Abstract
Measles virus (MV) is one of the candidates for the application of oncolytic virotherapy (OVT). Although an advanced clinical study has been reported on a T-cell lymphoma, the potential of MV OVT against B-cell lymphomas remains to be clarified. We found that an EBV-transformed B lymphoblastoid cell line, a model for diffuse large B-cell lymphoma, and EBV-positive Burkitt's lymphoma cells bearing type III latency were highly susceptible to the cytolysis induced by an MV vaccine strain CAM-70. As analyzed by EBV-positive and -negative counterparts of the same cytogenetic background, type III EBV latency, not type I, was shown to augment the susceptibility of B lymphoma cells to MV-induced cytolysis. Cell surface levels of CD150/signaling lymphocytic activation molecule, a receptor of MV, were upregulated in B lymphoma cell lines with type III EBV latency by 3.8-fold, on average. The cytolytic activity of CD150-tropic WT MV was akin to that of CD46- and CD150-tropic CAM-70, suggesting that CD150 is critical for the susceptibility to MV-induced cytolysis. Among EBV-encoded genes, latent membrane protein 1 was responsible for the CD150 upregulation. It was notable that the majority of B lymphoma cell lines of type III EBV latency showed higher susceptibility to the non-Edmonston-derived CAM-70 than to the Edmonston-derived Schwarz strain. This is the first report indicating the potential of non-Edmonston MV strain for the application of OVT. Furthermore, a cellular regulator of MV replication was implicated that functions in a vaccine strain-specific fashion. Altogether, the MV OVT should serve as an alternative therapy against EBV-positive diffuse large B-cell lymphoma with type III EBV latency.
Keywords: B-cell lymphoma, CD150/signaling lymphocytic activation molecule, Epstein–Barr virus, latent membrane protein 1, measles virus oncolytic virotherapy
Virus is an attractive tool to develop alternative therapies against cancer, adopting its ability to infect specific cell types and to transfer genetic materials. One of the most promising approaches is an OVT that aims to kill cancer cells by virus that replicates preferentially in transformed cells.(1,2) This idea was exemplified by an adenoviral vector that specifically replicates in p53-deficient cells, a genetic feature often associated with cancer cells.(3–5) The OVT has been considered as a salvage therapy for some malignancies for which effective therapies have not been developed, including metastatic melanoma, recurrent tumor, drug-resistant tumors, and glioblastoma. Phase II clinical trials of OVT have been carried out to treat some malignancies, and the results appeared promising.(6) Advanced clinical studies of OVT are underway based on reovirus, vaccinia virus, herpes virus, or Seneca valley virus targeting metastatic melanoma,(7,8) liver cancer,(9) and lung carcinoma (NCT01017601).
For the success of OVT, the safety of the virus is critical. Measles virus is an enveloped RNA virus that belongs to the Paramyxoviridae family of the genus Morbillivirus. Measles vaccine, containing an attenuated virus, has contributed significantly to the reduced incidence of measles worldwide, and the safety of the measles vaccine has been well established. Vaccine strains of MV use CD46/MCP as a receptor, which is upregulated in leukemic cells.(10–12) Thus, the vaccine strains of MV are attractive for the application to OVT for their potent cytolytic activity through apoptosis.(13–15) Among nine MV vaccine strains, seven are originated from the Edmonston strain.(16) The MV OVT has been explored using Edmonston-derived vaccine strains.(13,17,18) Therapeutic potential of MV on lymphomas was historically implicated by the cases with spontaneous regression of leukemia, BL, or HD after WT MV infection.(19–23) Subsequently, the therapeutic effect of the MV vaccine has been recognized against leukemia and HD.(24–26) The potential of MV OVT has been explored in animal models and showed promising results.(27–30) More recently, a clinical trial of MV OVT was carried out on cutaneous T-cell lymphoma and was shown to be effective.(13) Furthermore, a clinical trial of MV OVT has been reported against ovarian cancer.(17) However, the benefit of MV OVT on BCL remains to be assessed.
Epstein–Barr virus, a B cell-tropic gamma herpesvirus, serves as a risk factor for several human malignancies.(31–33) The virus establishes a latency in the infected cells.(34) According to the repertorie of viral latent gene expressions, EBV latency was classified into three, types I, II, and III (alternatively, groups I, II, and III).(34) Epstein–Barr virus establishes type I latency in BL, characterized by the expression of EBNA1 and EBER. Type II latency is detected in T-cell lymphomas and HD in which LMP1 is expressed in addition to type I latency genes. Type III EBV latency is established in DLBCL, including post-transplantation lymphoprolifarative disorders and AIDS lymphoma, in which all the latency-associated genes are expressed. Epstein–Barr virus immortalizes primary B cells, which serves as an in vitro model to study the B cell lymphomagenesis by EBV. In the EBV-transformed BLCL, EBV establishes type III latency. Measles virus replicates well in lymphoid cells, as a lymphoid cell surface marker, CD150/SLAM, is expressed that serves as an MV receptor for both vaccine and WT strains.(35) In fact, CD150 was discovered in B95-8, a BLCL originated from marmoset.(35) In our preliminary studies, we found that human BLCL has a high susceptibility to MV-mediated cytolysis, suggesting the possibility of MV OVT against DLBCL. However, it remains to be clarified whether the EBV latency contributes to susceptibility to MV infection. In this study, we aimed to assess the therapeutic potential of non-Edmonston MV vaccine strain and the possible contribution of EBV latency to the susceptibility of B lymphoma cells to MV infection.
Materials and Methods
Cells
Cells were maintained in RPMI-1640 medium (Wako Pure Chemical Industries, Osaka, Japan) supplemented with 10% FBS (Japan Bioserum, Tokyo, Japan), 100 U/mL penicillin, and 100 mg/mL streptomycin (Wako Pure Chemical Industries), at 37°C in a humidified 5% CO2 atmosphere. Cells and their types of EBV latency are summarized in Table 1. The inhibitor of IκB phosphorylation, BAY 11-7082, was also purchased from Wako Pure Chemical Industries.
Table 1.
Cell lines and types of Epstein–Barr virus (EBV) latency
| Name | Type of EBV latency | References |
|---|---|---|
| EBV-negative B cell lymphoma cell line | ||
| BJAB | – | Menezes et al.(55) |
| EBV-negative Burkitt's lymphoma cell line | ||
| Akata | – | Shimizu et al.(56) |
| BL-41 | – | Lenoir et al.(57) |
| Daudi | – | Nanbo et al.(40) |
| Mutu | – | Nanbo et al.(40) |
| EBV-positive B-cell lymphoma cell line | ||
| BJAB/B95-8 | III | Miyauchi et al.(37) |
| EBV-positive Burkitt's lymphoma cell line | ||
| Akata | I | Takada et al.(38) |
| BL-41/B95-8 | III | Carter et al.(36) |
| Daudi | I | Klein et al.(39) |
| Jijoye | III | Hinuma et al.(58) |
| Mutu | I and III | Gregory et al.(41) |
| Namalwa | III | Strander et al.(59) |
| P3HR-1 | III | Hinuma et al.(58) |
| Raji | III | Epstein et al.(60) |
| EBV-transformed BLCL | ||
| BLCL | III | Miyauchi et al.(37) |
| LMP1-transduced cell line | ||
| BJAB LMP1 | – | Nakayama et al.(47) |
BLCL, B lymphoblastoid cell line; LMP1, latent membrane protein 1.
Measles virus
The vaccine strains of measles CAM-70 (Biken, Osaka, Japan) and Schwarz (Takeda Pharmaceutical Co., Osaka, Japan) were propagated from the vaccine formulations in B95a cells, a subclone of B95-8 with adherent phenotype, and the infectious titer was assessed as 50% tissue culture infectious dose on B95a cells. The field isolate MV190112 of genotype D5 propagated in B95a was used as a WT MV.
Cytolytic activity
Target cells were plated in a 96-well U-bottomed plate at 2500 cells per well and the MV was inoculated at MOI 0.01–10. The surviving live cells were quantified by CellTiter Glo (Promega, Madison, WI, USA), which measures the ATP as an indicator of metabolically active cells. The MV infection produces cell syncytia, which remains metabolically active for a few days. The assay was carried out at 7 days post-infection, which appeared optimal to detect the MV-induced cell death in our experimental setting. The MV titer yielding the 50% reduction of viable cells (LD50) was determined.
Flow cytometry
The cell surface levels of MV receptors were analyzed by flow cytometry (FACSCalibur; BD Biosciences, San Jose, CA, USA) using the following antibodies purchased from BioLegend (San Diego, CA, USA): FITC anti-human CD150 antibody, FITC anti-human CD46 antibody, FITC Mouse IgG1 Kappa Isotype Control antibody.
Results
Cytolytic activities of MV strains on B lymphoma cells latently infected with EBV
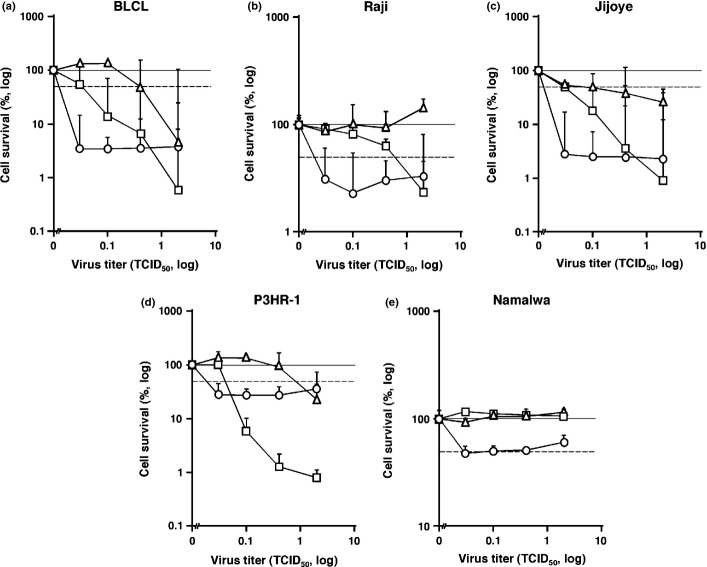
The cytolytic activity of three MV strains was examined in BLCL, a historical model of EBV-positive DLBCL, including a WT MV and two vaccine strains either non-Edmonston-derived CAM-70 or Edmonston-derived Schwarz. The LD50 at 7 days post-infection by CAM-70, Schwarz, and the WT MV were <0.03, 0.4, and 0.04 in BLCL, respectively. These data suggest that CAM-70 is potent in infecting and killing BLCL cells and, to our surprise, more potent than Schwarz (Fig. 1a). To verify these findings, we examined two representative B lymphoma cell lines showing type III EBV latency, Raji and Jijoye. The LD50 values in Raji were <0.03, >2.0, and 0.6 by CAM-70, Schwarz, and the WT MV, respectively (Fig. 1b). Similarly, in Jijoye, the LD50 values were estimated as <0.03, 0.1, and 0.03 by CAM-70, Schwarz, and the WT MV, respectively (Fig. 1c). We examined P3HR-1, a subclone from Jijoye latently infected with a defective EBV lacking EBNA2 and a part of EBNA-leader protein (LP) genes as yet expressing LMP1 at low levels.(34) The LD50 values of P3HR-1 were <0.03, 0.9, and 0.04 by CAM-70, Schwarz, and the WT MV, respectively, comparable to Jijoye (Fig. 1d). Additionally, Namalwa cells were susceptible only to the CAM-70 strain (Fig. 1e). These data suggest that CAM-70 has a greater potency in killing B lymphoma cells bearing type III EBV latency.
Fig. 1.
Susceptibility of B cell lines with type III Epstein–Barr virus (EBV) latency to measles virus (MV)-induced cytolysis. Efficiency of MV-induced cytolysis on B cell lines were assessed by cell viabilities measured at 7 days after infection. B cell lines bearing type III EBV latency are shown, including EBV-transformed B lymphoblastoid cell line (BLCL) (a), and Burkitt's lymphoma cell lines Raji (b), Jijoye (c), P3HR-1 (d), and Namalwa (e). The x-axis represents the MOI of MV. The y-axis represents the relative cell survival to the MV-uninfected control. Dotted line indicates the half survival level of the uninfected control. The error bar represents the SD of triplicate wells. Circle, non-Edmonston vaccine strain CAM-70; rectangle, WT MV; triangle, Edmonston-strain-derived vaccine strain Schwarz.
Link between MV's cytolytic activity and type of EBV latency of target cells
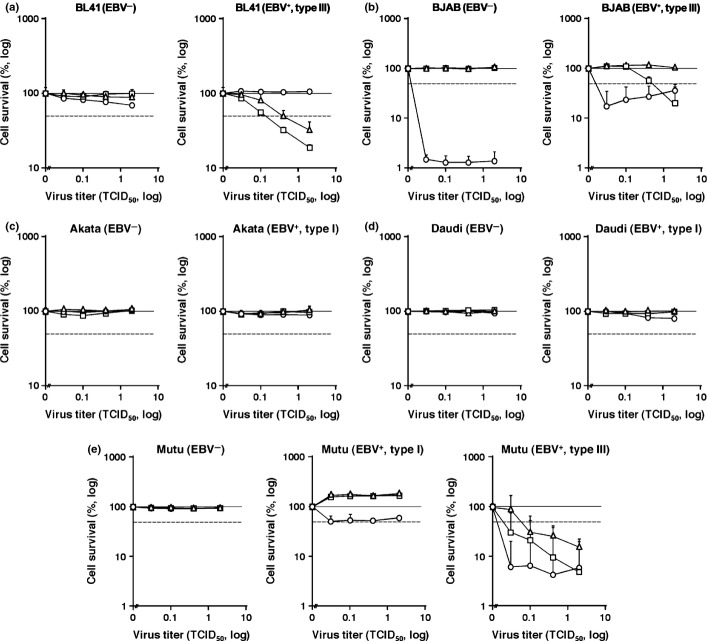
To assess the effect of EBV latency on MV susceptibility, we examined the efficiencies of MV-induced cytolysis in two EBV-negative B lymphoma cell lines BL41 and BJAB, and compared them with their EBV-positive counterparts. Infection of EBV 95-8 strain yields type III latency in both cell lines.(36,37) In BL41 cells, the LD50 values of all MV strains were above the limit of detection (Fig. 2a). In contrast, EBV-positive BL41 cells showed susceptibilities to Schwarz and the WT MV, with LD50 values of 0.4 and 0.15, respectively (Fig. 2a). In BJAB cells, Schwarz and the WT MV were not able to kill cells. However, CAM-70 showed substantial cytolytic activity (LD50 < 0.02) in BJAB cells (Fig. 2b). In accordance with BL41 cells, EBV-positive BJAB cells were more susceptible to MV than EBV-negative counterparts as represented by the enhanced susceptibility to WT MV (LD50 2.0) (Fig. 2b). These data suggested that type III EBV latency in B lymphoma cells augments the susceptibility to MV-induced cytolysis, although the magnitude of augmentation varies among cell types and the MV strains.
Fig. 2.
Contribution of Epstein–Barr virus (EBV) latency to cellular susceptibility to measles virus (MV)-induced cell lytic activity. Efficiency of MV-induced cytolysis on B lymphoma cell lines were assessed by cell viabilities measured at 7 days after infection, including BL41 (a), BJAB (b), Akata (c), Daudi (d), and Mutu (e). The EBV status and the type of latency are shown on each panel. The x-axis represents the MOI of MV. The y-axis represents the relative cell survival to the MV-uninfected control. Dotted line indicates the half survival level of the uninfected control. The error bar represents the SD of triplicate wells. Circle, non-Edmonston vaccine strain CAM-70; rectangle, WT MV; triangle, Edmonston-strain-derived vaccine strain Schwarz.
Next we examined Akata and Daudi cells in which EBV establishes type I latency.(38,39) We measured the LD50 values in EBV-positive Akata and Daudi cells and compared them with their EBV-negative counterparts isolated by limiting dilution cloning.(7,40) Measles virus-induced cytolytic activities were not detected in either cell line, regardless of EBV status (Fig. 2c,d). These data suggested that type I EBV latency did not confer the susceptibility of B lymphoma cells to MV infection.
To solidify these findings, we examined Mutu cells that yielded clones with type I and type III EBV latencies and a clone without EBV infection.(40,41) This allowed us to critically assess the effect of EBV latency on the same cytogenetic background. The cytolytic activity of MV was not observed in Mutu cells negative for EBV, nor in cells with type I EBV latency (Fig. 2e). In contrast, substantial cytolytic activity of MV was detected in type III Mutu cells where LD50 values in CAM-70, Schwarz, and the WT MV were <0.02, 0.05, and <0.02, respectively (Fig. 2e). These data, along with the data shown above, suggest that type III EBV latency, but not type I, in B lymphoma cells augments the cytolytic activity of MV.
Determinant of enhanced cellular susceptibility to MV infection by type III EBV latency
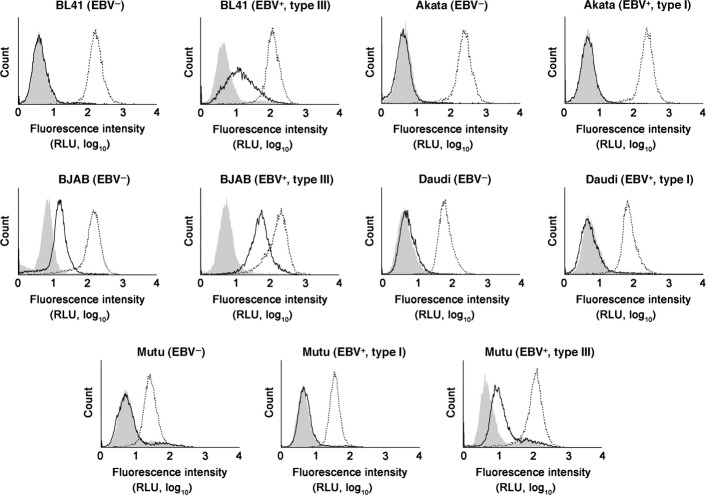
The cell surface levels of MV receptors determine the cellular susceptibility to MV infection. In lymphoid cells, two MV receptors are expressed, CD46/MCP(10,11) and CD150/SLAM.(35) We investigated whether the cell surface levels of these molecules were upregulated in cells with type III latency compared with those without EBV infection or with type I latency (Fig. 3). All cell lines were positive for CD46. However, CD150 was barely detected in EBV-negative Akata, Daudi, BL-41, and Mutu cells. Type III latency cells showed higher levels of CD150 than type I counterparts in BL41, BJAB, and Mutu cells. The average magnitude of CD150 upregulation by type III latency was 3.8-fold (3.2-, 3.4-, and 4.7-fold in BL41, BJAB, and Mutu cells, respectively). In contrast, a similar effect was not observed in CD46 except that the levels of CD46 was upregulated in type III latency in Mutu cells by 4.6-fold. Such effects were not observed in Akata and Daudi cells, suggesting that the CD150 upregulation was restricted to type III EBV latency. In agreement with our findings, increased levels of CD150 on type III Mutu cells were reported previously.(42) The vaccine strains of MV use both CD46 and CD150 as receptors. However, the WT MV used in this study was CD46-blind. Given that the cytolytic activity of the WT MV was largely paralleled to that of CAM-70, and the increased susceptibility to MV's cytolytic activity was observed only in the cells with type III EBV latency, it was likely the major determinant of MV's enhanced cytolytic activity was the cell surface CD150. Historically, it was emphasized that MV OVT targets the increased levels of CD46 on tumor cells using vaccine strains of MV.(12) However, our data point out that CD150 plays a major role in the killing of B lymphoma cells by MV. It is noted that BJAB cells were unique for their susceptibility to CAM-70 (Fig. 2b) due to the expression of CD150 at low levels in the absence of EBV latent infection, unlike other B lymphoma cell lines (Fig. 3).
Fig. 3.
Upregulation of CD150/signaling lymphocytic activation molecule (SLAM) in B lymphoma cells with type III Epstein–Barr virus (EBV) latency. The flow cytometric profiles of CD46/membrane cofactor protein and CD150/SLAM on various B lymphoma cell lines are shown. The dotted line indicates CD46/membrane cofactor protein, the bold line CD150/SLAM, and the shaded the isotype control. RLU, relative light unit.
Upregulation of CD150 by EBV-encoded oncogene LMP1
Finally, we sought the EBV latent gene responsible for the upregulation of CD150. We excluded EBNA1 and EBER because no CD150 upregulation was observed in cells with type I EBV latency. Raji and P3HR-1, highly susceptible to MV infection, are infected with defective EBV genetically devoid of EBNA2 and EBNA3, suggesting that these viral proteins are unlikely to be responsible. The signals from interleukin-1β, Toll-like receptor, and CD40, activating both the NF-κB and AP1 pathways, have been reported to upregulate CD150 expression.(43–45) Thus, we focused on the viral oncogene LMP1 because LMP1 is expressed in type III EBV latency and has been shown to activate both NF-κB and AP1.(6,46) The flow cytometric analysis revealed that BJAB cells stably expressing LMP1(46) expressed higher levels of CD150 than the EBV-negative counterparts (Fig. 2b vs Fig. 4a). These findings are consistent with a previous report demonstrating that LMP1 expression resulted in the upregulation of CD150 in a BL cell line Eli.(42) To directly test this working hypothesis, we measured the cell surface levels of CD150 on BJAB LMP1 cells after cells were treated with NF-κB inhibitors that limit the phosphorylation of IκB. Expression of CD150 on BJAB-LMP1 cells was downregulated in a dose-dependent manner after exposure to BAY 11-7082 (Fig. 4b). These data suggest that the upregulation of CD150 was partly due to the transcriptional activation by NF-κB, which was induced by LMP1.
Fig. 4.
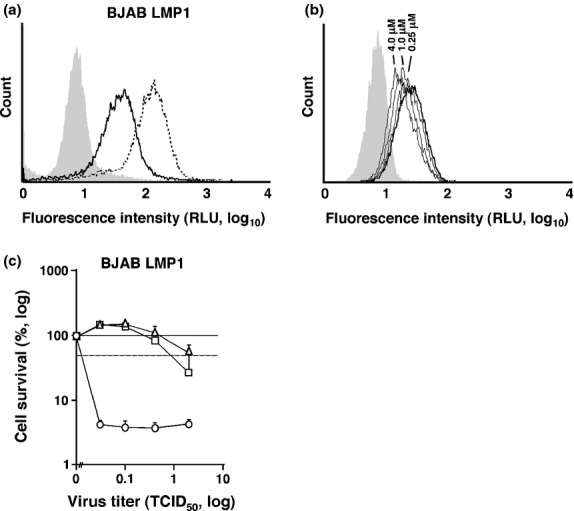
Epstein–Barr virus-encoded oncoprotein latent membrane protein 1 (LMP1) upregulates expression of CD150/signaling lymphocytic activation molecule (SLAM). (a) Flow cytometric analysis of CD46/membrane cofactor protein and CD150/SLAM on BJAB constitutively expressing LMP1. The dotted line indicates CD46/membrane cofactor protein, the bold line CD150/SLAM, and the shaded the isotype control. (b) Flow cytometric analysis of CD150/SLAM on BJAB constitutively expressing LMP1 treated with BAY 11-7082 for 1 h at 0.25–4.0 μM (thin lines) assayed at 16 h post-exposure. The bold line indicates the solvent control (DMSO) and the shaded indicates the isotype control. (c) BJAB LMP1 cells were infected with measles virus (MV) strains and the cell viabilities were measured 7 days after infection. The x-axis represents the MOI of MV. The y-axis represents the relative cell survival to the MV-uninfected control. Dotted line indicates the half survival level of the uninfected control. The error bar represents the SD of triplicate wells. Circle, non-Edmonston vaccine strain CAM-70; rectangle, WT MV; triangle, Edmonston-strain-derived vaccine strain Schwarz. RLU, relative light unit; TCID50, 50% tissue culture infectious dose.
The LD50 of MV on BJAB-LMP1 cells was measured to test whether the cellular susceptibility to MV was correlated with the upregulation of CD150 by LMP1. The LD50 values of BJAB-LMP1 cells were <0.02, 1.0, and >2.0 by CAM-70, Schwarz, and the WT MV, respectively, akin to those in EBV-positive BJAB cells (Fig. 4c). These data suggest that the upregulation of CD150 by LMP1 is responsible for the augmented susceptibility to MV-induced cytolytic activities in B lymphoma cells.
Discussion
Using a panel of B lymphoma cell lines with distinct EBV latency, we showed that type III EBV latency in B lymphoma cells enhances cellular susceptibility to the oncolytic activity of MV that infects lymphoma cells through CD150 upregulated by LMP1. These data suggest that the B-cell malignancies with EBV type III latency are attractive targets of MV OVT. This is a novel biological phenotype associated with LMP1. The oncoprotein LMP1 is expressed in type II EBV latency, which has been detected in HD and Natural Killer (NK)/T-cell lymphomas.(31,33,34) Expression of CD150 has been demonstrated in clinical specimens of HD.(48) Historically, it was emphasized that MV OVT targets the increased levels of CD46 on tumor cells.(12) However, our data indicate that CD150 plays the major role in the killing of B lymphoma cells by MV. Given that effective molecular therapies remain to be developed, it is feasible that MV OVT serves as an option against these lymphomas associated with EBV.
We detected a difference in oncolytic potency between Edmonston- and non-Edmonston-derived MV vaccine strains. Among 11 EBV-positive B lymphoma cell lines, CAM-70 showed higher efficacy in killing cells in 10 cell lines (90.9%) than Schwarz, indicating that CAM-70 has a higher therapeutic value to apply for MV OVT against B-cell lymphomas. This is the first report showing the potential of non-Edmonston MV strains for use in MV OVT.
The EBV-positive BL41 cell line was the only one in this study that showed a higher susceptibility to Schwarz than to CAM-70. This is not fully explained by the levels of CD46 and CD150, suggesting that unknown cellular factors are involved in the regulation of MV replication. It is also possible that an MV strain suitable for certain malignancies may differ from MV strains effective against other cancers. CAM-70 is an attenuated MV derived from the field isolate in Japan and genetically the most remote from the vaccine strains originated from the Edmonston strain.(16) CAM-70 and Schwarz share their ability to enter cells using CD46/CD150/Nectin as receptors. However, the syncitium formation by Schwarz is more robust than that by CAM-70 (J. Komano, unpublished data, 2012). In agreement with these data, CAM-70 was shown to have a lower efficacy to bind receptors.(49) It is not clear which viral genetic factor explains the difference in oncolytic activity between CAM-70 and Schwarz. Identification of viral and cellular factors that influence the cytolytic function of MV may help us to deepen our understanding of MV replication and virus–host interaction at molecular levels, and may facilitate the production of genetically engineered MV more effective in the MV OVT against DLBCL.
Measles virus OVT was successful in clinical studies in which enrolled subjects should have been immunized with MV vaccine or infected with the WT MV.(13) This suggests that the host immunity against MV does not necessarily have a negative impact on therapeutic effect. On the contrary, it was assumed that the host immunity might have enhanced therapeutic effects by killing the virus-infected cancer cells.(50) One of the approaches to increase the efficacy of OVT is to engineer the virus to arm the anticancer functions.(8,51–54) In MV OVT, the recombinant virus has been developed that delivers a drug-sensitizing gene to cancer cells.(18) Overall, although much is to be explored, our data and others indicate that the EBV-associated lymphomas are attractive targets for MV OVT.
Acknowledgments
This work was supported by the Japan Health Science Foundation, the Japanese Ministry of Health, Labor, and Welfare and the Japanese Ministry of Education, Culture, Sports, Science and Technology.
Glossary
- BCL
B-cell lymphoma
- BL
Burkitt's lymphoma
- BLCL
B lymphoblastoid cell line
- DLBCL
diffuse large B-cell lymphoma
- EBER
Epstein–Barr virus-encoded small RNA
- EBNA
Epstein–Barr nuclear antigen
- EBV
Epstein–Barr virus
- HD
Hodgkin's disease
- LMP
latent membrane protein
- MCP
membrane cofactor protein
- MV
measles virus
- NF-κB
nuclear factor-κB
- OVT
oncolytic virotherapy
- SLAM
signaling lymphocytic activation molecule
Disclosure Statement
The authors have no conflict of interest.
References
- 1.Russell SJ, Peng KW, Bell JC. Oncolytic virotherapy. Nat Biotechnol. 2012;30:658–70. doi: 10.1038/nbt.2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zeyaullah M, Patro M, Ahmad I, et al. Oncolytic viruses in the treatment of cancer: a review of current strategies. Pathol Oncol Res. 2012;18:771–81. doi: 10.1007/s12253-012-9548-2. [DOI] [PubMed] [Google Scholar]
- 3.Barker DD, Berk AJ. Adenovirus proteins from both E1B reading frames are required for transformation of rodent cells by viral infection and DNA transfection. Virology. 1987;156:107–21. doi: 10.1016/0042-6822(87)90441-7. [DOI] [PubMed] [Google Scholar]
- 4.Bischoff JR, Kirn DH, Williams A, et al. An adenovirus mutant that replicates selectively in p53-deficient human tumor cells. Science. 1996;274:373–6. doi: 10.1126/science.274.5286.373. [DOI] [PubMed] [Google Scholar]
- 5.Heise C, Sampson-Johannes A, Williams A, McCormick F, Von Hoff DD, Kirn DH. ONYX-015, an E1B gene-attenuated adenovirus, causes tumor-specific cytolysis and antitumoral efficacy that can be augmented by standard chemotherapeutic agents. Nat Med. 1997;3:639–45. doi: 10.1038/nm0697-639. [DOI] [PubMed] [Google Scholar]
- 6.Rowan K. Oncolytic viruses move forward in clinical trials. J Natl Cancer Inst. 2010;102:590–5. doi: 10.1093/jnci/djq165. [DOI] [PubMed] [Google Scholar]
- 7.Senzer NN, Kaufman HL, Amatruda T, et al. Phase II clinical trial of a granulocyte-macrophage colony-stimulating factor-encoding, second-generation oncolytic herpesvirus in patients with unresectable metastatic melanoma. J Clin Oncol. 2009;27:5763–71. doi: 10.1200/JCO.2009.24.3675. [DOI] [PubMed] [Google Scholar]
- 8.Galanis E, Markovic SN, Suman VJ, et al. Phase II trial of intravenous administration of Reolysin((R)) (Reovirus Serotype-3-dearing Strain) in patients with metastatic melanoma. Mol Ther. 2012;20:1998–2003. doi: 10.1038/mt.2012.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heo J, Reid T, Ruo L, et al. Randomized dose-finding clinical trial of oncolytic immunotherapeutic vaccinia JX-594 in liver cancer. Nat Med. 2013;19:329–36. doi: 10.1038/nm.3089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dorig RE, Marcil A, Chopra A, Richardson CD. The human CD46 molecule is a receptor for measles virus (Edmonston strain) Cell. 1993;75:295–305. doi: 10.1016/0092-8674(93)80071-l. [DOI] [PubMed] [Google Scholar]
- 11.Naniche D, Varior-Krishnan G, Cervoni F, et al. Human membrane cofactor protein (CD46) acts as a cellular receptor for measles virus. J Virol. 1993;67:6025–32. doi: 10.1128/jvi.67.10.6025-6032.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hara T, Suzuki Y, Semba T, Hatanaka M, Matsumoto M, Seya T. High expression of membrane cofactor protein of complement (CD46) in human leukaemia cell lines: implication of an alternatively spliced form containing the STA domain in CD46 up-regulation. Scand J Immunol. 1995;42:581–90. doi: 10.1111/j.1365-3083.1995.tb03700.x. [DOI] [PubMed] [Google Scholar]
- 13.Heinzerling L, Kunzi V, Oberholzer PA, Kundig T, Naim H, Dummer R. Oncolytic measles virus in cutaneous T-cell lymphomas mounts antitumor immune responses in vivo and targets interferon-resistant tumor cells. Blood. 2005;106:2287–94. doi: 10.1182/blood-2004-11-4558. [DOI] [PubMed] [Google Scholar]
- 14.Msaouel P, Iankov ID, Dispenzieri A, Galanis E. Attenuated oncolytic measles virus strains as cancer therapeutics. Curr Pharm Biotechnol. 2013;13:1732–41. doi: 10.2174/138920112800958896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Esolen LM, Park SW, Hardwick JM, Griffin DE. Apoptosis as a cause of death in measles virus-infected cells. J Virol. 1995;69:3955–8. doi: 10.1128/jvi.69.6.3955-3958.1995. Jun. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bankamp B, Takeda M, Zhang Y, Xu W, Rota PA. Genetic characterization of measles vaccine strains. J Infect Dis. 2011;204(Suppl 1):S533–48. doi: 10.1093/infdis/jir097. [DOI] [PubMed] [Google Scholar]
- 17.Galanis E, Hartmann LC, Cliby WA, et al. Phase I trial of intraperitoneal administration of an oncolytic measles virus strain engineered to express carcinoembryonic antigen for recurrent ovarian cancer. Cancer Res. 2010;70:875–82. doi: 10.1158/0008-5472.CAN-09-2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zaoui K, Bossow S, Grossardt C, et al. Chemovirotherapy for head and neck squamous cell carcinoma with EGFR-targeted and CD/UPRT-armed oncolytic measles virus. Cancer Gene Ther. 2012;19:181–91. doi: 10.1038/cgt.2011.75. [DOI] [PubMed] [Google Scholar]
- 19.Pasquinucci G. Possible effect of measles on leukaemia. Lancet. 1971;1:136. doi: 10.1016/s0140-6736(71)90869-5. [DOI] [PubMed] [Google Scholar]
- 20.Zygiert Z. Hodgkin's disease: remissions after measles. Lancet. 1971;1:593. doi: 10.1016/s0140-6736(71)91186-x. [DOI] [PubMed] [Google Scholar]
- 21.Bluming AZ, Ziegler JL. Regression of Burkitt's lymphoma in association with measles infection. Lancet. 1971;2:105–6. doi: 10.1016/s0140-6736(71)92086-1. [DOI] [PubMed] [Google Scholar]
- 22.Mota HC. Infantile Hodgkin's disease: remission after measles. Br Med J. 1973;2:421. doi: 10.1136/bmj.2.5863.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ziegler JL. Spontaneous remission in Burkitt's lymphoma. Natl Cancer Inst Monogr. 1976;44:61–5. [PubMed] [Google Scholar]
- 24.Hansen RM, Libnoch JA. Remission of chronic lymphocytic leukemia after smallpox vaccination. Arch Intern Med. 1978;138:1137–8. [PubMed] [Google Scholar]
- 25.Greentree LB. Hodgkin's disease: therapeutic role of measles vaccine. Am J Med. 1983;75:928. doi: 10.1016/0002-9343(83)90865-3. [DOI] [PubMed] [Google Scholar]
- 26.Schattner A. Therapeutic role of measles vaccine in Hodgkin's disease. Lancet. 1984;1:171. doi: 10.1016/s0140-6736(84)90112-0. [DOI] [PubMed] [Google Scholar]
- 27.Grote D, Russell SJ, Cornu TI, et al. Live attenuated measles virus induces regression of human lymphoma xenografts in immunodeficient mice. Blood. 2001;97:3746–54. doi: 10.1182/blood.v97.12.3746. [DOI] [PubMed] [Google Scholar]
- 28.Peng KW, Ahmann GJ, Pham L, Greipp PR, Cattaneo R, Russell SJ. Systemic therapy of myeloma xenografts by an attenuated measles virus. Blood. 2001;98:2002–7. doi: 10.1182/blood.v98.7.2002. [DOI] [PubMed] [Google Scholar]
- 29.Peng KW, Facteau S, Wegman T, O'Kane D, Russell SJ. Non-invasive in vivo monitoring of trackable viruses expressing soluble marker peptides. Nat Med. 2002;8:527–31. doi: 10.1038/nm0502-527. [DOI] [PubMed] [Google Scholar]
- 30.Phuong LK, Allen C, Peng KW, et al. Use of a vaccine strain of measles virus genetically engineered to produce carcinoembryonic antigen as a novel therapeutic agent against glioblastoma multiforme. Cancer Res. 2003;63:2462–9. [PubMed] [Google Scholar]
- 31.Thompson MP, Kurzrock R. Epstein-Barr virus and cancer. Clin Cancer Res. 2004;10:803–21. doi: 10.1158/1078-0432.ccr-0670-3. [DOI] [PubMed] [Google Scholar]
- 32.Klein E, Kis LL, Klein G. Epstein-Barr virus infection in humans: from harmless to life endangering virus-lymphocyte interactions. Oncogene. 2007;26:1297–305. doi: 10.1038/sj.onc.1210240. [DOI] [PubMed] [Google Scholar]
- 33.Rickinson AB, Kieff E. Epstin-Barr Virus. In: Knipe DM, Howley PM, editors. Fields Virology. 5th edn. Philadelphia, PA: Lippincott Williams & Wilkins; 2007. pp. 2655–700. [Google Scholar]
- 34.Kieff E, Rickinson AB. Epstin-Barr Virus and Its Replication. In: Knipe DM, Howley PM, editors. Fields Virology. 5th edn. Philadelphia, PA: Lippincott Williams & Wilkins; 2007. pp. 2603–54. [Google Scholar]
- 35.Tatsuo H, Ono N, Tanaka K, Yanagi Y. SLAM (CDw150) is a cellular receptor for measles virus. Nature. 2000;406:893–7. doi: 10.1038/35022579. [DOI] [PubMed] [Google Scholar]
- 36.Carter KL, Cahir-McFarland E, Kieff E. Epstein-barr virus-induced changes in B-lymphocyte gene expression. J Virol. 2002;76:10427–36. doi: 10.1128/JVI.76.20.10427-10436.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miyauchi K, Urano E, Yoshiyama H, Komano J. Cytokine signatures of transformed B cells with distinct Epstein-Barr virus latencies as a potential diagnostic tool for B cell lymphoma. Cancer Sci. 2011;102:1236–41. doi: 10.1111/j.1349-7006.2011.01924.x. [DOI] [PubMed] [Google Scholar]
- 38.Takada K, Horinouchi K, Ono Y, et al. An Epstein-Barr virus-producer line Akata: establishment of the cell line and analysis of viral DNA. Virus Genes. 1991;5:147–56. doi: 10.1007/BF00571929. [DOI] [PubMed] [Google Scholar]
- 39.Klein G, Pearson G, Nadkarni JS, et al. Relation between Epstein-Barr viral and cell membrane immunofluorescence of Burkitt tumor cells. I. Dependence of cell membrane immunofluorescence on presence of EB virus. J Exp Med. 1968;128:1011–20. doi: 10.1084/jem.128.5.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nanbo A, Inoue K, Adachi-Takasawa K, Takada K. Epstein-Barr virus RNA confers resistance to interferon-alpha-induced apoptosis in Burkitt's lymphoma. EMBO J. 2002;21:954–65. doi: 10.1093/emboj/21.5.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gregory CD, Rowe M, Rickinson AB. Different Epstein-Barr virus-B cell interactions in phenotypically distinct clones of a Burkitt's lymphoma cell line. J Gen Virol. 1990;71(Pt 7):1481–95. doi: 10.1099/0022-1317-71-7-1481. [DOI] [PubMed] [Google Scholar]
- 42.Nagy N, Maeda A, Bandobashi K, et al. SH2D1A expression in Burkitt lymphoma cells is restricted to EBV positive group I lines and is downregulated in parallel with immunoblastic transformation. Int J Cancer. 2002;100:433–40. doi: 10.1002/ijc.10498. [DOI] [PubMed] [Google Scholar]
- 43.Sidorenko SP, Clark EA. Characterization of a cell surface glycoprotein IPO-3, expressed on activated human B and T lymphocytes. J Immunol. 1993;151:4614–24. [PubMed] [Google Scholar]
- 44.Kruse M, Meinl E, Henning G, et al. Signaling lymphocytic activation molecule is expressed on mature CD83 + dendritic cells and is up-regulated by IL-1 beta. J Immunol. 2001;167:1989–95. doi: 10.4049/jimmunol.167.4.1989. [DOI] [PubMed] [Google Scholar]
- 45.Farina C, Theil D, Semlinger B, Hohlfeld R, Meinl E. Distinct responses of monocytes to Toll-like receptor ligands and inflammatory cytokines. Int Immunol. 2004;16:799–809. doi: 10.1093/intimm/dxh083. [DOI] [PubMed] [Google Scholar]
- 46.Kilger E, Kieser A, Baumann M, Hammerschmidt W. Epstein-Barr virus-mediated B-cell proliferation is dependent upon latent membrane protein 1, which simulates an activated CD40 receptor. EMBO J. 1998;17:1700–9. doi: 10.1093/emboj/17.6.1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nakayama T, Fujisawa R, Izawa D, Hieshima K, Takada K, Yoshie O. Human B cells immortalized with Epstein-Barr virus upregulate CCR6 and CCR10 and downregulate CXCR4 and CXCR5. J Virol. 2002;76:3072–7. doi: 10.1128/JVI.76.6.3072-3077.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yurchenko MY, Kovalevska LM, Shlapatska LM, Berdova GG, Clark EA, Sidorenko SP. CD150 regulates JNK1/2 activation in normal and Hodgkin's lymphoma B cells. Immunol Cell Biol. 2010;88:565–74. doi: 10.1038/icb.2010.14. [DOI] [PubMed] [Google Scholar]
- 49.Kato S, Ohgimoto S, Sharma LB, et al. Reduced ability of hemagglutinin of the CAM-70 measles virus vaccine strain to use receptors CD46 and SLAM. Vaccine. 2009;27:3838–48. doi: 10.1016/j.vaccine.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 50.Prestwich RJ, Harrington KJ, Pandha HS, Vile RG, Melcher AA, Errington F. Oncolytic viruses: a novel form of immunotherapy. Expert Rev Anticancer Ther. 2008;8:1581–8. doi: 10.1586/14737140.8.10.1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu TC, Kirn D. Systemic efficacy with oncolytic virus therapeutics: clinical proof-of-concept and future directions. Cancer Res. 2007;67:429–32. doi: 10.1158/0008-5472.CAN-06-2871. [DOI] [PubMed] [Google Scholar]
- 52.Opyrchal M, Aderca I, Galanis E. Phase I clinical trial of locoregional administration of the oncolytic adenovirus ONYX-015 in combination with mitomycin-C, doxorubicin, and cisplatin chemotherapy in patients with advanced sarcomas. Methods Mol Biol. 2009;542:705–17. doi: 10.1007/978-1-59745-561-9_35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Harrington KJ, Vile RG, Melcher A, Chester J, Pandha HS. Clinical trials with oncolytic reovirus: moving beyond phase I into combinations with standard therapeutics. Cytokine Growth Factor Rev. 2010;21:91–8. doi: 10.1016/j.cytogfr.2010.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Karapanagiotou EM, Roulstone V, Twigger K, et al. Phase I/II trial of carboplatin and paclitaxel chemotherapy in combination with intravenous oncolytic reovirus in patients with advanced malignancies. Clin Cancer Res. 2012;18:2080–9. doi: 10.1158/1078-0432.CCR-11-2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Menezes J, Leibold W, Klein G, Clements G. Establishment and characterization of an Epstein-Barr virus (EBC)-negative lymphoblastoid B cell line (BJA-B) from an exceptional, EBV-genome-negative African Burkitt's lymphoma. Biomedicine. 1975;22:276–84. [PubMed] [Google Scholar]
- 56.Shimizu N, Tanaba-Tochikura A, Kuroiwa Y, Takada K. Isolation of Epstein-Barr virus (EBV)-negative cell clones from the EBV-positive Burkitt's lymphoma (BL) line Akata: malignant phenotypes of BL cells are dependent on EBV. J Virol. 1994;68:6069–73. doi: 10.1128/jvi.68.9.6069-6073.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lenoir GM, Vuillaume M, Bonnardel C. The use of lymphomatous and lymphoblastoid cell lines in the study of Burkitt's lymphoma. IARC Sci Publ. 1985;60:309–18. [PubMed] [Google Scholar]
- 58.Hinuma Y, Konn M, Yamaguchi J, Wudarski DJ, Blakeslee JR, Jr, Grace JT., Jr Immunofluorescence and herpes-type virus particles in the P3HR-1 Burkitt lymphoma cell line. J Virol. 1967;1:1045–54. doi: 10.1128/jvi.1.5.1045-1051.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Strander H, Mogensen KE, Cantell K. Production of human lymphoblastoid interferon. J Clin Microbiol. 1975;1:116–7. doi: 10.1128/jcm.1.1.116-117.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Epstein MA, Achong BG, Barr YM, Zajac B, Henle G, Henle W. Morphological and virological investigations on cultured Burkitt tumor lymphoblasts (strain Raji) J Natl Cancer Inst. 1966;37:547–59. [PubMed] [Google Scholar]