Abstract
Gastric cancer (GC) is one of the most common malignancies worldwide. In particular, scirrhous type GC is highly metastatic and is characterized clinically by rapid disease progression and poor prognosis. MicroRNAs (miRNAs) play crucial roles in cancer development and progression. In the present study, we identified several miRNAs that are expressed at higher levels in scirrhous type GC than in non-scirrhous type GC by miRNA microarray analysis. Among these, microRNA-143 (miR-143) expression was higher in scirrhous type GC than in non-scirrhous types of GC. In situ hybridization and quantitative RT-PCR analysis showed that miR-143 is expressed by stromal fibroblasts but not by cancer cells. In stromal cells, miR-143 enhanced collagen type III expression in normal gastric fibroblasts and cancer-associated fibroblasts through activation of transforming growth factor-β)/SMAD signaling. Furthermore, high miR-143 expression in GC was associated with worse cancer-specific mortality (P = 0.0141). Multivariate analysis revealed that miR-143 was an independent prognostic factor. Treatment of GC cell lines with 5-aza-2′-deoxycytidine restored the expression of miR-143, and precursor miR-143 caused the inhibition of cancer cell invasion. These data suggest that miR-143 regulates fibrosis of scirrhous type GC through induction of collagen expression in stromal fibroblasts and that miR-143 expression serves as a prognostic marker of GC.
Keywords: Collagen type III, fibroblast, gastric cancer, microRNA-143, transforming growth factor-β
Gastric cancer (GC) is one of the most common malignancies worldwide. Although improved diagnosis and treatment have resulted in good long-term survival for patients with early GC, outcomes for those with advanced GC remain poor.(1) Gastric cancer can be subdivided into two major classifications, intestinal-type GC and diffuse-type GC.(2) Scirrhous type GC, composed mainly of diffuse-type GC cells, forms a Borrmann type 4 lesion and is characterized by highly metastatic potential and rapid proliferation.(3–5) Histologically, scirrhous type GC shows diffuse infiltration into the gastric wall with extreme stromal fibrosis. Transforming growth factor-β (TGF-β), produced by cancer cells, has been reported to activate stromal fibroblasts to stimulate collagen synthesis in scirrhous type GC.(6,7) Increasing matrix rigidity may lead to the activation of proliferation, and interstitial pressure by fibrosis in the cancer stroma may interfere with drug delivery to cancer cells.(8–11) Reflecting such characteristics, scirrhous type GC carries an extremely poor patient prognosis in comparison with other types of GC. Therefore, better knowledge of the pathological and biological basis of scirrhous type GC is necessary to improve diagnosis and treatment.
MicroRNAs (miRNAs) are small non-coding RNAs of 19–25 nucleotides in length that play important regulatory roles in posttranscriptional repression.(12,13) Through inhibition of target gene translation, miRNAs regulate many cellular processes including development, differentiation, stress response, apoptosis, and proliferation. Aberrant miRNA expression is found in a range of cancers, suggesting novel roles as oncogenes or tumor-suppressor genes.(14) Several reports indicated significant correlations between the histological classification of cancers and miRNA expression patterns.(14,15) We have previously reported that the two histological types of GC, intestinal-type and diffuse-type, show different miRNA signatures.(16) However, there is only one report focusing on scirrhous type GC, which found that miR-516a-3p participated in inhibition of peritoneal metastasis.(17)
In this study, we aimed to identify novel miRNAs in scirrhous type GC by comparing miRNA expression profiles of GC tissues and found that miR-143 expression levels in scirrhous type GC were higher than in other types of GC. It has been shown that miR-143 expression is induced by TGF-β signaling, and it regulates vascular smooth muscle cell differentiation.(18) Moreover, several lines of evidence support the importance of miR-143 in proliferation, invasion, and metastasis of various malignancies.(19–22) By in situ hybridization, miR-143 was localized in stromal fibroblasts but not in GC cells of scirrhous type GC tissue. Here, we investigated the function of miR-143 in stromal cells in scirrhous type GC, particularly in collagen type III synthesis by stromal fibroblasts. The expression of collagen type III was positively regulated by miR-143 through the TGF-β/SMAD signaling pathway. We also examined the correlation between miR-143 expression and patient prognosis using clinicopathological characteristics.
Materials and Methods
MicroRNA microarray hybridization
Total RNA was isolated from frozen tissue using Isogene (Nippon Gene, Tokyo, Japan). Short-strand RNA was purified from total RNA with RNeasy MinElute Cleanup Kit (Qiagen, Valencia, CA, USA). The oligonucleotide array we used contained Genopal-MICH07 DNA chips (Mitsubishi Rayon, Tokyo, Japan) comprising 188 oligonucleotide DNA probes. Details are described in Data S1.
Tissue samples
In all, 138 primary gastric tumors and 30 corresponding non-neoplastic mucosa were collected from patients diagnosed as having GC. Details are described in Data S1.
Cell culture
Nine cell lines derived from human GC and four human normal gastric fibroblasts (NFs), NF-33, -34, -35, and -38, and four cancer-associated fibroblasts (CaFs), CaF-33, 34, 35, and 38, were used. These cell lines were maintained as described previously.(23,24) Additional information on the NFs and CaFs is provided in Table S1, and details are described in Data S1.
Quantitative RT-PCR and western blots
Quantification of levels of collagen type III mRNA, α-smooth muscle actin (α-SMA) mRNA, β-actin mRNA, TGF-β variants mRNA, miR-143, and U6B was carried out using real-time fluorescence detection. For Western blot analysis, cells were lysed as described previously.(25) Details are described in Data S1.
In situ hybridization for miR-143 in combination with immunofluorescence staining and immunostaining of collagen
In situ hybridization was carried out as described by Nuovo et al.(26) with minor adjustments. A Dako EnVision+ Mouse Peroxidase Detection Kit (Dako, Carpinteria, CA, USA) was used for immunohistochemical analysis. Details are described in Data S1.
Cell transfection and TGF-β1 treatment
Transfection of cells was carried out with Lipofectamine RNAiMAX Reagent (Invitrogen, Carlsbad, CA, USA), according to the manufacturer's instructions. Fibroblasts were incubated in DMEM containing 10 ng/mL TGF-β1 (R&D Systems, Minneapolis, MO, USA). Details are described in Data S1.
Cell growth and in vitro invasion assay
The cells were seeded at a density of 2000 cells per well in 96-well plates. Cell growth was monitored after 1, 2, and 4 days by MTT assay.(27) Modified Boyden chamber assays were carried out to examine invasiveness as described previously.(28)
Immunofluorescence staining for cell lines
For cell staining, the cells were incubated with anti-collagen type III antibody (Daiichi Fine Chemical, Toyama, Japan), followed by incubations with Alexa Fluor 488-conjugated goat anti-mouse IgG (Molecular Probes, Eugene, OR, USA). Details are described in Data S1.
Coculture of CaF with scirrhous type GC cell line and BrdU incorporation assay
HSC-44PE, scirrhous type GC cell line, was cocultured with CaF-38, and proliferation activity was assessed by percentage of BrdU/CAM5.2-positive cells. Details are described in Data S1.
Statistical analysis
The Mann–Whitney U-test was used to calculate the significance of differences between two samples. Statistical differences between miRNA expression levels in GC samples and non-neoplastic mucosa samples were evaluated using the Wilcoxon matched pair test. The correlation between expression levels of miR-143 and clinicopathological parameters was analyzed with Fisher's exact test. A log–rank test and Kaplan–Meier plots were constructed for the miR-143 high and low groups, based on one-third of the miR-143 expression level. Univariate and multivariate analysis of factors influencing survival were carried out using the Cox proportional hazards model. Parameters for multivariate analysis were selected by the stepwise method. A P-value of less than 0.05 was considered statistically significant.
Results
Expression of miR-143 is greater in scirrhous type GC than in non-scirrhous type GC
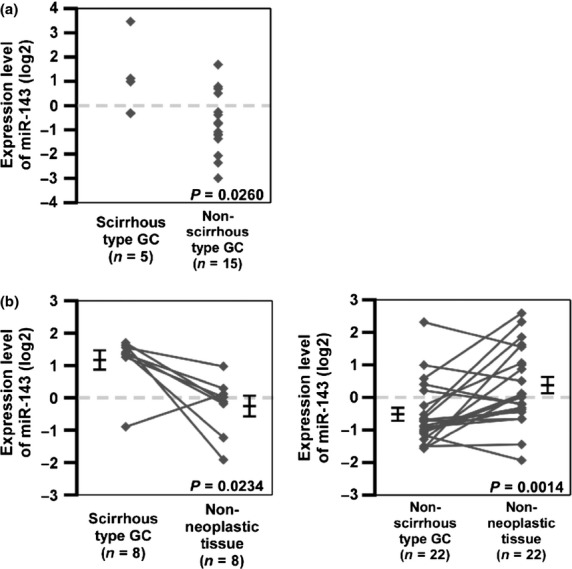
To identify miRNAs with altered expression levels among different histological types of GC, expression levels of 188 individual miRNAs were compared between five scirrhous type GCs and 15 non-scirrhous type GCs in miRNA microarray profiling. Expression levels of six miRNAs were significantly higher in scirrhous type GC than in non-scirrhous type GC (Table 1). Among these, miR-143 was expressed at the highest level in scirrhous type GC. To confirm the microarray data, we carried out quantitative RT-PCR (qRT-PCR) in 20 frozen GC tissue samples. As shown in Figure 1(a), miR-143 expression in scirrhous type GC was significantly higher than that in non-scirrhous type GC.
Table 1.
Summary of significantly increased miRNAs in scirrhous type GC, compared with non-scirrhous type GC
| miRBase ID | miRBase Accession No. | Intensity ave. |
Fold change | P value | |
|---|---|---|---|---|---|
| Scirrhous | Non-scirrhous | ||||
| hsa-miR-143 | MIMAT0000435 | 21598.00 | 4937.81 | 4.37 | 0.0060 |
| hsa-miR-145 | MIMAT0000437 | 19051.80 | 3668.44 | 5.19 | 0.0049 |
| hsa-miR-125b | MIMAT0000423 | 2076.80 | 556.50 | 3.73 | 0.0031 |
| hsa-miR-99a | MIMAT0000097 | 908.00 | 231.25 | 3.93 | 0.0036 |
| hsa-miR-100 | MIMAT0000098 | 792.60 | 211.69 | 3.74 | 0.0019 |
| hsa-miR-17-3p | MIMAT0000071 | 156.00 | 41.44 | 3.76 | 0.0498 |
Fig. 1.
MicroRNA-143 (miR-143) expression in gastric cancer (GC) and non-neoplastic tissue. (a) Expression levels of miR-143 in GC tissue samples (n = 20) were measured by quantitative RT-PCR analysis. (b) MicroRNA-143 expression levels in 30 formalin-fixed paraffin-embedded GC tissue samples and non-neoplastic tissue samples as determined by quantitative RT-PCR were compared. Statistical differences were evaluated using the Wilcoxon matched pair test. Bars and error bars indicated median and standard error.
MicroRNA-143 can act as a tumor suppressor gene, and its expression is decreased in tumor tissues relative to normal tissues.(19,20,29) To assess miR-143 expression between cancer tissue and non-neoplastic tissue from the same patients, we carried out qRT-PCR analyses of miR-143 using 30 formalin-fixed paraffin-embedded GC tissue samples and corresponding non-neoplastic gastric mucosa samples. Although miR-143 expression levels were significantly lower in non-scirrhous type GC tissues than in non-neoplastic gastric mucosa, the expression levels in scirrhous type GC were significantly higher than those in the corresponding non-neoplastic mucosa (Fig. 1b). These data suggest that miR-143 expression is downregulated in GCs but is sustained or increased in scirrhous type GC.
Analysis of miR-143 localization in scirrhous type GC
To elucidate why scirrhous-type GC possesses high miR-143 expression, we first investigated the localization of miR-143 expression in scirrhous type GC tissue by in situ hybridization of miR-143 in combination with immunostaining using markers for epithelial cells (CAM5.2), stromal fibroblasts (vimentin), and CaFs (α-SMA; Fig. 2a–c, Fig. S1).(30) Cancer fibroblasts, also termed myofibroblasts or activated fibroblasts, are well known as a major component of cancer stroma and play an important role in the regulation of cancer cell proliferation and metastasis.(5,30) In cancerous regions, double staining revealed that expression of miR-143 was observed in α-SMA- or vimentin-positive fibroblastic cells (Fig. 2a,b) but was not colocalized with CAM5.2-positive cancer cells (Fig. 2c). However, in non-neoplastic regions, miR-143 was highly expressed in normal epithelial cells, but the expression was faint or not present in stromal fibroblasts in non-neoplastic tissue (data not shown). Expression of miR-143 was also examined in 9 GC cell lines, as well as in NFs and CaFs. Expression of miR-143 was evident in NFs and CaFs, but was undetectable in GC cell lines (Fig. 2d). Moreover, the expression levels of miR-143 were higher in CaFs than in NFs, and CaFs derived from scirrhous type GC showed a tendency toward higher expression of miR-143 (Fig. 2d). These data indicated that miR-143 is localized to epithelial cells in normal gastric tissue, but its localization is changed to surrounding stromal fibroblasts, but not cancer cells, in scirrhous type GC.
Fig. 2.
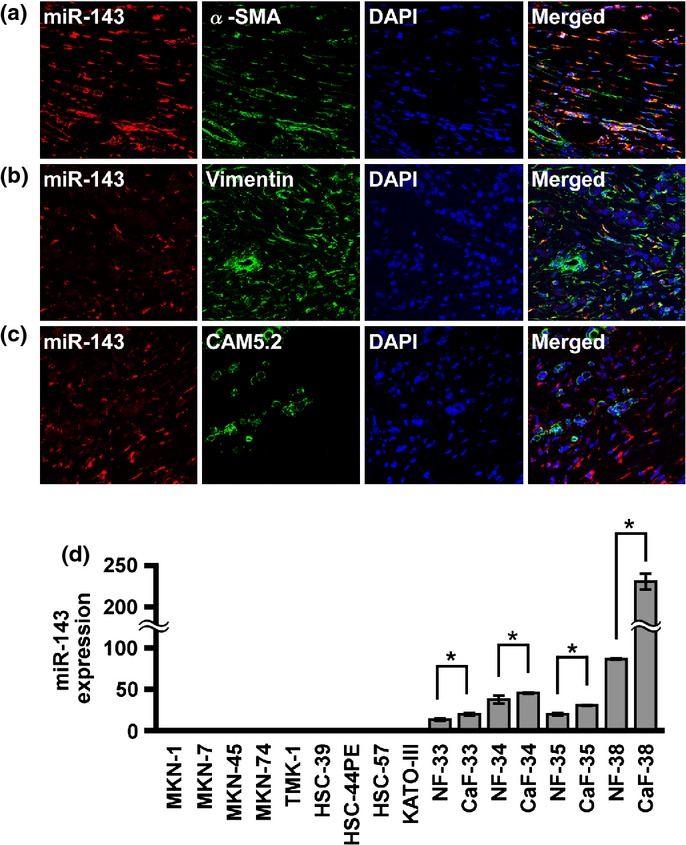
MicroRNA-143 (miR-143) expression in scirrhous type gastric cancer (GC) tissue and cell lines. In situ hybridization of miR-143 was carried out in combination with immunofluorescence staining in scirrhous type GC. MicroRNA-143 labeling was revealed by Cy3-conjugated streptavidin (red). (a) α-Smooth muscle actin (α-SMA), (b) vimentin, or (c) CAM5.2 labeling was revealed by FITC-conjugated secondary antibody (green). DNA was counterstained with DAPI (blue). MicroRNA-143 expression was localized to α-SMA-positive and vimentin-positive stromal fibroblasts. (d) MicroRNA-143 expression levels were evaluated in GC cell lines and fibroblasts. Bars and error bars indicate median and standard error, respectively. *P < 0.05. CaF, cancer-associated fibroblasts; NF, normal gastric fibroblasts.
MicroRNA-143 regulates collagen type III expression in stromal fibroblasts of scirrhous type GC
Because miR-143 was found to be expressed in stromal fibroblasts but not in cancer cells, we sought to investigate the function of miR-143 in stromal fibroblasts. Scirrhous type GC produces abundant collagen and thus promotes fibrosis.(31,32) We previously reported that collagen type III expression is associated with scirrhous type GC.(6,7) We first examined collagen type III expression in scirrhous type GC tissue by immunostaining and, as expected, collagen type III was detected in fibrillar bundles of scirrhous type GC (Fig. 3a). Collagen type III mRNA expression was examined in GC and fibroblasts by qRT-PCR. High levels of collagen type III mRNA expression were observed in stromal fibroblasts that retained high miR-143 expression (Fig. 3b). To assess the relation between collagen type III and miR-143, NF-38 and CaF-38 were selected because they had the highest miR-143 and collagen type III mRNA expression (Fig. 3b). Transfection of miR-143 inhibitor significantly suppressed collagen type III expression (Fig. 3c–e). In contrast, transfection of miR-143 precursor sustained or increased collagen type III expression (Fig. 3c–e). These data suggest that miR-143 positively regulates collagen type III expression in stromal fibroblasts of scirrhous type GC.
Fig. 3.
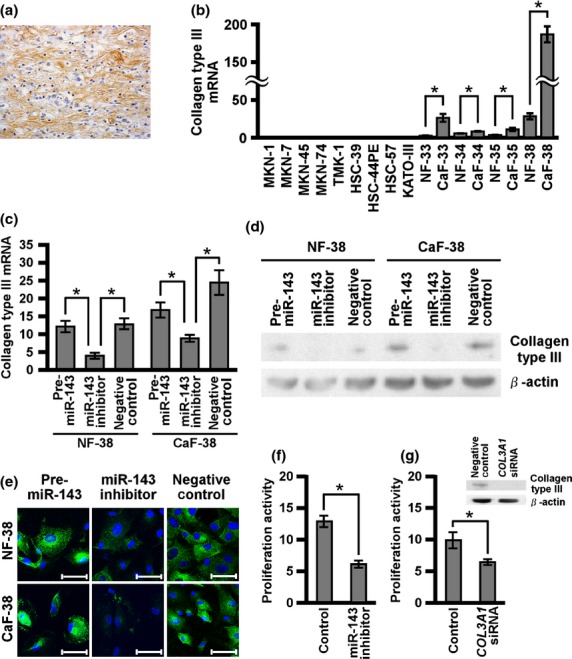
Regulation of collagen type III expression by microRNA-143 (miR-143). (a) Collagen type III expression was assessed by immunohistochemical analysis of scirrhous type gastric cancer (GC) tissue. (b) Collagen type III mRNA expression levels were evaluated in GC and fibroblasts. Normal gastric fibroblasts and cancer-associated fibroblasts (NF-38 and CaF-38, respectively) were transfected with negative control miRNA or precursor miR-143 or miR-143 inhibitor, and (c) quantitative RT-PCR, (d) Western blot, and (e) cell staining were carried out for collagen type III expression. Scale bars: 50 μm. (f) Proliferation activity after coculture of HSC-44PE GC cells and CaF-38 with miR-143 inhibitor or negative control. Proliferation activity of HSC-44PE was assessed by percentages of BrdU/CAM5.2-positive cells. (g) Proliferation activity after coculture of HSC-44PE and CaF-38 with COL3A1 siRNA or negative control. Collagen type III expression level was determined by Western blot analysis. Results are mean ± SE of triplicate measurements. *P < 0.05.
To address the biological significance of collagen type III induction by miR-143 in CAFs, HSC-44PE was directly cocultured with CaF-38 that was treated with miR-143 inhibitor or collagen type III siRNA. We used HSC-44PE because it was derived from scirrhous type GC patients. Proliferation of HSC-44PE cells was significantly repressed in coculture with miR-143- or collagen type III-inhibited CaF-38 (Fig. 3f,g). These data indicated that both miR-143 and collagen type III expression in stromal fibroblast could affect cancer cell proliferation.
Transforming growth factor-β regulates collagen type III expression through miR-143
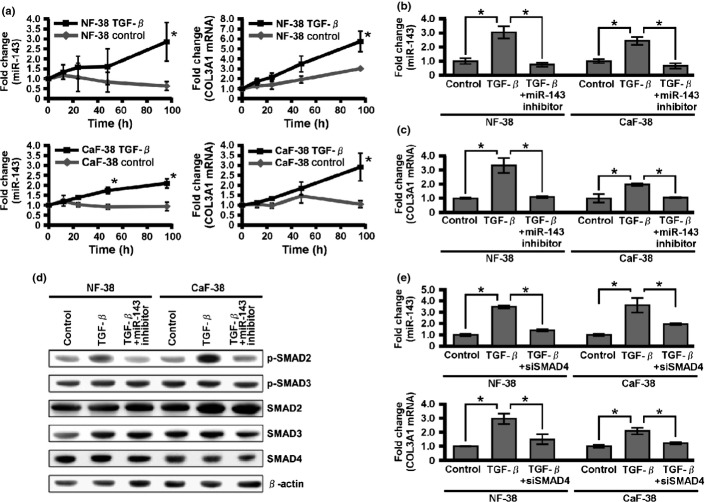
Scirrhous type GC secretes a larger amount of active form TGF-β than non-scirrhous type GC does,(32) and TGF-β has an important pathological and biological role in scirrhous type GC.(5,7,33,34) To investigate the effect of TGF-β1 on miR-143 and collagen type III expression, NF-38 and CaF-38 were treated with TGF-β1, and their miR-143 expression levels were monitored by qRT-PCR. There were no differences in the expression of endogenous TGF-β1, TGF-β2, or TGF-β3 between NF-38 and CaF-38 (Fig. S2). Treatment with TGF-β1 resulted in strong induction of miR-143 and collagen type III mRNA expression within 96 h in both fibroblasts (Fig. 4a). However, the induction of collagen type III mRNA in fibroblasts by TGF-β1 was significantly suppressed by pretreatment with miR-143 inhibitor (Fig. 4b,c). Interestingly, miR-143 inhibitor significantly suppressed the expression of p-SMAD2 (Fig. 4d), and SMAD4 siRNA significantly downregulated miR-143 and collagen type III mRNA expression in each fibroblast (Fig. 4e). These data imply that miR-143 is deeply involved in the regulation of collagen type III expression by TGF-β/SMAD signaling in NF and CaF.
Fig. 4.
Effect of transforming growth factor-β (TGF-β) on microRNA-143 (miR-143) and collagen type III expression. (a) Quantitative RT-PCR analysis of miR-143 and collagen type III mRNA in normal gastric fibroblasts (NF-38) and cancer-associated fibroblasts (CaF-38) after the indicated times of TGF-β1 treatment. (b) MicroRNA-143 expression levels in NF-38 and CaF-38 in the absence or presence of TGF-β1 with or without miR-143 inhibitor. (c) Collagen type III mRNA expression levels in NF-38 and CaF-38 in the absence or presence of TGF-β with or without miR-143 inhibitor. (d) Expression levels of TGF-β family signal components were determined by Western blot analysis. (e) Quantitative RT-PCR analysis of miR-143 and collagen type III mRNA in NF-38 and CaF-38 with SMAD4 siRNA. Results are mean ± SE of triplicate measurements. *P < 0.05.
Correlation between miR-143 expression and clinicopathological characteristics of GC
To investigate the relation between miR-143 expression and clinicopathological parameters, we examined miR-143 expression levels in 68 formalin-fixed paraffin-embedded samples of primary GC by qRT-PCR. The miR-143 expression levels were significantly higher in scirrhous type GC cases than in non-scirrhous type GC cases (P = 0.0167; Fig. 5a). As shown in Table 2, miR-143-positive GC cases showed advanced tumor stage (P = 0.0013) and tended to have scirrhous type histology (P = 0.0428) in comparison with miR-143-negative cases. Kaplan–Meier analysis showed that GC patients with high miR-143 expression correlated significantly with worse cancer-specific mortality (P = 0.0002, log–rank test; Fig. 5b). When limited to the cases with scirrhous type GC or non-scirrhous type GC, miR-143 expression had no prognostic effect (data not shown). Univariate and multivariate analysis showed miR-143 to be an independent prognostic marker of GC cases (P = 0.0141; Table 3).
Fig. 5.
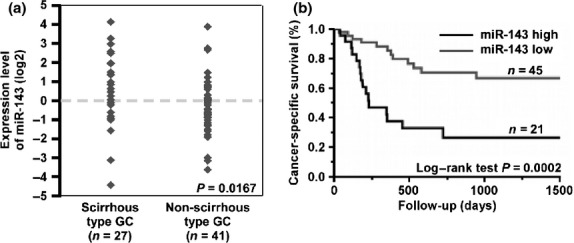
Relation between microRNA-143 (miR-143) expression and patient prognosis. (a) Expression levels of miR-143 in formalin-fixed paraffin-embedded gastric cancer (GC) tissue samples (n = 68) were measured by quantitative RT-PCR analysis. (b) Cancer-specific survival of 68 patients with GC based on expression levels of miR-143 was examined. The expression levels of miR-143 were divided into two groups, high and low expression of miR-143, based on one-third of the miR-143 expression level (cut-off line = one-third of miR-143 expression level in this group).
Table 2.
Relationship between microRNA-143 (miR-143) expression and clinicopathological characteristics in 68 patients with gastric cancer
| miR-143 expression |
P-value† | ||
|---|---|---|---|
| High (%) | Low | ||
| Age, years | |||
| <60 | 7 (25.9) | 20 | 0.2640 |
| ≥60 | 16 (39.0) | 25 | |
| Sex | |||
| Male | 14 (29.2) | 34 | 0.2086 |
| Female | 9 (45.0) | 11 | |
| Stage | |||
| I/II | 3 (11.1) | 24 | 0.0013 |
| III/IV | 20 (48.8) | 21 | |
| Histological classification | |||
| Well differentiated | 8 (27.6) | 21 | 0.3485 |
| Poorly differentiated | 15 (38.5) | 24 | |
| Non-scirrhous | 10 (24.4) | 31 | 0.0428 |
| Scirrhous | 13 (48.1) | 14 | |
Fisher's exact test.
Table 3.
Univariate and multivariate analysis of factors influencing survival in 68 patients with gastric cancer
| Univariate analysis |
Multivariate analysis |
|||
|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | |
| Age, years | ||||
| <60 | 1 (Ref.) | 0.4585 | ||
| ≥60 | 1.34 (0.63–3.01) | |||
| Sex | ||||
| Female | 1 (Ref.) | 0.4116 | ||
| Male | 1.18 (0.78–1.72) | |||
| Stage | ||||
| I/II | 1 (Ref.) | <0.0001 | 1 (Ref.) | 0.0004 |
| III/IV | 7.95 (2.78–33.47) | 6.20 (2.10–26.52) | ||
| Histological type | ||||
| Well differentiated | 1 (Ref.) | 0.9513 | ||
| Poorly differentiated | 0.98 (0.46–2.11) | |||
| Non-scirrhous | 1 (Ref.) | 0.1435 | ||
| Scirrhous | 1.75 (0.82–3.70) | |||
| Expression of miR-143 | ||||
| Low | 1 (Ref.) | 0.0005 | 1 (Ref.) | 0.0141 |
| High | 3.81 (1.80–8.29) | 2.62 (1.21–5.80) | ||
CI, confidence interval; HR, hazard ratio; miR-143, microRNA-143; Ref., reference.
DNA methylation may suppress miR-143 expression, which regulates scirrhous type GC cell invasion
Expression of miR-143 was detected in epithelial cells of non-neoplastic tissue, but its expression was suppressed in cancer cells (Fig. 2). To pursue the cause of miR-143 suppression in GC cell lines, we treated MKN-1 and HSC-44PE cell lines with 0–3 μmol/L 5-aza-2′-deoxycytidine for 4 days and analyzed sequential changes in miR-143 expression by qRT-PCR. Expression of miR-143 was restored by 5-aza-2′-deoxycytidine treatments (Fig. S3a), suggesting that DNA methylation may cause transcriptional inactivation of miR-143 in GC cells.
Next, to confirm the possible antiproliferative or anti-invasive effects of miR-143 on cancer cells, MTT and Transwell invasion assays were carried out using MKN-1 and HSC-44PE GC cell lines with forced miR-143 expression by miR-143 precursor transfection. Cell growth of each GC cell line with forced miR-143 expression did not differ from that of cells transfected with control miRNA (data not shown). However, the invasiveness of cell lines with miR-143 forced expression was reduced compared to that of the negative control miRNA-transfected cell lines (Fig. S3b). These data verified that exogenous recovery of miR-143 expression could suppress the invasiveness of cancer cells but not the proliferative activity.
Discussion
We report here that miR-143 expression was conserved in scirrhous type GC tissues and that the main source of miR-143 was stromal fibroblasts. MicroRNA-143 could regulate TGF-β/SMAD signaling to mediate the expression of collagen type III in stromal fibroblast of scirrhous type GC. We further showed that miR-143 maintenance or overexpression was significantly associated with advanced stage and poor clinical survival in GC. To our knowledge, this is the first report that refers to the importance of miR-143 in stromal fibroblasts of cancer.
Cancer cells and stromal fibroblasts interact with each other through various growth factors.(5) Among such growth factors, TGF-β signaling plays an important role in progression of scirrhous type GC.(5) It has been reported that some miRNAs regulate tissue fibrosis in various organs.(35–38) In the present study, we have identified a novel role for miR-143 in the regulation of collagen type III expression. Although TGF-β treatment induced collagen type III mRNA expression, downregulation of miR-143 expression significantly suppressed the induction of collagen type III in fibroblasts. The classic TGF-β signaling pathway involves the SMAD protein family.(39) The SMAD-dependent regulation of collagens by TGF-β has well-established mechanisms,(40–43) and collagen type III transcription is targeted by SMAD3.(40,42) The induction of collagen type III in gastric fibroblasts could be regulated by the same mechanism because SMAD4 siRNA could inhibit collagen type III expression in NFs and CaFs. Although we detected that miR-143 only affected the activation of SMAD2, the expression of p-SMAD3 seemed to be high enough for the regulation of collagen type III expression. It is well known that both p-SMAD2 and p-SMAD3 are essential for forming complexes with SMAD4, and this complex is crucial for activation of TGF-β/SMAD signaling.(39) Taken together, it could be speculated that activation of SMAD2, regulated by miR-143, is a trigger for enhancing collagen type III expression in NFs and CaFs. It has been generally accepted that miRNAs repress the translation of their targets.(44,45) Based on present results, it could be suspected that miR-143 regulates collagen type III expression by targeting some inhibitor of TGF-β/SMAD2 signaling. Further investigations are required to elucidate the underlying mechanism of collagen type III regulation by miR-143 in cancer stromal fibroblasts.
It has been reported that miR-143 acts as a tumor suppressor gene, and its downregulation is correlated with worse patient outcome.(20,46) However, these previous reports focused on the role of miR-143 in cancer cells and normal cells, and the function of miR-143 in stromal cells surrounding tumor cells has not been discussed. In the present study, we suggest that promoter hypermethylation is involved in downregulation of miR-143 expression in cancer cells. In addition, forced expression of miR-143 in GC cell lines significantly inhibited their invasiveness. Taken together, there is very strong evidence that the tumor suppressive role of miR-143 is downregulated by promoter hypermethylation in cancer cells. However, we also showed that miR-143 expression was localized within stromal fibroblasts, but not in cancer cells in scirrhous type GC, and contributed to the regulation of collagen type III expression and cancer cell proliferation. We also showed that miR-143 expression was higher in scirrhous type GC than in non-scirrhous type GC. In addition, its expression correlated significantly with advanced pathological stage and poor patient prognosis. Stromal fibrosis in cancer leads to increased matrix rigidity and subsequent activation of cancer cell proliferation through integrin-MAPK signaling.(11) It is common knowledge that fibrosis in cancer tissue can also lead to an increase in interstitial pressure that inhibits efficient drug delivery, resulting in chemoresistance.(8–10) Taken together, miR-143 expression in stromal fibroblasts of scirrhous type GC may participate in cancer progression and lead to poor clinical outcome.
In conclusion, our data position miR-143 as a critical mediator of collagen type III expression in scirrhous type GC. Consequently, miR-143 in cancer stroma may support the progression of scirrhous type GC through fibrillar formation, consistent with the effect of certain oncogenes. Full elucidation of the molecular mechanisms of miR-143 in stromal fibroblasts surrounding cancer cells may improve our understanding of tumor progression in scirrhous type GC.
Acknowledgments
We thank Mr. Shinichi Norimura for excellent technical assistance. This work was carried out with the kind cooperation of the Research Center for Molecular Medicine, Faculty of Medicine, Hiroshima University (Hiroshima, Japan). We thank the Analysis Center of Life Science, Hiroshima University, for the use of their facilities. This work was supported by Grants-in-Aid for Research from the Ministry of Education, Culture, Science, Sports, and Technology of Japan, and, in part, by a Grant-in-Aid for the Third Comprehensive 10-Year Strategy for Cancer Control and for Cancer Research from the Ministry of Health, Labour and Welfare of Japan, and by The National Institute of Biomedical Innovation (Program for Promotion of Fundamental Studies in Health Sciences). This work was also supported in part by a Research Fellowship from the Japan Society for the Promotion of Science and the National Cancer Center Research and Development Fund (23-A-9).
Disclosure Statement
The authors have no conflict of interest.
Supporting Information
Additional supporting information may be found in the online version of this article:
Fig. S1. α-Smooth muscle actin mRNA expression in normal gastric fibroblasts and cancer-associated fibroblasts.
Fig. S2. Transforming growth factor-β expression in normal gastric fibroblasts and cancer-associated fibroblasts.
Fig. S3. MicroRNA-143 expression might be suppressed by DNA methylation and effects on cell invasion of gastric cancer cell lines.
Table S1. Information on fibroblasts.
Data S1. Supplementary materials and methods.
References
- 1.Hohenberger P, Gretschel S. Gastric cancer. Lancet. 2003;362:305–15. doi: 10.1016/s0140-6736(03)13975-x. [DOI] [PubMed] [Google Scholar]
- 2.Lauren P. The two histological main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma. an attempt at a histo-clinical classification. Acta Pathol Microbiol Scand. 1965;64:31–49. doi: 10.1111/apm.1965.64.1.31. [DOI] [PubMed] [Google Scholar]
- 3.Otsuji E, Kuriu Y, Okamoto K, et al. Outcome of surgical treatment for patients with scirrhous carcinoma of the stomach. Am J Surg. 2004;188:327–32. doi: 10.1016/j.amjsurg.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 4.Ikeguchi M, Miyake T, Matsunaga T, et al. Recent results of therapy for scirrhous gastric cancer. Surg Today. 2009;39:290–4. doi: 10.1007/s00595-008-3860-1. [DOI] [PubMed] [Google Scholar]
- 5.Yashiro M, Hirakawa K. Cancer-stromal interactions in scirrhous gastric carcinoma. Cancer Microenviron. 2010;3:127–35. doi: 10.1007/s12307-010-0036-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yamamoto M, Sumiyoshi H, Nakagami K, Taniyama K, Tahara E. Distribution of collagen types I and III and basal lamina in human gastric carcinoma: an immunohistochemical and electron microscopic study. Virchows Arch A Pathol Anat Histopathol. 1984;403:313–22. doi: 10.1007/BF00737282. [DOI] [PubMed] [Google Scholar]
- 7.Yoshida K, Yokozaki H, Niimoto M, Ito H, Ito M, Tahara E. Expression of TGF-beta and procollagen type I and type III in human gastric carcinomas. Int J Cancer. 1989;44:394–8. doi: 10.1002/ijc.2910440303. [DOI] [PubMed] [Google Scholar]
- 8.Jain RK. Barriers to drug delivery in solid tumors. Sci Am. 1994;271:58–65. doi: 10.1038/scientificamerican0794-58. [DOI] [PubMed] [Google Scholar]
- 9.Heldin CH, Rubin K, Pietras K, Ostman A. High interstitial fluid pressure – an obstacle in cancer therapy. Nat Rev Cancer. 2004;4:806–13. doi: 10.1038/nrc1456. [DOI] [PubMed] [Google Scholar]
- 10.Nakajima TE, Yanagihara K, Takigahira M, et al. Antitumor effect of SN-38-releasing polymeric micelles, NK012, on spontaneous peritoneal metastases from orthotopic gastric cancer in mice compared with irinotecan. Cancer Res. 2008;68:9318–22. doi: 10.1158/0008-5472.CAN-08-2822. [DOI] [PubMed] [Google Scholar]
- 11.Worthley DL, Giraud AS, Wang TC. The extracellular matrix in digestive cancer. Cancer Microenviron. 2010;3:177–85. doi: 10.1007/s12307-010-0053-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–97. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 13.Ambros V. The functions of animal microRNAs. Nature. 2004;431:350–5. doi: 10.1038/nature02871. [DOI] [PubMed] [Google Scholar]
- 14.Calin GA, Croce CM. MicroRNA signatures in human cancers. Nat Rev Cancer. 2006;6:857–66. doi: 10.1038/nrc1997. [DOI] [PubMed] [Google Scholar]
- 15.Li X, Luo F, Li Q, et al. Identification of new aberrantly expressed miRNAs in intestinal-type gastric cancer and its clinical significance. Oncol Rep. 2011;26:1431–9. doi: 10.3892/or.2011.1437. [DOI] [PubMed] [Google Scholar]
- 16.Ueda T, Volinia S, Okumura H, et al. Relation between microRNA expression and progression and prognosis of gastric cancer: a microRNA expression analysis. Lancet Oncol. 2010;11:136–46. doi: 10.1016/S1470-2045(09)70343-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takei Y, Takigahira M, Mihara K, Tarumi Y, Yanagihara K. The metastasis-associated microRNA miR-516a-3p is a novel therapeutic target for inhibiting peritoneal dissemination of human scirrhous gastric cancer. Cancer Res. 2011;71:1442–53. doi: 10.1158/0008-5472.CAN-10-2530. [DOI] [PubMed] [Google Scholar]
- 18.Long X, Miano JM. Transforming growth factor-β1 (TGF-β1) utilized distinct pathways for the transcriptional activation of microRNA 143/145 in human coronary artery smooth muscle cells. J Biol Chem. 2011;286:30119–29. doi: 10.1074/jbc.M111.258814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen X, Guo X, Zhang H, et al. Role of miR-143 targeting KRAS in colorectal tumorigenesis. Oncogene. 2009;28:1385–92. doi: 10.1038/onc.2008.474. [DOI] [PubMed] [Google Scholar]
- 20.Dou L, Zheng D, Li J, et al. Methylation-mediated repression of microRNA-143 enhances MLL-AF4 oncogene expression. Oncogene. 2012;31:507–17. doi: 10.1038/onc.2011.248. [DOI] [PubMed] [Google Scholar]
- 21.Pagliuca A, Valvo C, Fabrizi E, et al. Analysis of the combined action of miR-143 and miR-145 on oncogenic pathways in colorectal cancer cells reveals a coordinate program of gene repression. Oncogene. 2012;5:495. doi: 10.1038/onc.2012.495. [DOI] [PubMed] [Google Scholar]
- 22.Noguchi S, Yasui Y, Iwasaki J, et al. Replacement treatment with microRNA-143 and -145 induces synergistic inhibition of the growth of human bladder cancer cells by regulating PI3K/Akt and MAPK signaling pathways. Cancer Lett. 2013;328:353–61. doi: 10.1016/j.canlet.2012.10.017. [DOI] [PubMed] [Google Scholar]
- 23.Fuyuhiro Y, Yashiro M, Noda S, et al. Cancer-associated orthotopic myofibroblasts stimulates the motility of gastric carcinoma cells. Cancer Sci. 2012;103:797–805. doi: 10.1111/j.1349-7006.2012.02209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Naito Y, Oue N, Hinoi T, et al. Reg IV is a direct target of intestinal transcriptional factor CDX2 in gastric cancer. PLoS ONE. 2012;7:e47545. doi: 10.1371/journal.pone.0047545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yasui W, Ayhan A, Kitadai Y, et al. Increased expression of p34cdc2 and its kinase activity in human gastric and colonic carcinomas. Int J Cancer. 1993;53:36–41. doi: 10.1002/ijc.2910530108. [DOI] [PubMed] [Google Scholar]
- 26.Nuovo GJ, Elton TS, Nana-Sinkam P, Volinia S, Croce CM, Schmittgen TD. A methodology for the combined in situ analyses of the precursor and mature forms of microRNAs and correlation with their putative targets. Nat Protoc. 2009;4:107–15. doi: 10.1038/nprot.2008.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alley MC, Scudiero DA, Monks A, et al. Feasibility of drug screening with panels of human tumor cell lines using a microculture tetrazolium assay. Cancer Res. 1988;48:589–601. [PubMed] [Google Scholar]
- 28.Sentani K, Oue N, Naito Y, et al. Upregulation of HOXA10 in gastric cancer with the intestinal mucin phenotype: reduction during tumor progression and favorable prognosis. Carcinogenesis. 2012;33:1081–8. doi: 10.1093/carcin/bgs121. [DOI] [PubMed] [Google Scholar]
- 29.Takagi T, Iio A, Nakagawa Y, Naoe T, Tanigawa N, Akao Y. Decreased expression of microRNA-143 and -145 in human gastric cancers. Oncology. 2009;77:12–21. doi: 10.1159/000218166. [DOI] [PubMed] [Google Scholar]
- 30.Kalluri R, Zeisberg M. Fibroblasts in cancer. Nat Rev Cancer. 2006;6:392–401. doi: 10.1038/nrc1877. [DOI] [PubMed] [Google Scholar]
- 31.Niitsu Y, Ito N, Kohda K, et al. Immunohistochemical identification of type I procollagen in tumour cells of scirrhous adenocarcinoma of the stomach. Br J Cancer. 1988;57:79–82. doi: 10.1038/bjc.1988.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mahara K, Kato J, Terui T, et al. Transforming growth factor beta 1 secreted from scirrhous gastric cancer cells is associated with excess collagen deposition in the tissue. Br J Cancer. 1994;69:777–83. doi: 10.1038/bjc.1994.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kawajiri H, Yashiro M, Shinto O, et al. A novel transforming growth factor beta receptor kinase inhibitor, A-77, prevents the peritoneal dissemination of scirrhous gastric carcinoma. Clin Cancer Res. 2008;14:2850–60. doi: 10.1158/1078-0432.CCR-07-1634. [DOI] [PubMed] [Google Scholar]
- 34.Shinto O, Yashiro M, Kawajiri H, et al. Inhibitory effect of a TGFbeta receptor type-I inhibitor, Ki26894, on invasiveness of scirrhous gastric cancer cells. Br J Cancer. 2010;102:844–51. doi: 10.1038/sj.bjc.6605561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chung AC, Huang XR, Meng X, Lan HY. miR-192 mediates TGF-beta/Smad3-driven renal fibrosis. J Am Soc Nephrol. 2010;21:1317–25. doi: 10.1681/ASN.2010020134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Qin W, Chung AC, Huang XR, et al. TGF-beta/Smad3 signaling promotes renal fibrosis by inhibiting miR-29. J Am Soc Nephrol. 2011;22:1462–74. doi: 10.1681/ASN.2010121308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Honda N, Jinnin M, Kajihara I, et al. TGF-beta-mediated downregulation of microRNA-196a contributes to the constitutive upregulated type I collagen expression in scleroderma dermal fibroblasts. J Immunol. 2012;188:3323–31. doi: 10.4049/jimmunol.1100876. [DOI] [PubMed] [Google Scholar]
- 38.Yang S, Cui H, Xie N, et al. miR-145 regulates myofibroblast differentiation and lung fibrosis. FASEB J. 2013;27:2382–91. doi: 10.1096/fj.12-219493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature. 2003;425:577–84. doi: 10.1038/nature02006. [DOI] [PubMed] [Google Scholar]
- 40.Verrecchia F, Chu ML, Mauviel A. Identification of novel TGF-beta/Smad gene targets in dermal fibroblasts using a combined cDNA microarray/promoter transactivation approach. J Biol Chem. 2001;276:17058–62. doi: 10.1074/jbc.M100754200. [DOI] [PubMed] [Google Scholar]
- 41.Verrecchia F, Mauviel A, Farge D. Transforming growth factor-beta signaling through the Smad proteins: role in systemic sclerosis. Autoimmun Rev. 2006;5:563–9. doi: 10.1016/j.autrev.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 42.Kim HJ, Kim MY, Jin H, et al. Peroxisome proliferator-activated receptor delta regulates extracellular matrix and apoptosis of vascular smooth muscle cells through the activation of transforming growth factor-{beta}1/Smad3. Circ Res. 2009;105:16–24. doi: 10.1161/CIRCRESAHA.108.189159. [DOI] [PubMed] [Google Scholar]
- 43.Zong L, Qu Y, Xu MY, Dong YW, Lu LG. 18alpha-glycyrrhetinic acid down-regulates expression of type I and III collagen via TGF-Beta1/Smad signaling pathway in human and rat hepatic stellate cells. Int J Med Sci. 2012;9:370–9. doi: 10.7150/ijms.4395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Esquela-Kerscher A, Slack FJ. Oncomirs – microRNAs with a role in cancer. Nat Rev Cancer. 2006;6:259–69. doi: 10.1038/nrc1840. [DOI] [PubMed] [Google Scholar]
- 45.Iorio MV, Croce CM. microRNA involvement in human cancer. Carcinogenesis. 2012;33:1126–33. doi: 10.1093/carcin/bgs140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu R, Liao J, Yang M, et al. The cluster of miR-143 and miR-145 affects the risk for esophageal squamous cell carcinoma through co-regulating fascin homolog 1. PLoS ONE. 2013;7:e33987. doi: 10.1371/journal.pone.0033987. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. α-Smooth muscle actin mRNA expression in normal gastric fibroblasts and cancer-associated fibroblasts.
Fig. S2. Transforming growth factor-β expression in normal gastric fibroblasts and cancer-associated fibroblasts.
Fig. S3. MicroRNA-143 expression might be suppressed by DNA methylation and effects on cell invasion of gastric cancer cell lines.
Table S1. Information on fibroblasts.
Data S1. Supplementary materials and methods.