Abstract
RUNX3 is a tumor suppressor for a variety of cancers. RUNX3 suppresses the canonical Wnt signaling pathway by binding to the TCF4/β-catenin complex, resulting in the inhibition of binding of the complex to the Wnt target gene promoter. Here, we confirmed that RUNX3 suppressed Wnt signaling activity in several gastric cancer cell lines; however, we found that RUNX3 increased the Wnt signaling activity in KatoIII and SNU668 gastric cancer cells. Notably, RUNX3 expression increased the ratio of the Wnt signaling-high population in the KatoIII cells. although the maximum Wnt activation level of individual cells was similar to that in the control. As found previously, RUNX3 also binds to TCF4 and β-catenin in KatoIII cells, suggesting that these molecules form a ternary complex. Moreover, the ChIP analyses revealed that TCF4, β-catenin and RUNX3 bind the promoter region of the Wnt target genes, Axin2 and c-Myc, and the occupancy of TCF4 and β-catenin in these promoter regions is increased by the RUNX3 expression. These results suggest that RUNX3 stabilizes the TCF4/β-catenin complex on the Wnt target gene promoter in KatoIII cells, leading to activation of Wnt signaling. Although RUNX3 increased the Wnt signaling activity, its expression resulted in suppression of tumorigenesis of KatoIII cells, indicating that RUNX3 plays a tumor-suppressing role in KatoIII cells through a Wnt-independent mechanism. These results indicate that RUNX3 can either suppress or activate the Wnt signaling pathway through its binding to the TCF4/β-catenin complex by cell context-dependent mechanisms.
Keywords: β-catenin, gastric cancer, RUNX3, TCF4, Wnt signaling
RUNX3 is a member of the runt-related transcription factor RUNX family and was originally identified as a tumor suppressor of gastric cancer development.(1–4) In approximately 80% of gastric cancers, RUNX3 expression is lost due to epigenetic silencing and mislocalization in the cytoplasm.(1,4,5) Moreover, expression of RUNX3 in gastric cancer cells results in suppression of tumorigenicity, while expression of the mutant form of RUNX3 R122C found in human gastric cancer does not affect tumorigenicity.(1,6) Consistently, gastric epithelial cells derived from Runx3 −/− p53 −/− mice form explanted tumors in nude mice.(7) The functional inactivation of RUNX3 is frequently observed in other solid tumors, including colon, pancreatic and lung cancers.(3,4) Taken together, these results indicate that RUNX3 plays a tumor suppressing role in a variety of cancers.
RUNX3 has multiple partners and is involved in diverse signaling pathways.(3,4) Wnt signaling suppresses phosphorylation of β-catenin by GSK-3β, leading to the accumulation of β-catenin in nuclei.(8) Accumulated β-catenin forms a complex with TCF4, which induces the transcription of Wnt target genes by binding to the promoter regions of these genes. The constitutive activation of Wnt signaling by genetic alteration leads to gastrointestinal tumor development.(9–11) It has previously been demonstrated in colon cancer cells that RUNX3 binds to TCF4 through the runt domain, forming a ternary complex of RUNX3, TCF4 and β-catenin, which inhibits the binding of the complex to the promoter region of Wnt target genes, thereby suppressing Wnt signaling.(12) The expression of Wnt target genes is significantly increased in Runx3 −/− mouse intestinal mucosa without any alteration of the expression levels of TCF4 and β-catenin, and Runx3 + /− mice develop intestinal tumors.(12) Notably, the association of the mutant RUNX3 R122C with TCF4 is weaker than wild-type RUNX3; thus, R122C cannot suppress Wnt signaling in Runx3 −/− gastric tumor cells.(13) These results indicate that Wnt activation by RUNX3 downregulation contributes to tumorigenicity.
In contrast to these findings, we present the unexpected finding that RUNX3 activates Wnt signaling in KatoIII and SNU668 gastric cancer cells. Interestingly, RUNX3 binds TCF4 and β-catenin also in the KatoIII cells, and binding of the complex to Wnt target gene promoter is more stable in the presence of RUNX3, which may cause Wnt signaling activation. Accordingly, it is possible that RUNX3 can either suppress or activate Wnt signaling activity by binding to the TCF4/β-catenin complex, and the direction of Wnt signaling modulation may be regulated by a cell context-dependent mechanism.
Materials and Methods
Cell culture experiments
Human gastric cancer cell lines, AGS (ATCC), AZ521, MKN45, KatoIII, (RIKEN, BioResource Center, Tsukuba, Japan), SNU216, SNU484, SNU601, SNU638, SNU668 and SNU719 (Korean Cell Line Bank, Seoul, Korea) were cultured in RPMI1640 supplemented with 10% FBS. The cell proliferation rate was examined using the Alamar Blue Cell Viability Reagent (Invitrogen, Carlsbad, CA, USA). For the soft agar colony formation assay, cells were suspended in 0.33% agarose contained in the medium and seeded on 0.5% bottom agar. After 21 days of culture, soft agar was stained with Giemsa solution (Wako, Osaka, Japan) and colony numbers were scored.
Cells were transfected with pcDNA3, pcDNA-Flag-RUNX3 or pcDNA-Flag-RUNX3(R122C) vector.(6) KatoIII-R3 stable cell line was constructed by transfection with pcDNA-RUNX3 and selected with G418 (Wako) at 100 μg/mL. To knock down gene expression, cells were transfected with Silencer Select siRNA for RUNX3 or β-catenin (Ambion, Cambridge, MA, USA).
To examine the Wnt activation level, cells were cotransfected with super 8× TOPflash or Super 8× FOPflash (Addgene, Cambridge, MA, USA), together with pcDNA3, pcDNA-Flag-RUNX3 or pcDNA-Flag-RUNX3(R122C).(6) At 24 h after transfection, the luciferase activity was measured using a Luciferase assay system (Promega, Madison, WI, USA).
Wnt suppression and activation
To inhibit Wnt signaling, cells were treated with 10 μg/mL of C59 (provided by Dr David Virshup), which inhibits porcupine, a membrane-bound O-acyltransferase required for Wnt palmitoylation.(14) To activate Wnt signaling, conditioned media including Wnt3a and Rspondin were prepared from L cells expressing Wnt3a and 293T cells expressing Rspondin, respectively (provided by Dr Marc Leushacke), and the conditioned media were supplemented at 10% volume in the culture medium.
Western blotting
A total of 10 μg of protein samples were separated in 10% SDS-polyacrylamide gels. Antibodies for RUNX3(5) or unphosphorylated β-catenin (Millipore, Billerica, MA, USA) were used as the primary antibodies. The anti-β-actin antibody (Sigma, St. Louis, MO, USA) was used as an internal control, and the ECL detection system (GE Healthcare, Buckinghamshire, UK) was used to detect the signals.
Real-time RT-PCR
Total RNA was extracted using ISOGEN (Nippon Gene, Tokyo, Japan) and cDNA was constructed using the Prime Script RT Reagent Kit (Takara, Tokyo, Japan). Real-time RT-PCR was performed using the SYBR Premix Ex TaqII (Takara) and Stratagene Mx3000P (Agilent Technologies, Santa Clara, CA, USA). The primers were purchased from Takara.
Flow cytometry analysis
To examine the intracellular RUNX3 and β-catenin levels, permeabilized cells were incubated with the primary antibodies for total β-catenin (Sigma) or RUNX3,(5) followed by the secondary antibodies for rabbit IgG-conjugated with Alexa 488 (Molecular Probes, Grand Island, NY, USA) or mouse IgG-conjugated with Alexa 633 (Invitrogen), and examined using FACS Canto II (BD Biosciences, San Jose, CA, USA). Cells were transfected with a pcDNA-RUNX3-IRES-mGFP expression vector, in which internal ribosome entry site (IRES) fragment from pTRE3G-IRES (Clontech Laboratories, Mountain View, CA, USA) and maxGFP cDNA from pmaxGFP (LONZA, Allendale, NJ, USA) were subcloned to pcDNA-Flag-RUNX3, and RUNX3-expressing cells were isolated using the FACS Aria cell sorter (BD Biosciences, San Jose, CA, USA) to collect GFP-expressing cells.
Immunocytochemistry
The cells were seeded on cover slips and fixed in 4% paraformaldehyde, then permeabilized with 0.5% Triton X-100 in PBS. Antibodies against total β-catenin (Sigma) or RUNX3 were used as the primary antibodies, and anti-rabbit IgG Alexa 594 or anti-mouse IgG Alexa 488 (Molecular Probes) were used as the secondary antibodies.(5)
Immunoprecipitation
KatoIII cells were transfected with the pcDNA-Flag-RUNX3 or pcDNA-Flag, and the cell lysates were used for immunoprecipitation with anti-FLAG M2 agarose (Sigma). Western blotting was performed using antibodies against unphosphorylated β-catenin (Millipore), TCF4 (Santa Cruz Biotechnology, Santa Cruz, CA), FLAG peptide or β-actin (Sigma).
ChIP
The cells were treated with formaldehyde solution (Wako) for crosslinking. ChIP was performed using the ChIP Assay kit EZ ChIP (Millipore) and antibodies against TCF4 (Santa Cruz Biotechnology), unphosphorylated β-catenin (Millipore), RUNX3(5) and mouse normal IgG. The primer sequences for the c-Myc promoter were: 5′-TTGCTGGGTTATTTTAATCAT-3′ and 5′-ACTGTTTGACAAACCGCATCC-3′.(15) For the Axin2 promoter, conserved TCF/LEF binding sites are localized in intron 1,(16) and Simple ChIP Human Axin2 Intron 1 Primers (Cell Signaling, Danvers, MA, USA) were used.
Statistical analysis
Statistical analyses were performed using the unpaired Student's t-test, with P-values <0.05 considered significant.
Results
Wnt activation by RUNX3 expression in KatoIII and SNU668 cells
RUNX3 expression was detected by western blotting in AZ521 and MKN45 cells, while it was not detected in other gastric cancer cell lines (Fig. 1a). Consistently, high levels of RUNX3 mRNA were detected in AZ521 and MKN45 cells by RT-PCR (Fig. S1). We transiently transfected the RUNX3 expression vector to all gastric cell lines, and examined Wnt signaling activity by luciferase reporter analysis. The Wnt signaling activity was significantly decreased in SNU216, SNU601, SNU638 and SNU719 cells, which was consistent with the previous results.(12) However, RUNX3 expression increased Wnt signaling activity in KatoIII and SNU668 cells (Fig. 1b). Importantly, the R122C mutant form of RUNX3 that is defective in the RUNX3 function did not change Wnt signaling activity in these cells.(1,6) The KatoIII-R3, stable RUNX3-expressing cells (Fig. S2) also exhibited an increased luciferase activity, which was suppressed by RUNX3 siRNA transfection (Fig. 1c). Moreover, Wnt activation levels increased gradually in accordance with the amount of the RUNX3 expression vector (Fig. 1d). Consistently, the expression levels of Wnt target genes, Sox4 and Axin2, increased significantly in KatoIII and SNU668 cells by RUNX3 expression (Fig. 1e).
Fig. 1.
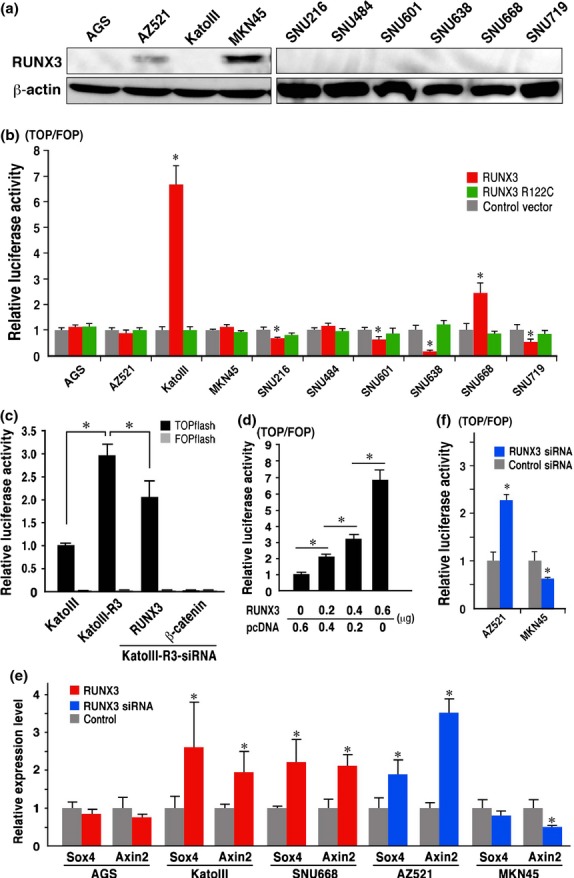
RUNX3-induced Wnt signaling activation. (a) Western blotting of the RUNX3 expression in gastric cancer cells. (b) Relative luciferase activity of TOPflash/FOPflash in the respective gastric cancer cells transfected with RUNX3 or R122C mutant RUNX3 vector to the control vector-transfected cells. (c) Luciferase activity of the TOPflash and FOPflash in KatoIII-R3 cells and siRNA-transfected KatoIII-R3 cells relative to the level in the parental KatoIII cells. (d) Relative luciferase activity of TOPflash/FOPflash in KatoIII cells transfected with RUNX3 expression vector. (e) Relative expression levels of the Wnt target genes, SOX4 and Axin2, detected by RT-PCR in the RUNX3 vector-transfected cells (red) or RUNX3 siRNA-transfected cells (blue) to the control level. (f) Relative luciferase activity of TOPflash/FOPflash in AZ521 and MKN45 cells transfected with RUNX3 siRNA to the control level. (b–f) Bar graphs are shown as mean ± SD. *P < 0.05 versus control levels otherwise indicated.
Notably, inhibition of endogenous RUNX3 expression by siRNA in AZ521 significantly increased Wnt signaling activity, whereas RUNX3 siRNA transfection partially suppressed Wnt signaling in MKN45 cells (Fig. 1e,f). These results, taken together, suggest that RUNX3 suppresses or activates the Wnt signaling in a cell context-dependent mechanism.
Saturation of ligand-induced β-catenin stabilization in KatoIII cells
We further examined the RUNX3-induced Wnt activation mechanism using KatoIII cells. In KatoIII cells, Wnt signaling is activated by β-catenin gene amplification.(17) Treatment of control KatoIII cells with a Wnt ligand secretion inhibitor C59 significantly suppressed the endogenous Wnt signaling, indicating that Wnt ligand stimulation maintains the basal Wnt activation level (Fig. 2a). C59 treatment also decreased the luciferase activity in the RUNX3-expressing KatoIII cells. However, the ratio of the RUNX3-induced increase of luciferase activity in the C59-treated cells was similar to that in the control cells; that is, approximately 4.5-fold the control levels. Accordingly, it is possible that RUNX3 increases Wnt signaling activity in KatoIII cells through a ligand-independent mechanism.
Fig. 2.
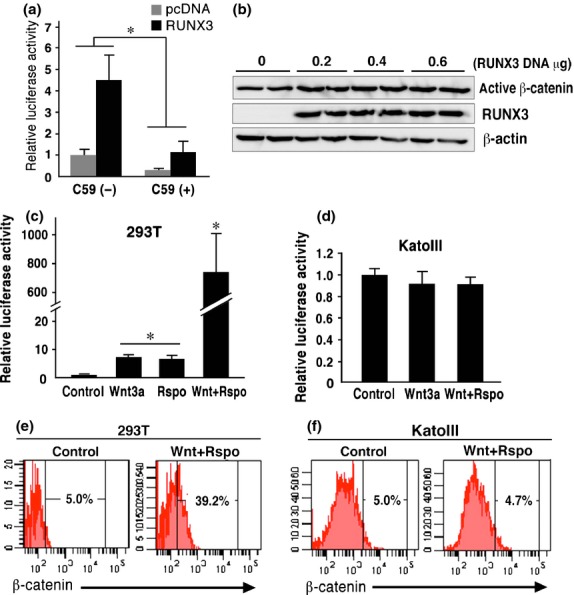
Saturation of ligand-induced β-catenin stabilization in KatoIII cells. (a) Luciferase activity of TOPflash/FOPflash in KatoIII cells transfected with RUNX3 or control vector in the absence or presence of the C59 relative to the control cell level (mean ± SD). *P < 0.05. (b) Western blotting for active β-catenin and RUNX3 in KatoIII cells transfected with RUNX3 vector. (c,d) Relative luciferase activity of TOPflash/FOPflash in the 293T cells (c) and KatoIII cells (d) treated with Wnt3a and/or Rspondin. (mean ± SD). *P < <0.05 versus control level. (e,f) Flow cytometry analyses of β-catenin in the control cells (left) and cells treated with Wnt3a/Rspondin (right) in 293T (e) and KatoIII cells (f). The proportion (%) of the β-cateninHi population (top 5% level of the control) is indicated.
We next examined the β-catenin levels of the RUNX3-transfected cells by western blotting. Although the active β-catenin levels were slightly increased both in the KatoIII-R3 cells and the RUNX3 vector-transiently transfected KatoIII cells (Fig. 2b, Fig. S2 and S3), the increase was not sufficient to explain the marked increase of the TOPflash activity (Fig. 1b).
The Wnt activation level increased significantly in 293T cells following treatment with Wnt3a and/or Rspondin (Fig. 2c). However, treatment of KatoIII cells with Wnt3a/Rspondin did not change the luciferase activity (Fig. 2d). Consistently, the β-catenin high population measured by flow cytometry was significantly increased in the 293T cells following Wnt3a/Rspondin treatment, while the β-catenin high population in the KatoIII cells did not change following stimulation with Wnt3a/Rspondin (Fig. 2e,f). Therefore, it is possible that the Wnt ligand-induced β-catenin stabilization level is saturated in KatoIII cells, and RUNX3 activates Wnt signaling through mechanisms other than β-catenin stabilization.
Increase of β-cateninHi population by RUNX3 expression in KatoIII cells
We previously found that the Wnt activation level in individual KatoIII cells oscillates between a Wnt-high and Wnt-low state.(18) We thus examined the β-catenin levels of the RUNX3-expressing cells using flow cytometry. When the RUNX3 vector was transfected, the β-cateninHi population (top 50% level of the control in Fig. 3a, [left]) increased significantly in the RUNX3Hi cells (78%: Q2/(Q1 + Q2)), but not in the RUNX3Lo cells (45%: Q4/(Q3 + Q4)) (Fig. 3a [right], b). Notably, the maximum β-catenin level in the RUNX3-transfected cells was similar to that observed in the control (Fig. 3a). We confirmed that transfection efficiency was not different between β-cateninHi and β-cateninLo KatoIII cells (Fig. S4). Immunocytochemistry of the RUNX3 vector-transfected KatoIII cells showed that approximately 73% of the RUNX3-positive cells were also positive for β-catenin, whereas approximately 44% of the RUNX3-negative cells were β-catenin positive (Fig. 3c). These results indicate that RUNX3 expression increases the number of cells in the Wnt-high population of KatoIII cells.
Fig. 3.
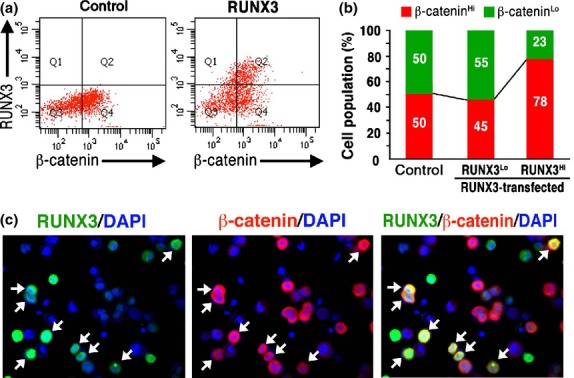
The increased β-cateninHi population in RUNX3-expressing KatoIII cells. (a) Flow cytometry analyses of β-catenin and RUNX3 expression in the control (left) and RUNX3-transfected (right) KatoIII cells. The β-cateninHi population (Q2 and Q4) was set as the top 50% level in the control KatoIII cells. (b) Proportion of the β-cateninHi and β-cateninLo cells in the control cells (control) or RUNX3Lo and RUNX3Hi of the RUNX3-transfected cells. (c) Immunocytochemistry for RUNX3 (green, left) and β-catenin (red, center) and a merged image (right) with DAPI staining (blue) of RUNX3-transfected KatoIII cells. Arrows, RUNX3 and β-catenin double-positive cells.
Tumor suppressing function of RUNX3 in KatoIII cells
It has been reported that RUNX3 suppresses the tumorigenicity of KatoIII cells.(19) Therefore, we reexamined the role of RUNX3 in the tumorigenicity of KatoIII cells. The RUNX3 vector-transfected KatoIII cells exhibited slight but significant suppression of cell proliferation at 48 and 72 h after seeding (Fig. 4a), and RUNX3 siRNA increased proliferation KatoIII-R3 cells at 48 h after seeding (Fig. S5).
Fig. 4.
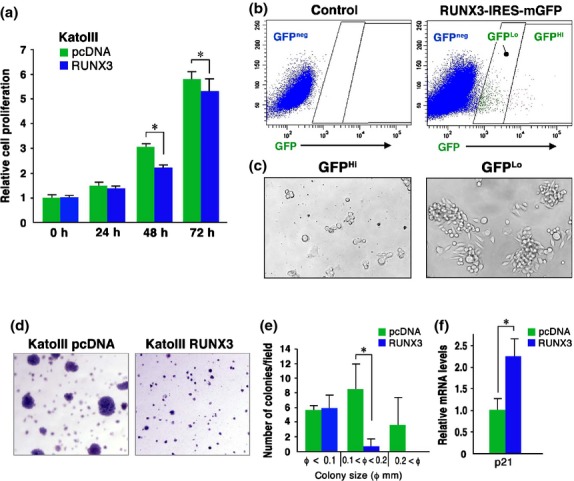
Suppression of tumorigenicity of KatoIII cells by RUNX3. (a) Relative cell proliferation rates of RUNX3 vector-transfected KatoIII cells and control vector-transfected cells at the indicated time points (mean ± SD). *P < 0.05. (b) Flow cytometry analyses for GFP expression in the control (left) and RUNX3-IRES-mGFP-transfected (right) KatoIII cells. (c) Representative photographs of GFPHi (left) and GFPLo (right) cells sorted from RUNX3-IRES-mGFP-transfected KatoIII cells after culture for 6 days. (d) Representative photographs of the soft agar colonies of the control (left) and RUNX3-transfected KatoIII cells (right). (e) The number of soft agar colonies per dissecting microscopic field. *P < 0.05. (f) Relative p21 mRNA expression level in RUNX3 vector-transfected KatoIII cells to the control cells (mean ± SD). *P < 0.05.
We next transfected the RUNX3-IRES-mGFP expression vector to KatoIII cells, and GFPHi and GFPLo cells were isolated by cell sorting, corresponding to RUNX3Hi-expressing and RUNX3Lo-expressing cells, respectively (Fig. 4b). Importantly, proliferation of GFPHi cells was significantly suppressed compared with that of the GFPLo cells (Fig. 4c), and we were unable to establish a GFPHi (RUNX3Hi) cell line due to the limited proliferation. Moreover, RUNX3-transfected KatoIII cells exhibited significant suppression of soft agar colony formation (Fig. 4d), and the number of colonies larger than 0.1 mm in diameter was significantly decreased to 5.6% of the control (Fig. 4e). Moreover, expression of cell cycle inhibitor p21WAF1/Cip1 was increased significantly in KatoIII cells by RUNX3 expression (Fig. 4f), which was consistent with previous report.(20) These results indicate that RUNX3 plays a tumor-suppressing role in KatoIII cells, and that RUNX3-dependent Wnt activation is not sufficient to maintain the tumorigenicity of RUNX3Hi KatoIII cells.
Binding of the RUNX3 and TCF4 complex to the Wnt target gene promoters in KatoIII cells
It has previously been shown that RUNX3 binds to TCF4/β-catenin complex, which suppresses the binding of the complex to the Wnt target gene promoters.(12,13) Notably, immunoprecipitation experiments revealed that RUNX3 bound β-catenin and TCF4 also in the RUNX3-transfected KatoIII cells (Fig. 5a), suggesting that RUNX3, TCF4 and β-catenin form a ternary complex also in KatoIII cells.
Fig. 5.
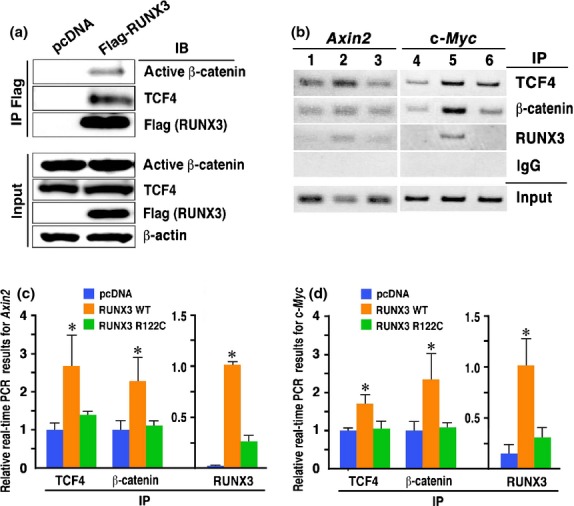
Binding of the RUNX3, TCF4 and β-catenin complex to Wnt target gene promoters. (a) Immunoprecipitation for β-catenin and TCF4 with an anti-Flag (RUNX3) antibody. (b) ChIP analyses of the Axin2 (left) and c-Myc (right) promoter regions using lysates precipitated (IP) with anti-TCF4, anti-β-catenin and anti-RUNX3 antibodies. Lanes 1 and 4, control KatoIII cells; Lanes 2 and 5, RUNX3-expressing KatoIII cells; and Lanes 3 and 6, R122C mutant RUNX3-expressing KatoIII cells. (c,d) ChIP-based real-time PCR for the Axin2 (c) and c-Myc (d) promoters (mean ± SD). ChIP samples were precipitated (IP) with anti-TCF4, anti-unphosphorylated β-catenin or anti-RUNX3 antibodies from control cells (blue), RUNX3-expressing KatoIII cells (orange) or R122C mutant RUNX3-expressing KatoIII cells (green), and examined by real-time PCR. *P < 0.05 versus control.
We next performed ChIP analyses to examine whether the binding of the TCF4/β-catenin complex to the promoter of the Wnt target genes was suppressed in the RUNX3-expressing KatoIII cells. In the control KatoIII cells, TCF4 and β-catenin bound to the Axin2 and c-Myc gene promoters (Fig. 5b, Lanes 1 and 4). Importantly, TCF4 and β-catenin also bound to the Axin2 and c-Myc gene promoters in the RUNX3-expressing KatoIII cells, and their band intensities were higher than those of the control cells (Fig. 5b, Lanes 2 and 5). Consistently, ChIP-based real-time PCR analysis showed that the quantified DNA amount of the Axin2 and c-Myc gene promoters that bound TCF4 and β-catenin was significantly higher in the RUNX3-expressing KatoIII cells compared with the control cells (Fig. 5c,d). Moreover, we confirmed that RUNX3 bound the Axin2 and c-Myc gene promoters in the RUNX3-expressing KatoIII cells (Fig. 5b, Lanes 2 and 5, and Fig. 5c,d). In contrast, the R122C mutant RUNX3 did not significantly increase the binding of TCF4 and β-catenin to the Axin2 and c-Myc gene promoters (Fig. 5b, Lanes 3 and 6, and Fig. 5c,d). These results indicate that the complex consisting of RUNX3, TCF4 and β-catenin binds to the promoter of Wnt target genes in KatoIII cells in a more stable fashion than the TCF4/β-catenin complex, which may increase the proportion of the Wnt-high cells in the RUNX3-expressing KatoIII cells.
Discussion
It has been demonstrated that RUNX3 binds to TCF4 and β-catenin, resulting in suppression of the binding of TCF4 to the Wnt target gene promoters, thereby suppressing the Wnt signaling.(12) In the present study, we found that RUNX3 increased the Wnt activity in KatoIII and SNU668 cells. RUNX3 bound TCF4 and β-catenin also in the KatoIII cells, and the complex was stabilized to bind Wnt target gene promoter, which is in contrast to the previous findings.(12,13)
The important question is how RUNX3 activates Wnt signaling in KatoIII and SNU668 cells. It is known that β-catenin replaces the transcriptional repressor, Groucho, from TCF in the nucleus, and recruits cofactors such as Bcl9 and pygopus to the TCF4/β-catenin complex, inducing target gene transcription.(8,21,22) It has also been reported that additional factors are required for the recruitment of β-catenin to the target gene promoters. Transducin β-like protein 1 (TBL1) and its related family member, TBLR1, recruit β-catenin to TCF on the Wnt target gene promoter.(22,23) TBL1 can bind both TCF4 and β-catenin, suggesting that it strengthens their physical association, which may contribute to Wnt activation. Jerky also recruits β-catenin to chromatin, and promotes the association of β-catenin and LEF1, resulting in the induction of Wnt target gene expression.(22,24) We found that RUNX3 increases the occupancy of the TCF4/β-catenin complex in KatoIII cells, suggesting that RUNX3 plays a role in the stabilization of the TCF4/β-catenin complex on the Wnt target gene promoter like TBL1 and Jerky. It is also possible that RUNX3 is involved in the recruitment of β-catenin to TCF4 on the Wnt target gene promoter like these molecules. We have previously shown that the Wnt activation levels are oscillating in the individual KatoIII cells,(18) and in this study, we showed that the RUNX3 expression increases the ratio of the Wnt-high population. Accordingly, it is conceivable that RUNX3 maintains the Wnt activation at a high level, suppressing the decrease of Wnt activity by stabilizing the TCF4/β-catenin complex on the Wnt target gene promoters.
Another unsolved question is how the RUNX3/TCF4 complex can bind to the DNA of the Wnt target gene promoter in KatoIII cells. As previously described, RUNX3-bound TCF4 cannot bind chromatin, possibly due to the competition for the DNA binding region of TCF4 with RUNX3. It is possible that cofactor(s) in the complex affect the conformation of the RUNX3/TCF4/β-catenin complex. Genetic polymorphisms of such cofactor(s) may cause conformational changes of the complex, which allows RUNX3-bound TCF4 to bind the Wnt target gene promoter. It would be worth examining the DNA sequences of cofactors of the TCF4/β-catenin complex in KatoIII and SNU668 cells to understand the molecular mechanism(s) responsible for the RUNX3-associated Wnt regulation.
In the present study, we also confirmed that RUNX3 transfection significantly suppressed the proliferation and tumorigenicity of KatoIII cells with induction of p21 expression. Moreover, RUNX3 has been shown to induce the apoptosis of gastric cancer cells by upregulating apoptosis-related genes.(25) It is thus possible that these gene products suppressed the proliferation and survival of RUNX3Hi-expressing KatoIII cells, and that RUNX3-induced Wnt activation is not sufficient to protect KatoIII cells from RUNX3-induced apoptosis. It remains to be investigated whether RUNX3 suppresses tumorigenicity also in SNU668 cells. However, it has been reported that RUNX3 is upregulated and functions as an oncogene in head and neck cancer cells by increasing the proliferation and sphere formation.(26,27) Accordingly, the RUNX3-associated tumor suppressing functions are likely inactivated in head and neck cancer cells, and, therefore, it is possible that RUNX3-associated Wnt activation contributes to tumorigenesis in such cancer cells.
The present results, together with previous findings,(12,13) indicate that RUNX3 can either suppress or activate Wnt signaling through binding to the TCF4/β-catenin complex. Although it remains to be investigated, cofactor(s) that bind the RUNX3/TCF4/β-catenin complex may be involved in the decision regarding the direction of Wnt signaling modulation; that is, suppression or activation.
Acknowledgments
We thank Dr Marc Leushacke for providing Wnt3a/Pspondin cells, and Dr David Virshup for providing C59. We thank Manami Watanabe and Ayako Tsuda for technical assistance. This work was supported by Grants-in-Aid for Scientific Research on Innovative Areas from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
Disclosure Statement
The authors have no conflict of interest.
Supporting Information
Additional supporting information may be found in the online version of this article:
Fig. S1 Relative RUNX3 mRNA levels in gastric cancer cell lines examined by RT-PCR.
Fig. S2 Western blotting results of RUNX3 and active form of β-catenin in KatoIII cells and KatoIII-R3 cells.
Fig. S3 Relative band intensities calculated from the Western blotting results (Fig. 2b) of active β-catenin and RUNX3 in the RUNX3 expression vector-transfected KatoIII cells.
Fig. S4 Flow cytometry analyses of β-catenin and GFP in the control KatoIII cells and GFP expression vector-transfected KatoIII cells.
Fig. S5 Cell proliferation rates of RUNX3 siRNA-transfected KatoIII-R3 cells.
References
- 1.Li QL, Ito K, Sakakura C, et al. Causal relationship between the loss of RUNX3 expression and gastric cancer. Cell. 2002;109:113–24. doi: 10.1016/s0092-8674(02)00690-6. [DOI] [PubMed] [Google Scholar]
- 2.Blyth K, Cameron ER, Neil JC. The RUNX genes: gain or loss of function in cancer. Nat Rev Cancer. 2005;5:376–87. doi: 10.1038/nrc1607. [DOI] [PubMed] [Google Scholar]
- 3.Chuang LSH, Ito K, Ito Y. RUNX family: regulation and diversification of roles through interacting proteins. Int J Cancer. 2013;132:1260–71. doi: 10.1002/ijc.27964. [DOI] [PubMed] [Google Scholar]
- 4.Chuang LSH, Ito Y. RUNX3 is multifunctional in carcinogenesis of multiple solid tumors. Oncogene. 2010;29:2605–15. doi: 10.1038/onc.2010.88. [DOI] [PubMed] [Google Scholar]
- 5.Ito K, Liu Q, Salto-Tellez M, et al. RUNX3, a novel tumor suppressor, is frequently inactivated in gastric cancer by protein mislocalization. Cancer Res. 2005;65:7743–50. doi: 10.1158/0008-5472.CAN-05-0743. [DOI] [PubMed] [Google Scholar]
- 6.Guo WH, Weng LQ, Ito K, et al. Inhibition of growth of mouse gastric cancer cells by Runx3, a novel tumor suppressor. Oncogene. 2002;21:8351–5. doi: 10.1038/sj.onc.1206037. [DOI] [PubMed] [Google Scholar]
- 7.Fukamachi H, Ito K, Ito Y. Runx3−/− gastric epithelial cells differentiate into intestinal type cells. Biochem Biophys Res Commun. 2004;321:58–64. doi: 10.1016/j.bbrc.2004.06.099. [DOI] [PubMed] [Google Scholar]
- 8.Clevers H, Nusse R. Wnt/β-catenin signaling and disease. Cell. 2012;149:1192–205. doi: 10.1016/j.cell.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 9.Oshima M, Oshima H, Kitagawa K, Kobayashi M, Itakura C, Taketo M. Loss of Apc heterozygosity and abnormal tissue building in nascent intestinal polyps in mice carrying a truncated Apc gene. Proc Natl Acad Sci USA. 1995;92:4482–6. doi: 10.1073/pnas.92.10.4482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harada N, Tamai Y, Ishikawa T, et al. Intestinal polyposis in mice with a dominant stable mutation of the β-catenin gene. EMBO J. 1999;18:5931–42. doi: 10.1093/emboj/18.21.5931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oshima H, Matsunaga A, Fujimura T, Tsukamoto T, Taketo MM, Oshima M. Carcinogenesis in mouse stomach by simultaneous activation of the Wnt signaling and prostaglandin E2 pathway. Gastroenterology. 2006;131:1086–95. doi: 10.1053/j.gastro.2006.07.014. [DOI] [PubMed] [Google Scholar]
- 12.Ito K, Lim AC, Salto-Tellez M, et al. RUNX3 attenuates β-catenin/T cell factors in intestinal tumorigenesis. Cancer Cell. 2008;14:226–37. doi: 10.1016/j.ccr.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 13.Ito K, Chuang LSH, Ito T, et al. Loss of Runx3 is a key event in inducing precancerous state of the stomach. Gastroenterology. 2011;140:1536–46. doi: 10.1053/j.gastro.2011.01.043. [DOI] [PubMed] [Google Scholar]
- 14.Proffitt KD, Madan B, Ke Z, et al. Pharmacological inhibition of the Wnt acyltransferase PORCN prevents growth of WNT-driven mammary cancer. Cancer Res. 2012;73:502–7. doi: 10.1158/0008-5472.CAN-12-2258. [DOI] [PubMed] [Google Scholar]
- 15.Nateri AS, Spencer-Dene B, Behrens A. Integration of phosphorylated c-Jun with TCF4 regulates intestinal cancer development. Nature. 2005;473:281–5. doi: 10.1038/nature03914. [DOI] [PubMed] [Google Scholar]
- 16.Jho EH, Zhang T, Domon C, Joo CK, Freund JN, Costantini F. Wnt/β-catenin/Tcf signaling induces the transcription of Axin2, a negative regulator of the signaling pathway. Mol Cell Biol. 2002;22:1172–83. doi: 10.1128/MCB.22.4.1172-1183.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nojima M, Suzuki H, Toyota M, et al. Frequent epigenetic inactivation of SFRP genes and constitutive activation of Wnt signaling in gastric cancer. Oncogene. 2007;26:4699–713. doi: 10.1038/sj.onc.1210259. [DOI] [PubMed] [Google Scholar]
- 18.Oguma K, Oshima H, Aoki M, et al. Activated macrophages promotes Wnt signalling through tumour necrosis factor-α in gastric tumour cells. EMBO J. 2008;27:1671–81. doi: 10.1038/emboj.2008.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sakakura C, Hasegawa K, Miyagawa K, et al. Possible involvement of RUNX3 silencing in the peritoneal metastases of gastric cancer. Clin Cancer Res. 2005;11:6479–88. doi: 10.1158/1078-0432.CCR-05-0729. [DOI] [PubMed] [Google Scholar]
- 20.Chi XZ, Yang JO, Lee KY, et al. RUNX3 suppresses gastric epithelial cell growth by inducing p21WAF1/Cip1 expression in cooperation with transforming growth factor β-ctivated SMAD. Mol Cell Biol. 2005;25:8097–107. doi: 10.1128/MCB.25.18.8097-8107.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cadigan KM, Waterman ML. TCF/LEFs and Wnt signaling in nucleus. Cold Spring Harb Perspect Biol. 2012;4:a007906. doi: 10.1101/cshperspect.a007906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cadigan KM. TCFs and Wnt/β-catenin signaling: more than one way to throw the switch. Curr Top Dev Biol. 2012;98:1–34. doi: 10.1016/B978-0-12-386499-4.00001-X. [DOI] [PubMed] [Google Scholar]
- 23.Li J, Wang CY. TBL1-TBLR1 and β-catenin recruit each other to Wnt target-gene promoter for transcription activation and oncogenesis. Nat Cell Biol. 2008;10:160–9. doi: 10.1038/ncb1684. [DOI] [PubMed] [Google Scholar]
- 24.Benchabane H, Xin N, Tian A, et al. Jerky/Earthbound facilitates cell-specific Wnt/Wngless signaling by modulating β-catenin-TCF activity. EMBO J. 2011;30:1444–58. doi: 10.1038/emboj.2011.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nagahama Y, Ishimaru M, Osaki M, et al. Apoptotic pathway induced by transduction of RUNX3 in the human gastric carcinoma cell line MKN-1. Cancer Sci. 2008;99:23–30. doi: 10.1111/j.1349-7006.2007.00650.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tsunematsu T, Kudo Y, Iizuka S, et al. RUNX3 has an oncogenic role in head and neck cancer. PLoS ONE. 2009;4:e5892. doi: 10.1371/journal.pone.0005892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kudo Y, Tsunematsu T, Takata T. Oncogenic role of RUNX3 in head and neck cancer. J Cell Biochem. 2011;112:387–93. doi: 10.1002/jcb.22967. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 Relative RUNX3 mRNA levels in gastric cancer cell lines examined by RT-PCR.
Fig. S2 Western blotting results of RUNX3 and active form of β-catenin in KatoIII cells and KatoIII-R3 cells.
Fig. S3 Relative band intensities calculated from the Western blotting results (Fig. 2b) of active β-catenin and RUNX3 in the RUNX3 expression vector-transfected KatoIII cells.
Fig. S4 Flow cytometry analyses of β-catenin and GFP in the control KatoIII cells and GFP expression vector-transfected KatoIII cells.
Fig. S5 Cell proliferation rates of RUNX3 siRNA-transfected KatoIII-R3 cells.


