Abstract
GC33 is a humanized mAb against human glypican-3 (GPC3). In the first-in-human study carried out in the USA, GC33 was well tolerated and showed preliminary antitumor activity in patients with advanced hepatocellular carcinoma. This study aimed to assess the safety, tolerability, and pharmacokinetic characteristics of GC33 in Japanese patients with advanced hepatocellular carcinoma. The study design was a conventional 3 + 3 dose-escalation design to determine the maximum tolerated dose of GC33 given i.v. at 5, 10, or 20 mg/kg weekly. Immunohistochemistry was carried out on tumor biopsies to evaluate GPC3 expression. Thirteen patients were enrolled across the three dose levels, and no patients observed any dose-limiting toxicity up to the highest planned dose of 20 mg/kg. The most common adverse events were decreased lymphocyte count, decreased natural killer cell count, increased C-reactive protein, and pyrexia. Grade 3 adverse events (increased blood pressure, decreased lymphocyte count, and decreased platelet count) were observed in two or more patients. The AUCinf showed a dose-proportional increase from the 5 mg/kg dose group to the 20 mg/kg dose group. The trough concentrations of GC33 appeared to reach a steady state after the fourth to the sixth dose. Seven of the 13 patients showed stable disease, the other six showed progressive disease. Furthermore, three patients showed long-term stable disease of more than 5 months. In conclusion, GC33 given at up to 20 mg/kg weekly was well tolerated in Japanese patients with advanced hepatocellular carcinoma.
Keywords: GC33, glypican-3, hepatocellular carcinoma, Japanese patients, phase I study
Hepatocellular carcinoma (HCC) is the sixth most common cancer in the world in terms of incidence, accounting for approximately 630 000 new patients per year, and the third most common cause of cancer death, with more than 600 000 people dying of HCC each year.(1,2) Most patients present with the advanced stage of disease; however, the only approved therapy for advanced HCC is sorafenib, an oral multikinase inhibitor, and the benefits of sorafenib remain modest.(3–5)
Glypican-3 (GPC3) is a member of the glypican family. Glypicans are proteoglycans that are attached to the cell surface by a glycosyl–phosphatidylinositol anchor, and play an important role in cellular growth, cell differentiation, and cell migration.(6–8) Immunohistochemical studies have shown that GPC3 is highly expressed in >70% of HCC tissues, whereas it is less detectable or not detectable in adjacent non-tumoral lesions.(9) It has been reported that GPC3 expression is correlated with poor prognosis in HCC because membranous GPC3-positive HCC patients have a significantly lower disease-free survival rate than GPC3-negative HCC patients after surgical resection.(10) Therefore, GPC3 represents a specific tumor marker and a potential therapeutic target in HCC.(11)
A recombinant humanized mAb against the C-terminal region of human GPC3,(12) GC33 induced antibody-dependent cellular cytotoxicity against GPC3-positive HCC cell lines and caused tumor growth inhibition in human liver cancer xenograft models.(13)
The first-in-human (FIH) study of GC33 carried out in the USA was a phase I, open-label, multicenter, dose-escalation study in which GC33 was given at 2.5, 5, 10, or 20 mg/kg as monotherapy to patients with advanced HCC. GC33 was well tolerated, and no dose-limiting toxicity (DLT) up to the highest planned dose level was detected.(14) In consideration of variations in the natural history and HCC treatment practices in Western countries versus Asian populations, and possible pharmacogenomic differences,(15–18) in the current study we evaluated the safety, tolerability, and pharmacokinetic (PK) characteristics of GC33 in Japanese patients. Additionally, we assessed the preliminary antitumor activity, explored biomarkers, and compared a fully automated GPC3 immunohistochemistry (IHC) test with a more laborious GPC3 IHC test that used previously.
Materials and Methods
Patients
Eligible patients had histologically or cytologically confirmed HCC (not including the fibrolamellar subtype) with no preferred alternative treatment, were ≥20 years old, had life expectancy of ≥3 months after enrolment, had an Eastern Cooperative Oncology Group performance status (ECOG PS) of 0 or 1, had a Child–Pugh class of A or B, and had at least one radiographically evident lesion. Patients were required to have adequate hematologic, hepatic, and renal function as evidenced by a platelet count of ≥50 000/μL, absolute neutrophil count of ≥1500/μL, transaminase (aspartic aminotransferase and alanine aminotransferase) levels of ≤5.0 × the upper limit of normal (ULN), total bilirubin level of ≤3.0 × ULN, prothrombin time-international normalized ratio of ≤2.0, alkaline phosphatase level of ≤5.0 × ULN, and serum creatinine level of ≤2.0 × ULN. Tumor samples also had to be available, biopsied within 12 months before the required informed consent, for GPC3 IHC testing.
Patients were excluded if they were HIV antibody positive or had active infection requiring treatment, except for hepatitis B virus and hepatitis C virus. Exclusion criteria also included a history of transplantation, patients with brain metastases with symptoms, central nervous system diseases (including psychiatric diseases), other concurrent malignancies within the last 5 years, central nervous system manifestations of hepatic encephalopathy, moderate or massive ascites, and a history of hypersensitivity to similar agents such as mAbs. Patients who had received surgery, locoregional therapy (ablation or transcatheter arterial [chemo] embolization) for HCC, chemotherapy other than sorafenib, radiotherapy, hormone therapy, immunotherapy, or any other investigational drug within 4 weeks prior to enrolment (2 weeks for sorafenib) were also excluded.
This trial was carried out in accordance with the Declaration of Helsinki and Good Clinical Practice. The trial protocol was approved by the Japanese regulatory agency PMDA and the institutional review board of each investigation site. Patients provided written informed consent according to institutional guidelines before enrolment.
Study design
The primary objective was to determine the safety and tolerability of GC33 in Japanese patients with advanced HCC, and the secondary objectives were to evaluate the PK, efficacy, and biomarkers. This trial was carried out at three sites across Japan, namely, the National Cancer Center Hospital East (Chiba), the Kanagawa Cancer Center Hospital (Kanagawa), and the National Cancer Center Hospital (Tokyo).
The patients enrolled were treated with GC33 at 5, 10, or 20 mg/kg given every week by i.v. infusion over a period of 30–90 min. Each cycle was defined by approximately 4 weeks of treatment. GC33 treatments were scheduled to continue until progressive disease (PD), occurrence of unacceptable toxicities, or the patient's withdrawal of consent. The starting dose of 5 mg/kg was chosen based on the dose of GC33 estimated from xenograft mouse models that was expected to still have potential for some antitumor activity,(13) while taking into account the safety and PK results from the phase I study carried out in the USA.
This study was a conventional 3 + 3 dose-escalation design. At least three patients in each of the 5 and 10 mg/kg cohorts, and at least six patients in the 20 mg/kg cohort were to be treated and monitored from the first dosing of GC33 until prior to the fifth dosing (cycle 1), as described previously.(14) Dose-limiting toxicity was defined as any grade 3 or higher toxicity occurring during cycle 1 (as categorized by the NCI's Common Terminology Criteria for Adverse Events version 4.03)(19) other than grade 3 infusion reaction, transient electrolyte abnormalities, grade 3 decreased lymphocyte count, grade 3 decreased platelet count not requiring platelet transfusion, and <10× ULN increase in hepatic enzymes (e.g., increased alanine aminotransferase, increased aspartic aminotransferase, increased alkaline phosphatase), grade 3 hepatic function abnormal (decreased serum albumin, increased total bilirubin, increased prothrombin time-international normalized ratio), and any non-drug-related toxicities. A medical monitor and an independent Data Safety Monitoring Board reviewed the safety data of each cohort and dose escalation.
Study assessment
Types and frequency of adverse events (AEs) and causal relationships between AEs and GC33 were evaluated. Incidence and severity of AEs were collected and graded according to the NCI's Common Terminology Criteria for Adverse Events version 4.03. Particular attention was paid to infusion-associated symptoms possibly as a result of GC33 infusion. Laboratory evaluations during the study included hematology, blood biochemistry, blood coagulation, and natural killer (NK) cell count. Anti-GC33 antibodies were measured at screening and during the study.
Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 was used to assess overall response and progression-free survival (PFS).(20) Tumor burden and response to treatment were evaluated at the baseline and every 8 weeks by imaging (computed tomography or MRI). α-Fetoprotein (AFP) and des-γ-carboxy prothrombin (DCP) were also measured at the baseline and during the study.
Tumoral expression of GPC3 was centrally examined and evaluated in biopsied specimens by two IHC methods, each using mouse anti-human GPC3 mAbs.(21) Method 1, which was the method used in the previous FIH study, was carried out at Charles River Laboratories Inc. (Reno, NV, USA) and assessed by three qualified pathologists blinded to clinical outcomes using the scoring criteria shown in Table 1.(14) Method 2, a fully automated IHC assay, was carried out at Ventana Medical Systems Inc. (Tucson, AZ, USA) using anti-glypican 3 (GC33) mouse monoclonal primary antibody (Catalog number 790–4564; Ventana Medical Systems) according to the manufacturer's instructions. The immunostaining resulting from method 2 was assessed by two qualified pathologists blinded to clinical outcomes. Staining intensity was assessed in the cytoplasm and at the cell membrane separately. Staining intensity was scored on a four-point scale: 0, no staining was observed; 1 (weak), an intensity identified by the pathologist as higher than background and specific to the cells of interest; 2, moderate staining; and 3, maximal (strong) staining. The percentage of cells with specific antibody reactivity was scored for the tumor component of the specimen. The H score was derived by summing the percentage of cell staining at each intensity (weak, moderate, strong) multiplied by the weighted intensity of staining.(22) Following the evaluation of GPC3 IHC staining intensity, the percentage of tumor cells stained, and the pattern of membrane and/or cytoplasmic positivity, a clinical score for method 2 was assigned according to the criteria described in Table 2.
Table 1.
Scoring system of immunohistochemical (IHC) staining of glypican-3 (GPC3) in hepatocellular tumor specimens using method 1
| Score | |
|---|---|
| Positive cell rate (PR) | |
| No staining | 0 |
| <20% | 1 |
| 20–49% | 2 |
| ≥50% | 3 |
| Staining intensity (SI)† | |
| Cytoplasm (SI-Cp) | |
| No staining | 0 |
| Minimal staining | 1 |
| Cell membrane (SI-Cm) | |
| Weak staining | 2 |
| Moderate staining | 3 |
| Strong staining | 4 |
| Staining pattern of cell membrane (SP-Cm)‡ | |
| No cell membrane pattern | 0 |
| Incomplete or partly complete (<20%) | 1 |
| Focally complete (20–49%) | 2 |
| Rather complete (≥50%) | 3 |
| GPC3 staining scoring | IHC total = PR + SI-Cp + SI-Cm + SP-Cm |
Scoring method 1, used in a previous first-in-human study, involved assessment by three qualified pathologists blinded to clinical outcomes using the scoring criteria shown here. †GPC3 staining categories: minimal, slight staining recognized at low-power objective, ×10; weak, slight staining recognized at low-power objective, ×4; moderate, staining found easily at ×4, but weaker than that of strong staining; strong, staining apparent strong with low-power objective, ×4.
Complete staining, circular (ring-like) staining of tumor cells; incomplete staining, focal staining of rim of cells.
Table 2.
Scoring criteria of immunohistochemical staining of glypican-3 in hepatocellular tumor specimens using method 2
| Clinical score | Membrane staining | Cytoplasmic staining | |
|---|---|---|---|
| 0 | Negative | and | Positive staining in <10% of tumor cells |
| 1 | Positive staining in <10% of tumor cells | and/or | Positive staining in ≥10% of tumor cells |
| 2 | Weak or moderate staining in ≥10% of tumor cells | with or without | Positive staining in ≥10% of tumor cells |
| 3 | Strong staining in ≥10% of tumor cells | or | Strong staining in ≥50% of tumor cells |
Scoring method 2 used a fully automated immunohistochemical assay, with results assessed by two qualified pathologists blinded to clinical outcomes.
Blood samples used for serum GC33 measurements were drawn at pre-dose, at the end of the first infusion, and at 1, 8, 24, 48, 72, and 120 h after the end of the first infusion. In addition, trough concentrations of serum GC33 were measured prior to each subsequent infusion until the end of cycle 3. GC33 in the serum was assayed as described previously.(14) Inter-assay accuracy and precision was within ±20%, and the coefficient of variation was <20%. Pharmacokinetic parameters for GC33 in serum were calculated by non-compartmental methods using data obtained after the first dosing.
Statistical analysis
All 13 patients received at least one full dose level and are included in the safety, PK, and antitumor activity patient population. Descriptive statistics were used to summarize the patient characteristics and for the safety analysis. All patients had one or more radiographically evident lesions and are included in the analysis of objective response and PFS by RECIST version 1.1. P-values were calculated by t-test. Spearman's correlation was used to compare the two IHC methods.
Results
Patient characteristics
From October 2010–November 2011, a total of 13 patients were enrolled across the three cohorts. The median age was 66 years, with 12 male patients (92%) and one female patient (8%). Ten patients (77%) were of Child–Pugh class A and three patients (23%) were of Child–Pugh class B7. Nine patients (69%) had an ECOG PS of 0, and four patients (31%) had an ECOG PS of 1. The median AFP and DCP levels before the first treatment were 361.1 ng/mL and 3.619 U/mL, respectively. All 13 patients had had at least one course of systemic chemotherapy prior to the study (among them, 12 had had sorafenib). The baseline patient characteristics are listed in Table 3.
Table 3.
Patient characteristics at baseline
| Characteristic | No. of patients (%) |
|---|---|
| Age, years | |
| Median | 66 |
| Range | 48–78 |
| Weight, kg | |
| Median | 62.6 |
| Range | 55.2–81.5 |
| Sex | |
| Male | 12 (92) |
| Female | 1 (8) |
| ECOG PS | |
| 0 | 9 (69) |
| 1 | 4 (31) |
| Disease stage | |
| Stage III | 4 (31) |
| Stage IVA | 3 (23) |
| Stage IVB | 6 (46) |
| Degree of differentiation | |
| Well differentiated | 3 (23) |
| Moderately differentiated | 8 (62) |
| Poorly differentiated | 2 (15) |
| Child-Pugh | |
| A | 10 (77) |
| B7 | 3 (23) |
| Etiology | |
| HBV positive | 4 (31) |
| HCV positive | 8 (62) |
| Alcohol liver diease | 1 (8) |
| Vascular invasion | |
| Yes | 7 (54) |
| No | 6 (46) |
| Extra-hepatic metastasis | |
| Yes | 6 (46) |
| No | 7 (54) |
| Laboratory values | |
| AFP, ng/mL | |
| Median | 361.1 |
| Range | 4.9–23.476 |
| DCP, U/mL | |
| Median | 3.619 |
| Range | 0.128–57.048 |
| Previous therapy | |
| Surgical resection | 4 (31) |
| RFA/PEI | 1 (8) |
| TACE/TAE | 10 (77) |
| TAI | 5 (38) |
| Other | 2 (15) |
| Systemic chemotherapy | 13 (100) |
| No. of prior regimens | |
| 1 | 6 (46) |
| 2 | 5 (38) |
| 3 | 1 (8) |
| 4 | 1 (8) |
| GPC3 IHC clinical score (method 2) | |
| 0 | 1 (8) |
| 1 | 6 (46) |
| 2 | 4 (31) |
| 3 | 2 (15) |
AFP, α-fetoprotein; DCP, des-γ-carboxy prothrombin; ECOG PS, Eastern Cooperative Oncology Group Performance Status; HBV, Hepatitis B virus; HCV, Hepatitis C virus; PEI, Percutaneous ethanol injection; RFA, Radiofrequency ablation; TACE, Transcatheter arterial chemoembolization; TAE, Transcatheter arterial embolization; TAI, Transcatheter arterial infusion.
Dose and duration of therapy
Of the 13 enrolled patients, four were assigned to cohort 1 (5 mg/kg), three were assigned to cohort 2 (10 mg/kg), and six were assigned to cohort 3 (20 mg/kg). Among the 13 patients, 12 were included in the DLT evaluation, and one patient was excluded as a result of withdrawing due to PD during the DLT evaluation period (in cohort 1). The mean duration of exposure was 99.0 days (range, 29–280 days) and the mean number of doses administered was 14.4 (4–40). Dose intensity was high across all patients in cohorts 1–3, with a mean relative dose intensity of 95.4% (range, 79.5–100%). Good compliance of administration was observed as relative dose intensities were over 90.0% in all three cohorts.
Safety and tolerability
GC33 up to 20 mg/kg/week was considered to be well tolerated as no DLTs were reported in any cohort and a maximum tolerated dose (MTD) was not reached. A summary of the AEs occurring in over 30% of patients and grade 3 AEs by dose group are listed in Tables 4 and 5. All patients had at least one AE, and the most common AEs were lymphocyte count decreased (77%), NK cell count decreased (77%), C-reactive protein increased (69%), and pyrexia (62%). Grade 3 AEs were reported in 54% of patients, and grade 3 AEs occurring in two or more patients were blood pressure increased (23%), lymphocyte count decreased (23%), and platelet count decreased (15%). No grade 4 or 5 AEs were reported. No AEs led to death or discontinuation.
Table 4.
Summary of common adverse events occuring in over 30% of patients with advanced hepatocellular carcinoma treated with GC33, a humanized antibody against glypican-3 (n = 13)
| Adverse events | 5 mg/kg n = 4 | 10 mg/kg n = 3 | 20 mg/kg n = 6 | Total no. of patients n (%) |
|---|---|---|---|---|
| Any adverse events | 4 | 3 | 6 | 13 (100) |
| Lymphocyte count decreased | 4 | 3 | 3 | 10 (77) |
| Natural killer cell count decreased | 2 | 3 | 5 | 10 (77) |
| C-reactive protein increased | 3 | 2 | 4 | 9 (69) |
| Pyrexia | 2 | 2 | 4 | 8 (62) |
| Aspartate aminotransferase increased | 1 | 2 | 3 | 6 (46) |
| Blood alkaline phosphatase increased | 1 | 1 | 4 | 6 (46) |
| Nausea | 2 | 0 | 4 | 6 (46) |
| Cancer pain | 1 | 2 | 3 | 6 (46) |
| Alanine aminotransferase increased | 1 | 2 | 2 | 5 (38) |
| Blood lactate dehydrogenase increased | 1 | 3 | 1 | 5 (38) |
| Blood albumin decreased | 1 | 0 | 3 | 4 (31) |
| Blood pressure increased | 1 | 1 | 2 | 4 (31) |
| Fatigue | 0 | 3 | 1 | 4 (31) |
| Decreased appetite | 1 | 0 | 3 | 4 (31) |
Table 5.
Summary of grade 3 adverse events in patients with advanced hepatocellular carcinoma treated with GC33, a humanized antibody against glypican-3 (n = 13)
| Adverse events | 5 mg/kg n = 4 | 10 mg/kg n = 3 | 20 mg/kg n = 6 | Total no. of patients n (%) |
|---|---|---|---|---|
| Any adverse events | 3 | 0 | 4 | 7 (54) |
| Blood pressure increased | 1 | 0 | 2 | 3 (23) |
| Lymphocyte count decreased | 2 | 0 | 1 | 3 (23) |
| Platelet count decreased | 1 | 0 | 1 | 2 (15) |
| Aspartate aminotransferase increased | 0 | 0 | 1 | 1 (8) |
| Blood phosphorus decreased | 1 | 0 | 0 | 1 (8) |
| Blood sodium decreased | 0 | 0 | 1 | 1 (8) |
| Lipase increased | 0 | 0 | 1 | 1 (8) |
| Weight increased | 1 | 0 | 0 | 1 (8) |
| Cholangitis | 1 | 0 | 0 | 1 (8) |
| Hepatic function abnormal | 0 | 0 | 1 | 1 (8) |
| Anaemia | 0 | 0 | 1 | 1 (8) |
| Oedema, peripheral | 1 | 0 | 0 | 1 (8) |
| Diabetes mellitus | 0 | 0 | 1 | 1 (8) |
| Pathological fracture | 1 | 0 | 0 | 1 (8) |
| Cancer pain | 0 | 0 | 1 | 1 (8) |
Infusion reactions were reported in 62% (8 out of 13 patients) and all of them were medically manageable. The most common infusion-associated symptoms were pyrexia (five patients, 38%), increased C-reactive protein (four patients, 31%), and nausea (three patients, 23%), and grade 3 increased blood pressure was reported in two patients in the 20 mg/kg cohort. The other infusion-associated symptoms were either grade 1 or 2. Almost all of the infusion-associated symptoms occurred on the third day after the first infusion. The investigators or sub-investigators provided premedication to prevent infusion reactions and provided appropriate treatment for any infusion reactions (acetaminophen, dexchlorpheniramine, dexamethasone sodium phosphate, loxoprofen sodium hydrate, prochlorperazine maleate). All infusion-associated symptoms were resolved.
The incidence of AEs seemed not to be dose-dependent. There was no evidence of cumulative toxicity and there was no difference in the incidence of AEs by GPC3 expression level. No anti-GC33 antibodies were detected in any of the patients at pre-treatment or post-treatment.
Pharmacokinetics
The PK data were evaluated for all 13 patients in all cohorts. The PK parameters of GC33 are shown in Table 6. At doses of 5, 10, and 20 mg/kg, the mean half-life (t1/2) was 4.17, 7.01, and 6.13 days and the mean total clearance was 0.566, 0.373, and 0.510 L/day, respectively. The PK exposure (Cmax and AUCinf) after the first dose increased as the dose increased. The AUCinf showed a dose-proportional increase from the 5 mg/kg dose group to the 20 mg/kg dose group. The trough concentrations of GC33 appeared to reach a steady state after the fourth to the sixth dose. The trough concentrations after reaching a steady state in all patients in all cohorts were over 30 μg/mL, which was the predicted effective concentration in the xenograft models.
Table 6.
Pharmacokinetics parameters of GC33, a humanized antibody against glypican-3
| t1/2 (day) | CL (L/day) | Cmax (μg/mL) | AUCinf (day μg/mL) | Vd,ss (L) | Ctrough (week 4; μg/mL) | Ctrough (week 6; μg/mL) | |
|---|---|---|---|---|---|---|---|
| 5 mg/kg | |||||||
| No. of patients | 3 | 3 | 4 | 3 | 3 | 3 | 2 |
| Mean (min.–max.) | 4.17 (2.90–5.39) | 0.566 (0.452–0.746) | 141 (114–186) | 584 (419–759) | 3.13 (2.56–3.80) | 61.7 (38.9–73.9) | 61.2 (33.9–88.4) |
| CV (%) | 29.9 | 27.9 | 22.3 | 29.2 | 20.0 | 32.0 | 63.0 |
| 10 mg/kg | |||||||
| No. of patients | 3 | 3 | 3 | 3 | 3 | 3 | 3 |
| Mean (min.–max.) | 7.01 (6.40–7.66) | 0.373 (0.327–0.432) | 258 (237–270) | 2020 (1790–2390) | 3.70 (3.53–3.97) | 259 (221–299) | 288 (246–353) |
| CV (%) | 9.02 | 14.3 | 7.16 | 16.0 | 6.55 | 15.1 | 19.9 |
| 20 mg/kg | |||||||
| No. of patients | 6 | 6 | 6 | 6 | 6 | 5 | 5 |
| Mean (min.–max.) | 6.13 (3.80–8.02) | 0.510 (0.308–0.816) | 395 (326–505) | 2670 (1480–3580) | 4.12 (3.23–5.31) | 406 (325–532) | 477 (379–616) |
| CV (%) | 28.9 | 33.9 | 17.2 | 25.7 | 17.6 | 19.5 | 23.8 |
PK parameters for GC33 in serum were calculated by non-compartmental methods. Pharmacokinetic parameters estimated following the first GC33 infusion are: t1/2, elimination half-life; CL, serum clearance; Cmax, maximum drug concentration; AUCinf, area under the concentration time curve from time 0 to infinity; and Vd,ss, volume of distribution at steady state. In addition, Ctrough (predose concentrations) at week 4 and 6 are summarized. CV, coefficient of variation.
Tumoral GPC3 expression
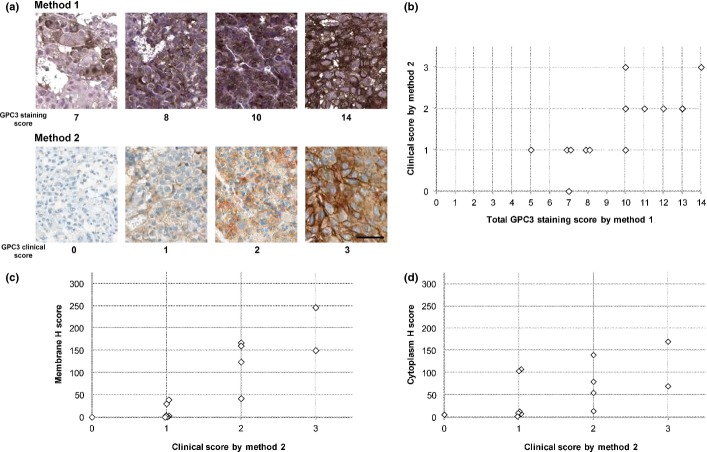
All 13 unique core-needle biopsied specimens taken from tumor lesions in the liver were stained by both methods and were evaluated by using their respective scoring criteria. The representative GPC3-IHC features for both staining methods are shown in Figure 1(a) and all cases are shown in Figure S1. Twelve patients had a total GPC3 staining score of 7 or more by method 1, and 12 patients had positive clinical scores by method 2 (Fig. 1b). Both staining methods produced a similar staining pattern in the majority of specimens. The GPC3 staining score of method 1 was highly correlated with the clinical score of method 2 (Spearman's correlation coefficient, 0.76; P = 0.00255). Higher clinical scores appeared to be associated with increased H scores for both the membrane and cytoplasm in this limited number of samples (Fig. 1c,d).
Fig. 1.
Immunohistochemistry (IHC) of glypican-3 (GPC3) in hepatocellular carcinoma. (a) Representative GPC3 staining features observed in matched specimens evaluated using two IHC methods. Method 1 was used in a previous first-in-human study; method 2 was a fully automated IHC assay. Scores are indicated under each photograph. Bar = 50 μm (b) Comparison of total GPC3 staining score by method 1 (0–14) and clinical score by method 2 (0–3). Spearman's correlation was 0.76 (P = 0.00255). (c) Comparison of clinical score and membrane H score. (d) Comparison of clinical score and cytoplasm H score. There was a positive association between the H scores and the GPC3 clinical scores, with R2 values of 0.75 and 0.33, respectively.
Antitumor activity
The efficacy analysis population consisted of all 13 patients who were treated with GC33. There were no patients whose best overall response was complete or partial response. Seven out of 13 patients (54%) showed stable disease (SD) (cohort 1, two out of four patients; cohort 2, two out of three patients; cohort 3, three out of six patients), the others showed PD. Three patients who had SD had received treatment for more than 5 months before progress (Fig. 2) and had a high GPC3 IHC score by method 1 (IHC score ≥7), similar to the previous phase I study.(14) The median PFS for all patients was 2.1 months (1.6–3.5 months) (95% confidence interval). There was no significant difference in the best overall response or PFS among the three cohorts. Seven out of 11 patients (64%) evaluated showed a decrease in AFP (six patients with 20% reduction), and 11 out of 13 patients (85%) showed a decrease in DCP (six patients with 40% reduction) compared to their baseline levels (Fig. 3a,b). Moreover, computed tomography imaging showed shrinkage in one patient's lung metastasis, in whom AFP and DCP levels were decreased (Fig. 3c). Two out of 13 patients were excluded from the AFP figure as their baseline AFP level was <10 ng/mL and deemed to be within the normal range.
Fig. 2.
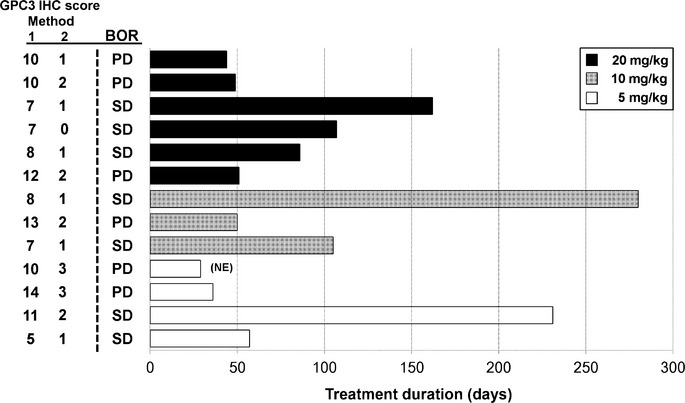
Treatment duration in patients with advanced hepatocellular carcinoma treated with humanized antibody against glypican-3 (n = 13). The GPC3 immunohistochemistry (IHC) scores evaluated using two IHC methods (method 1, score 0–14; method 2, score 0–3) and best overall response (BOR) are described to the left of the chart. NE, not evaluable; PD, progressive disease; SD, stable disease.
Fig. 3.
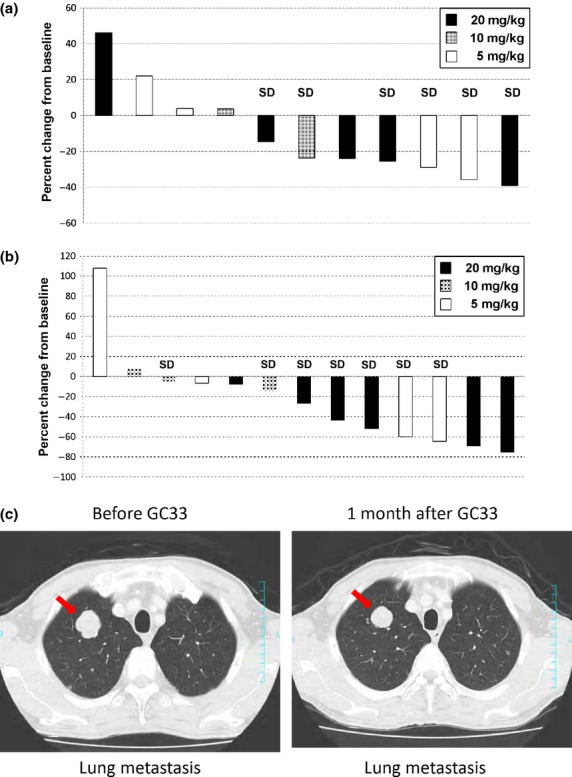
Best changes from baseline in α-fetoprotein (AFP) (a) and des-γ-carboxy prothrombin (DCP) (b) in patients with advanced hepatocellular carcinoma treated with humanized antibody against glypican-3 (GC33). Best changes in AFP (11 patients) or DCP (13 patients) are shown as best percent changes from their baseline values. Two patients were excluded from AFP changes because their baseline AFP levels were within the normal range. (c) Computed tomography imaging of lung metastasis before and after (arrow) treatment with GC33. SD, Stable disease.
Discussion
This is the first study to evaluate the safety and tolerability of GC33 in Japanese patients. The results showed that GC33 was generally well tolerated, as no DLTs were reported in any cohort and an MTD was not reached. This safety profile is similar to that reported in the FIH study in which most AEs were mild to moderate and no DLTs were experienced.(14)
The pharmacokinetics of GC33 showed that the clearance was larger and the half-life shorter at dose levels of 5.0 mg/kg than at dose levels of 10.0 mg/kg and 20.0 mg/kg suggesting non-linear elimination, which is similar to that found in the FIH.(14) In general, antibody elimination is connected with non-specific linear elimination from the reticuloendothelial system and with antigen-specific non-linear elimination.(23) Although non-linear elimination at 5 mg/kg was observed, the elimination of GC33 may be attributable to non-specific linear elimination because a dose-proportional increase in AUCinf was also observed. In addition, similar to the results of the previous phase I study,(14) a steady state appears to have been reached following four to six doses, and steady state trough concentrations of above 30 μg/mL were achieved in all patients in the 5–20 mg/kg groups. Preclinical data showed that a 5 mg/kg dose of GC33 had an effect on tumor growth inhibition,(13) which was associated with trough serum antibody concentrations of 30 μg/mL.
In this study, no patient had a best response of complete or partial response, although three patients showed long SD of more than 5 months. Also in this study, 7 of 11 patients (64%) showed a decrease in AFP and 11 of 13 patients (85%) showed a decrease in DCP. In the FIH study of GC33, AFP values were either decreased or stabilized in patients who showed long SD.(14) In the case of sorafenib, an early decrease of AFP has been postulated to be a good predictor of efficacy.(24) In our study, however, no pharmacodynamic analysis was carried out as no post-treatment biopsy of the tumors was performed.
For the purposes of further developing a companion diagnostic test, we also used a fully automated IHC method and compared the results of that method with those of the method used previously in the FIH study. The results obtained from preclinical work and the FIH study of GC33 suggested that GPC3 expression would be a key parameter for antitumor activity of GC33. Both IHC methods used the same mouse mAb against human GPC3 and both produced similar staining patterns in the majority of the specimens. Furthermore, the scoring system used in the previous method is based on the pattern and distribution of GC33 staining in both the membrane and cytoplasm, as is the new method for obtaining a clinical score that considers both membrane and cytoplasmic staining patterns and staining intensities.(14) However, the scoring system of the previous method is somewhat more complex than that of the new method, in that the range of scoring is 0–14 in the previous method whereas in the fully automated method the clinical score range is 0–3. In this study, in addition to patients whose tumors had a high GPC3 IHC clinical score (2 and 3), there were patients whose tumor specimens had a GPC3 IHC clinical score of 1 but who showed long SD of more than 5 months and/or a decrease in tumor marker levels. Therefore, no definitive conclusions can be made at present, and a cut-off level for the GPC3 IHC clinical score to predict efficacy of GC33 has not been determined.
Recently, another therapeutic approach for HCC that targets GPC3 is being developed. A phase I clinical trial of a vaccine composed of two GPC3-derived peptides has been carried out in advanced HCC patients. The vaccination was well tolerated and induced GPC3-specific T cell responses that would be correlated with survival.(25) As a therapeutic antibody, it can induce adaptive immunity against tumor-associated antigens and act to promote vaccine-like effects as well as direct activation of NK cells or macrophages to show Fc-mediated antitumor activity.(26)
In conclusion, because no DLTs were reported for doses up to 20 mg/kg/week and because an MTD was not reached, GC33 is considered generally well tolerated in Japanese patients with advanced HCC. The correlation between antitumor activity and GPC3 expression is not clear, due to the limited sample sizes. Currently, a global, placebo-controlled, double-blind, multicenter phase II trial in previously treated patients with advanced HCC is being carried out that will provide more data with which to clarify the correlation between GC33 efficacy and GPC3 expression, as measured by the fully automated GPC3 IHC method in tumor specimens obtained by core needle biopsy.
Acknowledgments
We thank all of the participating patients and their families, as well as the investigators, research nurses, study coordinators, and operations staff. The authors thank the pathologists Drs. Janine Feng and Bharathi Vennapusa (Ventana Medical Systems Inc., USA), Dr. Kevin S. McDorman (Charles River Laboratories Inc., USA), Dr. Vikram Deshpande (Massachusetts General Hospital, USA), and Dr. Hiroaki Kataoka (University of Miyazaki, Japan), Norihisa Ohishi for the PK analysis and discussion, and Toshihiko Ohtomo, Atsuhiko Kato, and Zhaodi Gu for their assistance.
Disclosure Statement
The study was designed under the responsibility of Chugai Pharmaceutical Co., Ltd, in conjunction with the steering committee. GC33 was provided by Chugai Pharmaceutical Co., Ltd. Takuji Okusaka and Junji Furuse have received Honoraria and Research Funding from Chugai Pharmaceutical Co., Ltd. Ikue Suzuki and Shunsuke Yamamoto are from Chugai Pharmaceutical Co., Ltd. The other authors have no conflict of interest.
Supporting Information
Additional supporting information may be found in the online version of this article:
Fig. S1 Immunohistochemical staining of glypican-3 (GPC3) in tumor biopsy specimens from patients with advanced hepatocellular carcinoma (n = 13) evaluated by two scoring methods, method 1 (score 0–14) (a) and method 2 (score 0–3) (b). Bar = 50 μm.
References
- 1.Alves RC, Alves D, Guz B, et al. Advanced hepatocellular carcinoma. Review of targeted molecular drugs. Ann Hepatol. 2011;10:21–7. [PubMed] [Google Scholar]
- 2.Ferenci P, Fried M, Labrecque D, et al. Hepatocellular carcinoma (HCC): a global perspective. J Clin Gastroenterol. 2010;44:239–45. doi: 10.1097/MCG.0b013e3181d46ef2. [DOI] [PubMed] [Google Scholar]
- 3.Zhu AX. New agents on the horizon in hepatocellular carcinoma. Ther Adv Med Oncol. 2013;5:41–50. doi: 10.1177/1758834012458480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25–34. doi: 10.1016/S1470-2045(08)70285-7. [DOI] [PubMed] [Google Scholar]
- 5.Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. New Engl J Med. 2008;359:378–90. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 6.Farooq M, Hwang SY, Park MK, Kim JC, Kim MK, Sung YK. Blocking endogenous glypican-3 expression releases Hep 3B cells from G1 arrest. Mol Cells. 2003;15:356–60. [PubMed] [Google Scholar]
- 7.Filmus J, Selleck SB. Glypicans: proteoglycans with a surprise. J Clin Invest. 2001;108:497–501. doi: 10.1172/JCI13712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li M, Choo B, Wong ZM, Filmus J, Buick RN. Expression of OCI-5/glypican 3 during intestinal morphogenesis: regulation by cell shape in intestinal epithelial cells. Exp Cell Res. 1997;235:3–12. doi: 10.1006/excr.1997.3629. [DOI] [PubMed] [Google Scholar]
- 9.Capurro M, Wanless IR, Sherman M, et al. Glypican-3: a novel serum and histochemical marker for hepatocellular carcinoma. Gastroenterology. 2003;125:89–97. doi: 10.1016/s0016-5085(03)00689-9. [DOI] [PubMed] [Google Scholar]
- 10.Yorita K, Takahashi N, Takai H, et al. Prognostic significance of circumferential cell surface immunoreactivity of glypican-3 in hepatocellular carcinoma. Liver Int. 2011;31:120–31. doi: 10.1111/j.1478-3231.2010.02359.x. [DOI] [PubMed] [Google Scholar]
- 11.Ho M, Kim H. Glypican-3: a new target for cancer immunotherapy. Eur J Cancer. 2011;47:333–8. doi: 10.1016/j.ejca.2010.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nakano K, Orita T, Nezu J, et al. Anti-glypican 3 antibodies cause ADCC against human hepatocellular carcinoma cells. Biochem Biophys Res Commun. 2009;378:279–84. doi: 10.1016/j.bbrc.2008.11.033. [DOI] [PubMed] [Google Scholar]
- 13.Ishiguro T, Sugimoto M, Kinoshita Y, et al. Anti-glypican 3 antibody as a potential antitumor agent for human liver cancer. Cancer Res. 2008;68:9832–8. doi: 10.1158/0008-5472.CAN-08-1973. [DOI] [PubMed] [Google Scholar]
- 14.Zhu AX, Gold PJ, El-Khoueiry AB, et al. First-in-man phase I study of GC33, a novel recombinant humanized antibody against glypican-3, in patients with advanced hepatocellular carcinoma. Clin Cancer Res. 2013;19:920–8. doi: 10.1158/1078-0432.CCR-12-2616. [DOI] [PubMed] [Google Scholar]
- 15.Okuda K, Takayasu K. Hepatobiliary disease. In: Okuda K, Mitchell DG, Itai Y, Ariyama J, editors. Primary Malignant Tumors of the Liver. London: Blackwell Science; 2001. pp. 343–89. [Google Scholar]
- 16.Bruix J, Sherman M, Llovet JM, et al. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol. 2001;35:421–30. doi: 10.1016/s0168-8278(01)00130-1. [DOI] [PubMed] [Google Scholar]
- 17.The Liver Cancer Study Group of Japan. Primary liver cancer in Japan: clinicopathologic features and results of surgical treatments. Ann Surg. 1990;211:277–87. [PMC free article] [PubMed] [Google Scholar]
- 18.Bruix J, Llovet JM. Prognostic prediction and treatment strategy in hepatocellular carcinoma. Hepatology. 2002;35:519–24. doi: 10.1053/jhep.2002.32089. [DOI] [PubMed] [Google Scholar]
- 19.2009. Cancer Therapy Evaluation Program, Common Terminology Criteria for Adverse Events (CTCAE), Version 4.0 (v4.03: June 14, 2010), DCTD, NCI, NIH, DHHS. [Cited 11 Nov 2013.] Available from URL: http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf.
- 20.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–47. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 21.Takai H, Kato A, Ishiguro T, et al. Optimization of tissue processing for immunohistochemistry for the detection of human glypican-3. Acta Histochem. 2010;112:240–50. doi: 10.1016/j.acthis.2008.11.025. [DOI] [PubMed] [Google Scholar]
- 22.McCarty KS, Jr, Szabo E, Flowers JL, et al. Use of a monoclonal anti-estrogen receptor antibody in the immunohistochemical evaluation of human tumors. Cancer Res. 1986;46(Suppl. 8):4244s–8s. [PubMed] [Google Scholar]
- 23.Mager DE, Jusko WJ. General pharmacokinetic model for drugs exhibiting target-mediated drug disposition. J Pharmacokinet Pharmacodyn. 2001;28:507–32. doi: 10.1023/a:1014414520282. [DOI] [PubMed] [Google Scholar]
- 24.Kuzuya T, Asahina Y, Tsuchiya K, et al. Early decrease in α-fetoprotein, but not des-γ-carboxy prothrombin, predicts sorafenib efficacy in patients with advanced hepatocellular carcinoma. Oncology. 2011;81:251–8. doi: 10.1159/000334454. [DOI] [PubMed] [Google Scholar]
- 25.Sawada Y, Yoshikawa T, Nobuoka D, et al. Phase I trial of a glypican-3-derived peptide vaccine for advanced hepatocellular carcinoma: immunological evidence and potential for improving overall survival. Clin Cancer Res. 2012;18:3686–96. doi: 10.1158/1078-0432.CCR-11-3044. [DOI] [PubMed] [Google Scholar]
- 26.Hilchey SP, Hyrien O, Mosmann TR, et al. Rituximab immunotherapy results in the induction of a lymphoma idiotype-specific T-cell response in patients with follicular lymphoma: support for a “vaccinal effect” of rituximab. Blood. 2009;113:3809–12. doi: 10.1182/blood-2008-10-185280. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 Immunohistochemical staining of glypican-3 (GPC3) in tumor biopsy specimens from patients with advanced hepatocellular carcinoma (n = 13) evaluated by two scoring methods, method 1 (score 0–14) (a) and method 2 (score 0–3) (b). Bar = 50 μm.