Abstract
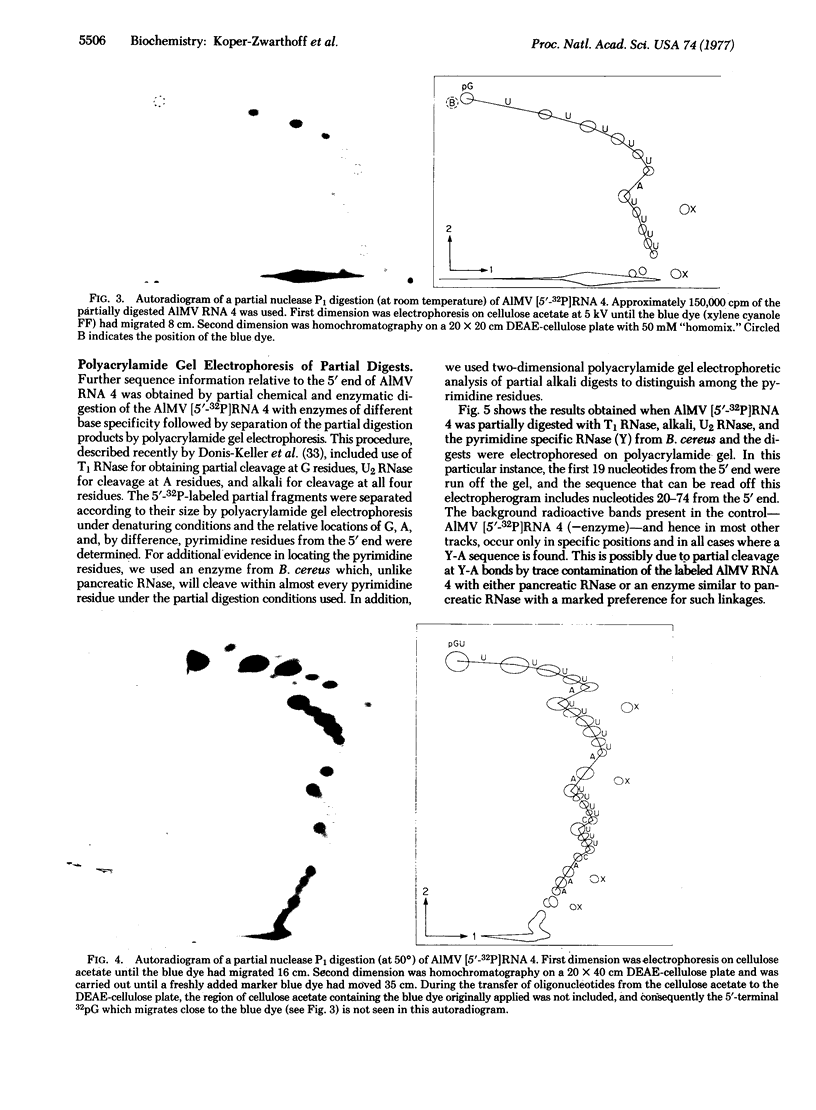
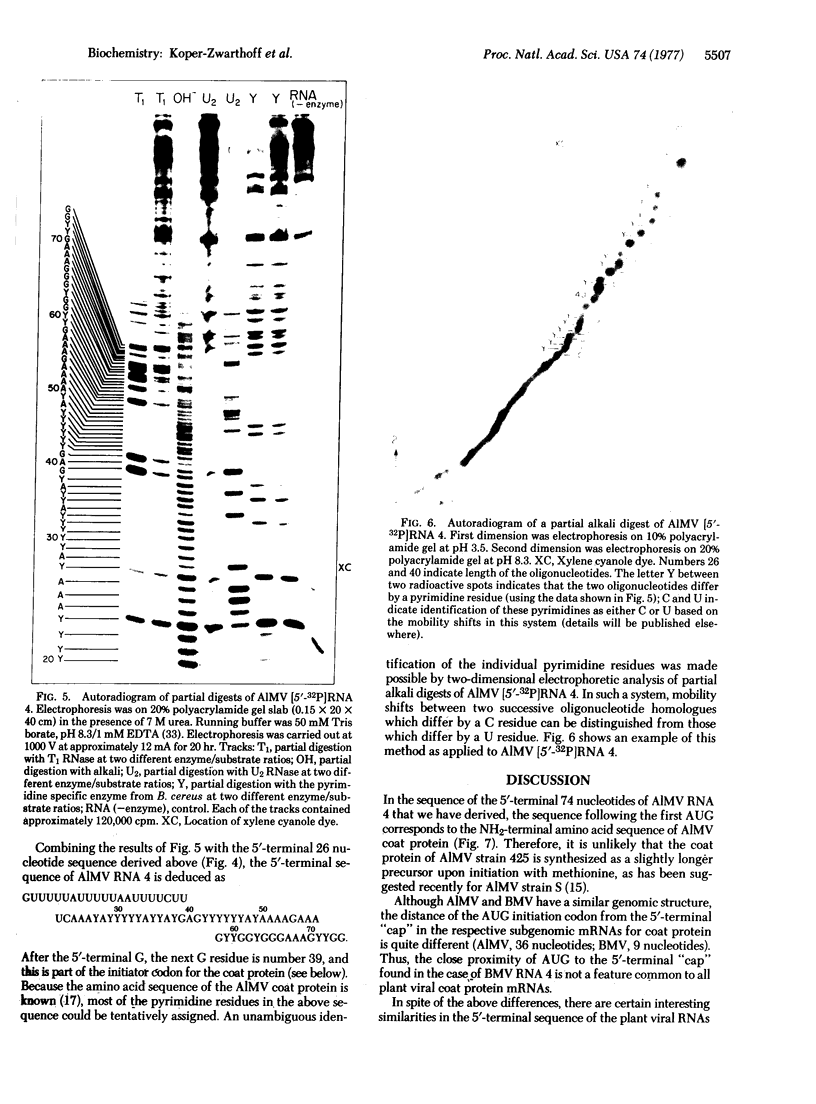
The sequence of the 5'-terminal 74 nucleotides of alfalfa mosaic virus RNA 4, the mRNA for the viral coat protein, has been deduced by using various new techniques for labeling the RNA at the 5' end with 32P and for sequencing the 5'-32P-labeled RNA. The sequence is NpppGUUUUUAUUUUUAAUUUUCUUUCAAAUACUUCCAUCAUGAGUUCUUCACAAAAGAAAGCUGGUGGGAAAGCUGG. The AUG initiator codon is located 36 nucleotides in from the 5' end; the nucleotide sequence beyond corresponds to the amino acid sequence of the coat protein. This 5' noncoding region is rich in U (58% U); except for the 5'-terminal G, the next G in is part of the initiator AUG codon.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baralle F. E. Structure-function relationship of 5' non-coding sequence of rabbit alpha- and beta-globin mRNA. Nature. 1977 May 19;267(5608):279–281. doi: 10.1038/267279a0. [DOI] [PubMed] [Google Scholar]
- Bol J. F., Brederode F. T., Janze G. C., Rauh D. K. Studies on sequence homology between the RNA's of alfalfa mosaic virus. Virology. 1975 May;65(1):1–15. doi: 10.1016/0042-6822(75)90002-1. [DOI] [PubMed] [Google Scholar]
- Bol J. F., van Vloten-Doting L. Function of top component a RNA in the initiation of infection by alfalfa mosaic virus. Virology. 1973 Jan;51(1):102–108. doi: 10.1016/0042-6822(73)90370-x. [DOI] [PubMed] [Google Scholar]
- Bol J. F., van Vloten-Doting L., Jaspars E. M. A functional equivalence of top component a RNA and coat protein in the initiation of infection by alfalfa mosaic virus. Virology. 1971 Oct;46(1):73–85. doi: 10.1016/0042-6822(71)90007-9. [DOI] [PubMed] [Google Scholar]
- Both G. W., Furuichi Y., Muthukrishnan S., Shatkin A. J. Effect of 5'-terminal structure and base composition on polyribonucleotide binding to ribosomes. J Mol Biol. 1976 Jul 5;104(3):637–658. doi: 10.1016/0022-2836(76)90126-1. [DOI] [PubMed] [Google Scholar]
- Castel A., Kraal B., Kerklaan P. R., Klok J., Bosch L. Initiation of polypeptide synthesis with various NH2-blocked aminoacyl-tRNAs under the direction of alfalfa mosaic virus RNA 4. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5509–5513. doi: 10.1073/pnas.74.12.5509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasgupta R., Shih D. S., Saris C., Kaesberg P. Nucleotide sequence of a viral RNA fragment that binds to eukaryotic ribosomes. Nature. 1975 Aug 21;256(5519):624–628. doi: 10.1038/256624a0. [DOI] [PubMed] [Google Scholar]
- Donis-Keller H., Maxam A. M., Gilbert W. Mapping adenines, guanines, and pyrimidines in RNA. Nucleic Acids Res. 1977 Aug;4(8):2527–2538. doi: 10.1093/nar/4.8.2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efstratiadis A., Kafatos F. C., Maniatis T. The primary structure of rabbit beta-globin mRNA as determined from cloned DNA. Cell. 1977 Apr;10(4):571–585. doi: 10.1016/0092-8674(77)90090-3. [DOI] [PubMed] [Google Scholar]
- Gerlinger P., Mohier E., Le Meur M. A., Hirth L. Monocistronic translation of alfalfa mosaic virus RNAs. Nucleic Acids Res. 1977 Apr;4(4):813–826. doi: 10.1093/nar/4.4.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillum A. M., Urquhart N., Smith M., RajBhandary U. L. Nucleotide sequence of salmon testes and salmon liver cytoplasmic initiator tRNA. Cell. 1975 Nov;6(3):395–405. doi: 10.1016/0092-8674(75)90189-0. [DOI] [PubMed] [Google Scholar]
- Glynn I. M., Chappell J. B. A simple method for the preparation of 32-P-labelled adenosine triphosphate of high specific activity. Biochem J. 1964 Jan;90(1):147–149. doi: 10.1042/bj0900147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haseltine W. A., Maxam A. M., Gilbert W. Rous sarcoma virus genome is terminally redundant: the 5' sequence. Proc Natl Acad Sci U S A. 1977 Mar;74(3):989–993. doi: 10.1073/pnas.74.3.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heijtink R. A., Houwing C. J., Jaspars E. M. Molecular weights of particles and RNAs of alfalfa mosaic virus. Number of subunits in protein capsids. Biochemistry. 1977 Oct 18;16(21):4684–4693. doi: 10.1021/bi00640a024. [DOI] [PubMed] [Google Scholar]
- Jaspars E. M. Plant viruses with a multipartite genome. Adv Virus Res. 1974;19:37–149. doi: 10.1016/s0065-3527(08)60659-4. [DOI] [PubMed] [Google Scholar]
- Jay E., Bambara R., Padmanabhan R., Wu R. DNA sequence analysis: a general, simple and rapid method for sequencing large oligodeoxyribonucleotide fragments by mapping. Nucleic Acids Res. 1974 Mar;1(3):331–353. doi: 10.1093/nar/1.3.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M., Shatkin A. J. Sequences of two 5'-terminal ribosome-protected fragments from reovirus messenger RNAs. J Mol Biol. 1977 May 5;112(1):75–96. doi: 10.1016/s0022-2836(77)80157-5. [DOI] [PubMed] [Google Scholar]
- Lockhard R. E., Rajbhandary U. L. Nucleotide sequences at the 5'termini of rabbit alpha and beta globin mRNA. Cell. 1976 Dec;9(4 Pt 2):747–760. doi: 10.1016/0092-8674(76)90138-0. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry R. P. Processing of RNA. Annu Rev Biochem. 1976;45:605–629. doi: 10.1146/annurev.bi.45.070176.003133. [DOI] [PubMed] [Google Scholar]
- Pinck L. The 5'-end groups of alfalfa mosaic virus RNAs are m7G5'ppp5'Gp. FEBS Lett. 1975 Nov 1;59(1):24–28. doi: 10.1016/0014-5793(75)80332-2. [DOI] [PubMed] [Google Scholar]
- Richards K., Guilley H., Jonard G., Keith G. Leader sequence of 71 nucleotides devoid of G in tobacco mosaic virus RNA. Nature. 1977 Jun 9;267(5611):548–550. doi: 10.1038/267548a0. [DOI] [PubMed] [Google Scholar]
- Richardson C. C. Phosphorylation of nucleic acid by an enzyme from T4 bacteriophage-infected Escherichia coli. Proc Natl Acad Sci U S A. 1965 Jul;54(1):158–165. doi: 10.1073/pnas.54.1.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roman R., Brooker J. D., Seal S. N., Marcus A. Inhibition of the transition of a 40 S ribosome-Met-tRNA-i-Met complex to an 80 S ribosome-Met-tRNA-i-Met- complex by 7-Methylguanosine-5'-phosphate. Nature. 1976 Mar 25;260(5549):359–360. doi: 10.1038/260359a0. [DOI] [PubMed] [Google Scholar]
- Rose J. K. Nucleotide sequences of ribosome recgonition sites in messenger RNAs of vesicular stomatitis virus. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3672–3676. doi: 10.1073/pnas.74.9.3672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Donelson J. E., Coulson A. R., Kössel H., Fischer D. Use of DNA polymerase I primed by a synthetic oligonucleotide to determine a nucleotide sequence in phage fl DNA. Proc Natl Acad Sci U S A. 1973 Apr;70(4):1209–1213. doi: 10.1073/pnas.70.4.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shatkin A. J. Capping of eucaryotic mRNAs. Cell. 1976 Dec;9(4 Pt 2):645–653. doi: 10.1016/0092-8674(76)90128-8. [DOI] [PubMed] [Google Scholar]
- Shine J., Czernilofsky A. P., Friedrich R., Bishop J. M., Goodman H. M. Nucleotide sequence at the 5' terminus of the avian sarcoma virus genome. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1473–1477. doi: 10.1073/pnas.74.4.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silberklang M., Prochiantz A., Haenni A. L., Rajbhandary U. L. Studies on the sequence of the 3'-terminal region of turnip-yellow-mosaic-virus RNA. Eur J Biochem. 1977 Feb;72(3):465–478. doi: 10.1111/j.1432-1033.1977.tb11270.x. [DOI] [PubMed] [Google Scholar]
- Stoll E., Billeter M. A., Palmenberg A., Weissmann C. Avian myeloblastosis virus RNA is terminally redundant: implications for the mechanism of retrovirus replication. Cell. 1977 Sep;12(1):57–72. doi: 10.1016/0092-8674(77)90185-4. [DOI] [PubMed] [Google Scholar]
- Van de Voorde A., Contreras R., Rogiers R., Fiers W. The initiation region of the SV40 VP1 gene. Cell. 1976 Sep;9(1):117–120. doi: 10.1016/0092-8674(76)90057-x. [DOI] [PubMed] [Google Scholar]
- Vloten-Doting L. V. Similarities and differences between viruses with a tripartite genome. Ann Microbiol (Paris) 1976 Jan;127A(1):119–129. [PubMed] [Google Scholar]
- van Beynum G. M., de Graaf J. M., Castel A., Kraal B., Bosch L. Structural studies on the coat protein of alfalfa mosaic virus. The complete primary structure. Eur J Biochem. 1977 Jan 3;72(1):63–78. doi: 10.1111/j.1432-1033.1977.tb11225.x. [DOI] [PubMed] [Google Scholar]