associated with ultraviolet (UV) light plays a central role in the pathogenesis of cataracts.(20–22) Therefore, if the antioxidative function is deficient at younger ages, early-onset cataracts may occur because of oxidatively-damaged DNA can not be repaired, leading to a higher cancer susceptibility for younger adults.
Another possible link between early-onset cataracts and cancer is genetic factors, relating to genetic polymorphisms, occurring in the general population for genes involved in a predisposition to carcinogenesis or cataractogenesis.(23–26) Among the several cancer-related polymorphic genes encoding for enzymes involved in free radical metabolism, the glutathione S-transferase (GST) gene system is one of the most well-known.(27,28) Saadat et al. find an odds ratio of 1.51 (P = 0.045) for cataracts in subjects with the null genotype of GSTM1.(26) GST are the family of phase II isoenzymes that protect against endogenous oxidative stress and exogenous potential toxins. They detoxify a variety of electrophilic compounds, generated by ROS damage to intracellular molecules.(29) UV light, chronic inflammation, hepatitis B and hepatitis C are all sources of ROS.(30) The polymorphisms of GSTM1, GSTT1, GSTP1 and GSTO2 have been associated with the risk of various cancers, such as breast cancer, hepatoma and skin carcinoma.(30–32) This may explain our result that the early-onset cataract cohort had significantly higher risk than the comparison cohort for all cancers, especially head and neck cancer, hepatoma and breast cancer. Hence, the polymorphism of GST may be a possible link between early-onset cataracts and carcinogenesis.
Our study has a few limitations. First, diagnoses of early-onset cataracts, cancer and other comorbid medical conditions are identified completely dependent on ICD codes. However, the NHI Bureau of Taiwan has established a mechanism to interview patients and reviews medical charts to verify diagnosis validity and quality of care. Hospitals receive heavy penalties from the NHI Bureau when discrepancies, overcharging and malpractice are discovered. To ensure the validity of the early-onset cataract diagnosis in this study, we ensured that all of the study cohort patients had at least three consensus diagnoses of early-onset cataracts. Second, a small number of patients may have undetected early-onset cataracts and would have been categorized as having non-early-onset cataracts, and, consequently, might have had a small chance of being selected as part of the comparison cohort. However, the sample sizes of both groups are large, which reduces the selection bias. In our study, patients with early-onset cataracts showed a significantly higher risk of cancer development. Further studies are necessary to confirm this association and the mechanisms involved. Third, those in the early-onset cataract cohort are more likely to have a history of hypertension, diabetes mellitus and hyperlipidemia. They were more likely to see a doctor and this might cause detection bias due to frequent examinations. Fourth, information on established risk factors of cancer, such as cigarette smoking, alcohol consumption, dietary habits and family history, are not available in the claims data. To minimize confounding from these factors, we have included in the data analysis smoking-related disorders such as stroke, ischemic heart disease, COPD and asthma, and alcohol related illnesses for adjustment. The overall measured adjusted cancer risk changed little from the crude risk. In addition, <5% women are smokers in Taiwan and the cancer risk associated with early onset cataracts was greater for women than for men.
In conclusion, this population-based study has demonstrated that early-onset cataracts are a significant predictor for subsequent cancer diagnosis after adjusting for possible confounding factors. Further studies should be conducted to see if our data can be replicated and to help clarify the underlying pathophysiological mechanisms of early-onset cataracts and their associations with cancer development.
Acknowledgments
Support was provided in part by the National Sciences Council, Executive Yuan (grant numbers SC99-2621-M-039-001), China Medical University Hospital (grant number 1MS1, DMR-103-065 and DMR-103-067), Taiwan Department of Health Clinical Trial and Research Center for Excellence (grant number DOH101-TD-B-111-004) and Cancer Research Center of Excellence (DOH101-TD-C-111-005).
Disclosure Statement
The authors have no conflict of interest.
References
- 1.Truscott RJ. Age-related nuclear cataract: a lens transport problem. Ophthalmic Res. 2000;32:185–94. doi: 10.1159/000055612. [DOI] [PubMed] [Google Scholar]
- 2.Spector A. Oxidation and aspects of ocular pathology. CLAO J. 1990;16:S8–10. [PubMed] [Google Scholar]
- 3.Augusteyn RC. Protein modification in cataract: possible oxidative mechanisms. In: Duncan G, editor. Mechanisms of Cataract Formation in the Human Lens. London: Academic Press; 1981. pp. 72–111. [Google Scholar]
- 4.Tsai SY, Hsu WM, Cheng CY, et al. Epidemiologic study of age-related cataracts among an elderly Chinese population in Shih-Pai Taiwan. Ophthalmology. 2003;110:1089–95. doi: 10.1016/S0161-6420(03)00243-4. [DOI] [PubMed] [Google Scholar]
- 5.Klaunig JE, Kamendulis LM, Hocevar BA. Oxidative stress and oxidative damage in carcinogenesis. Toxicol Pathol. 2010;38:96–109. doi: 10.1177/0192623309356453. [DOI] [PubMed] [Google Scholar]
- 6.Forte V, Pandey A, Abdelmessih R, et al. Obesity, diabetes, the cardiorenal syndrome, and risk for cancer. Cardiorenal Med. 2012;2:143–62. doi: 10.1159/000337314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen CL, Yang HI, Yang WS, et al. Metabolic factors and risk of hepatocellular carcinoma by chronic hepatitis B/C infection: a follow-up study in Taiwan. Gastroenterology. 2008;135:111–21. doi: 10.1053/j.gastro.2008.03.073. [DOI] [PubMed] [Google Scholar]
- 8.Farrell GC, Larter CZ. Nonalcoholic fatty liver disease: from steatosis to cirrhosis. Hepatology. 2006;43:S99–112. doi: 10.1002/hep.20973. [DOI] [PubMed] [Google Scholar]
- 9.Tan JS, Wang JJ, Mitchell P. Influence of diabetes and cardiovascular disease on the long term incidence of cataract: the Blue Mountains eye study. Ophthalmic Epidemiol. 2008;15:317–27. doi: 10.1080/09286580802105806. [DOI] [PubMed] [Google Scholar]
- 10.Paunksnis A, Bojarskiene F, Cimbalas A, et al. Relationship between cataract and metabolic syndrome and its components. Eur J Ophthalmol. 2007;17:605–14. doi: 10.1177/112067210701700420. [DOI] [PubMed] [Google Scholar]
- 11.Stitt AW. Advanced glycation: An important pathological event in diabetic and age related ocular disease. Br J Ophthalmol. 2001;85:746–53. doi: 10.1136/bjo.85.6.746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gomez-Ambrosi J, Salvador J, Fruhbeck G. Is hyperleptinemia involved in the development of age related lens opacities? Am J Clin Nutr. 2004;79:888–9. doi: 10.1093/ajcn/79.5.888. [DOI] [PubMed] [Google Scholar]
- 13.Narin F, Atabek ME, Karakukcu M, et al. The association of plasma homocysteine levels with serum leptin and apolipoprotein B levels in childhood obesity. Ann Saudi Med. 2005;25:209–14. doi: 10.5144/0256-4947.2005.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lindblad BE, Hakansson N, Philipson B, Wolk A. Metabolic syndrome components in relation to risk of cataract extraction: a prospective cohort study of women. Ophthalmology. 2008;115:1687–92. doi: 10.1016/j.ophtha.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 15.Balliet RM, Capparelli C, Guido C, et al. Mitochondrial oxidative stress in cancer-associated fibroblasts drives lactate production, promoting breast cancer tumor growth: understanding the aging and cancer connection. Cell Cycle. 2011;10:4065–73. doi: 10.4161/cc.10.23.18254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martinez-Outschoorn UE, Balliet RM, Rivadeneira DB, et al. Oxidative stress in cancer associated fibroblasts drives tumor-stroma co-evolution: a new paradigm for understanding tumor metabolism, the field effect and genomic instability in cancer cells. Cell Cycle. 2010;9:3256–76. doi: 10.4161/cc.9.16.12553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Whitaker-Menezes D, Martinez-Outschoorn UE, Flomenberg N, et al. Hyperactivation of oxidative mitochondrial metabolism in epithelial cancer cells in situ: visualizing the therapeutic effects of metformin in tumor tissue. Cell Cycle. 2011;10:4047–64. doi: 10.4161/cc.10.23.18151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pavlides S, Tsirigos A, Vera I, et al. Loss of stromal caveolin-1 leads to oxidative stress, mimics hypoxia and drives inflammation in the tumor microenvironment, conferring the “reverse Warburg effect”: a transcriptional informatics analysis with validation. Cell Cycle. 2010;9:2201–19. doi: 10.4161/cc.9.11.11848. [DOI] [PubMed] [Google Scholar]
- 19.Whitaker-Menezes D, Martinez-Outschoorn UE, Lin Z, et al. Evidence for a stromal-epithelial “lactate shuttle” in human tumors: MCT4 is a marker of oxidative stress in cancer-associated fibroblasts. Cell Cycle. 2011;10:1772–83. doi: 10.4161/cc.10.11.15659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ayala MN, Michael R, Soderberg PG. In vivo cataract after repeated exposure to ultraviolet radiation. Exp Eye Res. 2000;70:451–6. doi: 10.1006/exer.1999.0801. [DOI] [PubMed] [Google Scholar]
- 21.Ayala MN, Michael R, Soderberg PG. Influence of exposure time for UV radiation-induced cataract. Invest Ophthalmol Vis Sci. 2000;41:3539–43. [PubMed] [Google Scholar]
- 22.West SK, Longstreth JD, Munoz BE, et al. Model of risk of cortical cataract in theUSpopulation with exposure to increased ultraviolet radiation due to stratospheric ozone depletion. Am J Epidemiol. 2005;162:1080–8. doi: 10.1093/aje/kwi329. [DOI] [PubMed] [Google Scholar]
- 23.Hayes JD, Flanagan JU, Jowsey IR. Glutathione transferases. Annu Rev Pharmacol Toxicol. 2005;45:51–88. doi: 10.1146/annurev.pharmtox.45.120403.095857. [DOI] [PubMed] [Google Scholar]
- 24.Landi S. Mammalian class theta GST and differential susceptibility to carcinogens: a review. Mutat Res. 2000;463:247–83. doi: 10.1016/s1383-5742(00)00050-8. [DOI] [PubMed] [Google Scholar]
- 25.Salama SA, Abdel-Rahman SZ, Sierra-Torres CH, et al. Role of polymorphic GSTM1 and GSTT1 genotypes on NNK-induced genotoxicity. Pharmacogenetics. 1999;9:735–43. [PubMed] [Google Scholar]
- 26.Saadat I, Ahmadi Z, Farvardin-Jahromi M, Saadat M. Association between cataract and genetic polymorphisms of GSTM1, GSTT1, and GSTO2 with respect of work place. Mol Vis. 2012;18:1996–2000. [PMC free article] [PubMed] [Google Scholar]
- 27.Mannervik B. The isozymes of glutathione S-transferase. Adv Enzymol. 1985;57:357–417. doi: 10.1002/9780470123034.ch5. [DOI] [PubMed] [Google Scholar]
- 28.Lichtenstein P, Holm NV, Verkasalo PK, et al. Environmental and heritable factors in the causation of cancer-analyses of cohorts of twins from Sweden, Denmark, and Finland. N Engl J Med. 2000;343:78–85. doi: 10.1056/NEJM200007133430201. [DOI] [PubMed] [Google Scholar]
- 29.Vineis P. Cancer as an evolutionary process at the cell level: an epidemiological perspective. Carcinogenesis. 2003;24:1–6. doi: 10.1093/carcin/24.1.1. [DOI] [PubMed] [Google Scholar]
- 30.White DL, Li D, Nurgalieva Z, El-Serag HB. Genetic variants of glutathione S-transferase as possible risk factors for hepatocellular carcinoma: a HuGE systematic review and meta-analysis. Am J Epidemiol. 2008;167:377–89. doi: 10.1093/aje/kwm315. [DOI] [PubMed] [Google Scholar]
- 31.Sohail A, Kanwal N, Ali M, et al. Effects of glutathione-S-transferase polymorphisms on the risk of breast cancer: a population-based case-control study in Pakistan. Environ Toxicol Pharmacol. 2013;35:143–53. doi: 10.1016/j.etap.2012.11.014. [DOI] [PubMed] [Google Scholar]
- 32.Leite JL, Morari EC, Granja F, et al. Influence of the glutathione s-transferase gene polymorphisms on the susceptibility to basal cell skin carcinoma. Rev Med Chil. 2007;135:301–6. doi: 10.4067/s0034-98872007000300004. [DOI] [PubMed] [Google Scholar]


