Abstract
Temozolomide (TMZ), used to treat glioblastoma and malignant glioma, induces autophagy, apoptosis and senescence in cancer cells. We investigated fibrin glue (FG) as a drug delivery system for the local administration of high-concentration TMZ aimed at preventing glioma recurrence. Our high-power liquid chromatography studies indicated that FG containing TMZ (TMZ-FG) manifested a sustained drug release potential. We prepared a subcutaneous tumor model by injecting groups of mice with three malignant glioma cell lines and examined the antitumor effect of TMZ-FG. We estimated the tumor volume and performed immunostaining and immunoblotting using antibodies to Ki-67, cleaved caspase 3, LC3 and p16. When FG sheets containing TMZ (TMZ-FGS) were inserted beneath the tumors, their growth was significantly suppressed. In mice treated with peroral TMZ plus TMZ-FGS the tumors tended to be smaller than in mice whose tumors were treated with TMZ-FGS or peroral TMZ alone. The TMZ-FGS induced autophagy, apoptosis and senescence in subcutaneous glioma tumor cells. To assess the safety of TMZ-FG for normal brain, we placed it directly on the brain of living mice and stained tissue sections obtained in the acute and chronic phase immunohistochemically. In both phases, TMZ-FG failed to severely damage normal brain tissue. TMZ-FG may represent a safe new drug delivery system with sustained drug release potential to treat malignant glioma.
Keywords: Drug delivery system, fibrin glue, glioblastoma, malignant glioma, temozolomide
Glioblastoma multiforme (GBM) and anaplastic astrocytoma are primary malignant brain tumors with a poor prognosis. Glioblastoma multiforme accounts for 15.8% of primary brain and CNS tumors and for 54% of all gliomas.1 In a phase III randomized trial in patients with newly diagnosed GBM, radiation therapy with concurrent temozolomide (TMZ) was superior to radiation therapy alone in terms of overall survival. Currently, postoperative radiation therapy with concurrent TMZ chemotherapy is the standard treatment for patients with GBM. However, their median survival remains approximately 1–2 years.2,3
Temozolomide is an alkylating agent from the imidazotetrazine family. It induces various DNA adducts. Among them, O6-methylguanine is the most effective cytotoxin if not repaired by O6-methylguanine-DNA methyltransferase.4 DNA double-strand breaks induce various signaling proteins and pathways such as ataxia telangiectasia mutated (ATM) protein, death receptors and mitochondrial pathways.5–7
Fibrin glue (FG) is a human plasma product that mimics the final stage of blood coagulation. It changes into granulation tissue after tissue adhesion and then into collagen fibers; after 2 weeks, FG is almost completely replaced by collagen-rich granulation tissue.8 Fibrin glue is primarily applied to obtain hemostasis, sealing and adherence. In neurosurgery it is used as a sealant of the dura mater, for vascular anastomosis and to cap ventricles opened during brain tumor removal. Clinically, it has been reported to be safe for normal brain tissues.8–11 Fibrin glue has also been investigated as a delivery system for drugs, growth factors and as a gene delivery vector.12
In glioma removal surgery it is difficult to obtain an adequate margin in the normal brain. As 80% of GBM recur in the vicinity of the removal cavity, it is important to eradicate residual tumor cells around the cavity.13
In our in vitro experiments we analyzed the release of TMZ from FG. We then used FG as an in vivo drug delivery system to administer a high concentration of TMZ locally to glioblastoma tumor-bearing mice and assessed the antitumor potential of FG containing TMZ (TMZ-FG).
Materials and Methods
Chemicals
The TMZ was purchased from MSD Co. (Tokyo, Japan) and dissolved in distilled water. Fibrin glue (BOLHEAL), prepared by mixing solutions of fibrinogen and thrombin, was supplied by The Chemo-Sero-Therapeutic Research Institute (Kumamoto, Japan).
Glioblastoma cell line and human glioma-initiating cell lines
The human glioblastoma cell line U87MG was obtained from the American Type Culture Collection (Rockville, MD, USA). Anaplastic oligoastrocytoma (AOA) and GBM samples, obtained at Kumamoto University Hospital with the patients' consent and according to Research Ethics Committee guidelines, were used to establish the glioma cell lines. The glioma-initiating cells derived from AOA and GBM were named Kumamoto-glioma-initiating cells 1 (K-GIC1) and K-GIC2, respectively. The U87MG cells were cultured in DMEM/F-12 medium (Sigma Aldrich, St. Louis, MO, USA) supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin/amphotericin (Nacalaitesque, Kyoto, Japan). Samples from human tumors were dissociated with the Papain Dissociation System (Worthington Biochemical Corporation, Lakewood, NJ, USA). Dissociated cells were then cultured in serum-free medium with growth factor as previously described.14 All cells were incubated at 37°C in an atmosphere of 5% CO2. To confirm their tumor-forming potential, K-GIC1 and K-GIC2 cells were injected into the brain and flank of nude mice. Four weeks later, both cell lines had formed xenograft tumors at the injection sites. Pathologically they showed high cellularity and necrosis similar to the original tumor (Fig. S1).
Cell proliferation assay
Cell proliferation was determined using a WST-8 assay kit (Dojindo, Kumamoto, Japan). The U87MG cells, K-GIC1 and K-GIC2 were dissociated into single cells, seeded into the wells of 96-well plates and incubated with various concentrations of TMZ. Cell proliferation was measured every 24 h (0–72 h) according to the manufacturer's instructions.
High-performance liquid chromatography
Sample preparation
After mixing the TMZ solution with a thrombin solution, 0.5 mL of this solution and 0.5 mL of fibrinogen solution were admixed in 12-well plates (Corning Inc., New York, NY, USA) and left for a few minutes at room temperature until completely solidified. The wells were then filled with 1 mL of phosphate-buffered saline (PBS), which was exchanged every 24 h. Samples were collected at 24-h intervals (24–168 h). Sample preparation was as previously described.15 Sample aliquots (40 μL) were injected into HPLC columns for analysis.
Chromatography conditions
The HPLC system consisted of a LC-10ADvp pump and a PD-10vp UV/VIS absorbance detector (Shimadzu, Kyoto, Japan) set at 316 nm. It featured a 150 × 4.6-mm, 5-μm ZORBAX ODS column (Agilent Technologies, Santa Clara, CA, USA). The mobile phase consisted of 0.1% aqueous acetic acid-acetonitrile (90:10, v/v) and was delivered at 1.0 mL/min. The temperature was 40°C. Peak data were recorded with a chromatography management system (Shimadzu). A calibration curve was constructed using eight calibration standards prepared at concentrations of 0–2 mM TMZ. The amount of TMZ released from FG was based on the calibration curve standards.
Determination of the ratio of TMZ released from FG containing TMZ
The total amount of TMZ released from FG was the sum of TMZ released at 24-h intervals. The TMZ release ratio was calculated as:
Animal models
All mice were obtained from Charles River Laboratories (Yokohama, Japan) and all procedures were approved by the Animal Ethics Committee of Kumamoto University.
Transplantation of glioma cells to the flank of nude mice
We used 6-week-old female ICR-nu nu/nu mice. To prepare the subcutaneous tumor model, U87MG (2 × 106), K-GIC1 (5 × 105) and K-GIC2 (1 × 106) cells were suspended in 50 μL of PBS/BD Matrigel Matrix Growth Factor Reduced (BD Bioscience, Bedford, MA, USA) and injected into the flank of anesthetized mice.
Calculation of the subcutaneous tumor volume (TV)
The tumor size was measured every 2 days with calipers. When ulceration appeared on the surface of the tumor, the mice were killed. To calculate TV, we measured the greatest longitudinal (length) and transverse (width) diameter. The TV was calculated with the modified ellipsoidal formula:16
To calculate the relative TV we established day 6 as the standard because it was the day preceding TMZ treatment. We used the formula:
Preparation of FG sheet containing TMZ (TMZ-FGS)
We mixed 0.75 mL of thrombin solution containing TMZ with 0.75 mL of fibrinogen solution in six-well plates (Corning). The plates were left at room temperature until complete solidification was observed. There was no difference in the time required for solidification of FGS that did and did not contain TMZ.
Placement of FGS and administration of TMZ
Seven days after the subcutaneous transplantation of glioma cells, we randomly assigned the mice to six groups, with six mice in each group. For FGS placement, tumor-bearing mice were anesthetized, the skin over the tumor was cut and the FGS was inserted under the tumor. For peroral TMZ administration, TMZ (66 mg/kg) was dissolved in 100 μL of distilled water and then administered by oral gavage on post-transplantation days 7–11. Group 1 was the sham-operated control. Group 2 was FGS alone. In group 3 the inserted FGS contained 1 mM TMZ. Group 4 received only 100 μL of distilled water orally on post-transplantation days 7–11 and no FGS was inserted. Group 5 was treated with peroral TMZ only. Group 6 was treated with FGS containing 1 mM TMZ plus peroral TMZ.
Placement of FGS on the surface of the brain
We divided the 6-week-old female ICR mice into three groups, with three mice in each group. The first group was the sham-operated control. The second group was FGS without TMZ. The third group was FGS containing 1 mM TMZ. The mice were anesthetized and then we performed a 4-mm square craniotomy on the right side of the skull. The FGS was placed directly on the surface of the brain. The brains were removed two or 14 days after FGS placement.
Immunohistochemistry
The subcutaneous tumors and brains were formalin fixed overnight, embedded in paraffin and then cut into 4-μm-thick sections. Immunostaining was as previously described.17 The antibodies were rabbit anti-Ki-67 (1:100; Biocare Medical, Concord, CA, USA), rabbit anti-cleaved caspase 3 (1:200; Cell Signaling Technology, Danvers, MA, USA), mouse anti-p16 (1:50; Santa Cruz, Dallas, TX, USA), rabbit anti-microtubule-associated protein light chain 3 (LC3) (1:2000; Medical Biological Laboratories, Nagoya, Japan), rabbit anti-glial fibrillary acidic protein (GFAP) (1:1000; DAKO, Tokyo, Japan) and rabbit anti-cyclooxygenase 2 (COX-2) (1:50; Cayman Chemical, Ann Arbor, MI, USA). Some brain sections were dehydrated in 80% alcohol overnight at 37°C and stained with 0.1% cresyl violet for Nissl staining.
Western blot analysis
The subcutaneous tumors were divided into two parts (the side that was and the side that was not exposed to the FGS); the distance from the FGS margin was 5 mm. A piece from the FGS-exposed side was used for the extraction of protein. Western blotting was as described previously.18 The antibodies were rabbit anti-cleaved caspase 3 (1:500), rabbit anti-LC3 (1:5000), mouse anti-p16 (1:500) and mouse anti-β-tubulin (1:1000; Sigma Aldrich). Antibody binding was detected with the ECL kit (Amersham Biosciences, Buckinghamshire, UK) according to the manufacturer's instructions. The intensity of the bands was quantified using ImageJ (NIH Image analysis software v1.46, Bethesda, MD, USA) in a blinded fashion. For individual samples each value was corrected for the value of β-tubulin.
Statistical analysis
The values are shown as the mean ± SEM. Data were analyzed using anova followed by planned comparisons of multiple conditions or the unpaired two-tailed t-tests using graph pad prism version 5 software (Graph Pad Software, San Diego, CA, USA). A P < 0.05 was considered significant.
Results
Temozolomide inhibited glioma cell proliferation in a dose-dependent manner
The TMZ inhibited the proliferation of all cell lines in a dose-dependent manner (Fig.1a). At 1 mM TMZ, the glioma cell density and the number of colonies formed by glioma cells were decreased (Fig.1b).
Figure 1.
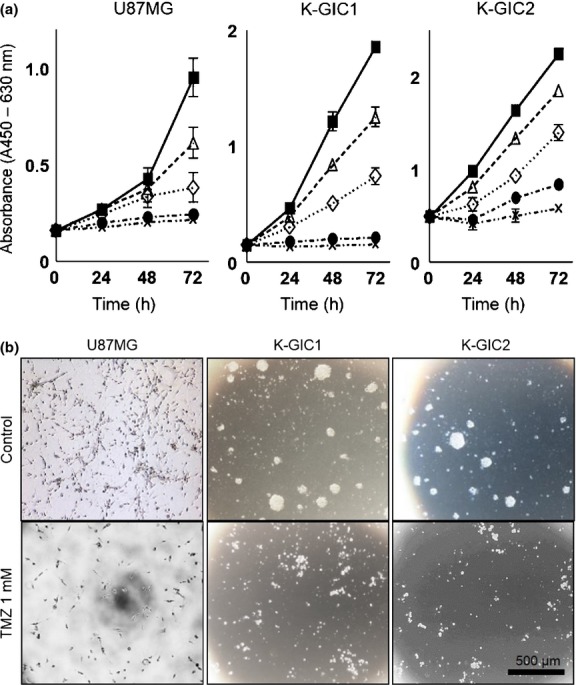
Dose-dependent effects of temozolomide (TMZ) on the growth of glioma cell lines. (a) Cell proliferation assay (n = 6). (▀), Control; (▵), 0.1 mM TMZ; (⋄), 0.4 mM TMZ; (•), 0.8 mM TMZ; (×), 1 mM TMZ. (b) Morphology of glioma cells incubated for 72 h with 1 mM TMZ and of the controls. Bar, 500 μm. K-GIC, Kumamoto-glioma-initiating cell.
Fibrin glue containing TMZ released TMZ gradually
We performed HPLC three times per sample to assay the release of TMZ from FGS containing 0.5 mM or 1 mM TMZ. The TMZ peak of the calibration curve standards and samples appeared at approximately 2.19 min (Fig.2a,b). The FGS containing 1 mM TMZ continued to release a greater amount of TMZ over the course of the 168-h observation period (Fig.2c). Up until 48 h the ratio of released TMZ was similar at the two TMZ concentrations; a significant difference was observed after 72 h (Fig.2d). The FGS containing 1 mM TMZ continued to release TMZ gradually over the entire 168-h observation period. This shows that TMZ was released gradually and that FGS containing the higher concentration of TMZ continued to release the drug at a higher concentration and for a longer period.
Figure 2.
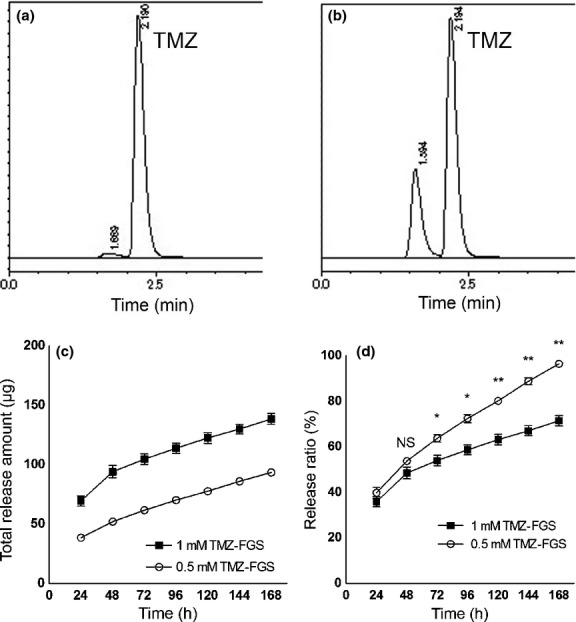
High-performance liquid chromatography (HPLC) of the temozolomide (TMZ) released from fibrin glue (FG). (a) HPLC of the calibration curve standards. (b) HPLC of PBS incubated for 24 h with FG sheets containing TMZ (TMZ-FGS). (c) Time-course of the total amount of TMZ released from FG (n = 3). (d) Ratio of TMZ released from FG in the course of 168 h (n = 3). For analysis we used the unpaired two-tailed t-test. *P < 0.05. **P < 0.001. NS, not significant.
TMZ-FGS exerted significant effects on subcutaneous tumors
We investigated the antitumor effects of TMZ-FGS in vivo. Seven days after the injection of glioma cells, 36 mice were divided into six equal groups. Based on the results of our in vitro experiments, we chose 1 mM as the concentration of TMZ to mix with the FG. The perorally adminstered TMZ dose in mice (66 mg/kg) is similar to the dose used to treat GBM patients (approximately 200 mg/m²).19,20
We measured the tumor size every 2 days for 26 days in U87MG- and for 28 days in K-GIC1- and K-GIC2-transplanted mice. As shown in Fig. S2, none manifested weight loss, infections, delayed healing or died during the observation period and at the end of this period all FGS had been almost completely absorbed.
At the end of the observation period the relative TV was significantly smaller in all TMZ-treated than TMZ-untreated mice (Fig.3a). Among the groups treated with TMZ, the TV in group 6 (TMZ-FGS plus peroral TMZ) was smaller than in the other two groups; the difference in the relative TV was significant in mice transplanted with U87MG cells (Fig.3b). The TV in group 3 (TMZ-FGS) was smaller than in group 5 (peroral TMZ) but the different was not statistically significant. There was no significant difference in the relative TV among the TMZ-untreated groups (Fig.3a,c; Table S1).
Figure 3.
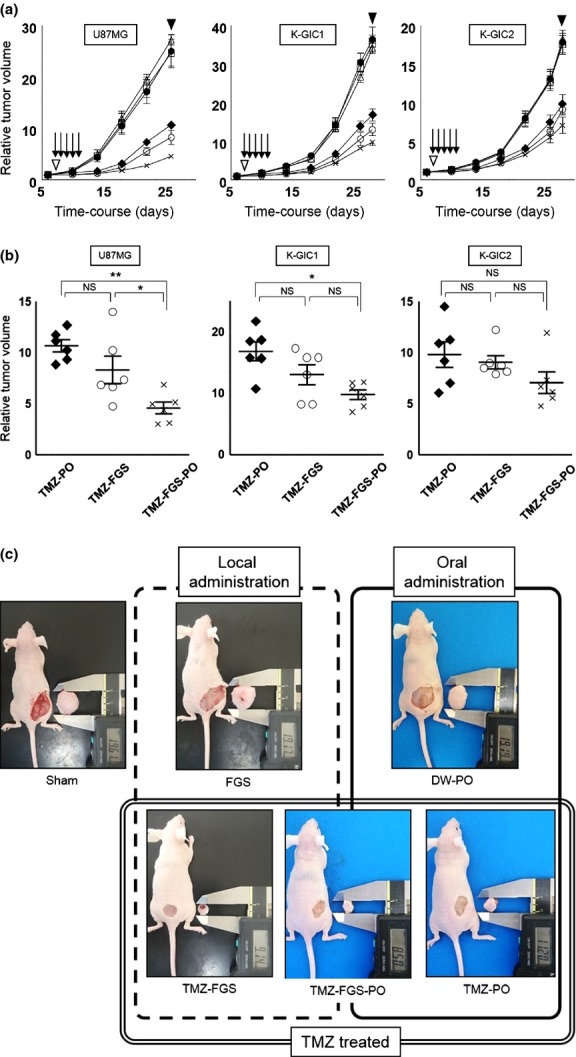
Growth curve and plots of the relative subcutaneous tumor volume. (●), Sham (group 1); (□), fibrin glue sheets (FGS) without temozolomide (TMZ) (FGS; group 2); (○), FGS containing TMZ (TMZ-FGS; group 3); (△), distilled water perorally (DW-PO; group 4); (♦), TMZ perorally (TMZ-PO; group 5); (×), TMZ-FGS plus TMZ perorally (TMZ-FGS-PO; group 6). (a) Growth curve (n = 6). (▽), FGS placement; (↓), oral administration of TMZ or distilled water; (▼), killed. (b) Plots of relative tumor volume in the three different TMZ-treated groups at the time the mice were killed (n = 6). Data were analyzed using anova. A P < 0.05 was considered statistically significant. *P < 0.05. **P < 0.001. (c) Images of mice bearing subcutaneous tumors induced with U87MG cells and the tumors removed at the time the mice were killed.
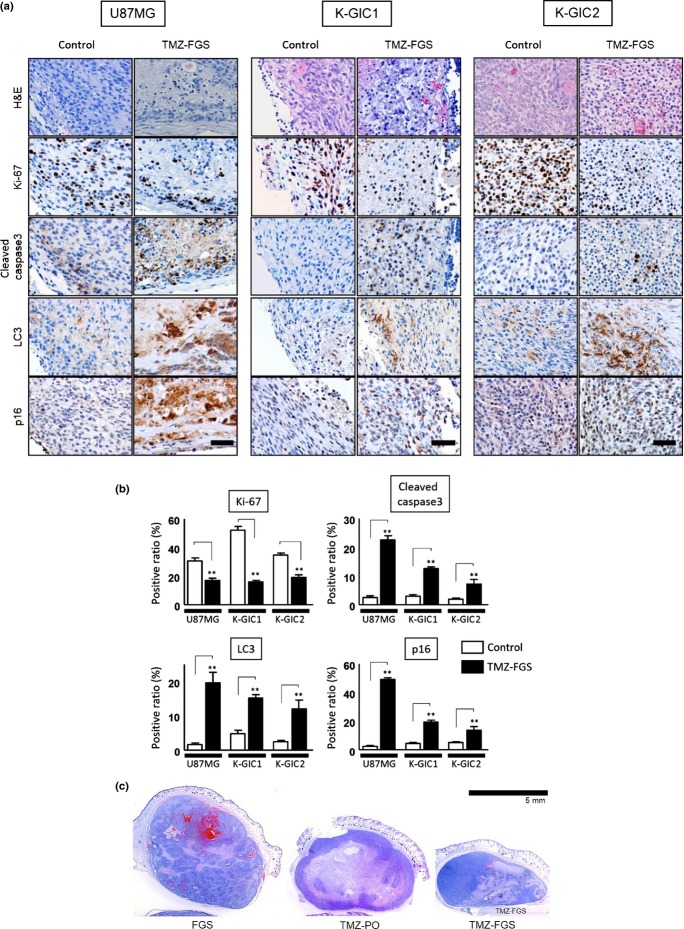
To detect the pathological changes induced by FGS containing 1 mM TMZ, we subjected subcutaneous tumor samples from mice treated for 7 days with FGS that did not (control) or did contain TMZ to immunohistochemical staining and immunoblotting.
We used Ki-67, cleaved caspase3, LC3 and p16 as markers for proliferation, apoptosis, autophagy and senescence. At high-power magnification, we counted immunohistochemically positive cells among the tumor cells around the FGS in 10 fields. The Ki-67-positive ratio was lower in all TMZ-treated mice than in the controls. In contrast, the ratio of cleaved caspase 3, LC3 and p16 positivity was higher in TMZ-treated mice than in the controls (Fig.4a,b; Table S2). Changes induced by TMZ were observed mainly on the tumor side covered with the TMZ-FGS. Such findings were not detected in mice treated with FGS only or with oral TMZ only (Fig.4c).
Figure 4.
(On the previous page) Immunohistochemical findings on the subcutaneous tumors. (a) Immunohistochemical staining of subcutaneous tumors treated for 7 days with fibrin glue sheets (FGS) that did not (control) and did contain temozolomide (TMZ-FGS). Original magnification, ×400; bar, 50 μm. (b) Positivity ratios of the antibody reactions (n = 3; 10 fields per sample). Analysis was with the unpaired two-tailed t-test. A P < 0.05 was considered statistically significant. **P < 0.001. (c) H&E staining of subcutaneous tumors elicited by transplanted U87MG cells. The mice were treated for 7 days with FGS alone, peroral TMZ (TMZ-PO) or TMZ-FGS. The FGS was placed under the tumors. Similar findings were obtained when Kumamoto-glioma-initiating cell 1 (K-GIC1) or K-GIC2 cells were transplanted (data not shown). Low magnification; bar, 5 mm.
Western blot analysis showed that the expression of cleaved caspase 3, LC3 and p16 was significantly higher in mice treated with TMZ-FGS than in the controls (Fig.5). An increase in the LC3-II/LC3-I level is correlated with an increase in autophagy.
Figure 5.
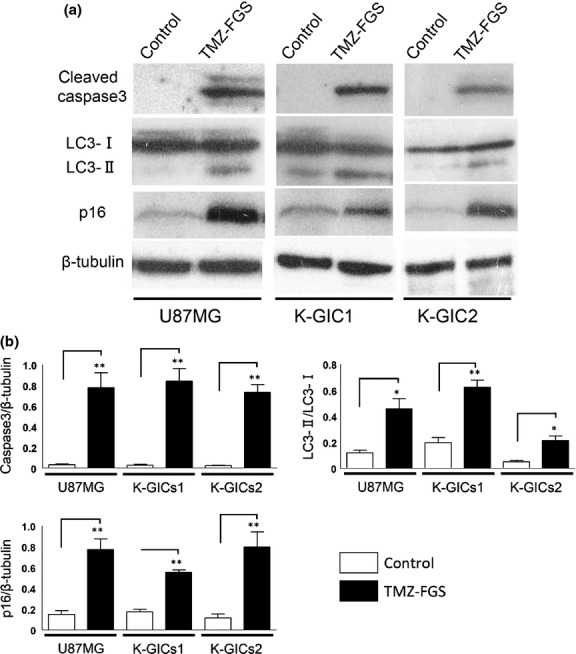
Western blot analysis of subcutaneous tumors. (a) Western blots of cleaved caspase 3, LC3-I, LC3-II, p16 and β-tubulin in subcutaneous tumors treated for 7 days with fibrin glue sheets (FGS) alone (control) or temozolomide (TMZ-FGS) (n = 3). (b) Graphs of the intensity of the bands. Analysis was with the unpaired two-tailed t-test. A P < 0.05 was considered statistically significant. *P < 0.05. **P < 0.001.
Our immunostaining and immunoblotting results document that TMZ-FGS inhibited the proliferation of tumor cells and that it induced autophagy, apoptosis and senescence.
TMZ-FGS was safe for normal brain tissue
To investigate the effect of FGS containing 1 mM TMZ and of FGS alone on the brain tissue of normal ICR mice in the acute and chronic phase, we stained brain tissue samples with H&E, GFAP, COX-2 and Nissl to investigate the inflammatory responses of astrocytes, the infiltration of other cells and demyelination. In the acute phase, GFAP and COX-2 staining around the FGS and TMZ-FGS was slightly stronger than in the controls; H&E and Nissl staining was not markedly different from the controls (Fig.6a). In the chronic phase we observed no staining difference (Fig.6b). Treatment with FGS containing TMZ did not result in severe edema, demyelination or inflammation of the normal brain in both phases after FGS placement. All FGS had retained their original shape at day 14. None of the mice manifested weight loss, infections or delayed healing and none died during the observation period (data not shown). Based on these findings we suggest that FGS containing TMZ is safe for normal brain tissue.
Figure 6.
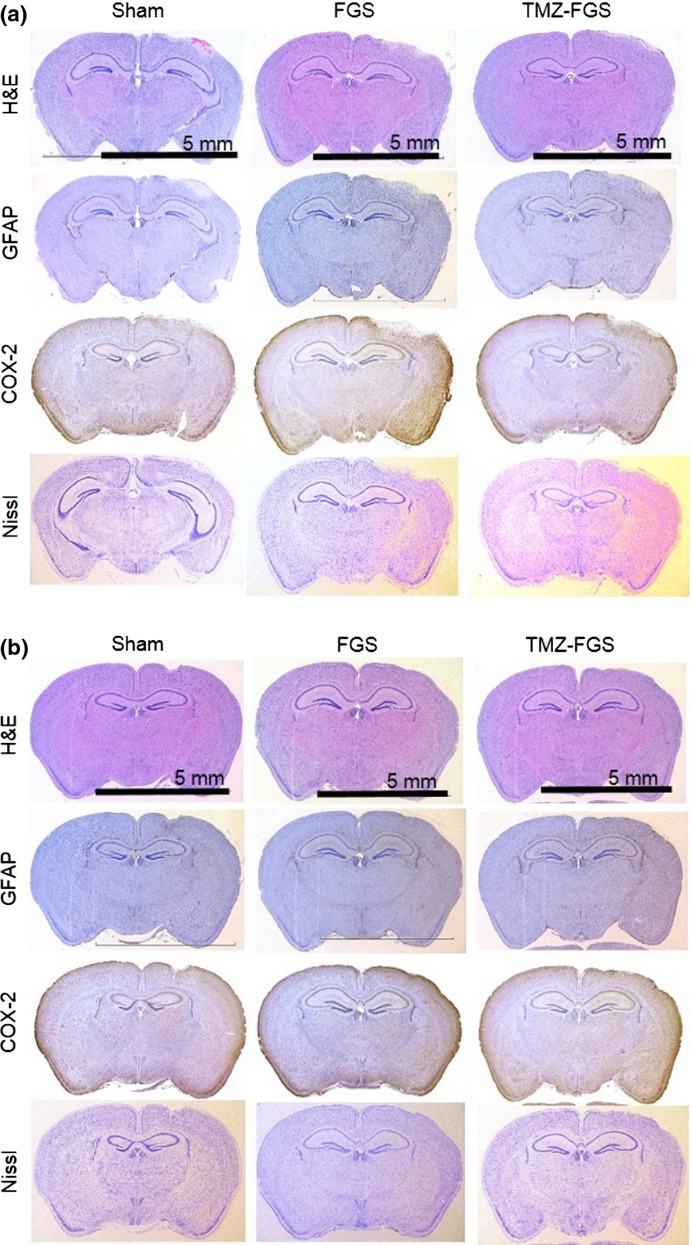
Adverse effects of fibrin glue sheets containing temozolomide (TMZ-FGS) on normal mouse brain. Effects in the acute (a) and chronic (b) phase are shown. Left, sham; middle, FGS alone; right, FGS containing 1 mM TMZ. Low magnification; bar, 5 mm.
Discussion
In their clinical study, Wick et al.13 found that 80% of GBM recur locally. To overcome tumor recurrence in patients with GBM, Gliadel wafers may represent a new therapeutic tool.21,22 In fact, the combination of Gliadel wafers and concomitant TMZ therapy using the Stupp protocol improved their median survival.23,24
In mice with subcutaneous tumors elicited by the transplantation of three glioma cell lines in the present study, FGS containing TMZ suppressed the TV growth significantly and more effectively than TMZ delivered perorally. Ostermann et al.25 who studied the area under the curve (AUC) for TMZ in cerebrospinal fluid (CSF) and in plasma reported that the AUCCSF/AUCplasma of the TMZ ratio was 20%. The systemic administration of TMZ elicits a chemical reduction in physiological pH. We hypothesized that a larger amount of TMZ is delivered directly to the tumor tissues by FG containing TMZ without the induction of a chemical reduction than by systemic TMZ administration.
We confirmed that FG containing TMZ induced autophagy, apoptosis and senescence in subcutaneous tumor cells. In earlier studies TMZ elicited autophagy in glioma cells,26 TMZ-induced G2 arrest led to glioma cell senescence27,28 and TMZ-induced DNA damage resulted in three types of glioma cell death (i.e. autophagy, apoptosis and senescence).7
In some cancer patients the systemic TMZ administration results in myelosuppression-induced pancytopenia, constipation and nausea.29,30 Because FG containing TMZ delivers a high concentration of TMZ directly to the target tissue and continues to release TMZ during more than 168 h, the incidence of adverse effects might be decreased. None of the mice in the present study died and we encountered no significant adverse effects in our in vivo model during the observation period. Norregaard et al.31 who used a slice culture model to investigate the cytotoxicity of various chemotherapeutic drugs in normal brain tissue reported that at specific concentrations and exposure times TMZ was not toxic.
Temozolomide is stable in acidic and unstable in alkaline environments.32,33 The pH of the fibrinogen and the thrombin solutions was 6.7 and 5.9, respectively. Because FG detains TMZ in an acidic condition, TMZ mixed with FG would remain stable for long periods at the target site. We found that FGS containing TMZ was effective at the target site and in an area approximately 5 mm distant from the FGS.
Others have also suggested FG as a delivery system for anticancer agents, growth factors and gene vectors and reported it to be safe for normal brain tissue, intracranial nerves and vessels.9–12,34 In addition, the drug concentration in FG can be changed easily and the shape of FG can be made to fit the target lesion. However, there may be a difference in the absorption of FG placed at intracranial and subcutaneous sites and while the proliferation and migration of fibroblasts and granulocytes appears to be related, details on the underlying mechanisms remain unclear.8
We found that 1 mM was the maximum TMZ concentration suitable for our experiments, because at higher concentrations the drug failed to dissolve completely and the FGS failed to solidify sufficiently. In contrast, a TMZ concentration of 1 mM is too high for a single in vitro exposure. We examined the release of TMZ from FG using HPLC as previously described35,36 and found that FG released TMZ gradually rather than all at once. For these reasons, we determined 1 mM TMZ as the adequate concentration mixed with FG for our in vivo experiments.
When we repeated the various experiments using a nude mouse intracranial tumor model, we were unable to obtain stable results. We could not prepare tumors stably near the brain surface to allow for direct contact with the TMZ-FGS. The placement of TMZ-FGS on the brain surface to treat intracerebral tumors yielded no significant findings. This suggests that the strongest antitumor effects of TMZ-FGS require direct contact with the tumor. In an effort to create TMZ-FG near the intracerebral tumor, we injected the thrombin-TMZ solution and the fibrinogen solution individually into the brain using a stereotactic technique. We found that solidification was insufficient and the concentration of TMZ in the TMZ-FG was inhomogeneous. These results indicate that the intracranial mouse model is suboptimal for these experiments.
The present study shows that FG containing TMZ exerts antitumor effects in subcutaneous tumor-bearing mice induced by the transplantation of malignant glioma cells. It also demonstrates that FG containing TMZ is safe for normal mouse brain tissue. As a drug delivery system, FG containing TMZ may facilitate the delivery of high concentrations of TMZ to sites harboring residual tumor cells. In future studies, we will perform experiments with different anticancer drugs and intracranial models using other animals.
Acknowledgments
This work was supported in part by a scholarship from the Graduate School of Medical Sciences, Kumamoto University, a Ministry of Education, Culture, Sports, Science and Technology KAKENHI Grant (25130710) and Japan Society for the Promotion of Science (JSPS) KAKENHI Grants (23390351 and 25462273). The authors thank M. Obata and K. Nakamura for technical assistance.
Disclosure Statement
The authors have no conflict of interest.
Funding information
Graduate School of Medical Sciences, Kumamoto University, Ministry of Education, Culture, Sports, Science and Technology KAKENHI (25130710). Japan Society for the Promotion of Science (JSPS) KAKENHI (23390351 and 25462273).
Supporting Information
Additional supporting information may be found in the online version of this article:
The primary culture cell lines form xenograft tumors in mice. Images are of H&E stains.
Bodyweight changes in the course of the observation period in mice injected subcutaneously with glioma cells.
Relative subcutaneous tumor volume at the end of the observation period in mice from the different groups. Values are mean ± SEM.
Positivity ratio (%) of each antibody reaction at day 7 of treatment.
References
- 1.Dolecek TA, Propp JM, Stroup NE, Kruchko C. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2005–2009. Neuro Oncol. 2012;14(Suppl. 5):v1–49. doi: 10.1093/neuonc/nos218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–96. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 3.Lawrence YR, Mishra MV, Werner-Wasik M, et al. Improving prognosis of glioblastoma in the 21st century: who has benefited most? Cancer. 2012;118:4228–34. doi: 10.1002/cncr.26685. [DOI] [PubMed] [Google Scholar]
- 4.Kaina B, Christmann M, Naumann S, Roos WP. MGMT: key node in the battle against genotoxicity, carcinogenicity and apoptosis induced by alkylating agents. DNA Repair (Amst) 2007;6:1079–99. doi: 10.1016/j.dnarep.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 5.Caporali S, Falcinelli S, Starace G, et al. DNA damage induced by temozolomide signals to both ATM and ATR: role of the mismatch repair system. Mol Pharmacol. 2004;66:478–91. doi: 10.1124/mol.66.3.. [DOI] [PubMed] [Google Scholar]
- 6.Ochs K, Kaina B. Apoptosis induced by DNA damage O6-methylguanine is Bcl-2 and caspase-9/3 regulated and Fas/caspase-8 independent. Cancer Res. 2000;60:5815–24. [PubMed] [Google Scholar]
- 7.Knizhnik AV, Roos WP, Nikolova T, et al. Survival and death strategies in glioma cells: autophagy, senescence and apoptosis triggered by a single type of temozolomide-induced DNA damage. PLoS ONE. 2013;8:e55665. doi: 10.1371/journal.pone.0055665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spotnitz WD. Commercial fibrin sealants in surgical care. Am J Surg. 2001;182:8S–14S. doi: 10.1016/s0002-9610(01)00771-1. [DOI] [PubMed] [Google Scholar]
- 9.de Vries J, Menovsky T, van Gulik S, Wesseling P. Histological effects of fibrin glue on nervous tissue: a safety study in rats. Surg Neurol. 2002;57:415–22. doi: 10.1016/s0090-3019(02)00736-x. discussion 22. [DOI] [PubMed] [Google Scholar]
- 10.Kassam A, Nemoto E, Balzer J, et al. Effects of Tisseel fibrin glue on the central nervous system of nonhuman primates. Ear Nose Throat J. 2004;83:246–8. 50, 52 passim. [PubMed] [Google Scholar]
- 11.Yasuda H, Kuroda S, Nanba R, et al. A novel coating biomaterial for intracranial aneurysms: effects and safety in extra- and intracranial carotid artery. Neuropathology. 2005;25:66–76. doi: 10.1111/j.1440-1789.2004.00590.x. [DOI] [PubMed] [Google Scholar]
- 12.Spicer PP, Mikos AG. Fibrin glue as a drug delivery system. J Controlled Release. 2010;148:49–55. doi: 10.1016/j.jconrel.2010.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wick W, Stupp R, Beule AC, et al. A novel tool to analyze MRI recurrence patterns in glioblastoma. Neuro Oncol. 2008;10:1019–24. doi: 10.1215/15228517-2008-058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Balenci L, Clarke ID, Dirks PB, et al. IQGAP1 protein specifies amplifying cancer cells in glioblastoma multiforme. Cancer Res. 2006;66:9074–82. doi: 10.1158/0008-5472.CAN-06-0761. [DOI] [PubMed] [Google Scholar]
- 15.Kim H, Likhari P, Parker D, et al. High-performance liquid chromatographic analysis and stability of anti-tumor agent temozolomide in human plasma. J Pharm Biomed Anal. 2001;24:461–8. doi: 10.1016/s0731-7085(00)00466-0. [DOI] [PubMed] [Google Scholar]
- 16.Jensen MM, Jorgensen JT, Binderup T, Kjaer A. Tumor volume in subcutaneous mouse xenografts measured by microCT is more accurate and reproducible than determined by 18F-FDG-microPET or external caliper. BMC Med Imaging. 2008;8:16. doi: 10.1186/1471-2342-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shinojima N, Tada K, Shiraishi S, et al. Prognostic value of epidermal growth factor receptor in patients with glioblastoma multiforme. Cancer Res. 2003;63:6962–70. [PubMed] [Google Scholar]
- 18.Muta D, Makino K, Nakamura H, Yano S, Kudo M, Kuratsu J. Inhibition of eIF4E phosphorylation reduces cell growth and proliferation in primary central nervous system lymphoma cells. J Neurooncol. 2011;101:33–9. doi: 10.1007/s11060-010-0233-6. [DOI] [PubMed] [Google Scholar]
- 19.Houghton PJ, Stewart CF, Cheshire PJ, et al. Antitumor activity of temozolomide combined with irinotecan is partly independent of O-6-methylguanine-DNA methyltransferase and mismatch repair phenotypes in xenograft models. Clin Cancer Res. 2000;6:4110–8. [PubMed] [Google Scholar]
- 20.Middlemas DS, Stewart CF, Kirstein MN, et al. Biochemical correlates of temozolomide sensitivity in pediatric solid tumor xenograft models. Clin Cancer Res. 2000;6:998–1007. [PubMed] [Google Scholar]
- 21.Brem H, Piantadosi S, Burger PC, et al. Placebo-controlled trial of safety and efficacy of intraoperative controlled delivery by biodegradable polymers of chemotherapy for recurrent gliomas. The Polymer-brain Tumor Treatment Group. Lancet. 1995;345:1008–12. doi: 10.1016/s0140-6736(95)90755-6. [DOI] [PubMed] [Google Scholar]
- 22.Westphal M, Hilt DC, Bortey E, et al. A phase 3 trial of local chemotherapy with biodegradable carmustine (BCNU) wafers (Gliadel wafers) in patients with primary malignant glioma. Neuro Oncol. 2003;5:79–88. doi: 10.1215/S1522-8517-02-00023-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McGirt MJ, Than KD, Weingart JD, et al. Gliadel (BCNU) wafer plus concomitant temozolomide therapy after primary resection of glioblastoma multiforme. J Neurosurg. 2009;110:583–8. doi: 10.3171/2008.5.17557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Noel G, Schott R, Froelich S, et al. Retrospective comparison of chemoradiotherapy followed by adjuvant chemotherapy, with or without prior gliadel implantation (carmustine) after initial surgery in patients with newly diagnosed high-grade gliomas. Int J Radiat Oncol Biol Phys. 2012;82:749–55. doi: 10.1016/j.ijrobp.2010.11.073. [DOI] [PubMed] [Google Scholar]
- 25.Ostermann S, Csajka C, Buclin T, et al. Plasma and cerebrospinal fluid population pharmacokinetics of temozolomide in malignant glioma patients. Clin Cancer Res. 2004;10:3728–36. doi: 10.1158/1078-0432.CCR-03-0807. [DOI] [PubMed] [Google Scholar]
- 26.Kanzawa T, Germano IM, Komata T, Ito H, Kondo Y, Kondo S. Role of autophagy in temozolomide-induced cytotoxicity for malignant glioma cells. Cell Death Differ. 2004;11:448–57. doi: 10.1038/sj.cdd.4401359. [DOI] [PubMed] [Google Scholar]
- 27.Filippi-Chiela EC, Thome MP, Bueno ESMM, et al. Resveratrol abrogates the Temozolomide-induced G2 arrest leading to mitotic catastrophe and reinforces the Temozolomide-induced senescence in glioma cells. BMC Cancer. 2013;13:147. doi: 10.1186/1471-2407-13-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hirose Y, Katayama M, Mirzoeva OK, Berger MS, Pieper RO. Akt activation suppresses Chk2-mediated, methylating agent-induced G2 arrest and protects from temozolomide-induced mitotic catastrophe and cellular senescence. Cancer Res. 2005;65:4861–9. doi: 10.1158/0008-5472.CAN-04-2633. [DOI] [PubMed] [Google Scholar]
- 29.Brada M, Judson I, Beale P, et al. Phase I dose-escalation and pharmacokinetic study of temozolomide (SCH 52365) for refractory or relapsing malignancies. Br J Cancer. 1999;81:1022–30. doi: 10.1038/sj.bjc.6690802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yung WK, Prados MD, Yaya-Tur R, et al. Multicenter phase II trial of temozolomide in patients with anaplastic astrocytoma or anaplastic oligoastrocytoma at first relapse. Temodal Brain Tumor Group. J Clin Oncol. 1999;17:2762–71. doi: 10.1200/JCO.1999.17.9.2762. [DOI] [PubMed] [Google Scholar]
- 31.Norregaard A, Jensen SS, Kolenda J, et al. Effects of chemotherapeutics on organotypic corticostriatal slice cultures identified by a panel of fluorescent and immunohistochemical markers. Neurotox Res. 2012;22:43–58. doi: 10.1007/s12640-011-9300-9. [DOI] [PubMed] [Google Scholar]
- 32.Andrasi M, Bustos R, Gaspar A, Gomez FA, Klekner A. Analysis and stability study of temozolomide using capillary electrophoresis. J Chromatogr B Analyt Technol Biomed Life Sci. 2010;878:1801–8. doi: 10.1016/j.jchromb.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 33.Beale P, Judson I, Moore S, et al. Effect of gastric pH on the relative oral bioavailability and pharmacokinetics of temozolomide. Cancer Chemother Pharmacol. 1999;44:389–94. doi: 10.1007/s002800050994. [DOI] [PubMed] [Google Scholar]
- 34.Yoshida H, Yamaoka Y, Shinoyama M, Kamiya A. Novel drug delivery system using autologous fibrin glue–release properties of anti-cancer drugs. Biol Pharm Bull. 2000;23:371–4. doi: 10.1248/bpb.23.371. [DOI] [PubMed] [Google Scholar]
- 35.Kim HK, Lin CC, Parker D, et al. High-performance liquid chromatographic determination and stability of 5-(3-methyltriazen-1-yl)-imidazo-4-carboximide, the biologically active product of the antitumor agent temozolomide, in human plasma. J Chromatogr B Biomed Sci Appl. 1997;703:225–33. doi: 10.1016/s0378-4347(97)00431-3. [DOI] [PubMed] [Google Scholar]
- 36.Shen F, Decosterd LA, Gander M, Leyvraz S, Biollax J, Lejeune F. Determination of temozolomide in human plasma and urine by high-performance liquid chromatography after solid-phase extraction. J Chromatogr B Biomed Appl. 1995;667:291–300. doi: 10.1016/0378-4347(95)00040-p. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The primary culture cell lines form xenograft tumors in mice. Images are of H&E stains.
Bodyweight changes in the course of the observation period in mice injected subcutaneously with glioma cells.
Relative subcutaneous tumor volume at the end of the observation period in mice from the different groups. Values are mean ± SEM.
Positivity ratio (%) of each antibody reaction at day 7 of treatment.