Abstract
Constitutive activation of the signal transducer and activator of transcription 3 (STAT3) or the nuclear factor-κB (NF-κB) pathway occurs frequently in cancer cells and contributes to oncogenesis. The activation of Janus kinase 2 (JAK2) and IκB kinase (IKK) are key events in STAT3 and NF-κB signaling, respectively. We have identified 2-methoxystypandrone (2-MS) from a traditional Chinese medicinal herb Polygonum cuspidatum as a novel dual inhibitor of JAK2 and IKK. 2-MS inhibits both interleukin-6-induced and constitutively-activated STAT3, as well as tumor necrosis factor-α-induced NF-κB activation. 2-MS specifically inhibits JAK and IKKβ kinase activities but has little effect on activities of other kinases tested. The inhibitory effects of 2-MS on STAT3 and NF-κB signaling can be eliminated by DTT or glutathione and can last for 4 h after a pulse treatment. Furthermore, 2-MS inhibits growth and induces death of tumor cells, particularly those with constitutively-activated STAT3 or NF-κB signaling. We propose that the natural compound 2-MS, as a potent dual inhibitor of STAT3 and NF-κB pathways, is a promising anticancer drug candidate.
Keywords: 2-Methoxystypandrone, anticancer drug, Janus kinase 2/signal transducer and activator of transcription 3 pathway, natural compound, tumor necrosis factor-α/nuclear factor-κB pathway
Signal transducer and activator of transcription 3 (STAT3) is a transcription factor that mediates cytokines and growth factors-directed transcription. An activated STAT3 by itself can cause cellular transformation and directly contributes to oncogenesis.(1,2) Constitutive activation of STAT3 signaling is associated with enhanced cell proliferation in many different tumors, including breast, prostate, head and neck, skin and colon cancer, and melanoma. (3–8) Small molecule inhibitors of Janus kinase 2 (JAK2)/STAT3 pathway are reported to be effective anticancer agents in both cancer cell lines and animal models.(9–14) Therefore, targeting the JAK2/STAT3 pathway is a promising strategy for treating human tumors.
Nuclear factor-κB (NF-κB) and its pathway regulators modulate the expression of genes involved in inflammation, cell survival and proliferation.(15) Constitutive activation of NF-κB pathway is a common feature of a variety of hematological and solid tumors, demonstrating that NF-κB plays an important role in carcinogenesis.(16) Given the importance of the NF-κB pathway in tumor development, many inhibitors of this pathway are under investigation for cancer therapy.(17)
The root of Polygonum cuspidatum Sieb. et Zucc. (Polygonaceae) is a well-known traditional Chinese medicinal herb that is used to treat various inflammatory diseases and cancer.(18) Although several active components such as emodin and resveratrol extracted from the root of P. cupidatum have been reported to exhibit anticancer activities,(19,20) the molecular targets and modes of action of P. cupidatum remain unclear.
In our previous study we reported that 2-methoxystypandrone (2-MS), isolated from the root of P. cupidatum, is a potent inhibitor of interleukin-6 (IL-6)/STAT3 signaling.(21) In the present study we investigate the molecular mechanisms of 2-MS and find that 2-MS inhibits both the IL-6-induced and the constitutively-activated STAT3 in human cancer cell lines through inhibition of the JAK2 kinase. In addition, 2-MS inhibits tumor necrosis factor-α (TNF-α)/NF-κB signaling pathway through inhibition of IκB kinase β (IKKβ). Furthermore, 2-MS inhibits growth and induces death of cancer cells. Our data strongly support the notion that 2-MS is a novel dual inhibitor of JAK2 and IKKβ kinases and is a promising anticancer drug candidate.
Materials and Methods
Reagents and antibodies
2-Methoxystypandrone was isolated from the root of P. cuspidatum Sieb, as reported previously;(21) DTT and MTT were purchased from Genebase (Shanghai, China); GSH was obtained from Shanghai Sibas Bioscience (Shanghai, China); IL-6 and IFN-α were from Peprotech (Saint Paul, MN, USA); TNF-α was from R&D Systems (Minneapolis, MN, USA); IFN-γ was obtained from Shanghai Clone (Shanghai, China); anti-phosphorylated STAT3 (Tyr705), anti-phophorylated JAK2 (Tyr1007/1008), anti-JAK2, anti-phophorylated JAK1 (Tyr1022/1023), anti-JAK1, anti-phophorylated TYK2(Tyr1054/1055), anti-TYK2, anti-phosphorylated IκB-α (Ser32/36), anti-IκB-α, anti-phosphorylated IKK-αβ (Ser176/180 for IKKα and Ser177/181 for IKKβ) and anti-IKK-α antibodies were obtained from Cell Signaling Technology (Boston, MA, USA); anti-STAT3, anti-GP130 and anti-α-Tubulin antibodies were from Santa Cruz Biotechnology (Dallas, TX, USA); anti-β-Actin antibody was from Abmart (Shanghai, China); anti-GAPDH antibody was obtained from Kang Chen Bioscience (Shanghai, China); secondary HRP-conjugated antibodies were from Multi Sciences Biotech (Hangzhou, China); and the Apoptosis Detection Kit was from Shanghai MaiYueEr Bioscience (Shanghai, China).
Cell lines and culture
HEK293/NF-κB cells, gifts from Professor Xin-Yuan Fu (National University of Singapore, Singapore), were HEK293 cells stably transfected with an NF-κB-responsive firefly luciferase reporter plasmid, and all other cell lines were obtained from the American Type Culture Collection. HEK293/NF-κB, HeLa, MCF-7, U87MG and SK-OV-3 cells were cultured in DMEM (Gibco, Grand Island, NY, USA) supplemented with 10% (v/v) FBS (Gibco), 100 μg/mL ampicillin and 100 μg/mL streptomycin. Jurkat, U937, THP-1, 786-0, Bel-7404, PC-3, BGC, A549 and H460 cells were grown in RPMI 1640 medium (Gibco) supplemented with 10% FBS, 100 μg/mL ampicillin and 100 μg/mL streptomycin. DU-145 cells were cultured in RPMI 1640 medium supplemented with 10% FBS, 2 mM L-glutamine, 100 μg/mL ampicillin and 100 μg/mL streptomycin. HepG2 cells were cultured in Minimum Essential Medium α medium (Gibco) supplemented with 10% (v/v) FBS, 100 μg/mL ampicillin and 100 μg/mL streptomycin. All cell lines were cultured at 37°C in a humidified atmosphere of 95% air and 5% CO2.
Luciferase assay
HEK293/NF-κB cells were seeded into 96-well cell culture plates and allowed to grow for 48 h and cells were then treated with 2-MS for 2 h followed by stimulation with 2 ng/mL TNF-α for 5 h. Luciferase activity was determined using the Promega luciferase kits according to the manufacturer's instruction (Promega, Madison, WI, USA). All luciferase assay experiments were repeated at least twice.
MTT assay
Cell growth rate was measured by MTT assay. Briefly, approximately 4500–12 000 cells were seeded into 96-well plates. After 24 h, cells were treated with vehicle control (DMSO) or compounds for 72 h. After treatment, 30 μL MTT (5 mg/mL) were added to the culture medium. After incubating for 3 h at 37°C, the cells were solubilized in 100 μL “Triplex Solution” (10% SDS-5% isobutanol-12 mM HCl) for 16 h, and then the absorbance of each well was measured at 595 nm with a spectrophotometer (TECAN infinite F200; TECAN, Männedorf, Switzerland).
Western blot analysis
Cells were lysed with ice-cold 1× Laemmli buffer (Sigma-Aldrich, Saint Louis, MO, USA) and then samples were boiled for 10 min. Proteins were separated by 8% sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to a nitrocellulose membrane (from Millipore, Darmstadt, Hesse, Germany). Membranes were blocked with 5% nonfat dry milk in TBST (1‰ Tween 20) for 1 h at room temperature. Then they were subjected to primary antibodies in 5% BSA in TBST at 4°C overnight. Membranes were then washed with TBST and incubated with peroxidase-conjugated secondary antibodies at room temperature for 1 h. After washing with TBST, immune complexes on the membranes were detected by enhanced chemiluminescence (Millipore).
Janus kinase 2 and IκB kinase β in vitro kinase assay
The JAK2 (IKKβ) in vitro kinase assay was performed using JAK2 (IKKβ) immunoprecipitates, an HTScan JAK2 (IKKβ) Kinase Assay Kit (Cell Signaling Technology) and Streptavidin coated 96-well plates (Sigma). Briefly, HeLa cell (in IKKβ assay, HEK293 cells were transfected with plasmids encoding IKKβ-FLAG 24 h before lysed) growth in 10-cm dish with 90% confluence was stimulated with 30 ng/mL IL-6 (in IKKβ assay, no stimulation) and then lysed with 1 mL lysis buffer (50 mM HEPES [pH 7.4], 150 mM NaCl, 0.15% Triton X-100, 0.5 mM DTT [no DTT in IKKβ assay], 2 mM sodium orthovanadate [Na3VO4], 2 mM sodium fluoride [NaF], 1 mM PMSF and protease inhibitor cocktail [Sigma, 1:1000]) on ice for 30 min. Cell lysates were collected and centrifuged to remove the pellet. The lysates were immunoprecipitated with 1:100 (v/v) JAK2 (Santa Cruz Biotechnology) or FLAG antibody (Abmart) at 4°C for 3 h and then 1:10 (v/v) Protein A/G Plus Agarose (Santa Cruz Biotechnology) was added to the lysates at 4°C for about 16 h. The JAK2 or IKKβ immunoprecipitates were then centrifuged and washed twice with lysis buffer and once with kinase reaction buffer (60 mM HEPES [pH 7.5], 5 mM MgCl2, 5 mM MnCl2, 25 μM Na3VO4, 200 μM ATP and 0.5 mM DTT [no DTT in IKKβ assay]). JAK2 or IKKβ immunoprecipitates were incubated with DMSO or 2-MS for 5 min and then subjected to kinase reaction at 25°C for 30 min in a final volume of 50 μL kinase reaction buffer containing 1.5 μM FLT3 (Tyr589) or IκB-(Ser32) biotinylated peptide substrate. Samples were then processed according to the protocol for the HTScan JAK2 (IKKβ) Kinase Assay Kit (Cell Signaling Technology).
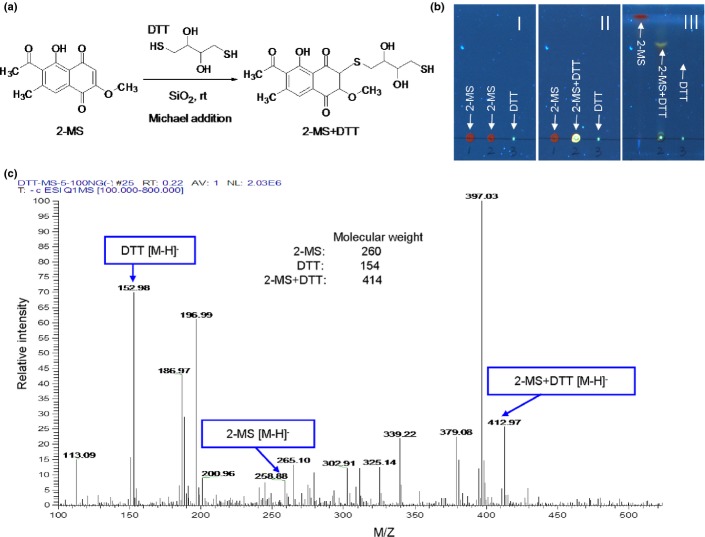
Thin layer chromatography and electrospray ionization mass spectrometry analysis
TLC was carried out on pre-coated silica gel F254 plates (Merck, Darmstadt, Germany); these plates were first run successively in methanol solvent system up to the upper edge in order to eliminate impurities. The plates then were dried, and subsequently reactivated by heating at 125°C for 60 min. 2-MS (0.3 mg) and DTT (0.7 mg) were dissolved in 100 μL of acetone, and applied quantitatively, either as spots, or in bands (1 × 20 cm) as with fine glass capillaries. TLC spots were developed in the CHCl3-MeOH (4:1, v/v) solvent system and scanned under UV-366 nm using the Camag TLC Visualizer (Muttenz, Switzerland). The yellow TLC bands of Michael adducts of DTT to 2-MS were scraped from the plates and eluted in acetonitrile. The resultant solution was filtered through a 45-μm filter for a negative-mode electrospray ionization mass spectrometry (ESI-MS) analysis. ESI-MS was performed on a Thermo TSQ Quantum Ultra LC-MS/MS instrument (Thermo Fisher Scientific, Waltham, MA, USA).
Results
2-Methoxystypandrone inhibited both interleukin-6-induced and constitutively-activated signal transducer and activator of transcription 3 in human tumor cells
We screened an extract library of Chinese medicinal herbs for their inhibitory effect on STAT3 signaling using a STAT3 responsive luciferase reporter gene-transfected HepG2 cell line and found 2-MS (Fig. 1), which inhibited STAT3 activity in both a luciferase assay using the HepG2 cells and a western blotting assay using MDA-MB-231 cells.(21) To confirm the inhibitory effects of 2-MS on the JAK2/STAT3 signaling pathway, we examined its effects on the IL-6-induced phosphorylation/activation of STAT3. 2-MS inhibited both the IL-6-induced and the constitutive phosphorylation/activation of STAT3 in a dose-dependent manner in HeLa cells (Fig. 2a,c). The inhibition was rapid. A 15-min treatment was sufficient to block the activation (Fig. 2b), suggesting direct inhibition of the STAT3 phosphorylation. 2-MS also significantly inhibited the constitutive activation of STAT3 in MCF-7, DU-145 and HepG2 cells (Fig. 2d). Therefore, 2-MS inhibited both the IL-6 induced as well as the constitutive activation of STAT3 in human tumor cells.
Fig. 1.
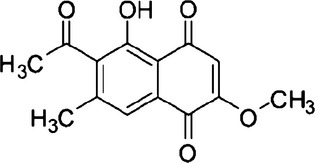
Chemical structure of 2-methoxystypandrone.
Fig. 2.
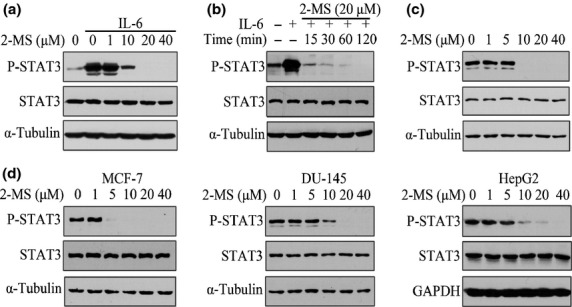
2-Methoxystypandrone (2-MS) inhibited both interleukin-6 (IL-6)-induced and constitutive activation of signal transducer and activator of transcription 3 (STAT3) in human tumor cell lines. (a) HeLa cells were incubated with indicated concentrations of 2-MS for 2 h before stimulation with IL-6 (30 ng/mL) for 15 min. Whole cell lysates were processed for western blot analysis and probed with anti-P-STAT3 (Y705) antibodies. Anti-STAT3 and anti-α-Tubulin antibodies were used as loading controls. (b) HeLa cells were pretreated with 20 μM 2-MS for indicated time before stimulation with IL-6 (30 ng/mL) for 15 min. Whole cell lysates were subjected to western blot analysis. (c) HeLa cells were incubated with indicated concentrations of 2-MS for 2 h. Whole cell lysates were processed for western blot analysis. (d) MCF-7, DU-145 and HepG2 cells were incubated with indicated concentrations of 2-MS for 2 h and whole cell lysates were subjected to western blot analysis.
2-Methoxystypandrone inhibited Janus kinase 2 and IκB kinase in vitro kinase activity
The inhibitory effect of 2-MS on STAT3 activation suggested that 2-MS might interfere with the upstream components of the STAT3 signaling pathway. Because JAK2 is the major kinase to activate STAT3 while GP130 is important for tyrosine phosphorylation of JAK2 in the IL-6/STAT3 pathway,(22) we first analyzed the effects of 2-MS on the expression and phosphorylation of JAK2 and the expression of GP130. In HeLa cells, 10 μM 2-MS significantly inhibited phosphorylation of JAK2 (Y1007/1008), while it had no effects on the expressions of JAK2 and GP130 (Fig. 3a). In addition, 10 μM 2-MS significantly inhibited the phosphorylation of JAK2 (Y1007/1008) in HeLa cells without IL-6 stimulation, further suggesting that JAK2 is the direct target of 2-MS (Fig. 3b).
Fig. 3.
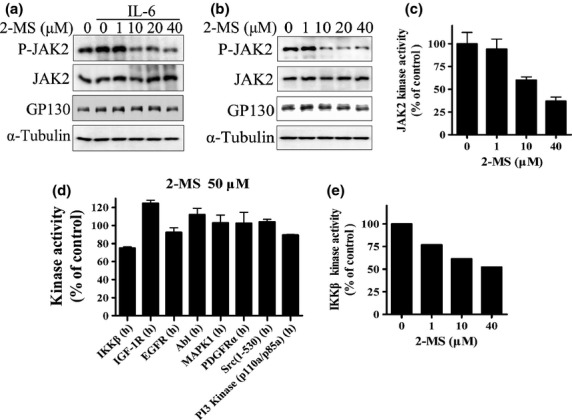
Effects of 2-methoxystypandrone (2-MS) on Janus kinase 2 (JAK2) and activities of other human kinases. (a) HeLa cells were incubated with indicated concentrations of 2-MS for 2 h before stimulation with interleukin-6 (IL-6) (30 ng/mL) for 15 min. Whole cell lysates were processed for western blot analysis and probed with anti-P-JAK2 (Y1007/1008), anti-JAK2 or anti-GP130 antibodies. Anti-α-Tubulin antibody was used as a loading control. (b) HeLa cells were incubated with indicated concentrations of 2-MS for 2 h before being processed for western blot analysis. (c) In vitro kinase assay of JAK2. JAK2 protein immunoprecipitated from HeLa cells was subjected to in vitro kinase assay in the presence of indicated concentrations of 2-MS. (d) Effects of 2-MS on activities of human kinases. In vitro kinase assays were processed by Millipore Kinase Services. (e) In vitro kinase assay of IKKβ. IKKβ protein immunoprecipitated from HEK293 cells was subjected to in vitro kinase assay in the presence of indicated concentrations of 2-MS.
We next analyzed whether 2-MS directly inhibit the JAK2 kinase activity in vitro. We immunoprecipitated the JAK2 kinase from HeLa cells and performed an in vitro kinase assay. We found that 10 μM 2-MS significantly inhibited JAK2 in vitro kinase activity (Fig. 3c), suggesting that 2-MS is a direct JAK2 kinase inhibitor. In order to investigate the specificity of 2-MS, we tested the effects of 2-MS on eight different human tyrosine or serine/threonine kinases. 50 μM 2-MS had almost no effect on them, except for a 25% inhibition on IKKβ (Fig. 3d). 2-MS also has no effect on epidermal growth factor signaling pathway in HeLa cells (Fig. S1). We next confirmed the inhibition effects of 2-MS on IKKβ kinase activity in vitro. We immunoprecipitated the IKKβ kinase from HEK293 cells and performed an in vitro kinase assay. As shown in Fig. 3(e), 2-MS inhibited IKKβ in vitro kinase activity in a dose-dependent manner. We also examined the effects of 2-MS on JAK1 and TYK2, which are two other kinases of the JAK family. As shown in Fig. S2, 10 μM 2-MS significantly inhibited the IFN-γ-induced phosphorylation of JAK1 (Tyr1022/1023) and the IFN-α-induced phosphorylation of TYK2 (Tyr1054/1055). Thus, 2-MS is likely a pan-JAK inhibitor.
2-Methoxystypandrone inhibited tumor necrosis factor-α-induced NF-κB signal transduction, phosphorylation of inhibitor of nuclear factor-κB-α (IκB-α) and IκB kinase in human tumor cells
Activation of IKK in response to stimuli of various cytokines is a key event in NF-κB signaling, which is important for tumor cell survival and growth. To fully understand the effects of 2-MS on tumor cells, we next investigated the effects of 2-MS on NF-κB signaling pathway.
We examined the effects of 2-MS on the NF-κB signaling pathway using an NF-κB responsive luciferase reporter gene-transfected HEK293 cell line. We found that 2-MS inhibited TNF-α-induced NF-κB responsive reporter gene activity in a dose-dependent manner, with a half-maximal inhibition dose of 3 μM (Fig. 4a). Parallel to inhibition of NF-κB signaling, 2-MS only slightly decreased the cell number of HEK293 cells (Fig. 4b), suggesting that the inhibition effect of 2-MS on NF-κB signaling was not caused by reduced cell number.
Fig. 4.
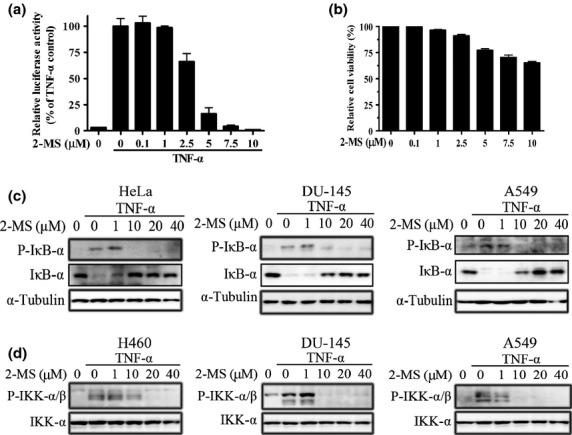
2-Methoxystypandrone (2-MS) inhibited tumor necrosis factor-α (TNF-α)-induced nuclear factor-κB (NF-κB) signal transduction, phosphorylation of IκB-α and IKK in human tumor cell lines. (a) 2-MS inhibited TNF-α-induced NF-κB regulated luciferase activity. HEK293/NF-κB cells were pretreated with indicated concentrations of 2-MS for 2 h. Luciferase activity was measured following stimulation with TNF-α (2 ng/mL) for 5 h. (b) Effects of 2-MS on cell survival. HEK293/NF-κB cells were treated with indicated concentrations of 2-MS for 7 h. Cell viability was determined by MTT assay. (c) 2-MS inhibited TNF-α-induced phosphorylation of IκB-α. HeLa, DU-145 and A549 cells were incubated with indicated concentrations of 2-MS for 2 h before stimulation with TNF-α (2 ng/mL) for 15 min. Whole cell lysates were subjected to western blot analysis and probed with anti-P-IκB-α (Ser32/36) and anti-IκB-α antibodies. Anti-α-Tubulin antibodies were used as a loading control. (d) 2-MS inhibited TNF-α-induced phosphorylation of IKK. H460, DU-145 and A549 cells were incubated with indicated concentrations of 2-MS for 2 h before stimulation with TNF-α (2 ng/ml) for 15 min. Whole cell lysates were subjected to western blot analysis and probed with anti-P-IKKα/β (Ser176/180 for IKKα and Ser177/181 for IKKβ) antibodies. Anti-IKK-α antibodies were used as a loading control.
Activation of NF-κB signaling requires phosphorylation of IκB-α. Hence, we examined the effects of 2-MS on the phosphorylation of IκB-α. As shown in Figure 4(c), 2-MS significantly inhibited the TNF-α-induced phosphorylation of IκB-α in HeLa, DU-145 and A549 cells at a concentration of 10 μM. After phosphorylation, IκB-α undergoes a proteasome-mediated degradation. Therefore, the changes of IκB-α were the opposite of that of phosphorlylated IκB-α (Fig. 4c). Because IKK is the major upstream kinase of IκB-α in TNF-α-induced NF-κB signaling, we analyzed the effects of 2-MS on the phosphorylation of IKK. In H460, DU-145 and A549 cells, 2-MS significantly inhibited the phosphorylation of IKKα/β (Ser176/180 for IKKα and Ser177/181 for IKKβ) at a concentration (10 μM) similar to that used for inhibition of the TNF-α-induced luciferase activity and the phosphorylation of IκB-α. Thus, 2-MS, as an IKKβ kinase inhibitor, inhibited the TNF-α-induced phosphorylation of IKK, IκB-α and NF-κB signal transduction in human tumor cells.
Effects of 2-methoxystypandrone on signal transducer and activator of transcription 3 and nuclear factor-κB signaling can be reversed by DTT or GSH and were irreversible
As shown in Fig. 1, 2-MS contains three α,β-unsaturated carbonyl groups which are reactive with thiols. The thiols of cysteines in JAK2 and IKKβ are reported to be important for their kinase activities and several compounds with thiol-reactive groups have been found to covalently bind to Cysteine 179 of IKKβ and inhibit its kinase activity.(23–26) Therefore, we investigated whether the inhibitory effects of 2-MS on JAK2 or IKKβ kinase activity were through interacting with their cysteine thiols. We used two thiol-containing compounds, DTT or GSH, to compete with JAK2 or IKKβ on interacting with 2-MS to test whether the inhibitory effects of 2-MS on the STAT3 or NF-κB pathway could be alleviated by the two thiol-containing compounds. As shown in Figure. 5(a) and (c), after incubation with DTT or GSH, the inhibitory effects of 2-MS on STAT3 and NF-κB pathways were abolished. Therefore, 2-MS may inhibit the STAT3 and NF-κB pathways through interacting with the thiols of JAK2 and IKKβ via its α,β-unsaturated carbonyl groups. To further demonstrate the 2-MS-thiol interaction, 2-MS was mixed with DTT to induce a chemical reaction of Michael addiction. The proposed reaction site of 2-MS is illustrated in Fig. 6(a). The Michael adducts of DTT to 2-MS were then examined using TLC and ESI-MS analysis, which detected one product at m/z 412.97 [2-MS + DTT-H]−, indicating the addition of DTT to 2-MS (Fig. 6b,c). The results strongly support the possibility that 2-MS reacts with the cysteine thiols of JAK2 and IKKβ.
Fig. 5.
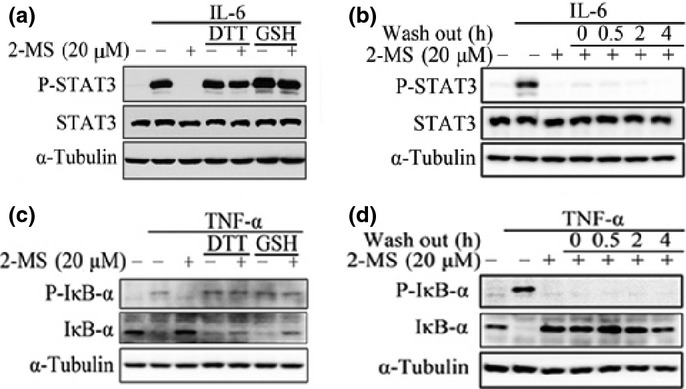
Effects of 2-methoxystypandrone (2-MS) on signal transducer and activator of transcription 3 (STAT3) and NF-κB signaling can be abolished by DTT or GSH treatment and were irreversible. (a) 20 μM 2-MS were incubated with or without 500 μM DTT or GSH at 37°C for 1 h before being added to HeLa cells for 2 h, followed by stimulation with interleukin-6 (IL-6) (30 ng/mL) for 15 min. Whole cell lysates were processed for western blot analysis and probed with anti-P-STAT3 (Y705) antibodies. Anti-STAT3 and anti-α-Tubulin antibodies were used as loading controls. (b) HeLa cells were pretreated with 20 μM 2-MS for 2 h and then washed with fresh medium for three times. After the indicated time periods, the cells were stimulated with IL-6 (30 ng/mL) for 15 min. Whole cell lysates were processed for western blot analysis. (c) 20 μM 2-MS were incubated with or without 500 μM DTT or GSH at 37°C for 1 h before being added to HeLa cells for 2 h, followed by stimulation with tumor necrosis factor-α (TNF-α) (2 ng/mL) for 15 min. Whole cell lysates were subjected to western blot analysis and probed with anti-P-IκB-α (Ser32/36) and anti-IκB-α antibodies. Anti-α-Tubulin antibodies were used as a loading control. (d) HeLa cells were pretreated with 20 μM 2-MS for 2 h and then washed with fresh medium three times. After the indicated time periods, the cells were stimulated with TNF-α (2 ng/mL) for 15 min. Whole cell lysates were processed for western blot analysis.
Fig. 6.
The covalent interaction of 2-methoxystypandrone (2-MS) with DTT. (a) The Michael addition reaction of α,β-unsaturated carbonyl of 2-MS with DTT; (b) TLC photography of 2-MS, DTT and Michael adducts of DTT to 2-MS under the ultraviolet light, 366 nm. TLC was performed on silica gel F254 TLC plates and was scanned using the Camag TLC visualizer. The No. III plate was developed in CHCl3-MeOH (4:1, v/v) solvent system, the spots in the No. III plate: 1: 2-MS (red); 2: 2-MS+DTT (yellow); 3: DTT. (c) Detection of a mixture of DTT, 2-MS and Michael adduct of DTT to 2-MS on the silica gel plate by a negative-mode electrospray ionization mass spectrometry (ESI-MS). The TLC bands of Michael adducts of DTT to 2-MS were scraped from the plates and eluted in acetonitrile. The resultant solution was filtered through a 45 μM filter and then collected for ESI-MS analysis.
We also found that the inhibition effects of 2-MS on the STAT3 and NF-κB pathways lasted as long as 4 h after a pulse treatment with the compound (Fig. 5b,d), indicating that 2-MS irreversibly inhibits JAK2 and IKKβ, probably through covalent modification of JAK2 and IKKβ.
Taken together, these data suggest that 2-MS may covalently modify JAK2 and IKKβ and inhibit their kinase activities.
2-Methoxystypandrone inhibited growth of human cancer cells
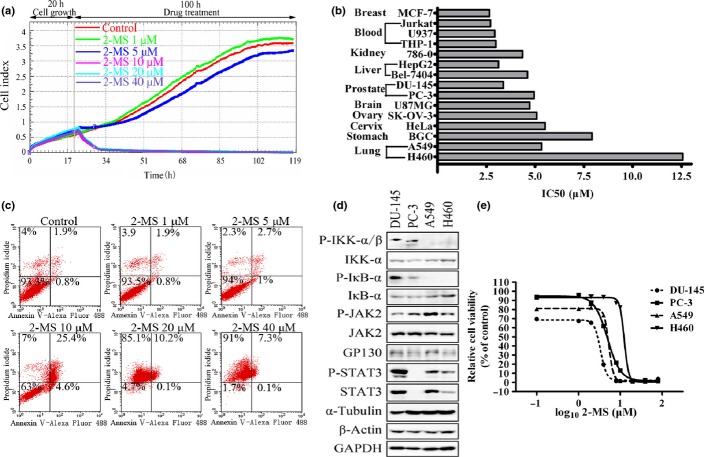
The STAT3 and NF-κB signaling pathways play an important role in tumor cell survival and growth. Therefore, we analyzed the effects of 2-MS on the growth and survival of a panel of human tumor cell lines. We first examined the 2-MS's effects on HeLa cells using a real-time cell analysis system. As shown in Figure. 7(a), 2-MS exhibited inhibitory effects on HeLa cells and 10 μM 2-MS totally inhibited the cell growth. 2-MS inhibited the growth of all human tumor cell lines we examined with an IC50 between 2.5 to 12.5 μM (Figure. 7b). Many STAT3 and NF-κB pathway inhibitors inhibit cell growth by inducing apoptosis.(9–14,24,25) Therefore, we tested the ability of 2-MS to induce apoptosis. As shown in Figure. 7(c), 2-MS mostly induced necrosis of the tumor cells, as indicated by the single PI staining. We also examined the effects of 2-MS on the PARP cleavage in the tumor cells. However, we did not detect the PARP cleavage caused by 2-MS (data not shown).
Fig. 7.
2-Methoxystypandrone (2-MS) inhibited growth of human cancer cells. (a) Real-time cell analysis (RTCA) system analysis of 2-MS's effects on HeLa cells. (b) IC50 of 2-MS on viability of different human tumor cell lines for 72 h. (c) HeLa cells were pretreated with indicated concentrations of 2-MS for 24 h. Cells were then stained with Annexin V-Alexa Fluor 488 Apoptosis Detection Kit before analysis of apoptosis through flow cytometry. (d) Lysates from four human prostate and lung cancer cell lines DU-145, PC-3, A549 and H460 were processed for western blot analysis using specific antibodies as indicated. (e) DU-145, PC-3, A549 and H460 cells were treated with 2-MS at various concentrations for 72 h and then processed for MTT assay.
As a kinase inhibitor of JAK2 and IKKβ, 2-MS effectively inhibits the STAT3 and NF-κB signaling pathways and growth of cancer cells. We examined whether the growth inhibition effects of 2-MS on cancer cells are related to the activation status of STAT3 or NF-κB pathway. As shown in Figure. 7(d) and (e), prostate cancer cell lines DU-145, PC-3 (which have constitutively activated NF-κB pathway) and non-small-cell lung cancer (NSCLC) cell line A549 (which has constitutively activated STAT3 pathway) cells are significantly more sensitive than H460 cells (NSCLC cell line which lacked constitutively activated NF-κB or STAT3 pathway) to growth inhibition of 2-MS. Taken together, these data demonstrate the potential of 2-MS to inhibit growth and induce cell death of human cancer cells, particularly those with constitutively activated STAT3 and/or NF-κB pathway.
Discussion
The root of P. cuspidatum is used as a traditional Chinese medicine to treat cancer.(18) Although many active components from the root of P. cupidatum have been reported to be kinase inhibitors,(27) only a few have been found to inhibit JAK2 or IKKβ.(20,28) Here we report the identification of a new dual JAK2 and IKKβ kinase inhibitor, 2-MS, from the root of P. cupidatum. Because both STAT3 and NF-κB pathways play important roles in oncogenesis and tumor growth,(1,2,16) elucidating the molecular mechanisms of 2-MS in regulating the STAT3 and NF-κB pathways will help us to understand and better use 2-MS in cancer treatment.
2-Methoxystypandrone is a potent inhibitor of the STAT3 and NF-κB pathways. It inhibited the IL-6-induced and the constitutive activation of STAT3 as well as the TNF-α-induced activation of NF-κB. 2-MS inhibited tumor cell growth, especially those with activated STAT3 and/or NF-κB pathway. The direct targets of 2-MS are JAK2 and IKKβ kinases. 2-MS specifically inhibited JAK2 and IKKβ kinase activities while had little effect on the activities of seven other kinases analyzed.
The exact mechanisms of 2-MS inhibiting the JAK2 and IKKβ kinases are not clear. However, we have several lines of evidence suggesting that 2-MS may inhibit the JAK2 and IKKβ kinase activities by covalently interacting with the JAK2 and IKKβ proteins. First, 2-MS has three α,β-unsaturated carbonyl groups which are thiol-reactive and formed covalent linkages with DTT. Second, the inhibitory effects of 2-MS on the STAT3 and NF-κB pathways were abolished after incubating with DTT or GSH, suggesting that the α,β-unsaturated carbonyl groups in 2-MS are active groups that may form adducts with the thiols of the cysteins in the JAK2 and IKKβ proteins. Third, the inhibition effects of 2-MS on STAT3 and NF-κB pathways lasted for as long as 4 h after a pulse treatment with the compound, suggesting that the inhibition effects of 2-MS were irreversible. Taken together, these data suggest that 2-MS may covalently modify JAK2 and IKKβ and inhibit their kinase activities.
Constitutively-activated STAT3 and/or NF-κB pathway contribute to malignant progression by preventing apoptosis.(2,29) Many small molecule inhibitors of the STAT3 or NF-κB pathway inhibit tumor cell growth and induce apoptosis.(9–14,24,25) However, our data demonstrated that 2-MS induced necrosis instead of apoptosis of HeLa cells, 2-MS also did not cause PARP cleavage in HeLa and some other cancer cells (data not shown). It is possible that 2-MS has other targets in addition to JAK2 and IKKβ kinases and the cell death induced by 2-MS is probably a result of the combination effects of these targets.(30–32)
In summary, our data reveals that 2-MS, a natural compound isolated from P. cupidatum, potently inhibits the STAT3 and NF-κB pathways through specifically inhibiting the JAK2 and IKKβ kinase activities. Furthermore, 2-MS demonstrates strong anticancer activity on a panel of cancer cell lines. These data strongly indicate that 2-MS is a new anticancer drug candidate.
Acknowledgments
This work was supported by China Ministry of Science and Technology Key New Drug Creation and Manufacturing Program Grant 2013ZX09102015, China Ministry of Science and Technology Research Grant 2013ZX10002010-009, China National Natural Science Foundation Grants 81373432, 31129004, 91129701 and 91213304, and National Science and Technology 973 Grants 2012CB910704 and 2013CB910900.
Disclosure Statement
The authors have no conflict of interest.
Supporting Information
Additional supporting information may be found in the online version of this article:
Fig. S1 Effects of 2-methoxystypandrone (2-MS) on epidermal growth factor signaling pathway in HeLa cells.
Fig. S2 2-methoxystypandrone (2-MS) inhibited phosphorylation of JAK1 and TYK2.
References
- 1.Bromberg JF, Wrzeszczynska MH, Devgan G, et al. Stat3 as an Oncogene. Cell. 1999;98:295–303. doi: 10.1016/s0092-8674(00)81959-5. [DOI] [PubMed] [Google Scholar]
- 2.Bowman T, Garcia R, Turkson J, et al. STATs in oncogenesis. Oncogene. 2000;19:2474–88. doi: 10.1038/sj.onc.1203527. [DOI] [PubMed] [Google Scholar]
- 3.Garcia R, Bowman TL, Guillian N, et al. Constitutive activation of Stat3 by the Src and JAK tyrosine kinases participates in growth regulation of human breast carcinoma cells. Oncogene. 2001;20:2499–513. doi: 10.1038/sj.onc.1204349. [DOI] [PubMed] [Google Scholar]
- 4.Mora LB, Buettner R, Seigne J, et al. Constitutive activation of STAT3 in human prostate tumors and cell lines direct inhibition of STAT3 signaling induces apoptosis of prostate cancer cells. Cancer Res. 2002;62:6659–66. [PubMed] [Google Scholar]
- 5.Kijima T, Niwa H, Steinman RA, et al. STAT3 activation abrogates growth factor dependence and contributes to head and neck squamous cell carcinoma tumor growth in vivo. Cell Growth Differ. 2002;13:355. [PubMed] [Google Scholar]
- 6.Chan KS, Carbajal S, Kiguchi K, et al. Epidermal growth factor receptor-mediated activation of Stat3 during multistage skin carcinogenesis. Cancer Res. 2004;64:2382–9. doi: 10.1158/0008-5472.can-03-3197. [DOI] [PubMed] [Google Scholar]
- 7.Corvinus FM, Orth C, Moriggl R, et al. Persistent STAT3 activation in colon cancer is associated with enhanced cell proliferation and tumor growth. Neoplasia. 2005;7:545. doi: 10.1593/neo.04571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Niu G, Bowman T, Huang M, et al. Roles of activated Src and Stat3 signaling in melanoma tumor cell growth. Oncogene. 2002;21:7001. doi: 10.1038/sj.onc.1205859. [DOI] [PubMed] [Google Scholar]
- 9.Blaskovich MA, Sun J, Cantor A, et al. Discovery of JSI-124 (cucurbitacin I), a selective Janus kinase/signal transducer and activator of transcription 3 signaling pathway inhibitor with potent antitumor activity against human and murine cancer cells in mice. Cancer Res. 2003;63:1270–9. [PubMed] [Google Scholar]
- 10.Iwamaru A, Szymanski S, Iwado E, et al. A novel inhibitor of the STAT3 pathway induces apoptosis in malignant glioma cells both in vitro and in vivo. Oncogene. 2007;26:2435–44. doi: 10.1038/sj.onc.1210031. [DOI] [PubMed] [Google Scholar]
- 11.Burger R, Le Gouill S, Tai Y-T, et al. Janus kinase inhibitor INCB20 has antiproliferative and apoptotic effects on human myeloma cells in vitro and in vivo. Mol Cancer Ther. 2009;8:26–35. doi: 10.1158/1535-7163.MCT-08-0149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin L, Hutzen B, Zuo M, et al. Novel STAT3 phosphorylation inhibitors exhibit potent growth-suppressive activity in pancreatic and breast cancer cells. Cancer Res. 2010;70:2445–54. doi: 10.1158/0008-5472.CAN-09-2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schust J, Sperl B, Hollis A, et al. Stattic: a small-molecule inhibitor of STAT3 activation and dimerization. Chem Biol. 2006;13:1235–42. doi: 10.1016/j.chembiol.2006.09.018. [DOI] [PubMed] [Google Scholar]
- 14.Hedvat M, Huszar D, Herrmann A, et al. The JAK2 inhibitor AZD1480 potently blocks Stat3 signaling and oncogenesis in solid tumors. Cancer Cell. 2009;16:487–97. doi: 10.1016/j.ccr.2009.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Perkins ND. Integrating cell-signaling pathways with NF-κB and IKK function. Nat Rev Mol Cell Biol. 2007;8:49–62. doi: 10.1038/nrm2083. [DOI] [PubMed] [Google Scholar]
- 16.Basseres D, Baldwin A. Nuclear factor-κB and inhibitor of κB kinase pathways in oncogenic initiation and progression. Oncogene. 2006;25:6817–30. doi: 10.1038/sj.onc.1209942. [DOI] [PubMed] [Google Scholar]
- 17.Karin M, Yamamoto Y, Wang QM. The IKK NF-κB system: a treasure trove for drug development. Nat Rev Drug Discov. 2004;3:17–26. doi: 10.1038/nrd1279. [DOI] [PubMed] [Google Scholar]
- 18.Lin Y-W, Yang F-J, Chen C-L, et al. Free radical scavenging activity and antiproliferative potential of Polygonum cuspidatum root extracts. J Nat Med. 2010;64:146–52. doi: 10.1007/s11418-009-0387-8. [DOI] [PubMed] [Google Scholar]
- 19.Srinivas G, Babykutty S, Sathiadevan PP, et al. Molecular mechanism of emodin action: transition from laxative ingredient to an antitumor agent. Med Res Rev. 2007;27:591–608. doi: 10.1002/med.20095. [DOI] [PubMed] [Google Scholar]
- 20.Savouret J, Quesne M. Resveratrol and cancer: a review. Biomed Pharmacother. 2002;56:84–7. doi: 10.1016/s0753-3322(01)00158-5. [DOI] [PubMed] [Google Scholar]
- 21.Liu J, Zhang Q, Chen K, et al. Small-molecule STAT3 signaling pathway modulators from Polygonum cuspidatum. Planta Med. 2012;78:1568–70. doi: 10.1055/s-0032-1315121. [DOI] [PubMed] [Google Scholar]
- 22.Narazaki M, Witthuhn BA, Yoshida K, et al. Activation of JAK2 kinase mediated by the interleukin 6 signal transducer gp130. Proc Nat Acad Sci USA. 1994;91:2285–9. doi: 10.1073/pnas.91.6.2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Duhé RJ, Evans GA, Erwin RA, et al. Nitric oxide and thiol redox regulation of Janus kinase activity. Proc Nat Acad Sci USA. 1998;95:126–31. doi: 10.1073/pnas.95.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ahmad R, Raina D, Meyer C, et al. Triterpenoid CDDO-Me blocks the NF-κB pathway by direct inhibition of IKKβ on Cys-179. J Biol Chem. 2006;281:35764–9. doi: 10.1074/jbc.M607160200. [DOI] [PubMed] [Google Scholar]
- 25.Pandey MK, Sung B, Kunnumakkara AB, et al. Berberine modifies cysteine 179 of IκBα kinase, suppresses nuclear factor-κB-regulated antiapoptotic gene products, and potentiates apoptosis. Cancer Res. 2008;68:5370–9. doi: 10.1158/0008-5472.CAN-08-0511. [DOI] [PubMed] [Google Scholar]
- 26.Pandey MK, Sandur SK, Sung B, et al. A tetrahydroxychalcone, inhibits nuclear factor (NF)-κB and NF-κB-regulated gene expression through direct inhibition of IκBα kinase β on cysteine 179 residue. J Biol Chem. 2007;282:17340–50. doi: 10.1074/jbc.M700890200. [DOI] [PubMed] [Google Scholar]
- 27.Jayatilake GS, Jayasuriya H, Lee E-S, et al. Kinase inhibitors from Polygonum cuspidatum. J Nat Prod. 1993;56:1805–10. doi: 10.1021/np50100a021. [DOI] [PubMed] [Google Scholar]
- 28.Muto A, Hori M, Sasaki Y, et al. Emodin has a cytotoxic activity against human multiple myeloma as a Janus–activated kinase 2 inhibitor. Mol Cancer Ther. 2007;6:987–94. doi: 10.1158/1535-7163.MCT-06-0605. [DOI] [PubMed] [Google Scholar]
- 29.Escarcega R, Fuentes-Alexandro S, Garcia-Carrasco M, et al. The transcription factor nuclear factor-kappa B and cancer. Clin Oncol. 2007;19:154–61. doi: 10.1016/j.clon.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 30.Chiou W, Liao J, Huang C, et al. 2-Methoxystypandrone represses RANKL-mediated osteoclastogenesis by down-regulating formation of TRAF6–TAK1 signaling complexes. Br J Pharmacol. 2010;161:321–35. doi: 10.1111/j.1476-5381.2010.00823.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Singh SB, Graham PL, Reamer RA, et al. Discovery, total synthesis, HRV 3C-protease inhibitory activity, and structure–activity relationships of 2-methoxystypandrone and its analogues. Bioorg Med Chem Lett. 2001;11:3143–6. doi: 10.1016/s0960-894x(01)00648-5. [DOI] [PubMed] [Google Scholar]
- 32.Li YB, Lin ZQ, Zhang ZJ, et al. Protective, antioxidative and antiapoptotic effects of 2-methoxy-6-acetyl-7-methyljuglone. Planta Med. 2011;77:354–61. doi: 10.1055/s-0030-1250385. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 Effects of 2-methoxystypandrone (2-MS) on epidermal growth factor signaling pathway in HeLa cells.
Fig. S2 2-methoxystypandrone (2-MS) inhibited phosphorylation of JAK1 and TYK2.