Abstract
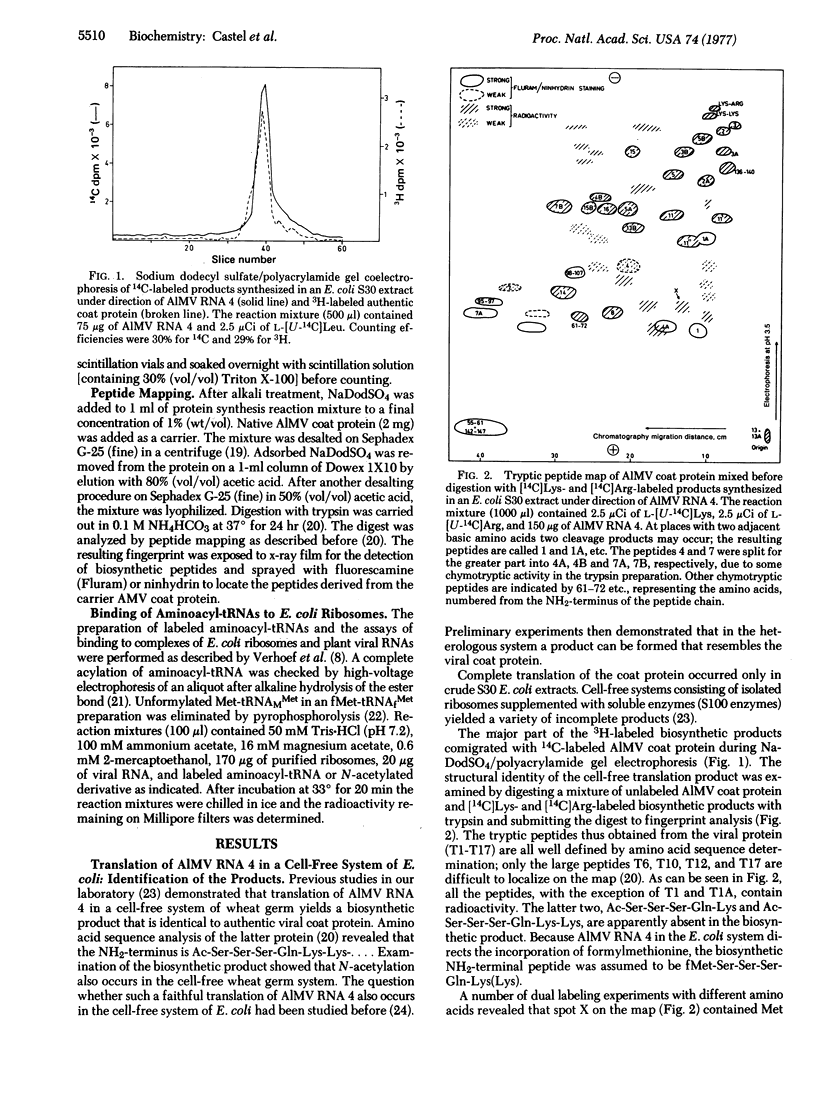
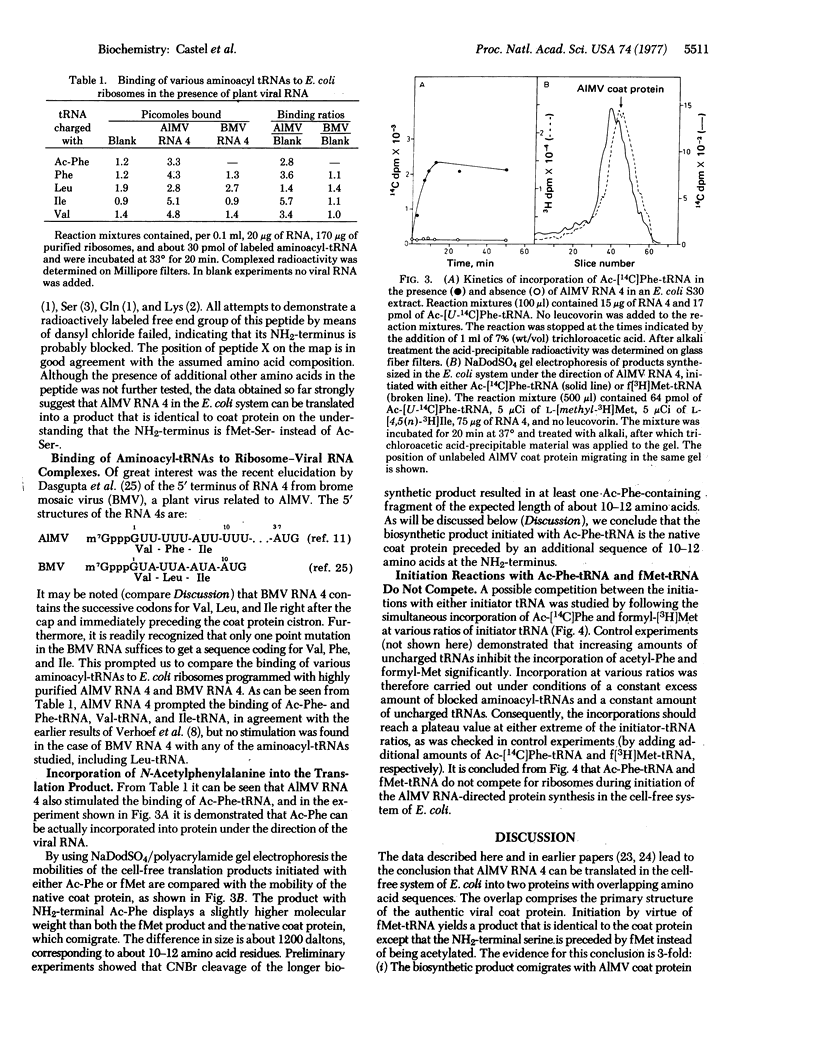
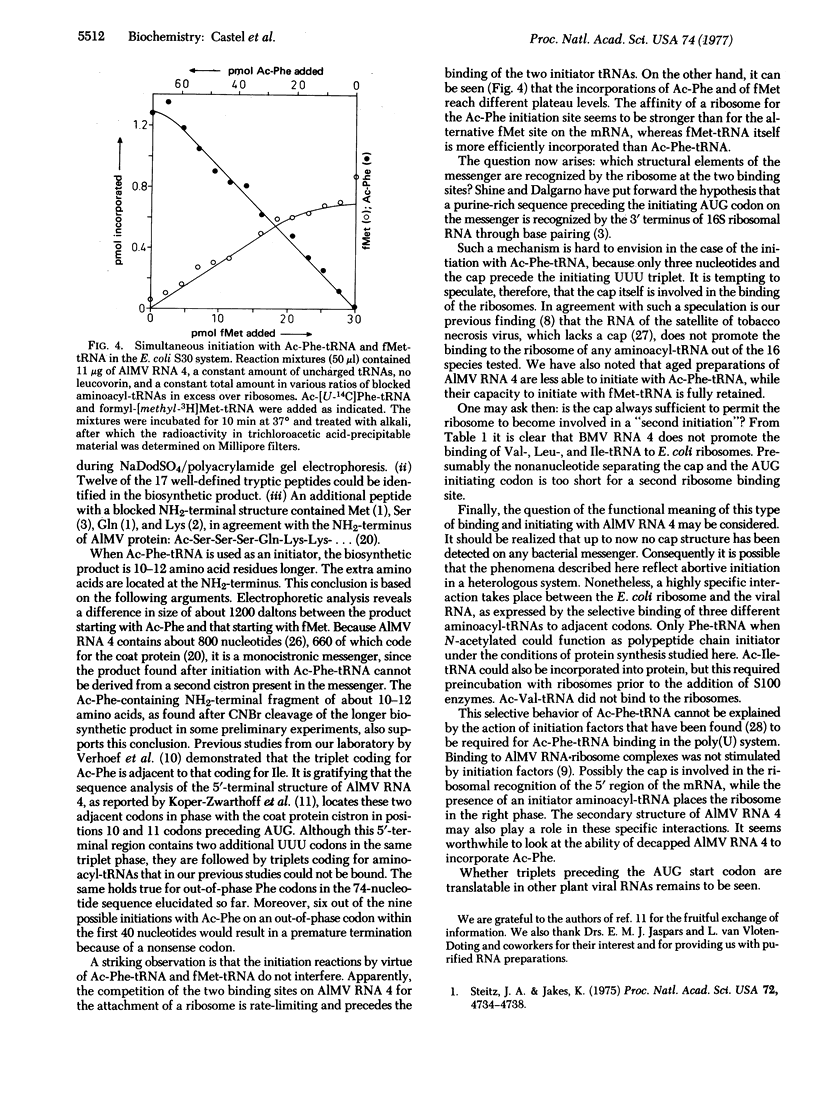
Initiation of polypeptide synthesis in a cell-free system of Escherichia coli directed by alfalfa mosaic virus RNA 4 was studied by using either fMet-tRNA or Ac-Phe-tRNA as initiator tRNA. Initiation with fMet-tRNA yielded a product that was identical to the authentic viral coat protein except that the NH2-terminal serine was preceded by fMet instead of being acetylated. When Ac-Phe-tRNA was used as initiator, the biosynthetic product was 10-12 amino acid residues longer, the extra amino acids being located at the NH2-terminus. fMet-tRNA and Ac-Phe-tRNA did not compete for ribosomes during initiation of protein synthesis, as became evident from incorporation studies using both initiator tRNAs simultaneously. It is concluded that E. coli ribosomes recognize two sites on the 5' end of alfalfa mosaic virus RNA 4 that are separated by a region of about 30 nucleotides. The results are in complete agreement with the 5'-terminal nucleotide sequence of this RNA [Koper-Zwarthoff, E. C., Lockhard, R. E., RajBhandary, U. L., Alzner-deWeerd, B. & Bol, J. F. (1977) Proc. Natl. Acad. Sci. USA 74, 5504-5508].
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bol J. F., van Vloten-Doting L., Jaspars E. M. A functional equivalence of top component a RNA and coat protein in the initiation of infection by alfalfa mosaic virus. Virology. 1971 Oct;46(1):73–85. doi: 10.1016/0042-6822(71)90007-9. [DOI] [PubMed] [Google Scholar]
- Dasgupta R., Shih D. S., Saris C., Kaesberg P. Nucleotide sequence of a viral RNA fragment that binds to eukaryotic ribosomes. Nature. 1975 Aug 21;256(5519):624–628. doi: 10.1038/256624a0. [DOI] [PubMed] [Google Scholar]
- Haenni A. L., Chapeville F. The behaviour of acetylphenylalanyl soluble ribonucleic acid in polyphenylalanine synthesis. Biochim Biophys Acta. 1966 Jan 18;114(1):135–148. doi: 10.1016/0005-2787(66)90261-9. [DOI] [PubMed] [Google Scholar]
- Heijtink R. A., Houwing C. J., Jaspars E. M. Molecular weights of particles and RNAs of alfalfa mosaic virus. Number of subunits in protein capsids. Biochemistry. 1977 Oct 18;16(21):4684–4693. doi: 10.1021/bi00640a024. [DOI] [PubMed] [Google Scholar]
- Koper-Zwarthoff E. C., Lockard R. E., Alzner-deWeerd B., RajBhandary U. L., Bol J. F. Nucleotide sequence of 5' terminus of alfalfa mosaic virus RNA 4 leading into coat protein cistron. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5504–5508. doi: 10.1073/pnas.74.12.5504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruseman J., Kraal B., Jaspars E. M., Bol J. F., Brederode F. T., Veldstra H. Molecular weight of the coat protein of alfalfa mosaic virus. Biochemistry. 1971 Feb 2;10(3):447–455. doi: 10.1021/bi00779a015. [DOI] [PubMed] [Google Scholar]
- Leung D. W., Gilbert C. W., Smith R. E., Sasavage N. L., Clark J. M., Jr Translation of satellite tobacco necrosis virus ribonucleic acid by an in vitro system from wheat germ. Biochemistry. 1976 Nov 2;15(22):4943–4950. doi: 10.1021/bi00667a030. [DOI] [PubMed] [Google Scholar]
- Lodish H. F. Specificity in bacterial protein synthesis: role of initiation factors and ribosomal subunits. Nature. 1970 May 23;226(5247):705–707. doi: 10.1038/226705a0. [DOI] [PubMed] [Google Scholar]
- Lucas-Lenard J., Lipmann F. Initiation of polyphenylalanine synthesis by N-acetylphenylalanyl-SRNA. Proc Natl Acad Sci U S A. 1967 Apr;57(4):1050–1057. doi: 10.1073/pnas.57.4.1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcker K. The formation of N-formyl-methionyl-sRNA. J Mol Biol. 1965 Nov;14(1):63–70. doi: 10.1016/s0022-2836(65)80230-3. [DOI] [PubMed] [Google Scholar]
- NIRENBERG M. W., MATTHAEI J. H. The dependence of cell-free protein synthesis in E. coli upon naturally occurring or synthetic polyribonucleotides. Proc Natl Acad Sci U S A. 1961 Oct 15;47:1588–1602. doi: 10.1073/pnas.47.10.1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neal M. W., Florini J. R. A rapid method for desalting small volumes of solution. Anal Biochem. 1973 Sep;55(1):328–330. doi: 10.1016/0003-2697(73)90325-4. [DOI] [PubMed] [Google Scholar]
- Ochoa S. Initiation of protein synthesis. Naturwissenschaften. 1976 Aug;63(8):347–355. doi: 10.1007/BF00607927. [DOI] [PubMed] [Google Scholar]
- Shine J., Dalgarno L. The 3'-terminal sequence of Escherichia coli 16S ribosomal RNA: complementarity to nonsense triplets and ribosome binding sites. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1342–1346. doi: 10.1073/pnas.71.4.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steitz J. A., Jakes K. How ribosomes select initiator regions in mRNA: base pair formation between the 3' terminus of 16S rRNA and the mRNA during initiation of protein synthesis in Escherichia coli. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4734–4738. doi: 10.1073/pnas.72.12.4734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VOORMA H. O., GOUT P. W., VAN DUIN, HOOGENDAM B. W., BOSCH L. THE READ-OUT OF TURNIP YELLOW MOSAIC VIRUS RIBONUCLEIC ACID AS A POLYCISTRONIC MESSENGER IN CELL-FREE SYSTEMS OF ESCHERICHIA COLI. Biochim Biophys Acta. 1965 Mar 15;95:446–460. doi: 10.1016/0005-2787(65)90191-7. [DOI] [PubMed] [Google Scholar]
- Van Vloten-Doting L., Jaspars E. M. The uncoating of alfalfa mosaic virus by its own RNA. Virology. 1972 Jun;48(3):699–708. doi: 10.1016/0042-6822(72)90154-7. [DOI] [PubMed] [Google Scholar]
- Verhoef N. J., Bosch L. Chain initiation during polypeptide synthesis in cell-free bacterial systems programmed with a plant viral messenger. Initiation with N-acetylated aminoacyl-tRNAs on adjacent codons. Virology. 1971 Jul;45(1):75–84. doi: 10.1016/0042-6822(71)90114-0. [DOI] [PubMed] [Google Scholar]
- Verhoef N. J., Kraal B., Bosch L. The binding of aminoacyl-tRNA to complexes of Escherichia coli ribosomes and plant viral RNA. Biochim Biophys Acta. 1968 Feb 26;155(2):456–464. doi: 10.1016/0005-2787(68)90191-3. [DOI] [PubMed] [Google Scholar]
- Verhoef N. J., Lupker J. H., Cornelissen M. C., Bosch L. Chain initiation during polypeptide synthesis in cell-free bacterial systems programmed with a plant viral messenger. The formation of N-acetylphenylalanylisoleucyl-tRNA on the messenger-ribosome complex. Virology. 1971 Jul;45(1):85–90. doi: 10.1016/0042-6822(71)90115-2. [DOI] [PubMed] [Google Scholar]
- Vermeer C., van Alphen W., van Knippenberg P., Bosch L. Initiation factor-dependent binding of MS2 RNA to 30-S ribosomes and the recycling of IF-3. Eur J Biochem. 1973 Dec 3;40(1):295–308. doi: 10.1111/j.1432-1033.1973.tb03197.x. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- van Beynum G. M., de Graaf J. M., Castel A., Kraal B., Bosch L. Structural studies on the coat protein of alfalfa mosaic virus. The complete primary structure. Eur J Biochem. 1977 Jan 3;72(1):63–78. doi: 10.1111/j.1432-1033.1977.tb11225.x. [DOI] [PubMed] [Google Scholar]
- van Dieijen G., van Knippenberg P. H., van Duin J. The specific role of ribosomal protein S1 in the recognition of native phage RNA. Eur J Biochem. 1976 May 1;64(2):511–518. doi: 10.1111/j.1432-1033.1976.tb10330.x. [DOI] [PubMed] [Google Scholar]
- van Knippenberg P. H. A possible role of the 5' terminal sequence of 16S ribosomal RNA in the recognition of initiation sequences for protein synthesis. Nucleic Acids Res. 1975 Jan;2(1):79–85. doi: 10.1093/nar/2.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]


