Abstract
Transcriptional GATA factors are known lineage selector genes and regulate a variety of biological processes including specification and differentiation of tissues. In the present study, we examined expression profiles of six GATA factor genes in invasive ductal carcinomas (IDC) of the breast using microarray analysis (n = 20) and found that GATA4 expression was closely correlated with recurrence in patients. Because the significance of GATA4 has remained largely unknown in breast carcinoma, we further immunolocalized GATA4 in ductal carcinoma in situ (DCIS) of the breast (n = 48) and IDC (n = 163). GATA4 immunoreactivity was detected in the nuclei of carcinoma cells and was positive in 27% of DCIS and 31% of IDC cases. GATA4 status was significantly associated with nuclear grade and van Nuys classification in DCIS and was positively associated with distant metastasis, histological grade and HER2 status, but negatively correlated with progesterone receptor labeling index in IDC. Subsequent multivariate analysis demonstrated that GATA4 status was an independent prognostic factor for both disease-free and breast cancer-specific survival of IDC patients. All of these results indicate that GATA4 plays important roles in the progression of breast carcinoma from an early stage and that immunohistochemical GATA4 status is considered a potent prognostic factor in human breast cancer patients.
Keywords: Breast, carcinoma, immunohistochemistry, metastasis, prognosis
Breast cancer is one of the most common malignancies in women. Invasive breast cancer is generally regarded as a disease that metastasizes in the early phase1 and metastasis is the major cause of death of breast cancer patients. Breast cancer patients frequently receive adjuvant therapies such as endocrine therapy and chemotherapy after surgical treatment. However, distant recurrence in patients treated with the anti-estrogen tamoxifen after surgery has been reported in 15% of patients at 10 years2 and results of 11 clinical trials revealed that 25% of patients who received adjuvant chemotherapy developed distant recurrence.3 Therefore, it is very important to examine the molecular mechanisms of recurrence in breast carcinoma to improve the clinical outcome of patients.
Zinc finger transcriptional factors of the GATA family bind to the consensus DNA sequence (A/T) GATA (A/G) and regulate a variety of biological processes including specification and differentiation of tissues (reviewed in Shimizu and Yamamoto4 and Kaneko et al.5). Six members of the GATA family have been identified.6–9 Expressions of GATA1, GATA2 and GATA3 are mainly restricted to hematopoietic and neuronal cell lineages (reviewed in Patient and McGhee10) and GATA factor switching from GATA2 to GATA1 contributes to erythroid differentiation.5,11 In contrast, GATA4, GATA5 and GATA6 are commonly expressed in heart and digestive organs and GATA4 plays important roles in cardiovascular development.12 The former three and latter three are often referred to as hematopoietic GATA factors and endodermal GATA factors, respectively, but much broader tissue distribution of GATA proteins has also been reported.10 GATA factors can function in undifferentiated progenitor cells and can direct the coordinated maturation and cell cycle withdrawal in terminally differentiating cells;13 alteration of GATA factor expression is suggested to contribute to the development of various human cancers.14
Among these GATA factors, GATA3 is expressed in mammary gland epithelium and plays a role as a key factor in the development of mammary epithelium.15 GATA3 was positive in 77–95% of estrogen receptor (ER)-positive breast carcinoma and was reported as a marker to determine response to endocrine therapy for breast cancer patients.16 However, significance of other GATA factors has remained largely unknown in breast carcinoma. Therefore, in the present study, we first evaluated expression profiles of six GATA factors in breast carcinoma tissues based on microarray data and demonstrated that GATA4 was closely correlated with recurrence in patients. Previously, Bertucci et al.17 reported an association between GATA4 immunoreactivity and HER2 status in breast carcinoma, but other clinicopathological features of GATA4, including whether it can represent a prognostic factor in breast cancer patients, have not yet been examined to the best of our knowledge. Therefore, in the present study we immunolocalized GATA4 in human breast carcinoma to clarify its significance.
Materials and Methods
Patients and tissues
Two sets of tissue specimens were evaluated in the present study. In the first set, 20 specimens of invasive ductal carcinoma (IDC) of the breast were obtained from women (age, 40–74 years) who underwent surgical treatment from 2001 or 2002 in the Department of Surgery, Tohoku University Hospital, Sendai, Japan. Among these, 18 patients received endocrine therapy and seven patients received adjuvant chemotherapy after surgery. Disease-free survival was defined as the time from surgery to the date of the first locoregional recurrence or first distant metastasis within the follow-up time after surgery (range, 8–137 months). These specimens were stored at −80°C for microarray analysis. Specimens fixed in 10% formalin and embedded in paraffin wax were available in 17 cases and these were used for immunohistochemistry for GATA4.
In the second set, 48 specimens of pure ductal carcinoma in situ (DCIS) of the breast and 163 specimens of IDC were obtained from female Japanese patients who underwent surgical treatment from 1998 to 2005 for DCIS and 1995 to 1999 for IDC in the Department of Surgery, Tohoku University Hospital, Sendai, Japan. The patients did not receive adjuvant therapy before surgery. A review of the charts of IDC patients revealed that 116 patients received adjuvant endocrine therapy and 111 patients received adjuvant chemotherapy following surgery. The clinical outcome was evaluated by disease-free and breast cancer-specific survival of stages I–III IDC patients according to a previous report18 and the mean follow-up time was 115 months (range, 1–175 months). Breast cancer-specific survival was defined as the time from surgery to death from the breast cancer. All specimens had been fixed in 10% formalin and embedded in paraffin wax.
Research protocols for the present study were approved by the Ethics Committee at Tohoku University School of Medicine.
Laser capture microdissection/microarray analysis
Gene expression profiles of breast carcinoma cells in the first set (n = 20) were examined using microarray analysis. Part of the gene expression profile data was assembled in our previous study.19,20 Briefly, approximately 5000 breast carcinoma cells were laser transferred from the frozen section and total RNA was subsequently extracted. Sample preparation and processing were performed as described in the Affymetrix GeneChip Expression Analysis Manual (Affymetrix, Inc., Santa Clara, CA, USA), with the exception that the labeled cRNA samples were hybridized to the complete human U133 GeneChip set (Affymetrix, Inc.), containing U133A (22 215 genes) and U133B (22 577 genes). In the present study we focused on expression of six GATA factor genes.
Immunohistochemistry
Goat polyclonal antibody for GATA4 (sc-1237) was purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Monoclonal antibodies for ER (ER1D5), progesterone receptor (PR) (MAB429) and Ki-67 (MIB1) were purchased from Immunotech (Marseille, France), Chemicon (Temecula, CA, USA) and DAKO (Carpinteria, CA, USA), respectively. Rabbit polyclonal antibody for HER2 (A0485) was obtained from DAKO.
A Histofine Kit (Nichirei Bioscience, Tokyo, Japan), which uses the streptavidin-biotin amplification method, was used in the present study. Antigen retrieval was performed by heating the slides in an autoclave at 120°C for 5 min in citric acid buffer (pH 6.0) for these antibodies. The antigen–antibody complex was visualized with 3,3′-diaminobenzidine and counterstained with hematoxylin. We used human ovarian tissue as a positive control21 and normal goat IgG instead of the primary antibody as a negative control for GATA4 immunostaining.
Scoring of immunoreactivity and statistical analysis
GATA4 immunoreactivity was detected in the nuclei of breast carcinoma cells and the cases that had more than 10% of the positive carcinoma cells were considered positive for GATA4 status. Immunoreactivity for ER, PR and Ki-67 was detected in the nuclei and was evaluated in more than 1000 carcinoma cells for each case. Subsequently, the percentage of immunoreactivity (labeling index [LI]) was determined. Cases with an ER LI of more than 1% were considered ER-positive breast carcinoma according to a previous report.22 HER2 immunoreactivity was evaluated according to the grading system proposed in the HercepTest (DAKO) and strongly circumscribed membrane immunoreactivity of HER2 present in more than 10% carcinoma cells (score 3+) was considered positive.
An association between immunohistochemical GATA4 status and clinicopathological factors was evaluated using the Student's t-test or a cross-table using the Chi-squared test. Disease-free and breast cancer-specific survival curves were generated according to the Kaplan–Meier method and statistical significance was calculated using the log-rank test. Uni- and multivariate analyses were evaluated using a proportional hazard model (Cox). P-values < 0.05 were considered significant in the present study.
Results
Gene expression profiles of GATA factors associated with recurrence in IDC patients
First we examined associations between gene expression of GATA factors and recurrence of 20 IDC cases using microarray analysis. The median with a min–max value of signal intensity of each GATA factor gene was as follows: GATA1, 44 (13–119); GATA2, 130 (64–230); GATA3, 573 (104–1650); GATA4, 18 (9–98); GATA5, 6 (3–87); and GATA6, 57 (7–134). As shown in Figure1, when we classified these cases into two groups according to the median value, GATA4 was significantly associated with an increased incidence of recurrence (P = 0.031) (Fig.1d). GATA1 tended to link to the increased recurrence (P = 0.18) (Fig.1a), while GATA2 tended to link to decreased recurrence (P = 0.13) (Fig.1b), but these did not reach statistical significance. GATA3 (Fig.1c), GATA5 (Fig.1e) and GATA6 (Fig.1f) were not associated with recurrence in patients in the present study (P = 0.50, P = 0.55 and P = 0.76, respectively).
Figure 1.
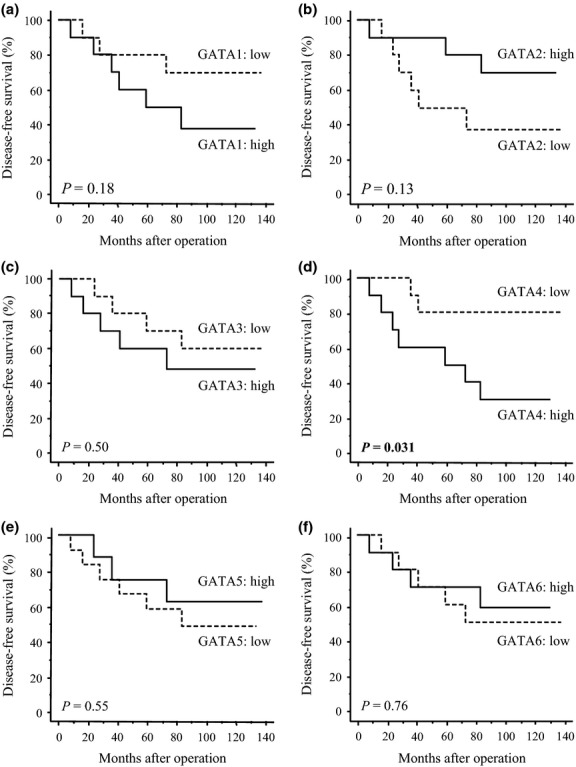
Association between expression of six GATA factor genes ([a] GATA1; [b] GATA2; [c] GATA3; [d] GATA4; [e] GATA5; and [f] GATA6) and recurrence of invasive ductal carcinoma of the breast using the Kaplan–Meier method (n = 20). The cases were categorized into two groups according to the median value of the signal intensity obtained from the microarray (i.e. high group [solid line] and low group [dashed line]). Statistical analysis was evaluated using the log-rank test. P-values < 0.05 were considered significant and are shown in bold.
Associations of the expression levels among these GATA factor genes are summarized in Table1. Inverse associations were detected between GATA2 and GATA5 expression levels (P = 0.048) and between GATA3 and GATA6 expression levels (P = 0.021). GATA1 tended to be positively associated with GATA2 (P = 0.084) and GATA6 (P = 0.087), and GATA2 tended to be associated with GATA6 (P = 0.13), although these did not reach statistical significance. GATA4 tended to be inversely associated with GATA3 (P = 0.18), but it was not associated with other GATA factors.
Table 1.
Association among expression levels of six GATA factor genes in 20 invasive ductal carcinoma of the breast cases
| GATA2 | GATA3 | GATA4 | GATA5 | GATA6 | |
|---|---|---|---|---|---|
| GATA1 | 0.084 (r = 0.40) | 0.80 | 0.53 | 0.34 | 0.087 (r = 0.39) |
| GATA2 | 0.44 | 0.45 | 0.048 (r = −0.45) | 0.13 (r = 0.35) | |
| GATA3 | 0.18 (r = −0.31) | 0.50 | 0.021 (r = −0.51) | ||
| GATA4 | 0.49 | 0.26 | |||
| GATA5 | 0.29 |
Data are presented as P-values. Statistical analysis was performed using correlation coefficient (r) and regression equation. P-values < 0.05 were considered significant and are shown in bold.
GATA4 immunolocalization in human breast carcinoma
GATA4 immunoreactivity was detected in the nuclei of carcinoma cells in both DCIS (Fig.2a) and IDC (Fig.2b) tissues, but was negative in non-neoplastic mammary glands and stroma (Fig.2c). In the positive control, GATA4 was immunolocalized in the ovarian antral follicle (Fig.2d), as reported previously.21 When we immunolocalized GATA4 in 17 IDC cases using the microarray analysis, the median value of GATA4 signal intensity was 1.8-fold higher in GATA4-positive cases (n = 7) than GATA4-negative cases (n = 10), although the P-value did not reach significance (P = 0.071) (Fig.2e).
Figure 2.
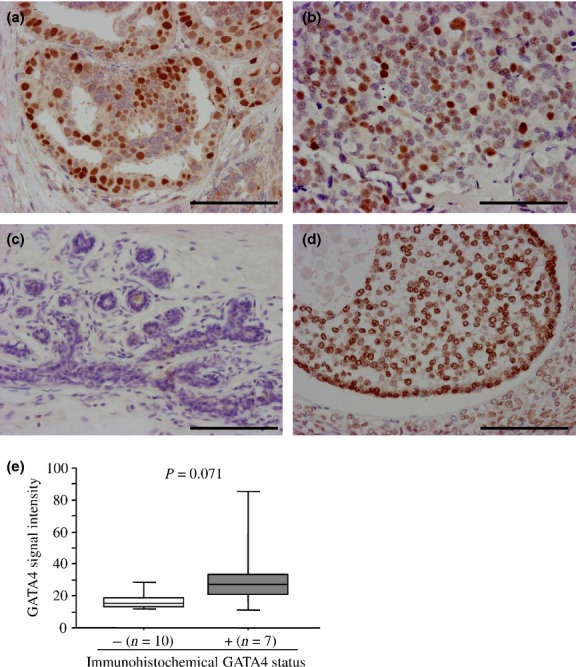
Immunohistochemistry for GATA4 in breast carcinoma. (a, b) GATA4 immunoreactivity was detected in the nucleus of carcinoma cells in ductal carcinoma in situ (DCIS) (a) and invasive ductal carcinoma (IDC) (b) tissues. (c) GATA4 immunoreactivity was not detected in the non-neoplastic mammary epithelium or stroma. (d) In the positive control section, GATA4 immunoreactivity was detected in granulosa cells of the antral follicle in the ovary. Bar, 100 μm, respectively. (e) Association between immunohistochemical GATA4 status and the signal intensity of the GATA4 gene obtained from microarray (n = 17). Data are represented as a box and whisker plot (open box, GATA4-negative group; and gray box, GATA4-positive group). The median value is represented by a horizontal line in each box and the 75th (upper margin) and 25th (lower margin) percentiles of the values are demonstrated. The upper and lower bars indicated the 90th and 10th percentiles, respectively. Statistical analysis was performed using the Mann–Whitney U-test.
Associations between immunohistochemical GATA4 status and clinicopathological parameters in DCIS are shown in Table2. The number of GATA4-immunopositive breast carcinomas was 13 out of 48 (27%) DCIS cases. GATA4 status was significantly associated with nuclear grade (P = 0.0085) and van Nuys classification (P = 0.026), while no significant association was detected between GATA4 status and patients' age, menopausal status, comedo necrosis, ER status and PR LI.
Table 2.
Association between immunohistochemical GATA4 status and clinicopathological parameters in 48 ductal carcinoma in situ of the breast cases
| GATA4 status |
P-value | ||
|---|---|---|---|
| + (n = 13) | − (n = 35) | ||
| Age† (years) | 59.9 ± 2.6 | 59.4 ± 1.7 | 0.86 |
| Menopausal status | |||
| Premenopausal | 3 | 6 | 0.64 |
| Postmenopausal | 10 | 29 | |
| Nuclear grade | |||
| 1 and 2 | 7 | 31 | 0.0085 |
| 3 | 6 | 4 | |
| Comedo necrosis | |||
| Absent | 5 | 10 | 0.51 |
| Present | 8 | 25 | |
| van Nuys classification | |||
| 1 | 3 | 9 | 0.026 |
| 2 | 4 | 22 | |
| 3 | 6 | 4 | |
| ER status | |||
| Positive | 12 | 33 | 0.80 |
| Negative | 1 | 2 | |
| PR LI† (%) | 45.4 ± 9.3 | 43.3 ± 5.2 | 0.84 |
Data are presented as mean ± SEM. All other values represent the number of cases. Statistical analysis was performed using the Student's t-test or a cross-table using the Chi-squared test. P-values < 0.05 were considered significant and are shown in bold. ER, estrogen receptor; LI, labeling index; PR, progesterone receptor.
Associations between GATA4 status and various clinicopathological parameters in IDC are summarized in Table3. Of 163 IDC cases examined in the present study, 51 cases (31%) were GATA4 positive. GATA4 status was positively associated with distant metastasis (P = 0.020), histological grade (P = 0.011) and HER2 status (P = 0.0022), while it was inversely correlated with PR LI (P = 0.0029). In contrast, no significant association was detected between GATA4 status and other factors such as patients' age, menopausal status, stage, pathological T factor (pT), lymph node metastasis, ER status and Ki-67 LI.
Table 3.
Association between GATA4 status and clinicopathological parameters in 163 invasive ductal carcinoma of the breast cases
| GATA4 status |
P-value | ||
|---|---|---|---|
| + (n = 51) | − (n = 112) | ||
| Age† (years) | 53.2 ± 1.5 | 55.3 ± 1.2 | 0.30 |
| Menopausal status | |||
| Premenopausal | 19 | 43 | 0.89 |
| Postmenopausal | 32 | 69 | |
| Stage | |||
| I | 10 | 31 | 0.12 |
| II | 23 | 53 | |
| III | 6 | 17 | |
| IV | 12 | 11 | |
| Pathological T factor (pT) | |||
| pT1 | 15 | 42 | 0.31 |
| pT2–4 | 36 | 70 | |
| Lymph node metastasis | |||
| Positive | 26 | 46 | 0.24 |
| Negative | 25 | 66 | |
| Distant metastasis | |||
| Positive | 12 | 11 | 0.020 |
| Negative | 39 | 101 | |
| Histological grade | |||
| 1 (well) | 6 | 34 | 0.011 |
| 2 (moderate) | 22 | 49 | |
| 3 (poor) | 23 | 29 | |
| ER status | |||
| Positive | 39 | 93 | 0.32 |
| Negative | 12 | 19 | |
| PR LI† (%) | 18.6 ± 3.6 | 33.9 ± 3.0 | 0.0029 |
| HER2 status | |||
| Positive | 18 | 16 | 0.0022 |
| Negative | 33 | 96 | |
| Ki-67 LI† (%) | 20.5 ± 1.9 | 19.0 ± 1.8 | 0.61 |
Data are presented as mean ± SEM. All other values represent the number of cases. Statistical analysis was performed using the Student's t-test or a cross-table using the Chi-squared test. P-values < 0.05 were considered significant and are shown in bold. ER, estrogen receptor; LI, labeling index; PR, progesterone receptor.
Association between GATA4 status and clinical outcome of IDC patients
As demonstrated in Figure3(a), GATA4 status was significantly associated with an increased incidence of recurrence in stage I–III patients (n = 140) (P = 0.0069 using the log-rank test). Association between GATA4 status and breast cancer-specific survival is summarized in Figure3(b) and a significant association was detected between GATA4 status and an adverse clinical outcome of patients (P = 0.0013 using the log-rank test). Interestingly, this association was also significant (P = 0.0032) in stage IV patients (n = 23) (data not shown). A tendency between GATA4 status and a worse prognosis was observed regardless of lymph node status in stage I–III cases (lymph node-negative group: P = 0.19 for disease-free survival [data not shown] and P = 0.0053 for breast cancer-specific survival [Fig.3c]; lymph node-positive group: P = 0.017 for disease-free survival [data not shown] and P = 0.067 for breast cancer-specific survival [data not shown]). Associations between GATA4 status and the clinical outcome of patients according to ER and HER2 status are shown in Figures S1 and S2, respectively.
Figure 3.
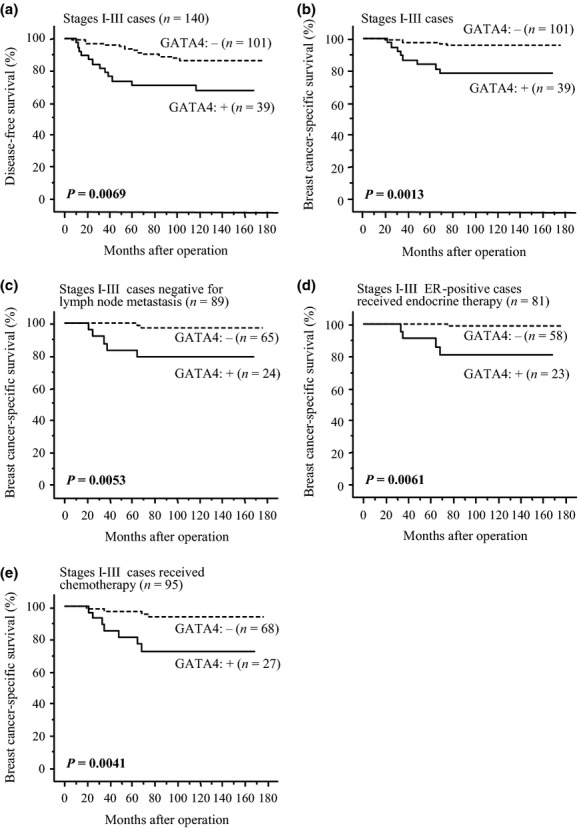
Disease-free (a) and breast cancer-specific survival (b–e) of stage I–III patients according to GATA4 status. The solid line shows GATA4-positive cases and the dashed line shows GATA4-negative cases. (a, b) Total cases (n = 140); (c) cases negative for lymph node metastasis (n = 89); (d) estrogen receptor (ER)-positive cases that received adjuvant endocrine therapy following surgery (n = 81); and (e) cases that received adjuvant chemotherapy after surgery (n = 95). Statistical analysis was performed using the log-rank test. P-values < 0.05 were considered significant and are shown in bold.
Eighty-one patients received adjuvant endocrine therapy after surgery in stage I–III ER-positive cases in the present study and GATA4 status was marginally (P = 0.064) associated with an increased risk of recurrence (data not shown) and significantly (P = 0.0061) associated with breast cancer-specific survival (Fig.3d) in these patients. GATA4 status was also significantly associated with a worse prognosis in stage I–III patients who received adjuvant chemotherapy (n = 95) (P = 0.0015 for disease-free survival [data not shown] and P = 0.0041 for breast cancer-specific survival [Fig.3e]).
Results of univariate analysis of disease-free survival using Cox (Table4), lymph node metastasis, GATA4 status, HER2 status and PR LI were demonstrated to be significant prognostic parameters for disease-free survival in stage I–III patients (n = 140). A multivariate analysis revealed that only lymph node metastasis (P = 0.0092) and GATA4 status (P = 0.047) were independent prognostic factors with relative risks over 1.00. In the univariate analysis for breast cancer-specific survival (Table5), GATA4 status (P = 0.0044), histological grade (P = 0.0053), Ki-67 LI (P = 0.026) and HER2 status (P = 0.047) were significant prognostic variables in stage I-III patients (n = 140), and a subsequent multivariate analysis revealed that only GATA4 status was an independent prognostic factor with a relative risk over 1.00 (P = 0.0094).
Table 4.
Univariate and multivariate analyses of disease-free survival in stage I–III breast cancer patients (n = 140)
| Variable | Univariate |
Multivariate |
||
|---|---|---|---|---|
| P-value | Relative risk (95% CI) | P-value | Relative risk (95% CI) | |
| Lymph node metastasis (positive/negative) | 0.0055 | 3.2 (1.4–7.4) | 0.0092 | 3.1 (1.3–7.1) |
| GATA4 status (positive/negative) | 0.010 | 2.9 (1.3–6.4) | 0.047 | 2.3 (1.0–5.3) |
| HER2 status (positive/negative) | 0.034 | 2.5 (1.1–5.9) | 0.19 | 1.9 (0.7–4.8) |
| PR LI (0–95%) | 0.044 | 1.0 (0.9–1.0) | 0.44 | 1.0 (1.0–1.0) |
| Adjuvant endocrine therapy (positive/negative) | 0.13 | 0.5 (0.2–1.2) | ||
| pT (pT1/pT2–4) | 0.23 | 0.6 (0.2–1.4) | ||
| Adjuvant chemotherapy (positive/negative) | 0.28 | 1.8 (0.6–5.4) | ||
| Ki-67 LI (0–82%) | 0.48 | 1.0 (1.0–1.0) | ||
| ER status (positive/negative) | 0.72 | 0.8 (0.3–2.2) | ||
| Histological grade (1,2/3) | 0.77 | 0.9 (0.4–2.1) | ||
Statistical analysis was performed using the proportional hazard model (Cox). Data considered significant (P < 0.05) in the univariate analyses are shown in bold and these were examined in the multivariate analyses. CI, confidence interval; ER, estrogen receptor; LI, labeling index; PR, progesterone receptor; pT, pathological T factor.
Table 5.
Univariate and multivariate analyses of breast cancer-specific survival in stage I–III breast cancer patients (n = 140)
| Variable | Univariate |
Multivariate |
||
|---|---|---|---|---|
| P-value | Relative risk (95% CI) | P-value | Relative risk (95% CI) | |
| GATA4 status (positive/negative) | 0.0044 | 5.7 (1.7–19.0) | 0.0094 | 2.3 (1.0–5.3) |
| Histological grade (1,2/3) | 0.0053 | 0.2 (0.06–0.06) | 0.15 | 0.4 (0.1–1.5) |
| Ki-67 LI (0–82%) | 0.026 | 1.0 (1.0–1.1) | 0.20 | 1.0 (1.0–1.1) |
| HER2 status (positive/negative) | 0.047 | 3.2 (1.0–10.1) | 0.78 | 1.2 (0.3–4.3) |
| PR LI (95–0%) | 0.10 | 1.0 (1.0–1.0) | ||
| Lymph node metastasis (positive/negative) | 0.11 | 2.5 (0.8–8.0) | ||
| pT (pT1/pT2–4) | 0.11 | 0.3 (0.1–1.3) | ||
| Adjuvant chemotherapy (positive/negative) | 0.18 | 4.1 (0.5–31.7) | ||
| ER status (negative/positive) | 0.19 | 0.4 (0.1–1.5) | ||
| Adjuvant endocrine therapy (positive/negative) | 0.35 | 0.6 (0.2–1.9) | ||
Statistical analysis was performed using the proportional hazard model (Cox). Data considered significant (P < 0.05) in the univariate analyses are shown in bold and these were examined in the multivariate analyses. CI, confidence interval; ER, estrogen receptor; LI, labeling index; PR, progesterone receptor; pT, pathological T factor.
Discussion
Results of the microarray analysis in the present study revealed that the GATA4 expression level was significantly associated with increased recurrence in IDC patients. A similar tendency was detected in GATA1, although the P-value did not reach significance, while GATA2 expression tended to be associated with a better prognosis. Among the GATA factors, Boidot et al.23 reported that GATA1 was overexpressed in breast carcinoma and possibly associated with tumor aggressiveness, which is consistent with our finding. The role of GATA2 is in dispute in breast carcinoma and Acosta et al.24 showed that GATA2 expression was decreased in breast carcinoma tissues relative to normal breast tissues, whereas Wang et al.25 reported that GATA2 was increased in breast carcinoma and negatively regulates PTEN (phosphatase and tensin homolog deleted on chromosome 10) transcription. Although GATA3 was the most abundantly expressed in breast carcinoma among the GATA factors in the present study, these data suggest that other GATA factors may also play roles in breast carcinoma. GATA4 was the most pronouncedly linked to recurrence in breast carcinoma patients, but to the best of our knowledge its clinicopathological significance has remained largely unknown.
In the present study, GATA4 immunoreactivity was detected in 27% of DCIS, which is generally regarded as a precursor lesion of IDC, and 31% of IDC cases, whereas it was negative in morphologically normal mammary glands. In previous studies, GATA4 immunoreactivity was detected in several human malignancies, such as ovarian carcinoma (12%26), breast carcinoma (27%17), glioblastoma of the brain (42%27), pancreatic carcinoma (68%28) and gastric carcinoma (93%29). Loss of GATA4 expression by the promoter methylation was reported in several carcinomas30,31 and GATA4 is suggested to function as a tumor suppressor in some aspects.32 In contrast, GATA4 was frequently expressed in neuroblastoma, but was negative in the developing nervous system.33 GATA4 promoter demethylation was induced by several factors including the mitogen-activated protein kinase pathway34 and MYC,35 and GATA4 promoted initiation of adrenocortical neoplasms in mice.36 The results in the present study suggest that GATA4 is overexpressed in breast carcinoma compared with normal breast tissues and plays important roles in breast carcinoma from an early stage. Interestingly, Karafin et al.28 found GATA4-positive pancreatic carcinoma was significantly higher in female than male patients and suggested functions of GATA4 as a gender-specific regulator.
The results in the present study demonstrated that GATA4 status was significantly associated with nuclear grade in DCIS and histological grade in IDC cases. Two different models have been proposed to explain the possible mechanisms of transition from DCIS to IDC. In the first model, low-grade DCIS lesions are considered to progress to high-grade DCIS lesions that then become IDC (i.e. linear progression theory)37,38 and in the latter model of the hypothesis, low-grade DCIS lesions progress to low-grade IDC and high-grade DCIS lesions progress to high-grade IDC (parallel disease theory).39,40 Accumulating data including chromosomal-alteration studies support the parallel disease theory41,42 and a great majority of molecular alterations detected in breast carcinoma can be detected already in DCIS.43,44 Taken together with similar GATA4 immunopositivities of DCIS (27%) and IDC (31%) cases examined, the present results seem to be more compatible with the parallel disease theory. Because GATA4 is a known lineage selector gene, GATA4 overexpression might be associated with dedifferentiation and higher-grade malignancy in breast carcinoma.
In the present study, GATA4 status was significantly associated with HER2 status and distant metastasis in IDC cases. HER2 plays important roles in the proliferation and metastasis of breast carcinoma.45 Previously, Bertucci et al.17 examined gene expression profiles of breast carcinomas and identified GATA4 as one of the 29 overexpressed genes differentially expressed in breast carcinomas associated with HER2 overexpression. They also showed a positive association between GATA4 immunoreactivity and HER2 status in breast carcinoma tissues, which is in good agreement with our finding. In addition, Hua et al.46 reported that the GATA4 gene was activated by HER2, whereas HER2 expression was repressed by GATA4 through its direct binding to the regulatory sequences, indicating a direct functional link between GATA4 and HER2 in breast carcinoma. In contrast, Barbosa et al.47 showed that GATA4 mRNA expression was more abundant in metastasizing adrenocortical tumors than non-metastasizing tumors and very recently Castro et al.35 demonstrated that GATA4 was necessary for MYC-induced metastasis in lung adenocarcinoma. Therefore, it is suggested that GATA4 is involved in oncogene-dependent signaling including HER2 in the development of distant metastasis with breast carcinoma. Considering that we did not detect a significant association between GATA4 status and lymph node metastasis in the present study, GATA4 might play an important role in the processes of hematogenous spread.
In the present study, GATA4 status was significantly associated with van Nuys classification in DCIS cases. The van Nuys classification was reported to be significantly associated with local recurrence of DCIS and has been established as a potent prognostic classification for DCIS patients.48 GATA4 status was significantly associated with recurrence and worse prognosis in IDC patients and a similar tendency was also detected in patients who received adjuvant therapies after surgery. Moreover, results of our present multivariate analyses clearly demonstrated that GATA4 status was an independent prognostic factor for both recurrence and breast cancer-specific survival in IDC patients. Therefore, GATA4 status is suggested to be a prognostic factor rather than a predictive marker for adjuvant therapies in breast carcinoma patients. Previously, Anttonen et al.21 reported that GATA4 expression was positively correlated with recurrence of ovarian granulosa cell tumors, which is consistent with the results of the present study. GATA4 has been reported to serve as a survival factor in carcinoma cells by regulating anti-apoptotic factors such as Bcl-2 and Bcl-x49,50 and overexpression of GATA4 prevented cardiac myocyte apoptosis induced by antracyclines including doxorubicin, which is frequently used in breast carcinoma.51 No information is available about the effects of endocrine therapy on GATA4 functions in breast carcinoma to our knowledge. However, considering that we did not find an association between GATA4 status and ER status in breast carcinoma and GATA4 expression was regulated by estrogen in osteoblasts but not in breast carcinoma cells,52 GATA4 might not directly regulate estrogen actions in breast carcinoma, which is different from GATA3.16 Because GATA4 possibly regulates a variety of biological functions of breast carcinoma cells as described in this section, residual carcinoma cells following surgical treatment in GATA4-positive breast carcinomas could still have the potential to rapidly recur despite adjuvant therapy. Further examinations are required to clarify the molecular functions and possible new therapeutic potential of GATA4 in human breast carcinoma. Replication studies with different sets and prospective studies are also needed to confirm the significance of GATA4 in breast carcinoma.
In summary, we examined the expression profiles of GATA factor genes in IDC cases using microarray analysis and demonstrated that GATA4 expression was closely associated with recurrence. Subsequent immunohistochemical analyses revealed that GATA4 immunoreactivity was detected in 27% of DCIS and 31% of IDC cases, and was significantly associated with nuclear grade and van Nuys classification of DCIS and distant metastasis, histological grade, PR LI and HER2 status in IDC. Multivariate analysis further demonstrated that GATA4 status was an independent prognostic factor in IDC patients. These findings suggest that GATA4 plays important roles in the progression of breast carcinoma from an early stage and immunohistochemical GATA4 status is a potent prognostic factor in breast cancer patients.
Disclosure Statement
The authors have no conflict of interest.
Funding Information
None declared.
Supporting Information
Additional supporting information may be found in the online version of this article:
Association between GATA4 status and disease-free or breast cancer-specific survival in stage I–III patients according to estrogen receptor status.
Association between GATA4 status and disease-free or breast cancer-specific survival in stage I–III patients according to HER2 status.
References
- 1.Hüsemann Y, Geigl JB, Schubert F, et al. Systemic spread is an early step in breast cancer. Cancer Cell. 2008;13:58–68. doi: 10.1016/j.ccr.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 2.Paik S, Shak S, Tang G, et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med. 2004;351:2817–26. doi: 10.1056/NEJMoa041588. [DOI] [PubMed] [Google Scholar]
- 3.Tevaarwerk AJ, Gray RJ, Schneider BP, et al. Survival in patients with metastatic recurrent breast cancer after adjuvant chemotherapy: little evidence of improvement over the past 30 years. Cancer. 2013;119:1140–8. doi: 10.1002/cncr.27819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shimizu R, Yamamoto M. Gene expression regulation and domain function of hematopoietic GATA factors. Semin Cell Dev Biol. 2005;16:129–36. doi: 10.1016/j.semcdb.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 5.Kaneko H, Shimizu R, Yamamoto M. GATA factor switching during erythroid differentiation. Curr Opin Hematol. 2010;17:163–8. doi: 10.1097/MOH.0b013e32833800b8. [DOI] [PubMed] [Google Scholar]
- 6.Yamamoto M, Ko LJ, Leonard MW, et al. Activity and tissue-specific expression of the transcription factor NF-E1 multigene family. Genes Dev. 1990;4:1650–62. doi: 10.1101/gad.4.10.1650. [DOI] [PubMed] [Google Scholar]
- 7.Arceci RJ, King AA, Simon MC, Orkin SH, Wilson DB. Mouse GATA-4: a retinoic acid-inducible GATA-binding transcription factor expressed in endodermally derived tissues and heart. Mol Cell Biol. 1993;13:2235–46. doi: 10.1128/mcb.13.4.2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kelley C, Blumberg H, Zon LI, Evans T. GATA-4 is a novel transcription factor expressed in endocardium of the developing heart. Development. 1993;118:817–27. doi: 10.1242/dev.118.3.817. [DOI] [PubMed] [Google Scholar]
- 9.Laverriere AC, MacNeill C, Mueller C, et al. GATA-4/5/6, a subfamily of three transcription factors transcribed in developing heart and gut. J Biol Chem. 1994;269:23177–84. [PubMed] [Google Scholar]
- 10.Patient RK, McGhee JD. The GATA family (vertebrates and invertebrates) Curr Opin Genet Dev. 2002;12:416–22. doi: 10.1016/s0959-437x(02)00319-2. [DOI] [PubMed] [Google Scholar]
- 11.Suzuki M, Kobayashi-Osaki M, Tsutsumi S, et al. GATA factor switching from GATA2 to GATA1 contributes to erythroid differentiation. Genes Cells. 2013;18:921–33. doi: 10.1111/gtc.12086. [DOI] [PubMed] [Google Scholar]
- 12.Zhou P, He A, Pu WT. Regulation of GATA4 transcriptional activity in cardiovascular development and disease. Curr Top Dev Biol. 2012;100:143–69. doi: 10.1016/B978-0-12-387786-4.00005-1. [DOI] [PubMed] [Google Scholar]
- 13.Chlon TM, Crispino JD. Combinatorial regulation of tissue specification by GATA and FOG factors. Development. 2012;139:3905–16. doi: 10.1242/dev.080440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bresnick EH, Katsumura KR, Lee HY, Johnson KD, Perkins AS. Master regulatory GATA transcription factors: mechanistic principles and emerging links to hematologic malignancies. Nucleic Acids Res. 2012;40:5819–31. doi: 10.1093/nar/gks281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kouros-Mehr H, Slorach EM, Sternlicht MD, Werb Z. GATA-3 maintains the differentiation of the luminal cell fate in the mammary gland. Cell. 2006;127:1041–55. doi: 10.1016/j.cell.2006.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fang SH, Chen Y, Weigel RJ. GATA-3 as a marker of hormone response in breast cancer. J Surg Res. 2009;157:290–5. doi: 10.1016/j.jss.2008.07.015. [DOI] [PubMed] [Google Scholar]
- 17.Bertucci F, Borie N, Ginestier C, et al. Identification and validation of an ERBB2 gene expression signature in breast cancers. Oncogene. 2004;23:2564–75. doi: 10.1038/sj.onc.1207361. [DOI] [PubMed] [Google Scholar]
- 18.Suzuki S, Takagi K, Miki Y, et al. Nucleobindin 2 in human breast carcinoma as a potent prognostic factor. Cancer Sci. 2012;103:136–43. doi: 10.1111/j.1349-7006.2011.02119.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nagasaki S, Suzuki T, Miki Y, et al. 17Beta-hydroxysteroid dehydrogenase type 12 in human breast carcinoma: a prognostic factor via potential regulation of fatty acid synthesis. Cancer Res. 2009;69:1392–9. doi: 10.1158/0008-5472.CAN-08-0821. [DOI] [PubMed] [Google Scholar]
- 20.Takagi K, Miki Y, Shibahara Y, et al. BUB1 immunolocalization in breast carcinoma: its nuclear localization as a potent prognostic factor of the patients. Horm Cancer. 2013;4:92–102. doi: 10.1007/s12672-012-0130-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anttonen M, Unkila-Kallio L, Leminen A, Butzow R, Heikinheimo M. High GATA-4 expression associates with aggressive behavior, whereas low anti-Müllerian hormone expression associates with growth potential of ovarian granulosa cell tumors. J Clin Endocrinol Metab. 2005;90:6529–35. doi: 10.1210/jc.2005-0921. [DOI] [PubMed] [Google Scholar]
- 22.Hammond ME, Hayes DF, Dowsett M, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer (unabridged version) Arch Pathol Lab Med. 2010;134:e48–72. doi: 10.5858/134.7.e48. [DOI] [PubMed] [Google Scholar]
- 23.Boidot R, Végran F, Jacob D, et al. The transcription factor GATA-1 is overexpressed in breast carcinomas and contributes to survivin upregulation via a promoter polymorphism. Oncogene. 2010;29:2577–84. doi: 10.1038/onc.2009.525. [DOI] [PubMed] [Google Scholar]
- 24.Acosta D, Suzuki M, Connolly D, et al. DNA methylation changes in murine breast adenocarcinomas allow the identification of candidate genes for human breast carcinogenesis. Mamm Genome. 2011;22:249–59. doi: 10.1007/s00335-011-9318-6. [DOI] [PubMed] [Google Scholar]
- 25.Wang Y, He X, Ngeow J, Eng C. GATA2 negatively regulates PTEN by preventing nuclear translocation of androgen receptor and by androgen-independent suppression of PTEN transcription in breast cancer. Hum Mol Genet. 2012;21:569–76. doi: 10.1093/hmg/ddr491. [DOI] [PubMed] [Google Scholar]
- 26.Cai KQ, Caslini C, Capo-chichi CD, et al. Loss of GATA4 and GATA6 expression specifies ovarian cancer histological subtypes and precedes neoplastic transformation of ovarian surface epithelia. PLoS ONE. 2009;4:e6454. doi: 10.1371/journal.pone.0006454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Agnihotri S, Wolf A, Munoz DM, et al. A GATA4-regulated tumor suppressor network represses formation of malignant human astrocytomas. J Exp Med. 2011;208:689–702. doi: 10.1084/jem.20102099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Karafin MS, Cummings CT, Fu B, Iacobuzio-Donahue CA. The developmental transcription factor Gata4 is overexpressed in pancreatic ductal adenocarcinoma. Int J Clin Exp Pathol. 2009;3:47–55. [PMC free article] [PubMed] [Google Scholar]
- 29.Yamamura N, Kishimoto T. Epigenetic regulation of GATA4 expression by histone modification in AFP-producing gastric adenocarcinoma. Exp Mol Pathol. 2012;93:35–9. doi: 10.1016/j.yexmp.2012.03.012. [DOI] [PubMed] [Google Scholar]
- 30.Guo M, Akiyama Y, House MG, et al. Hypermethylation of the GATA genes in lung cancer. Clin Cancer Res. 2004;10:7917–24. doi: 10.1158/1078-0432.CCR-04-1140. [DOI] [PubMed] [Google Scholar]
- 31.Hellebrekers DM, Lentjes MH, van den Bosch SM, et al. GATA4 and GATA5 are potential tumor suppressors and biomarkers in colorectal cancer. Clin Cancer Res. 2009;15:3990–7. doi: 10.1158/1078-0432.CCR-09-0055. [DOI] [PubMed] [Google Scholar]
- 32.Zheng R, Blobel GA. GATA transcription factors and cancer. Genes Cancer. 2010;1:1178–88. doi: 10.1177/1947601911404223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hoene V, Fischer M, Ivanova A, et al. GATA factors in human neuroblastoma: distinctive expression patterns in clinical subtypes. Br J Cancer. 2009;101:1481–9. doi: 10.1038/sj.bjc.6605276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liang Q, Wiese RJ, Bueno OF, et al. The transcription factor GATA4 is activated by extracellular signal-regulated kinase 1- and 2-mediated phosphorylation of serine 105 in cardiomyocytes. Mol Cell Biol. 2001;21:7460–9. doi: 10.1128/MCB.21.21.7460-7469.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Castro IC, Breiling A, Luetkenhaus K, et al. MYC-induced epigenetic activation of GATA4 in lung adenocarcinoma. Mol Cancer Res. 2013;11:161–72. doi: 10.1158/1541-7786.MCR-12-0414-T. [DOI] [PubMed] [Google Scholar]
- 36.Krachulec J, Vetter M, Schrade A, et al. GATA4 is a critical regulator of gonadectomy-induced adrenocortical tumorigenesis in mice. Endocrinology. 2012;153:2599–611. doi: 10.1210/en.2011-2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Carter CL, Corle DK, Micozzi MS, Schatzkin A, Taylor PR. A prospective study of the development of breast cancer in 16,692 women with benign breast disease. Am J Epidemiol. 1988;128:467–77. doi: 10.1093/oxfordjournals.aje.a114995. [DOI] [PubMed] [Google Scholar]
- 38.Lakhani SR, Chaggar R, Davies S, et al. Genetic alterations in ‘normal’ luminal and myoepithelial cells of the breast. J Pathol. 1999;189:496–503. doi: 10.1002/(SICI)1096-9896(199912)189:4<496::AID-PATH485>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 39.Sontag L, Axelrod DE. Evaluation of pathways for progression of heterogeneous breast tumors. J Theor Biol. 2005;232:179–89. doi: 10.1016/j.jtbi.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 40.Wiechmann L, Kuerer HM. The molecular journey from ductal carcinoma in situ to invasive breast cancer. Cancer. 2008;112:2130–42. doi: 10.1002/cncr.23430. [DOI] [PubMed] [Google Scholar]
- 41.Hwang ES, DeVries S, Chew KL, et al. Patterns of chromosomal alterations in breast ductal carcinoma in situ. Clin Cancer Res. 2004;10:5160–7. doi: 10.1158/1078-0432.CCR-04-0165. [DOI] [PubMed] [Google Scholar]
- 42.Irvine T, Fentiman IS. Biology and treatment of ductal carcinoma in situ. Expert Rev Anticancer Ther. 2007;7:135–45. doi: 10.1586/14737140.7.2.135. [DOI] [PubMed] [Google Scholar]
- 43.Nofech-Mozes S, Spayne J, Rakovitch E, Hanna W. Prognostic and predictive molecular markers in DCIS: a review. Adv Anat Pathol. 2005;12:256–64. doi: 10.1097/01.pap.0000184177.65919.5e. [DOI] [PubMed] [Google Scholar]
- 44.Burkhardt L, Grob TJ, Hermann I, et al. Gene amplification in ductal carcinoma in situ of the breast. Breast Cancer Res Treat. 2010;123:757–65. doi: 10.1007/s10549-009-0675-8. [DOI] [PubMed] [Google Scholar]
- 45.Freudenberg JA, Wang Q, Katsumata M, et al. The role of HER2 in early breast cancer metastasis and the origins of resistance to HER2-targeted therapies. Exp Mol Pathol. 2009;87:1–11. doi: 10.1016/j.yexmp.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hua G, Zhu B, Rosa F, et al. A negative feedback regulatory loop associates the tyrosine kinase receptor ERBB2 and the transcription factor GATA4 in breast cancer cells. Mol Cancer Res. 2009;7:402–14. doi: 10.1158/1541-7786.MCR-08-0175. [DOI] [PubMed] [Google Scholar]
- 47.Barbosa AS, Giacaglia LR, Martin RM, Mendonca BB, Lin CJ. Assessment of the role of transcript for GATA-4 as a marker of unfavorable outcome in human adrenocortical neoplasms. BMC Endocr Disord. 2004;4:3. doi: 10.1186/1472-6823-4-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Silverstein MJ, Poller DN, Waisman JR, et al. Prognostic classification of breast ductal carcinoma-in-situ. Lancet. 1995;345:1154–7. doi: 10.1016/s0140-6736(95)90982-6. [DOI] [PubMed] [Google Scholar]
- 49.Kyrönlahti A, Rämö M, Tamminen M, et al. GATA-4 regulates Bcl-2 expression in ovarian granulosa cell tumors. Endocrinology. 2008;149:5635–42. doi: 10.1210/en.2008-0148. [DOI] [PubMed] [Google Scholar]
- 50.Jääskeläinen M, Nieminen A, Pökkylä RM, et al. Regulation of cell death in human fetal and adult ovaries–role of Bok and Bcl-X(L) Mol Cell Endocrinol. 2010;330:17–24. doi: 10.1016/j.mce.2010.07.020. [DOI] [PubMed] [Google Scholar]
- 51.Kim Y, Ma AG, Kitta K, et al. Anthracycline-induced suppression of GATA-4 transcription factor: implication in the regulation of cardiac myocyte apoptosis. Mol Pharmacol. 2003;63:368–77. doi: 10.1124/mol.63.2.368. [DOI] [PubMed] [Google Scholar]
- 52.Miranda-Carboni GA, Guemes M, Bailey S. GATA4 regulates estrogen receptor-alpha-mediated osteoblast transcription. Mol Endocrinol. 2011;25:1126–36. doi: 10.1210/me.2010-0463. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Association between GATA4 status and disease-free or breast cancer-specific survival in stage I–III patients according to estrogen receptor status.
Association between GATA4 status and disease-free or breast cancer-specific survival in stage I–III patients according to HER2 status.


