Abstract
For breast cancer patients with a preoperative diagnosis of ductal carcinoma in situ (DCIS), sentinel lymph node (SN) biopsy has been proposed as an axillary staging procedure in selected patients with a higher likelihood of having occult invasive lesions. With detailed histological examination of primary tumors and molecular whole-node analysis of SNs, we aimed to validate whether this selective application accurately identifies patients with SN metastasis. The subjects were 336 patients with a preoperative needle-biopsy diagnosis of DCIS who underwent SN biopsy using the one-step nucleic acid amplification assay in the period 2009–2011. The incidence and preoperative predictors of upstaging to invasive disease on final pathology and SN metastasis, and their correlation, were investigated. Of the 336 patients, 113 (33.6%) had invasive disease, and 6 (1.8%) and 17 (5.0%) had macro- and micrometastasis in axillary nodes respectively. Of the 113 patients with invasive disease, 4 (3.5%) and 9 (8.0%) had macro- and micrometastasis. Predictors of invasive disease included palpability, mammographic mass, and calcifications (spread >20 mm), and intraductal solid structure, but no predictor was found for SN metastasis. Therefore, even though occult invasive disease was found at final pathology, most of the patients had no metastasis or only micrometastasis in axillary nodes. Predictors of invasive disease and SN metastasis were not completely consistent, so the selective SN biopsy for patients with a higher risk of invasive disease may not accurately identify those with SN metastasis. More accurate application of SN biopsy is required for patients with a preoperative diagnosis of DCIS.
Keywords: Breast cancer, ductal carcinoma in situ, one-step nucleic acid amplification assay, OSNA assay, sentinel lymph node biopsy
Ductal carcinoma in situ (DCIS) is the most common type of pre-invasive breast cancer. By definition, DCIS does not metastasize to the lymph nodes because the tumor is limited to the epithelial layer and does not extend to the lymphatic vessels. However, lesions initially diagnosed to be DCIS on needle biopsy are occasionally upstaged to invasive cancer after the final pathology report of the completely excised specimen.1 This is due to inherent limitations of biopsy sampling techniques, by which a small invasive lesion may fail to be detected in a large area of intraductal lesion. A meta-analysis has shown that this upstaging occurs in 25.9% of patients with a needle-biopsy diagnosis of DCIS.2
Sentinel node (SN) biopsy is the standard axillary staging procedure for clinically node-negative breast cancer.3 For patients with a preoperative diagnosis of DCIS, SN biopsy has been proposed for use in selected patients with a higher likelihood of developing occult invasive lesions (i.e. large, high-grade, comedo-type, or clinical/radiologic mass) and those undergoing mastectomy, because SN mapping cannot be carried out after an invasive tumor is identified.1,4 A meta-analysis has shown that the incidence of SN metastases is 7.4% in patients with a preoperative needle-biopsy diagnosis of DCIS.4 However, it has not been well validated whether this selective application of SN biopsy in patients with a higher risk of invasive disease accurately identifies patients with a higher likelihood of SN metastasis.5–9
The diagnostic accuracy of the primary tumor and nodal status is dependent on the rigor of the examination. The fewer samples of primary tumor or lymph node that are examined, the greater the risk that small invasive lesions or metastases may not be identified. In most of the previous reports on the upstaging to invasive cancer from preoperatively diagnosed DCIS, histological examination procedures for primary tumors have not been described in detail. Thus, the primary tumor status on final pathology may be underestimated.
Similarly, conventional histopathological examinations may lead to underestimation of nodal status: they are limited in their ability to detect metastases accurately due to the partial evaluation of a node. The one-step nucleic acid amplification (OSNA) assay (Sysmex, Kobe, Japan) was developed to overcome this limitation of histopathological lymph node examination. The OSNA assay is accepted and routinely used in more than 230 institutions in Spain, Japan, Italy, the UK, France, and elsewhere.10 This assay can assess whole lymph nodes, and the detection and amplification of cytokeratin 19 (CK19) mRNA can yield quantitative results for detecting clinically relevant nodal metastases (>0.2 mm in size).11 Calibration and validation studies11,12 have provided reasonable evidence that the CK19 mRNA copy number detected by the OSNA assay can provide good estimates of macrometastasis, micrometastasis, and no metastasis, including isolated tumor cells, as defined by the Cancer Staging Manual of the American Joint Committee on Cancer.13 The OSNA whole-node assay detects a greater number of micrometastases than routine histopathological examinations.14–16 In particular, we showed that the number of patients with a postoperative diagnosis of DCIS and SN metastasis increased significantly after the introduction of the OSNA assay for SN biopsies.17
Therefore, using detailed histological examination of primary tumors and the OSNA whole-node assay of SNs, the primary tumor and nodal status can be evaluated more accurately. In this single-center retrospective study, we investigated the incidence and predictors of invasive disease on final pathology and SN metastasis, and their correlation, in order to validate whether or not the selective SN biopsy for patients with a higher risk of invasive disease accurately identifies those with SN metastasis.
Materials and Methods
Patients and tumors
The study consisted of cN0 patients with a preoperative needle-biopsy diagnosis of DCIS who underwent SN biopsy with the OSNA assay between April 2009 and August 2011 at The Cancer Institute Hospital (Tokyo, Japan). Each eligible patient received both clinical and ultrasonographic examinations of the preoperative lymph node status. When there was a possibility of nodal metastasis on at least one of the examinations, the cN0 status was confirmed by ultrasonography-guided fine-needle aspiration cytology for the suspicious lymph node. The exclusion criteria were as follows: (i) SN mapping without the use of a radioisotope tracer; (ii) metastasis detected only in non-SNs; (iii) previous excision of a primary tumor; (iv) heterochronous ipsilateral breast cancer recurrence; (v) neoadjuvant drug therapy; and (vi) multicentric tumors of the unilateral breast. This study was approved by the Institutional Review Board of the Cancer Institute Hospital.
Presence of comedo necrosis, intraductal structure, and nuclear grade were histologically assessed in the preoperative needle-biopsy materials. The intraductal structures were classified into three patterns (i.e. flat/low papillary, cribriform/papillary, and solid subtypes) regardless of the presence or absence of comedo necrosis. Primary tumor and nodal status were classified according to the seventh edition of the American Joint Committee on Cancer Staging Manual.13 The postoperative pN status was classified on the basis of the overall axillary status including the SN and non-SN status in patients who underwent axillary dissection.
Sentinel lymph node biopsy using OSNA assay
All patients underwent SN mapping and identification with a radioisotope tracer plus/minus a vital dye. Whole SNs from each patient were evaluated using the OSNA assay without histopathological examination. When one or more SNs were positive, complete axillary lymph node dissection was carried out immediately. Since September 2009, we have also used the OSNA assay for non-SNs in patients who underwent axillary dissection following metastatic SN biopsy.
The OSNA assay procedure has been previously described in detail.11 Briefly, whole lymph nodes were homogenized with 4 mL lysis buffer solution (Lynorhag; Sysmex) and centrifuged at 10 000 g at room temperature. A 2-μL supernatant was analyzed with the RD-100i System (Sysmex), an automated molecular detection system that uses a reverse transcription loop-mediated isothermal amplification method,18 and the LynoampBC Kit (Sysmex). The degree of amplification was determined on the basis of a reaction by-product, pyrophosphate.19 The resultant change in turbidity after precipitation of magnesium pyrophosphate was then correlated with the CK19 mRNA copy number per microlitre of the original lysate using a standard curve established beforehand using three calibrators containing different CK19 mRNA copy numbers. The number of CK19 mRNA copies per microlitre was extrapolated from the standard curve for both the measurement sample and a 1:10 diluted sample. The cut-off for negative/positive was set at 250 copies/μL.11 Tumor burden ≥250–<5000 and ≥5000 copies/μL in a lymph node were considered equivalent to histopathological micro- and macrometastasis, respectively.11 In situations where the reaction was inhibited in the measurement sample, the copy numbers in the diluted sample were used.
Postoperative histological examinations of primary tumors
Partial mastectomy materials were sectioned continuously from the nipple side to the periphery at 5-mm intervals (Fig.1a). All sections were histologically examined with H&E staining. Total mastectomy materials were sectioned continuously from the nipple to the periphery at 5–7-mm intervals (Fig.1b). The sectioning was carried out to cover the entire tumor spread using macroscopic and radiologic findings as references. Most of the sections within the tumor spread were histologically confirmed with H&E staining.
Figure 1.
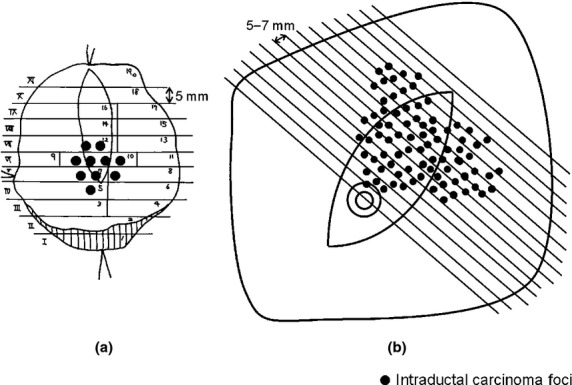
Histological examinations of primary breast tumors in patients with a preoperative needle-biopsy diagnosis of ductal carcinoma in situ who underwent partial mastectomy (a) or total mastectomy (b).
Statistical analyses
First, we used univariate logistic regression analysis to screen for potential predictors of invasive disease on final pathology and SN metastasis, respectively. Next, we used multivariate logistic regression analysis for the significant factors from the univariate analyses. Finally, combining the significant characteristics from the multivariate analysis, the incidence of invasive disease and SN metastasis were compared. Only preoperative factors were used for these analyses. P-values < 0.05 were considered statistically significant, and the confidence intervals were set at the 95% level. All statistical analyses were carried out using R statistical software (version 2.10.1; http://www.r-project.org/).20
Results
Patient characteristics
Between April 2009 and August 2011, 1746 breast cancer patients underwent SN biopsy using the OSNA assay; 1568 of them did not meet the exclusion criteria. Among them, 336 (21.4%) were diagnosed with DCIS using preoperative needle-biopsy materials. The demographic characteristics are presented in Table1. All patients were Asian women.
Table 1.
Correlation between patient characteristics and invasive disease on final pathology or sentinel node metastasis in patients preoperatively diagnosed with ductal carcinoma in situ (n = 336)
| Characteristics | No. | % | Invasive disease |
Sentinel node metastasis |
||
|---|---|---|---|---|---|---|
| No. | % | No. | % | |||
| No. of patients | 336 | 100.0 | 113 | 33.6 | 23 | 6.8 |
| Age (years) | ||||||
| ≤39 | 47 | 14.0 | 15 | 31.9 | 4 | 8.5 |
| 40–64 | 252 | 75.0 | 82 | 32.5 | 15 | 6.0 |
| ≥65 | 37 | 11.0 | 16 | 43.2 | 4 | 10.8 |
| Tumor location | ||||||
| Upper-outer | 151 | 44.9 | 55 | 36.4 | 13 | 8.6 |
| Upper-inner | 81 | 24.1 | 30 | 37.0 | 4 | 4.9 |
| Lower-outer | 66 | 19.6 | 18 | 27.3 | 2 | 3.0 |
| Lower-inner/central | 38 | 11.3 | 10 | 26.3 | 4 | 10.5 |
| Palpability | ||||||
| Non-palpable | 176 | 52.4 | 40 | 22.7 | 12 | 6.8 |
| Palpable | 160 | 47.6 | 73 | 45.6 | 11 | 6.9 |
| Mammographic finding | ||||||
| Calcifications, spread ≤20 mm | 80 | 23.8 | 12 | 15.0 | 5 | 6.3 |
| Calcifications, spread >20 mm | 85 | 25.3 | 30 | 35.3 | 7 | 8.2 |
| Mass, FAD, or distortion | 119 | 35.4 | 60 | 50.4 | 9 | 7.6 |
| None | 52 | 15.5 | 11 | 21.2 | 2 | 3.8 |
| Needle biopsy method | ||||||
| With vacuum-assist | 262 | 78.0 | 79 | 30.2 | 16 | 6.1 |
| Without vacuum-assist | 74 | 22.0 | 34 | 45.9 | 7 | 9.5 |
| No. of biopsy specimens | ||||||
| 1–2 | 113 | 33.6 | 43 | 38.1 | 8 | 7.1 |
| 3–5 | 114 | 33.9 | 43 | 37.7 | 10 | 8.8 |
| 6–17 | 109 | 32.4 | 27 | 24.8 | 5 | 4.6 |
| Period from breast biopsy to surgery (days) | ||||||
| ≤30 | 42 | 12.5 | 14 | 33.3 | 3 | 7.1 |
| 31–90 | 213 | 63.4 | 75 | 35.2 | 14 | 6.6 |
| ≥91 | 81 | 24.1 | 24 | 29.6 | 6 | 7.4 |
| Comedo necrosis | ||||||
| Non-comedo | 240 | 71.4 | 72 | 30.0 | 17 | 7.1 |
| Comedo | 96 | 28.6 | 41 | 42.7 | 6 | 6.3 |
| Intraductal structure | ||||||
| Low papillary/flat | 68 | 20.2 | 15 | 22.1 | 4 | 5.9 |
| Cribriform/papillary | 166 | 49.4 | 46 | 27.7 | 8 | 4.8 |
| Solid | 102 | 30.4 | 52 | 51.0 | 11 | 10.8 |
| Nuclear grade | ||||||
| 1 | 191 | 56.8 | 54 | 28.3 | 14 | 7.3 |
| 2 | 110 | 32.7 | 45 | 40.9 | 7 | 6.4 |
| 3 | 35 | 10.4 | 14 | 40.0 | 2 | 5.7 |
| Breast surgery | ||||||
| Partial mastectomy | 151 | 44.9 | 35 | 23.2 | 12 | 7.9 |
| Total mastectomy | 185 | 55.1 | 78 | 42.2 | 11 | 5.9 |
| Estrogen receptor status | ||||||
| + | 274 | 81.5 | 80 | 29.2 | 22 | 8.0 |
| − | 62 | 18.5 | 33 | 53.2 | 1 | 1.6 |
| Progesterone receptor status | ||||||
| + | 240 | 71.4 | 70 | 29.2 | 19 | 7.9 |
| − | 96 | 28.6 | 43 | 44.8 | 4 | 4.2 |
FAD, focal asymmetric density.
Distribution of postoperative pT and pN status
Of the 336 patients, 223 (66.4%) did not have an invasive lesion (pTis) on final pathology and 113 (33.6%) were upstaged to invasive disease (pT1/T2) (Fig.2a). Of the 113 patients, 85 (75.2%) had an invasive lesion ≤5 mm in size (pT1mi/T1a).
Figure 2.
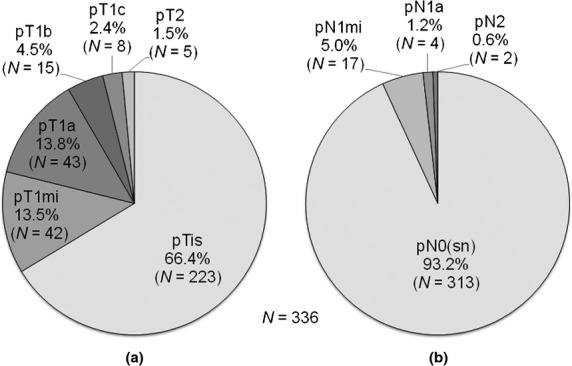
Distribution of pathological T status on final pathology (a) and overall pathological N status (b) in patients with a preoperative needle-biopsy diagnosis of ductal carcinoma in situ. is, in situ (intraductal carcinoma lesion); mi, micrometastasis; sn, sentinel node.
Of the 336 patients, 313 (93.2%) did not have SN metastasis (pN0) and 23 (6.8%) had SN metastasis (three with macrometastasis and 20 with micrometastasis). All the SN-positive patients underwent axillary dissection. For the non-SN evaluation, seven patients underwent single-section histology and 16 underwent OSNA assay. One of the three patients with SN macrometastasis had non-SN metastasis, and the non-SN metastasis was macrometastasis. Four of the 20 patients with SN micrometastasis had non-SN metastasis, and all the non-SN metastasis were micrometastasis. Considering non-SN status in addition to SN status, 17 (5.0%) and 6 (1.8%) of the 336 patients had only micrometastasis (pN1mi) and at least one macrometastasis (pN1a/N2), respectively (Fig.2b).
Correlation between postoperative pT and pN status
Of the 223 patients without invasive disease (pTis), 10 (4.5%) had nodal metastasis; 8 (3.6%) and 2 (0.9%) had pN1mi and pN1a/N2 disease, respectively (Fig.3). Of the 113 patients with detected invasive disease (pT1/T2), 13 (11.5%) had nodal metastasis; 9 (8.0%) and 4 (3.5%) had pN1mi and pN1a/N2 disease, respectively.
Figure 3.
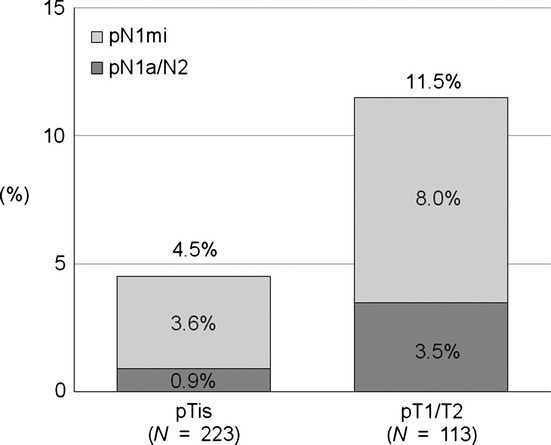
Correlation between pathological T status on final pathology and overall pathological N status in patients with a preoperative needle-biopsy diagnosis of ductal carcinoma in situ. is, in situ (intraductal carcinoma lesion); mi, micrometastasis.
Univariate analysis of invasive disease on final pathology and of SN metastasis
The following seven categories were significantly associated with invasive disease on final pathology (Table2): (i) palpability; (ii) mammographic findings; (iii) needle biopsy method; (iv) number of biopsy specimens; (v) comedo necrosis; (vi) intraductal structure; and (vii) nuclear grade. There was no significant factor predicting SN metastasis (Table2); thus, the multivariable analysis was not carried out.
Table 2.
Univariate analysis of invasive disease on final pathology and sentinel node metastasis in patients preoperatively diagnosed with ductal carcinoma in situ (n = 336)
| Characteristics | Invasive disease |
Sentinel node metastasis |
||||||
|---|---|---|---|---|---|---|---|---|
| Odds ratio | 95% CI |
P-value | Odds ratio | 95% CI |
P-value | |||
| Lower | Upper | Lower | Upper | |||||
| Age (years) | ||||||||
| ≤39 | 1.00 | 1.00 | ||||||
| 40–64 | 1.03 | 0.53 | 2.01 | 0.93 | 0.68 | 0.22 | 2.15 | 0.51 |
| ≥65 | 1.63 | 0.66 | 3.97 | 0.29 | 1.30 | 0.30 | 5.60 | 0.72 |
| Tumor location | ||||||||
| Upper-outer | 1.00 | 1.00 | ||||||
| Upper-inner | 1.03 | 0.59 | 1.80 | 0.93 | 0.55 | 0.17 | 1.75 | 0.31 |
| Lower-outer | 0.65 | 0.35 | 1.24 | 0.19 | 0.33 | 0.07 | 1.51 | 0.15 |
| Lower-inner/central | 0.62 | 0.28 | 1.38 | 0.24 | 1.25 | 0.38 | 4.07 | 0.71 |
| Palpability | ||||||||
| Non-palpable | 1.00 | 1.00 | ||||||
| Palpable | 2.85 | 1.78 | 4.57 | <0.01** | 1.01 | 0.43 | 2.36 | 0.98 |
| Mammographic finding | ||||||||
| Calcifications, spread ≤20 mm | 1.00 | 1.00 | ||||||
| Calcifications, spread >20 mm | 3.09 | 1.45 | 6.60 | <0.01** | 1.35 | 0.41 | 4.43 | 0.62 |
| Mass, FAD, or distortion | 5.76 | 2.83 | 11.74 | <0.01** | 1.23 | 0.40 | 3.81 | 0.72 |
| None | 1.52 | 0.61 | 3.76 | 0.36 | 0.60 | 0.11 | 3.21 | 0.55 |
| Needle biopsy method | ||||||||
| With vacuum-assist | 1.00 | 1.00 | ||||||
| Without vacuum-assist | 1.97 | 1.16 | 3.34 | 0.01* | 1.61 | 0.63 | 4.06 | 0.32 |
| No. of biopsy specimens | ||||||||
| 1–2 | 1.00 | 1.00 | ||||||
| 3–5 | 0.99 | 0.58 | 1.69 | 0.96 | 1.26 | 0.48 | 3.32 | 0.64 |
| 6–17 | 0.54 | 0.30 | 0.95 | 0.03* | 0.63 | 0.20 | 1.99 | 0.43 |
| Period from breast biopsy to surgery (days) | ||||||||
| ≤30 | 1.00 | 1.00 | ||||||
| 31–90 | 1.09 | 0.54 | 2.19 | 0.82 | 0.91 | 0.25 | 3.33 | 0.89 |
| ≥91 | 0.84 | 0.38 | 1.87 | 0.67 | 1.04 | 0.25 | 4.38 | 0.96 |
| Comedo necrosis | ||||||||
| Non-comedo | 1.00 | 1.00 | ||||||
| Comedo | 1.74 | 1.07 | 2.84 | 0.03* | 0.87 | 0.33 | 2.29 | 0.78 |
| Intraductal structure | ||||||||
| Low papillary/flat | 1.00 | 1.00 | ||||||
| Cribriform/papillary | 1.35 | 0.70 | 2.64 | 0.37 | 0.81 | 0.24 | 2.79 | 0.74 |
| Solid | 3.67 | 1.84 | 7.34 | <0.01** | 1.93 | 0.59 | 6.35 | 0.28 |
| Nuclear grade | ||||||||
| 1 | 1.00 | 1.00 | ||||||
| 2 | 1.76 | 1.07 | 2.88 | 0.03* | 0.86 | 0.34 | 2.20 | 0.75 |
| 3 | 1.69 | 0.80 | 3.57 | 0.17 | 0.77 | 0.17 | 3.53 | 0.73 |
P < 0.05
P < 0.01. CI, confidence interval; FAD, focal asymmetric density.
Multivariate analysis of invasive disease on final pathology
The following four characteristics were significantly associated with invasive disease (Table3): (i) palpable tumor; (ii) mammographic finding of calcification spread >20 mm; (iii) mammographic finding of mass, focal asymmetric density, or architectural distortion; and (iv) intraductal solid structure.
Table 3.
Multivariate analysis of invasive disease on final pathology in patients preoperatively diagnosed with ductal carcinoma in situ (n = 336)
| Characteristics | Odds ratio | 95% CI |
P-value | |
|---|---|---|---|---|
| Lower | Upper | |||
| Palpability | ||||
| Non-palpable | 1.00 | |||
| Palpable | 2.22 | 1.25 | 3.92 | <0.01** |
| Mammographic finding | ||||
| Calcifications, spread ≤20 mm | 1.00 | |||
| Calcifications, spread >20 mm | 3.54 | 1.55 | 8.08 | <0.01** |
| Mass, FAD, or distortion | 5.52 | 2.46 | 12.40 | <0.01** |
| None | 1.33 | 0.50 | 3.54 | 0.56 |
| Needle biopsy method | ||||
| With vacuum-assist | 1.00 | |||
| Without vacuum-assist | 1.21 | 0.63 | 2.33 | 0.56 |
| No. of biopsy specimens | ||||
| 1–2 | 1.00 | |||
| 3–5 | 0.99 | 0.53 | 1.85 | 0.97 |
| 6–17 | 0.59 | 0.29 | 1.20 | 0.14 |
| Comedo necrosis | ||||
| Non-comedo | 1.00 | |||
| Comedo | 1.75 | 0.90 | 3.39 | 0.10 |
| Intraductal structure | ||||
| Low papillary/flat | 1.00 | |||
| Cribriform/papillary | 0.77 | 0.35 | 1.68 | 0.51 |
| Solid | 3.20 | 1.41 | 7.27 | <0.01** |
| Nuclear grade | ||||
| 1 | 1.00 | |||
| 2 | 1.18 | 0.66 | 2.11 | 0.58 |
| 3 | 0.46 | 0.17 | 1.20 | 0.11 |
P < 0.01. CI, confidence interval; FAD, focal asymmetric density.
Combining these four significant characteristics as above, 12 patterns of incidence of invasive disease and SN metastasis were calculated (Table4). Four of the 12 patterns showed ≥50% incidence of invasive disease, but only one of these four patterns showed ≥10% incidence of SN metastasis. However, four of the 12 patterns showed ≥10% incidence of SN metastasis, but only one of these four patterns showed ≥50% incidence of invasive disease.
Table 4.
Correlation between incidence of invasive disease on final pathology and sentinel node metastasis according to each combination using significant predictors of invasive disease in patients preoperatively diagnosed with ductal carcinoma in situ (n = 336)
| Palpability | Mammographic finding | Intraductal structure | No. | Invasive disease |
Sentinel node metastasis |
||
|---|---|---|---|---|---|---|---|
| No. | % | No. | % | ||||
| Palpable | Calcifications, spread >20 mm | Solid | 13 | 12 | 92.3† | 0 | 0.0 |
| Others | 19 | 5 | 26.3 | 3 | 15.8‡ | ||
| Mass, FAD, or distortion | Solid | 23 | 15 | 65.2† | 2 | 8.7 | |
| Others | 60 | 31 | 51.7† | 6 | 10.0‡ | ||
| Others | Solid | 10 | 5 | 50.0† | 0 | 0.0 | |
| Others | 35 | 3 | 8.6 | 0 | 0.0 | ||
| Non-palpable | Calcifications, spread >20 mm | Solid | 13 | 5 | 38.5 | 2 | 15.4‡ |
| Others | 40 | 7 | 17.5 | 2 | 5.0 | ||
| Mass, FAD, or distortion | Solid | 9 | 4 | 44.4 | 0 | 0.0 | |
| Others | 27 | 7 | 25.9 | 1 | 3.7 | ||
| Others | Solid | 34 | 9 | 26.5 | 6 | 17.6‡ | |
| Others | 53 | 5 | 9.4 | 0 | 0.0 | ||
≥50%
10%. FAD, focal asymmetric density.
Discussion
The present study features a combination of detailed histological examination of primary breast tumors and whole lymph node analysis using the OSNA assay and a less biased selection of the eligible patients. First of all, to the best of our knowledge, this is the first report in patients with preoperatively diagnosed DCIS to accurately evaluate tumor burden in axillary lymph nodes using molecular whole-node analysis. In this study, all patients' SN(s) were rigorously identified using a radioisotope tracer, and each whole SN was evaluated using the OSNA assay. In addition, more than two-thirds of patients for whom axillary dissection was carried out after a metastatic SN biopsy underwent the OSNA assay for the evaluation of the non-SNs. Thus, the axillary nodal status was more accurately evaluated using the OSNA assay than in previous studies using histopathological examinations.
The second feature of the present study is the standardized and relatively detailed histological examination of primary breast tumors to identify occult invasive foci. In most previous reports on the upstaging to invasive cancer from preoperatively diagnosed DCIS, histological examination procedures for primary tumors have not been described in detail. Thus, the primary tumor status on final pathology may be underestimated. In the present study, 33.6% of the preoperative diagnosed DCIS were upstaged to invasive disease on final pathology, and this rate is slightly higher than that found in the previous meta-analysis (25.9%).2
Third, the indication for SN biopsy in patients with DCIS is relatively wide in our institution. Approximately 84% of patients with a postoperative diagnosis of DCIS underwent the SN biopsy.17 The previous studies on SN biopsy for preoperatively diagnosed DCIS may have a selection bias, because a subgroup of DCIS patients were selected for SN biopsy.5,7,8,21,22 The wider indication in the present study may lead to the lower node-positive rate. Despite the use of molecular whole-node analysis, the overall node-positive rate was 6.8%, which was slightly lower than that found in the previous meta-analysis (7.4%).4
In patients with preoperatively diagnosed DCIS, most of the patients have no metastasis or only micrometastasis in axillary nodes and the incidence of macrometastasis was low, even though occult invasive disease was found in the primary tumor at the final pathology. The OSNA assay can reproducibly distinguish between tumor burdens equivalent to macro- and micrometastasis according to the cut-off value for CK19 mRNA.11,12 Through stratification of the nodal tumor burden, more than two-thirds of metastases were micrometastases. The overall incidence of macro- and micrometastasis was found to be 1.8% and 5.0%, respectively. Moreover, in tumors detected invasive disease, the incidence of macro- and micrometastasis was 3.5% and 8.0%, respectively.
Most of the predictors of invasive disease from the univariate analysis were consistent with the conventional factors,2,4,8 that is, tumors showing palpable/mammographic mass, mammographic calcification spread >20 mm in size, comedo necrosis, intraductal solid structure, or high nuclear grade. However, no preoperative predictor of invasive disease was significantly correlated with SN metastasis. This result might be due to the low number of SN-positive patients. However, the odds ratios for SN metastasis were much lower than those for invasive disease. In particular, patients with tumors with comedo necrosis or tumors of high grade were less likely to have SN metastasis (odds ratio < 1.0). Moreover, the combination of the significant predictors of invasive disease was not able to identify those patients with a higher likelihood of SN metastasis. Thus, predictors of invasive disease are not completely consistent with those of nodal metastasis; therefore, the selective application of SN biopsy in patients with a higher risk of invasive disease may not accurately identify the patients with a higher likelihood of SN metastasis.
The conventional application of SN biopsy for patients with a preoperative diagnosis of DCIS can also be limited in terms of using clinical, radiological, and pathological factors. These factors can be difficult to standardize, and there can be an inter-observer variability in measuring these factors.23–26 A few studies have reported that profiles of gene and miRNA expression show valuable predictive associations with lymph node metastasis.27,28 Thus, the gene and miRNA expression profiles of primary tumors in needle-biopsy specimens might be useful for more accurately identifying patients with a higher likelihood of SN metastasis.
In conclusion, most patients with a preoperative diagnosis of DCIS had no metastasis or only micrometastasis in axillary nodes, even though invasive disease was found on final pathology. Predictors of invasive disease and SN metastasis were not completely consistent, so the selective application of SN biopsy in patients with a higher risk of invasive disease may not accurately identify the patients with a higher likelihood of SN metastasis. More accurate application of SN biopsy is required for patients with a preoperative diagnosis of DCIS.
Acknowledgments
This work was supported in part by a Grant-in-Aid for Young Scientists (B) (No. 21791264) from the Japanese Ministry of Education, Culture, Sports, Science, and Technology and a research grant from the Foundation for the Promotion of Cancer Research in Japan. We thank Masujiro Makita, Seiichiro Nishimura, Yumi Miyagi, Kotaro Iijima, Hidetomo Morizono, Takehiko Sakai, Akiko Ogiya, Kyoko Masumura-Shimoyama, Rie Gokan, and Naoya Gomi (Breast Oncology Center, The Cancer Institute of the Japanese Foundation for Cancer Research, Tokyo, Japan).
Disclosure Statement
The authors have no conflicts of interest.
Funding information
Japanese Ministry of Education, Culture, Sports, Science, and Technology (21791264). Foundation for the Promotion of Cancer Research in Japan.
References
- 1.McMasters KM, Chao C, Wong SL, Martin RC, III, Edwards MJ. Sentinel lymph node biopsy in patients with ductal carcinoma in situ: a proposal. Cancer. 2002;95:15–20. doi: 10.1002/cncr.10641. [DOI] [PubMed] [Google Scholar]
- 2.Brennan ME, Turner RM, Ciatto S, et al. Ductal carcinoma in situ at core-needle biopsy: meta-analysis of underestimation and predictors of invasive breast cancer. Radiology. 2011;260:119–28. doi: 10.1148/radiol.11102368. [DOI] [PubMed] [Google Scholar]
- 3.Lyman GH, Giuliano AE, Somerfield MR, et al. American Society of Clinical Oncology guideline recommendations for sentinel lymph node biopsy in early-stage breast cancer. J Clin Oncol. 2005;23:7703–20. doi: 10.1200/JCO.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 4.Ansari B, Ogston SA, Purdie CA, Adamson DJ, Brown DC, Thompson AM. Meta-analysis of sentinel node biopsy in ductal carcinoma in situ of the breast. Br J Surg. 2008;95:547–54. doi: 10.1002/bjs.6162. [DOI] [PubMed] [Google Scholar]
- 5.Yen TW, Hunt KK, Ross MI, et al. Predictors of invasive breast cancer in patients with an initial diagnosis of ductal carcinoma in situ: a guide to selective use of sentinel lymph node biopsy in management of ductal carcinoma in situ. J Am Coll Surg. 2005;200:516–26. doi: 10.1016/j.jamcollsurg.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 6.Meijnen P, Oldenburg HS, Loo CE, Nieweg OE, Peterse JL, Rutgers EJ. Risk of invasion and axillary lymph node metastasis in ductal carcinoma in situ diagnosed by core-needle biopsy. Br J Surg. 2007;94:952–6. doi: 10.1002/bjs.5735. [DOI] [PubMed] [Google Scholar]
- 7.Tan JC, McCready DR, Easson AM, Leong WL. Role of sentinel lymph node biopsy in ductal carcinoma-in-situ treated by mastectomy. Ann Surg Oncol. 2007;14:638–45. doi: 10.1245/s10434-006-9211-9. [DOI] [PubMed] [Google Scholar]
- 8.Han JS, Molberg KH, Sarode V. Predictors of invasion and axillary lymph node metastasis in patients with a core biopsy diagnosis of ductal carcinoma in situ: an analysis of 255 cases. Breast J. 2011;17:223–9. doi: 10.1111/j.1524-4741.2011.01069.x. [DOI] [PubMed] [Google Scholar]
- 9.Trentin C, Dominelli V, Maisonneuve P, et al. Predictors of invasive breast cancer and lymph node involvement in ductal carcinoma in situ initially diagnosed by vacuum-assisted breast biopsy: experience of 733 cases. Breast. 2012;21:635–40. doi: 10.1016/j.breast.2012.06.009. [DOI] [PubMed] [Google Scholar]
- 10.Sysmex Corporation. [Cited 16 Mar 2013.] Available from URL: http://lifescience.sysmex.co.jp/ls/products/osna/index.html.
- 11.Tsujimoto M, Nakabayashi K, Yoshidome K, et al. One-step nucleic acid amplification for intraoperative detection of lymph node metastasis in breast cancer patients. Clin Cancer Res. 2007;13:4807–16. doi: 10.1158/1078-0432.CCR-06-2512. [DOI] [PubMed] [Google Scholar]
- 12.Tamaki Y, Akiyama F, Iwase T, et al. Molecular detection of lymph node metastases in breast cancer patients: results of a multicenter trial using the one-step nucleic acid amplification assay. Clin Cancer Res. 2009;15:2879–84. doi: 10.1158/1078-0432.CCR-08-1881. [DOI] [PubMed] [Google Scholar]
- 13.Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. American Joint Committee on Cancer Cancer Staging Manual. 7th edn. New York, NY: Springer; 2010. [Google Scholar]
- 14.Remoundos DD, Ng VV, Wilson HA, Ahmed F, Chia Y, Cunnick GH. The use of one step nucleic-acid amplification (OSNA) in clinical practice: a single-centre study. Breast. 2013;22:162–7. doi: 10.1016/j.breast.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 15.Osako T, Iwase T, Kimura K, et al. Intraoperative molecular assay for sentinel lymph node metastases in early stage breast cancer: a comparative analysis between one-step nucleic acid amplification whole node assay and routine frozen section histology. Cancer. 2011;117:4365–74. doi: 10.1002/cncr.26060. [DOI] [PubMed] [Google Scholar]
- 16.Osako T, Iwase T, Kimura K, Yamashita K, Horii R, Akiyama F. Accurate staging of axillary lymph nodes from breast cancer patients using a novel molecular method. Br J Cancer. 2011;105:1197–202. doi: 10.1038/bjc.2011.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Osako T, Iwase T, Kimura K, Masumura K, Horii R, Akiyama F. Incidence and possible pathogenesis of sentinel node micrometastases in ductal carcinoma in situ of the breast detected using molecular whole lymph node assay. Br J Cancer. 2012;106:1675–81. doi: 10.1038/bjc.2012.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Notomi T, Okayama H, Masubuchi H, et al. Loop-mediated isothermal amplification of DNA. Nucleic Acids Res. 2000;28:E63. doi: 10.1093/nar/28.12.e63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mori Y, Nagamine K, Tomita N, Notomi T. Detection of loop-mediated isothermal amplification reaction by turbidity derived from magnesium pyrophosphate formation. Biochem Biophys Res Commun. 2001;289:150–4. doi: 10.1006/bbrc.2001.5921. [DOI] [PubMed] [Google Scholar]
- 20.Ihaka R, Gentleman R. R: a language for data analysis and graphics. J Comput Graph Stat. 1996;5:299–314. [Google Scholar]
- 21.Moore KH, Sweeney KJ, Wilson ME, et al. Outcomes for women with ductal carcinoma-in-situ and a positive sentinel node: a multi-institutional audit. Ann Surg Oncol. 2007;14:2911–7. doi: 10.1245/s10434-007-9414-8. [DOI] [PubMed] [Google Scholar]
- 22.Huo L, Sneige N, Hunt KK, Albarracin CT, Lopez A, Resetkova E. Predictors of invasion in patients with core-needle biopsy-diagnosed ductal carcinoma in situ and recommendations for a selective approach to sentinel lymph node biopsy in ductal carcinoma in situ. Cancer. 2006;107:1760–8. doi: 10.1002/cncr.22216. [DOI] [PubMed] [Google Scholar]
- 23.Theissig F, Kunze KD, Haroske G, Meyer W. Histological grading of breast cancer. Interobserver, reproducibility and prognostic significance. Pathol Res Pract. 1990;186:732–6. doi: 10.1016/S0344-0338(11)80263-3. [DOI] [PubMed] [Google Scholar]
- 24.Gilchrist KW, Kalish L, Gould VE, et al. Interobserver reproducibility of histopathological features in stage II breast cancer. An ECOG study. Breast Cancer Res Treat. 1985;5:3–10. doi: 10.1007/BF01807642. [DOI] [PubMed] [Google Scholar]
- 25.Elmore JG, Wells CK, Lee CH, Howard DH, Feinstein AR. Variability in radiologists' interpretations of mammograms. N Engl J Med. 1994;331:1493–9. doi: 10.1056/NEJM199412013312206. [DOI] [PubMed] [Google Scholar]
- 26.Kerlikowske K, Grady D, Barclay J, et al. Variability and accuracy in mammographic interpretation using the American College of Radiology Breast Imaging Reporting and Data System. J Natl Cancer Inst. 1998;90:1801–9. doi: 10.1093/jnci/90.23.1801. [DOI] [PubMed] [Google Scholar]
- 27.Huang E, Cheng SH, Dressman H, et al. Gene expression predictors of breast cancer outcomes. Lancet. 2003;361:1590–6. doi: 10.1016/S0140-6736(03)13308-9. [DOI] [PubMed] [Google Scholar]
- 28.Smeets A, Daemen A, Vanden Bempt I, et al. Prediction of lymph node involvement in breast cancer from primary tumor tissue using gene expression profiling and miRNAs. Breast Cancer Res Treat. 2011;129:767–76. doi: 10.1007/s10549-010-1265-5. [DOI] [PubMed] [Google Scholar]


