Abstract
Although tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) has shown efficacy in a phase 2 clinical trial, development of resistance to TRAIL by tumor cells is a major roadblock. We investigated whether quercetin, a flavonoid, can sensitize human ovarian cancer cells to TRAIL. Results indicate that quercetin sensitized cancer cells to TRAIL. The quercetin induced expression of death receptor DR5 but did not affect expression of DR4 in cancer cells. The induction of DR5 was mediated through activation of JNK and through upregulation of a transcription factor CCAAT enhancer-binding protein homologous protein (CHOP); as silencing of these signaling molecules abrogated the effect of quercetin. Upregulation of DR5 was mediated through the generation of reactive oxygen species (ROS), as ROS scavengers reduced the effect of quercetin on JNK activation, CHOP upregulation, DR induction, TRAIL sensitization, downregulated the expression of cell survival proteins and upregulated the proapoptotic proteins. Furthermore, quercetin enhances TRAIL mediated inhibition of tumor growth of human SKOV-3 xenograft was associated with induction of apoptosis, activation of caspase-3, CHOP and DR5. Overall, our data suggest that quercetin enhances apoptotic death of ovarian cancer cells to TRAIL through upregulation of CHOP-induced DR5 expression following ROS mediated endoplasmic reticulum-stress.
Keywords: CCAAT enhancer-binding protein homologous protein, death receptor 5, quercetin, reactive oxygen species, tumor necrosis factor-related apoptosis-inducing ligand
Tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL) has been thought to have the strongest antitumor activity in a variety of tumor cell types, while exhibiting minimal cytotoxicity in most normal cells.1,2 Death receptor 5 (DR5; TRAIL-R2) and death receptor 4 (DR4; TRAIL-R1) are members of the TNF family that are activated by TRAIL.3,4 TRAIL induces formation of death inducing signal complexes.5 However, recent reports have shown that some cancer cells are resistant to the apoptotic effects of TRAIL.6 TRAIL resistant cells can be sensitized by chemotherapeutic drugs in vitro, indicating that combined therapy may be useful to treat TRAIL resistant cancer cells. Several reports have shown that the DR5 plays an important role in sensitizing cancer cells to apoptosis induced by TRAIL and chemotherapeutic agents.7
Numerous signaling molecules are known to trigger DR5 induction, including activation of inositol-requiring enzyme 1 (IRE1), PKR-like ER kinase (PERK), and activating transcription factor (ATF6),8,9 and the binding of CCAAT/enhancer binding protein (C/EBP) homologous protein (CHOP) transcription factor to DR5 promoter.10 Reactive oxygen species (ROS), a byproduct of normal metabolic processes and generated by exogenous sources, are integral components of cell signaling pathways.11 Important downstream mediators of ROS-induced signaling are the IRE1,12 such as JNK. ROS have also been shown to induce CHOP expression.13 Thus the agents that can modulate the expression of these signaling molecules can induce DR5 expression and might offer potential as anticancer agents. One of the potential sources of such agents includes natural products derived from “Mother Nature.” Natural products have played a significant role over the years in the discovery of cancer drugs: more than 70% of drugs are of natural origin.14 Quercetin (Fig.1a) are polyphenol chemicals found naturally in fruits, vegetables, tea and other plant-derived foods and beverages. Experimental evidence suggests that quercetin have several potential anticarcinogenic characteristics, including antioxidant, antiestrogenic, antiproliferative and anti-inflammatory properties.15
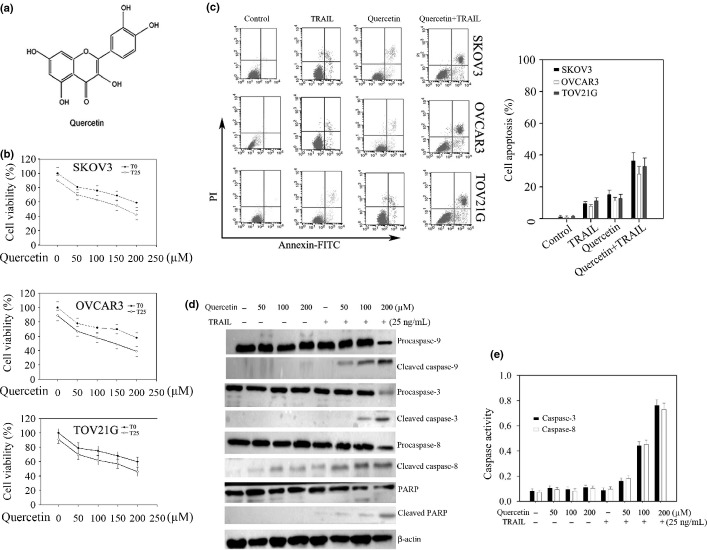
Figure 1.
Quercetin increases tumor necrosis factor-related apoptosis-inducing ligand- (TRAIL) induced apoptosis in human ovarian cancer cells. (a) Chemical structure of quercetin. (b,c) Cells were seeded at 4 × 104 cells/mL in a 96-well plate and treated with the indicated concentrations of quercetin for 24 h with or without TRAIL (25 ng/mL). (b) Cell viability. Cell viability was determined by cell counting kit as described in the “Materials and Methods”. (c) Flow cytometric analysis. Cells apoptosis were subjected to FACS analysis after staining with annexin-V/PI. (d) Expression of proapoptotic proteins. SKOV-3 cells treated were lysed for Western blot analysis. (e) Caspase-3 and 8 activities in vitro. SKOV-3 cell lysates were obtained from the cells treated as above. All the procedures were done according to the “Materials and Methods”.
Furthermore, quercetin is mediated by the dissociation of Bax from Bcl-xL and the activation of caspase families in human prostate cancer cells.16 Quercetin may have antitumor effects on human cervical cancer HeLa cells via AMP-activated protein kinase (AMPK) induced HSP70 and downregulation of epidermal growth factor receptor (EGFR).17 Quercetin induces apoptosis via AMPK activation and p53-dependent apoptotic cell death in HT-29 colon cancer cells.18 Although quercetin had been demonstrated to display a potent in vitro growth inhibition of several tumor cell lines, the molecular mechanism by which quercetin exerts anticancer effects has not been well understood. The combined results of previous studies and the present study show that quercetin can sensitize tumor cells to TRAIL by modulating signaling molecules that regulate apoptosis.
Materials and Methods
Cell culture and viability assay
SKOV-3, OVCAR-3, TOV-21G and HOSE cells were procured from China Center for Type Culture Collection (CCTCC, Wuhan, China), and cultured according to CCTCC guidelines. Cells (4 × 104 cells/mL) in 96-well plates (100 mL/well) were treated with appropriate amount of quercetin and TRAIL (25 ng/mL) for 24 h. Ten microliters of Cell Counting Kit (CCK) solution was added to each well and the plates were further incubated at 37°C for 1.5 h. Using Dojindo's highly water-soluble tetrazolium salt, the absorbance was measured at 450 nm with a reference wavelength at 650 nm using a microplate reader MR700 (Dynatech, Chantilly, VA, USA).
Reagents and materials
Antibody to caspase-3 was obtained from Imgenex (San Diego, CA, USA). Antibodies to caspase-9, c-FLIP, p-JNK, JNK, GRP78, and CHOP were purchased from Cell Signaling (Beverley, MA, USA). Antibody to p-eIF2α was from StressGen (Ann Arbor, MI, USA). Antibody to caspase-8 was obtained from Calbiochem (San Diego, CA, USA). All other antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). DMEM and fetal bovine serum were obtained from Gibco-BRL (Grand Island, NY, USA). Polyvinylidene difluoride (PVDF, 0.22 mm) membrane was purchased from Bio-Rad (Hercules, CA, USA). [g-32P] ATP was from BMS (Avenue, NY, USA). All the other reagents were obtained from Sigma (St. Louis, MO, USA).
Apoptosis analysis
Apoptosis was analyzed by FACS as previously described.8 After 5 × 105 cells/well were seeded to a 6-well plate for 24 h, the cells were then treated with or without quercetin and/or TRAIL (25 ng/mL) for 24 h. Then, the cells were harvested, washed with PBS, and stained with FITC-Annexin V (Sigma) and propidium iodide (PI). The apoptosis of cells was analyzed by flow cytometry using Cell Quest Software.
Measurement of reactive oxygen species generation
2′,7′-dichlorodihydrofluorescein diacetate (DCFH-DA) can be deacetylated by intracellular esterase to the non-fluorescent DCFH, which can be oxidized by ROS to the fluorescent compound 2′, 7′-dichloroflorescein (DCF). The fluorescence intensity of DCF is proportional to the amount of ROS produced by the cells. After 5 × 105 cells/well was seeded to a 6-well plate for 24 h, the cells were treated with quercetin for 24 h. The assay was conducted as described previously.11
Determination mitochondrial membrane potential (ΔΨm)
Cells (2 × 105 cells/well) in 12-well plate were treated with quercetin at various concentrations, and grown in 5% CO2 and 95% air at 37°C. At the end of incubation, cells from each treatment were harvested and washed twice by PBS, and then resuspended in 500 μL of DiOC6 (1 μM) for the level of ΔΨm. Then cells were incubated at 37°C in a dark room for 30 min and analyzed immediately by flow cytometry as described previously.19
Transfection with siRNA
We used HiPerFect transfection reagent for silencing CHOP. Scrambled siRNA was used as a siRNA control. Cells were plated and allowed to adhere for 24 h. On the day of transfection, 12 μL of transfection reagent was added to 25 nM siRNA in a final volume of 100 μL of culture medium. After 48 h, cells were treated with quercetin and then exposed to TRAIL for 24 h.
Analysis of gene expression by real-time PCR
Total RNAs were isolated using TRIzol reagent (Invitrogen) from tumor cells according to the manufacturer's instructions. Quantitative real-time PCR for the CHOP, DR5 and GAPDH genes was performed using procedures previously described.20 The primer sequences were as follows: CHOP, sense 5′-GCACCTCCCAGAGCCCTCACTCTCC-3′, antisense 5′-GTCTACTCCAAGCC TTCCCCCTGCG-3′; DR5, sense 5′-GTCTGCTCTGATCACCCAAC-3′, antisense 5′-CTGCAACTGTGACTCCTATG-3′; GAPDH, sense 5′-TCATTGACCTCAACTA CATGGTTT-3′, antisense 5′-GAAGATGGTGATGGGATTTC-3′. The results were expressed as the expression level of each gene relative to that of housekeeping gene GAPDH.
Western blot analysis
Following incubation of cells in the presence or absence of quercetin and/or TRAIL (25 ng/mL), cell were lysed or homogenized for western blot analysis as previously described.20 Primary antibodies and horseradish peroxidase conjugated secondary antibodies were purchased from StressGen (Ann Arbor, MI, USA), SantaCruz Biotechnology (Santa Cruz, CA, USA), Calbiochem (San Diego, CA, USA) and Cell Signaling (Beverley, MA, USA), respectively.
In vitro caspase-3 activity assay
After appropriate treatment, in vitro caspase activity was measured using a caspase activation kit according to the manufacturer's protocol (R&D Systems, Minneapolis, MN, USA). Active caspase cleaves the peptide and releases chromophore pNA that can be detected spectrophotometrically at a wavelength of 405 nm.
Xenograft assay
Four- to six-week-old female athymic nude mice were purchased from SLAC Laboratory Animal Company (Shanghai, China). The mice were maintained in the accredited animal facility of Tongji Medical College, and used for studies approved by the Animal Care and Use Committee of Tongji Medical College. About 5 × 106 SKOV-3 cells were injected subcutaneously into both right and left flanks. Thirty mice were assigned randomly to each group. Since each mouse was implanted with two xenografts, each group had 20 tumors. Two days after tumor implantation, mice in the control group received PBS, whereas mice in the treatment group received 2 mg/kg quercetin suspended in PBS by oral gavage and/without TRAIL (100 ng, i.p.) every day. Beginning on the 7th day after cell implantation, tumor volume was measured thrice a week using vernier calipers until day 30 as previously described by us.19 At the termination of the experiment, mice were killed at 24 h after the last administration of compound. Tumor samples were fixed in paraformaldehyde.
Immunohistochemistry
Muscle tissues from the inoculation sites of the treated mice were surgically excised, embedded in paraffin, and stained with hematoxylin and eosin for histopathologic evaluation. For indirect immunostaining, fresh tissues were embedded in an optimum cutting temperature solution and cut into 10-μm sections. The sections were fixed by acetone and incubated overnight at 4°C with rabbit anti-human caspase-3 or CHOP or DR5 monoclonal antibody diluted at 1/100. Biotinylated rat anti-rabbit immunoglobulin G was used as the secondary antibody, followed by streptavidin-conjugated horseradish peroxidase in the third step. The antibodies were purchased from Santa Cruz.
Intracellular ATP detection
After exposure of the cells to quercetin or/and TRAIL (25 ng/mL), equal amounts of cell lysates were subjected to the measurement of ATP level according to the manufacturer's protocol (Promega, Madison,WI, USA).
Statistical analysis
The results are expressed as the mean value ± SD and are interpreted by anova repeated measures test. Differences are considered statistically significant when P < 0.05.
Result
Quercetin showed potent cell growth inhibition of SKOV-3, OVCAR-3 and TOV-21G cells and sensitizes tumor cells to TRAIL-induced apoptosis
To investigate the effect of quercetin on TRAIL-induced apoptosis, SKOV-3, OVCAR-3 and TOV-21G cells were respectively exposed to 25 ng/mL of TRAIL with or without quercetin at various concentrations. It was found that combined treatment of the cells with both TRAIL and quercetin showed significantly higher cell death compared to TRAIL or quercetin alone (Fig.1b). The IC50 values for quercetin combined with or without TRAIL in ovarian cancer cells such as SKOV-3, OVCAR-3 and TOV-21G were found to be 222.1 ± 5.64 μM versus 153.3 ± 4.03 μM, 217.2 ± 4.89 μM versus 147.4 ± 3.86 μM, and 237.6 ± 6.07 μM versus 159.4 ± 3.64 μM, respectively.
However, 200 μM quercetin did not significantly affect cell viability in normal ovarian cells (HOSE cells) (Fig. S1a). Flow cytometric analysis and Hoechst 33 258 staining tests further supported that combination of both TRAIL and quercetin could dramatically enhance cell apoptosis (Fig.1c, Fig. S1b). The results indicated that apoptosis in SKOV-3, OVCAR-3 and TOV-21G cells was induced at 14.85 ± 2.93%, 11.99 ± 2.33%, and 12.67 ± 2.61% by quercetin, at 9.42 ± 1.75%, 8.53 ± 1.21%, and 10.82 ± 2.01% by TRAIL, and at 36.42 ± 5.04%, 30.34 ± 4.54%, and 33.29 ± 5.28% by the combination of the two agents (Fig.1c). In accordance with these results, activation of caspase-3, 8, and 9 and PARP cleavage were significantly increased when the SKOV-3 cells were exposed to both TRAIL and quercetin (Fig.1d,e). In Figure1e, after SKOV-3 cells were treated with 0, 50, 100, and 200 μM quercetin combined with or without TRAIL (25 ng/mL) for 24 h, the activation of caspase-3 in a dose-dependent manner was 0.14 ± 0.02 ng/mL and 0.15 ± 0.019 ng/mL, 0.18 ± 0.021 ng/mL and 0.22 ± 0.023 ng/mL, 0.17 ± 0.022 ng/mL and 0.51 ± 0.049 ng/mL, and 0.165 ± 0.018 ng/mL and 0.87 ± 0.06 ng/mL; the activation of caspase-8 in a dose-dependent manner was 0.13 ± 0.019 ng/mL and 0.16 ± 0.017 ng/mL, 0.17 ± 0.015 ng/mL and 0.23 ± 0.022 ng/mL, 0.155 ± 0.016 ng/mL and 0.52 ± 0.044 ng/mL, and 0.17 ± 0.017 ng/mL and 0.81 ± 0.68 ng/mL, respectively. These results indicate that quercetin increases TRAIL-induced apoptosis in ovarian cancer cells.
Combined quercetin and TRAIL treatment induces mitochondria dysfunction and downregulates the expression of various antiapoptotic proteins
Many of the mitochondrial cell survival proteins such as XIAP, Bcl-2, Bcl-xL, and cFLIP have been shown to cause TRAIL resistance.21,22 To see the intrinsic activity of quercetin in mitochondria, SKOV-3 cells were treated with quercetin and the level of intracellular ATP was measured. As expected, quercetin dose dependently reduced ATP formation (Fig.2a). Correspondingly, numerous pieces of evidence have shown that apoptosis is associated with the increase of intracellular ROS levels and the loss of ΔΨm, of mitochondria. The results indicated that quercetin significantly increased the intracellular ROS levels and decreased the levels of ΔΨm in SKOV-3 cells in a dose-dependent course (Fig.2b,c). These results indicate that quercetin significantly affects mithochondrial membrane potential. In addition, quercetin obviously inhibited Bcl-2, Bcl-xL, XIAP, and Survivin expression, whereas FLICE like inhibitory protein (cFLIP) was not significantly affected (Fig.2d). These results raised the possibility that quercetin induced mitochondrial dysfunction and downregulation of some cell survival proteins could contribute to the enhancement of TRAIL induced apoptosis (Fig. S2a–d).
Figure 2.
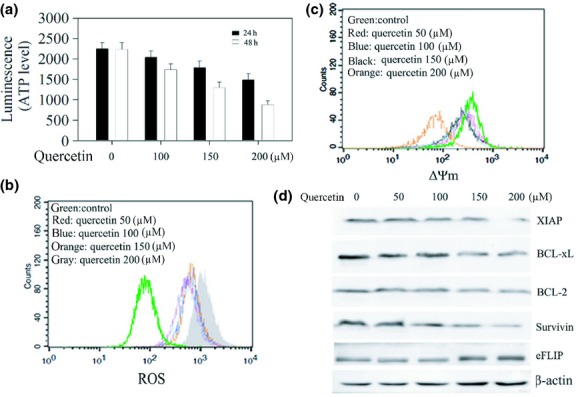
Quercetin induces mitochondrial dysfunction and downregulates antiapoptotic proteins. (a) Effect of quercetin on ATP production. SKOV-3 cells exposed to quercetin at varying concentrations were incubated for the indicated times. After lysis, equal amounts of cell lysates were used for the detection of ATP level as described in the “Materials and Methods”. (b) Increase of the reactive oxygen species (ROS) level in SKOV-3 cells showing a concentration dependent manner. (c) Decrease of the ΔΨm level in SKOV-3 cells showing a dose-related manner. (d) Western blotting analysis for the expression of antiapoptotic proteins. SKOV-3 cells treated were lysed for Western blot analysis..
ROS is essential for potentiation of TRAIL-induced apoptosis by Quercetin
Reactive oxygen species has been implicated in the induction of DR5 and plays an important role in sensitizing cancer cells to apoptosis induced by TRAIL and chemotherapeutic agents.7,8,11 First, we investigated whether quercetin induced upregulation of death receptors requires ROS. As expected, induced DR5 expression was observed by the quercetin treatment in ovarian cancer cells (Fig.3a). Moreover, quercetin induced DR5 expression and the level of ROS was suppressed by N-acetyl-L-cysteine (NAC), a ROS scavenger (Fig.3b,c). Next, we examined the role of ROS in potentiation of TRAIL-induced apoptosis by quercetin. SKOV-3 cells were treated with NAC before quercetin and TRAIL treatment, as shown in Figure3(d), the number of apoptotic cells induced by quercetin plus TRAIL was reduced from 39.5 ± 3.87% to 17.3% ± 2.01% when cells were pretreated with NAC. Overall, these results suggest that ROS play a critical role in potentiation of TRAIL-induced apoptosis by quercetin.
Figure 3.
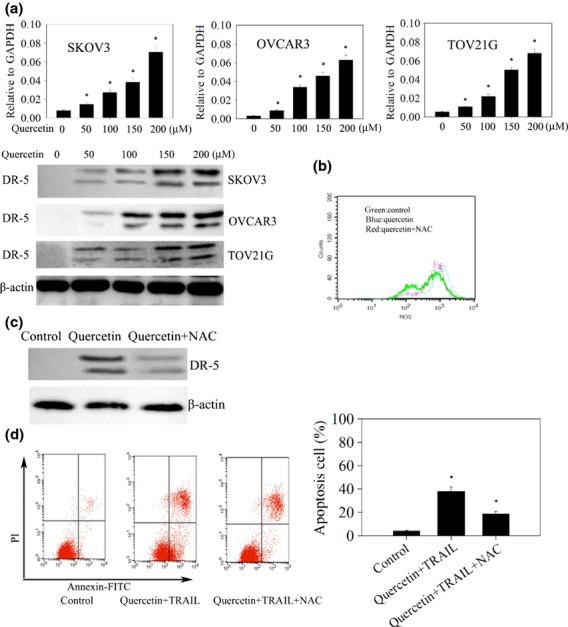
Quercetin upregulates DR5 through mediation of reactive oxygen species (ROS). (a) Quercetin enhances cell-surface expression of DR5. Cells were treated with 200 μM quercetin for 24 h, and cell-surface expression of DR5 identified by real time-polymerase chain reaction (PCR) (upper) and western blot (lower) analysis. *P < 0.05 when compared to control. (b–d) SKOV3 cells were pre-exposed to N-acetyl-L-cysteine (NAC) for 1 h, washed off, labeled with 2′,7′-dichlorodihydrofluorescein diacetate (DCFH-DA), and then exposed to quercetin for 24 h. (b) The intracellular ROS levels were then measured by flow cytometry. (c) The whole-cell extracts were analyzed by Western blotting using DR5 antibodies. (d) Apoptosis was measured by annexin-V/PI as described in Materials and Methods. *P < 0.05 when compared to control.
Effect of ROS on quercetin induced ER stress in ovarian cells
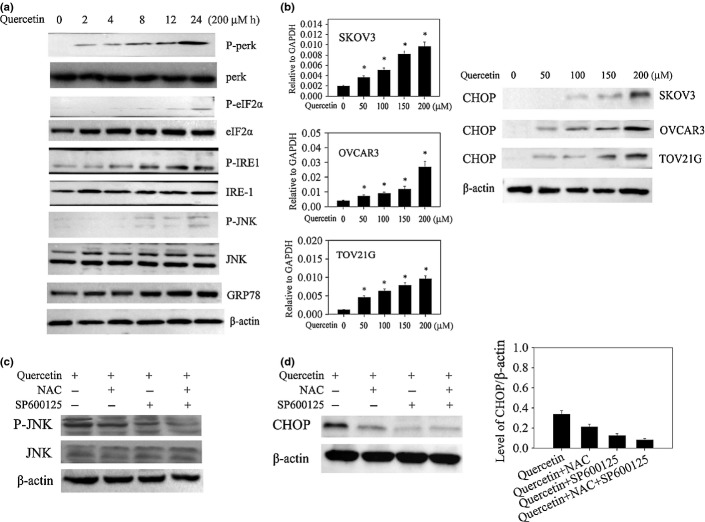
Mitochondrial stress is frequently accompanied by an endoplasmic reticulum stress (ER-stress) signaling.9 Upon ER-stress induction, three proteins could be activated: IRE1α, PERK, and ATF6. Phosphorylation of c-jun N-terminal kinase (JNK) and eIF2α also follows IRE1a and PERK activations, respectively. When the tumor cells were treated with quercetin for different times, it appeared that phosphorylation of IRE1α and JNK was significantly increased compared to other ER-associated proteins (Fig.4a). GRP78 and CHOP expressions were reported to occur downstream of IRE1α, our data showed that there was no effect on GRP78 expression while CHOP expression could be detected following quercetin treatment, suggesting that CHOP mediated pathway might be involved in quercetin-induced ER-stress and the subsequent apoptosis (Fig.4b).
Figure 4.
Quercetin induces endoplasmic reticulum (ER) stress signaling pathway through mediation of reactive oxygen species (ROS). (a) Cells were treated with quercetin at 200 μM for the indicated times, and equal amounts of cell lysates were subjected to western blot analysis with specific antibodies. (b) Cells were treated with quercetin for 24 h and expression of CCAAT enhancer-binding protein homologous protein (CHOP) identified by real time-polymerase chain reaction (PCR) (left) and western blot (right) analysis. *P < 0.05 when compared to control. (c,d) cells were pre-exposed to NAC and/or SP600125 for 1 h, washed off, labeled with 2′,7′-dichlorodihydrofluorescein diacetate (DCFH-DA), and then exposed to quercetin for 4 h. (c) JNK and p-JNKs were measured by western blotting. (d) Expression of CHOP identified by western blot analysis (left). The protein levels for each sample were determined as a ratio to their corresponding β-actin levels (the gray density of desired blots of CHOP/the gray density of desired blots of β-actin) (right). P < 0.05 when compared to control.
On the basis of the above data, we hypothesized that ER stress and apoptosis induced by quercetin in ovarian cancer cells could be due to ROS generation via JNK mediating CHOP. To verify this, when SKOV-3 cells were pretreated with NAC, quercetin induced JNK phosphorylation was inhibited (Fig.4c, Fig. S1c), which prevented CHOP production in SKOV-3 cells (Fig.4d). On the other hand, CHOP production and JNK phosphorylation induced by quercetin was also inhibited by a selective JNK inhibitor (SP600125) (Fig.4c,d). Moreover, only the joint of the JNK inhibitor and NAC completely abolished the effect of quercetin (Fig.4c,d, Fig. S1d,e). In Figure4d, The protein levels for each sample were determined as a ratio to their corresponding β-actin levels, compared with the control group (0.37 ± 0.04), CHOP was decreased at 0.23 ± 0.02 by the NAC group, at 0.17 ± 0.02 by the JNK inhibitor group, and at 0.12 ± 0.01 by the combination of the two agents. Therefore, these results indicate that ROS are implicated in quercetin induced upregulation of CHOP via JNK activation, which may result from the suppression of quercetin induced ER stress.
CHOP is crucial for upregulating DR5 expression and enhances the TRAIL mediated apoptosis effect by Quercetin
CHOP, a resident chaperone in the ER, is a key regulator of DR5.10,23 To determine in more detail how quercetin modulates the expression of DR5, SKOV-3 cells were treated with quercetin for 24 h and qPCR analysis was performed for the determination of CHOP expression. Quercetin significantly increased CHOP expression in a dose-dependent manner (Fig.4b). To further confirm that CHOP regulates DR5 expression, SKOV-3 cells were transfected with CHOP siRNA, followed by quercetin. Knockdown of CHOP in SKOV-3 cells diminished quercetin induced increased production of DR5 (Fig.5a). Moreover, CHOP knockdown greatly reduced caspase-3 and 9 activation as well as PARP cleavage in cells treated with both quercetin and TRAIL (Fig.5b). Fluorescence-activated cell sorting analyses showed also that cell apoptosis was restored upon the depletion of CHOP by siRNA treatment (Fig.5c). These findings suggest that CHOP upregulates DR5 expression, which could be one of the mechanisms by which quercetin enhances TRAIL induced apoptosis in ovarian cancer cells.
Figure 5.
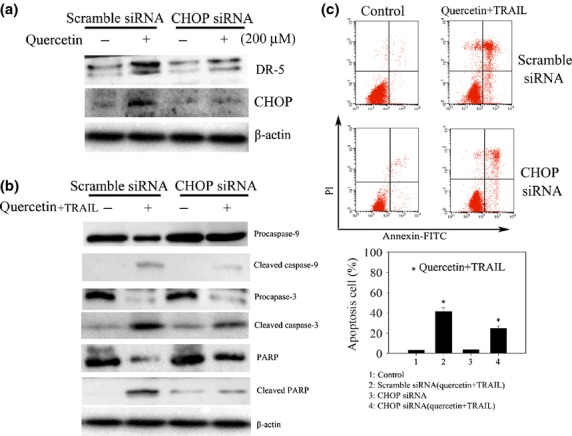
CCAAT enhancer-binding protein homologous protein (CHOP) plays a crucial role in enhancing apoptotic effect of quercetin in SKOV-3 cells treated with tumor necrosis factor-related apoptosis-inducing ligand (TRAIL). (a) Knockdown of CHOP with siRNA represses DR5 expression in SKOV-3 cells. Scrambled siRNA and CHOP siRNA were transfected into the cells followed by quercetin treatment for 24 h. Total cell lysates were subjected to Western blot analysis with specific antibodies to CHOP and DR5. (b) Western blotting analysis for the detection of caspase-3 and 9 activation and PARP cleavage. Cells treated with siRNA as in (a) were further challenged with quercetin (200 μM) and TRAIL (25 ng/mL) for 24 h and were lysed to be subjected to Western blot with the respective antibodies. (c) Flow cytometric analysis. Cells treated as in (b) were stained for the determination of annexin V/PI positive populations using flow cytometry. *P < 0.05 when compared to control.
Quercetin exhibits antitumor activity in ovarian cancer cell xenografts
Based on the above results, we next investigated whether quercetin enhances TRAIL induced ovarian tumor apoptosis in vivo. To test this, we injected 5 × 106 SKOV-3 cells subcutaneously on both right and left flanks of female athymic nude mice. The treated group received quercetin by oral gavage with or without TRAIL, while the control group received PBS. Tumor volume was recorded thrice a week using vernier calipers and weight of mice was recorded twice a week. Our results show that tumor growth was reduced by the quercetin treated group, whereas only quercetin in combination with TRAIL significantly suppressed the growth of ovarian tumors (Fig.6a). Interestingly, the weight of mice from both groups did not differ significantly suggesting that quercetin was not toxic to the mice (Fig.6b). To test whether quercetin enhances TRAIL-induced tumor apoptosis was associated with ER stress by CHOP/DR5 pathway in vivo. A feature of apoptosis was observed in the tumor sections stained with immunohistochemistry that detected cleaved-caspase-3, CHOP, and DR5 is significantly increased in combined quercetin and TRAIL treated tumor (Fig.6c). Overall, these findings demonstrate that quercetin enhances TRAIL-induced apoptosis in vivo and is associated with CHOP/DR5 pathway.
Figure 6.
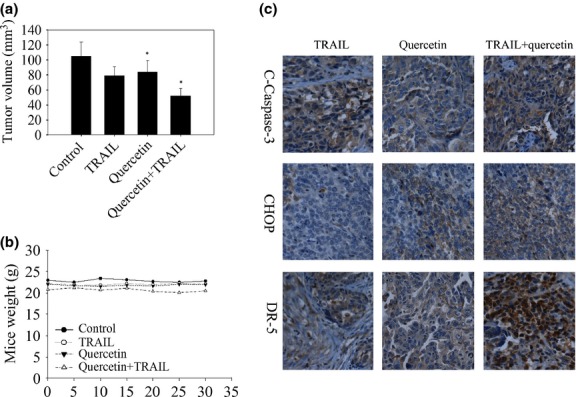
In vivo antitumor effects of quercetin treated with tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) in SKOV-3 xenografts. SKOV-3 tumor cells were implanted into athymic nude mice. Mice received 2 mg/kg quercetin by oral gavage with/or without TRAIL 100 ng/day as described in the Materials and Methods Section. (a) Effect of TRAIL, quercetin combination with or without TRAIL on ovarian tumor growth. *P < 0.05 when compared to control. (b) Animal weights during the course of the experiment. (c) Expression of clevead-caspase-3, CHOP, and DR5 in tumor tissues of SKOV-3 xenograft mouse model.
Discussion
Although TRAIL is one of the potent cytokines with the potential to kill cancer cells selectively, its use has limitations because of the resistance that certain cancer types develop.24 Therefore, agents that are safe and can sensitize cancer cells to TRAIL are needed. Here we provide evidence that quercetin, a bioactive plant flavonoid, can sensitize human ovarian cancer cells to TRAIL. Since overexpression of DR5 in TRAIL-resistant cancer cells restored TRAIL sensitivity.25 Our results showed that quercetin causes a significant increase in the levels of DR5 protein in ovarian cancer cells. Interestingly, quercetin treatment did not affect the levels of other IAP (XIAP, cIAP1, and cIAP2), emphasizing the specific effect of quercetin on DR5 expression. The increased level of DR5 expression, leading to stimulation of the death receptor pathway, seems to be the cause of the activation of caspase-3, caspase-8, caspase-9 and PARP. However, enhanced TRAIL-induced apoptosis by quercetin induced DR5 upregulation is associated with increased activation of the caspase pathway.26
In the present study, we demonstrated that quercetin, which was originally isolated as a H+–ATP synthase inhibitor,27 sensitized tumor cell killing by TRAIL. Flow cytometric assay showed that quercetin exposure can cause cell apoptosis of ovarian cancer cell, ΔΨm disruption, ROS generation, and cytochrome c release that, due to mitochondrial dynamics, affects mitochondrial function and vice versa. It was reported that mitochondrial dysfunction including as ROS overproduction, ΔΨm disruption, and ATP reduction could damage mitochondrial dynamics.19 In addition, in accordance with the previous reports demonstrating the relationship between quercetin-induced mitochondrial dysfunction and cell apoptosis,28 we also showed that quercetin repressed the mitochondrial survival proteins including Bcl-2, Bcl-xL, and Survivin, leading to the enhanced apoptosis of cancer cells to TRAIL. Considering the observations that overexpression of antiapoptotic Bcl-2, Bcl-xL, and Survivin has been shown to be linked to tumor cell resistance to TRAIL.29,30
We found that one of the most important upstream signals required for the upregulation of death receptors is ROS. The result shown that quercetin induced the generation of ROS in ovarian cancer cells, use of ROS scavengers downregulated the expression of DR5 induced by quercetin, the potentiation of TRAIL-induced apoptosis by quercetin was also abrogated by the use of ROS scavengers. Moreover, given the report by Sciarretta et al.30 demonstrating that ROS is an important genomic response to ER stress and is characterized by the activation of three distinct signal transduction pathways mediated by PERK, IRE1 and ATF6. ROS scavengers inactivated JNKs and downregulated CHOP. Thus ROS generation is an upstream event that leads to JNK activation, in particular IRE1α activation and CHOP induction, which in turn are responsible for the increased upregulation of TRAIL receptors. On the another hand, CHOP is thought to be one of the most important transcription factors that can directly bind to DR5 promoter region and regulate DR5 expression dynamically.10 Our observations further revealed that induction of DR5 by the quercetin is mediated through CHOP upregulation. These observations are supported by earlier reports showing the role of ROS in upregulating CHOP and enhancing the effects of TRAIL.31
Recent studies have shown that quercetin is a strong inducer of apoptosis in diverse cancer cells.15,17 However, it is unclear whether quercetin enhances TRAIL-induced apoptosis of ovarian cancer cells in vivo occurs in response to treatment with this compound in vitro. In the present study, our results show that induction of apoptosis by quercetin indeed occurred in vivo, which could be responsible for the inhibitory effects on tumor growth by quercetin. Furthermore, only TRAIL in combination with quercetin significantly suppressed the growth of ovarian tumors, suggesting that quercetin enhances sensitivity of cancer cells to the apoptotic agent TRAIL and its potential use as an anticancer agent or an adjunct to current cancer therapies. Consistent with the in vitro data, our in vivo results indicate that increasing of clevead-caspase-3, CHOP, and DR5 is closely correlated with the reduction of SKOV-3 tumor xenografts, further supporting our notion.
Taken together, our study demonstrates that mitochondrial proteins (ROS) are mediating the apoptotic process, downstream or in parallel, CHOP might be a crucial player for DR5 upregulation and cancer cell killing by quercetin. IRE1a-JNK signaling could also be considered to be the major ER-stress pathway for quercetin-induced CHOP expression, thus contributing to the enhanced apoptotic death of ovarian cancer cells to TRAIL. Hence, quercetin could be an attractive candidate for combined chemotherapy against cancer. Future studies using clinically relevant animal models, however, are needed to fully realize the potential of this fascinating molecule in the prevention and treatment of cancer.
Acknowledgments
This work was supported by the National Science Foundation of China (Nos. 81373433), National Science Foundation of Hubei province (Nos. 2012FFB01906), and Education Department of Hubei province for Young Talents Foundation (Nos. Q20132605).
Disclosure
The authors have no conflict of interest.
Funding information
National Science Foundation of China (81373433). National Science Foundation of Hubei province (2012FFB01906). Education Department of Hubei province for Young Talents Foundation (Q20132605).
Supporting Information
Additional supporting information may be found in the online version of this article:
Quercetin enhances TRAIL induced apoptosis in human ovarian cancer cells.
Fig. S2 Quercetin enhances TRAIL induced mitochondrial dysfunction and downregulation of some cell survival proteins in human ovarian cancer cells.
References
- 1.Bhardwaj A, Aggarwal BB. Receptor mediated choreography of life and death. J Clin Immunol. 2003;23:317–32. doi: 10.1023/a:1025319031417. [DOI] [PubMed] [Google Scholar]
- 2.Pan G, O'Rourke K, Chinnaiyan AM, et al. The receptor for the cytotoxic ligand TRAIL. Science. 1997;276:111–3. doi: 10.1126/science.276.5309.111. [DOI] [PubMed] [Google Scholar]
- 3.Kurbanov BM, Fecker LF, Geilen CC, et al. Resistance of melanoma cells to TRAIL does not result from upregulation of antiapoptotic proteins by NF-kappaB but is related to downregulation of initiator caspases and DR4. Oncogene. 2007;26:3364–77. doi: 10.1038/sj.onc.1210134. [DOI] [PubMed] [Google Scholar]
- 4.van Geelen CM, Pennarun B, Le PT, et al. Modulation of TRAIL resistance in colon carcinoma cells: different contributions of DR4 and DR5. BMC Cancer. 2011;11:39. doi: 10.1186/1471-2407-11-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aggarwal BB, Gupta SC, Kim JH. Historical perspectives on tumor necrosis factor and its superfamily: 25 years later, a golden journey. Blood. 2012;119:651–65. doi: 10.1182/blood-2011-04-325225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ndozangue TO, Sebbagh M, Merino D, et al. A mitochondrial block and expression of XIAP lead to resistance to TRAIL induced apoptosis during progression to metastasis of a colon carcinoma. Oncogene. 2008;27:6012–22. doi: 10.1038/onc.2008.197. [DOI] [PubMed] [Google Scholar]
- 7.Kim EH, Yoon MJ, Kim SU, et al. Arsenic trioxide sensitizes human glioma cells, but not normal astrocytes, to TRAIL induced apoptosis via CCAAT/enhancer-binding protein homologous protein dependent DR5 upregulation. Cancer Res. 2008;68:266–75. doi: 10.1158/0008-5472.CAN-07-2444. [DOI] [PubMed] [Google Scholar]
- 8.Kim JY, Kim EH, Park SS, et al. Quercetin sensitizes human hepatoma cells to TRAIL-induced apoptosis via Sp1 mediated DR5 upregulation and proteasome mediated c-FLIPS downregulation. J Cell Biochem. 2008;105:1386–98. doi: 10.1002/jcb.21958. [DOI] [PubMed] [Google Scholar]
- 9.Kraskiewicz H, FitzGerald U. InterfERing with endoplasmic reticulum stress. Trends Pharmacol Sci. 2012;33:53–63. doi: 10.1016/j.tips.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 10.Yamaguchi H, Wang HG. CHOP is involved in endoplasmic reticulum stress-induced apoptosis by enhancing DR5 expression in human carcinoma cells. J Biol Chem. 2004;279:45495–502. doi: 10.1074/jbc.M406933200. [DOI] [PubMed] [Google Scholar]
- 11.Gupta SC, Hevia D, Patchva S, et al. Upsides and downsides of reactive oxygen species for cancer: the roles of reactive oxygen species in tumorigenesis, prevention, and therapy. Antioxid Redox Signal. 2012;16:1295–322. doi: 10.1089/ars.2011.4414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Torres M, Forman HJ. Redox signaling and the MAP kinase pathways. BioFactors. 2003;17:287–96. doi: 10.1002/biof.5520170128. [DOI] [PubMed] [Google Scholar]
- 13.Tang JR, Nakamura M, Okura T, et al. Mechanism of oxidative stress-induced GADD153 gene expression in vascular smooth muscle cells. Biochem Biophys Res Commun. 2002;1:1255–9. doi: 10.1006/bbrc.2002.6336. [DOI] [PubMed] [Google Scholar]
- 14.Newman DJ, Cragg GM. Natural products as sources of new drugs over the 30 years from 1981 to 2010. J Nat Prod. 2012;75:311–35. doi: 10.1021/np200906s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gates MA, Tworoger SS, Hecht JL, et al. A prospective study of dietary flavonoid intake and incidence of epithelial ovarian cancer. Int J Cancer. 2007;121:2225–32. doi: 10.1002/ijc.22790. [DOI] [PubMed] [Google Scholar]
- 16.Lee DH, Szczepanski M, Lee YJ. Role of Bax in quercetin induced apoptosis in human prostate cancer cells. Biochem Pharmacol. 2008;75:2345–55. doi: 10.1016/j.bcp.2008.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jung YH, Heo J, Lee YJ, et al. Quercetin enhances TRAIL induced apoptosis in prostate cancer cells via increased protein stability of death receptor 5. Life Sci. 2010;86:351–7. doi: 10.1016/j.lfs.2010.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim HJ, Kim SK, Kim BS, et al. Apoptotic effect of quercetin on HT-29 colon cancer cells via the AMPK signaling pathway. J Agric Food Chem. 2010;58:8643–50. doi: 10.1021/jf101510z. [DOI] [PubMed] [Google Scholar]
- 19.Szabadkai G, Bianchi K, Varnai P, et al. Chaperone mediated coupling of endoplasmic reticulum and mitochondrial Ca2+ channels. J Cell Biol. 2006;175:901–11. doi: 10.1083/jcb.200608073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wei G, Zhang GM, Liu Y, et al. IFN-γ withdrawal after immunotherapy potentiates B16 melanoma invasion and metastasis by intensifying tumor integrin αvβ3 signaling. Int J Cancer. 2008;123:702–8. doi: 10.1002/ijc.23553. [DOI] [PubMed] [Google Scholar]
- 21.Bodmer JL, Holler N, Reynard S, et al. TRAIL receptor-2 signals apoptosis through FADD and caspase-8. Nat Cell Biol. 2000;2:241–3. doi: 10.1038/35008667. [DOI] [PubMed] [Google Scholar]
- 22.Seol DW, Li J, Seol MH, Park SY, Talanian RV, Billiar TR. Signaling events triggered by tumor necrosis factor related apoptosis inducing ligand (TRAIL): caspase-8 is required for TRAIL-induced apoptosis. Cancer Res. 2001;61:1138–43. [PubMed] [Google Scholar]
- 23.Oh YT, Liu X, Yue P, et al. ERK/ribosomal S6 kinase (RSK) signaling positively regulates death receptor 5 expression through co-activation of CHOP and Elk1. J Biol Chem. 2010;285:41310–9. doi: 10.1074/jbc.M110.153775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dida F, Li Y, Iwao A, et al. Resistance to TRAIL induced apoptosis caused by constitutional phosphorylation of Akt and PTEN in acute lymphoblastic leukemia cells. Exp Hematol. 2008;36:1343–53. doi: 10.1016/j.exphem.2008.04.011. [DOI] [PubMed] [Google Scholar]
- 25.Reuss DE, Mucha J, Hagenlocher C, et al. Sensitivity of malignant peripheral nerve sheath tumor cells to TRAIL is augmented by loss of NF1 through modulation of MYC/MAD and is potentiated by curcumin through induction of ROS. PLoS ONE. 2013;8:e57152. doi: 10.1371/journal.pone.0057152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tian X, Ye J, Alonso-Basanta M, et al. Modulation of CCAAT/enhancer binding protein homologous protein (CHOP) dependent DR5 expression by nelfinavir sensitizes glioblastoma multiforme cells to tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) J Biol Chem. 2011;286:29408–16. doi: 10.1074/jbc.M110.197665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee SM, Moon J, Cho Y, et al. Quercetin upregulates expressions of peroxisome proliferators activated receptor γ, liver X receptor α, and ATP binding cassette transporter A1 genes and increases cholesterol efflux in human macrophage cell line. Nutr Res. 2013;33:136–43. doi: 10.1016/j.nutres.2012.11.010. [DOI] [PubMed] [Google Scholar]
- 28.Zhang L, Fang B. Mechanisms of resistance to TRAIL induced apoptosis in cancer. Cancer Gene Ther. 2005;12:228–37. doi: 10.1038/sj.cgt.7700792. [DOI] [PubMed] [Google Scholar]
- 29.Song JJ, An JY, Kwon YT, Lee YJ. Evidence for two modes of development of acquired tumor necrosis factor related apoptosis inducing ligand resistance. Involvement of Bcl-xL. J Biol Chem. 2007;282:319–28. doi: 10.1074/jbc.M608065200. [DOI] [PubMed] [Google Scholar]
- 30.Sciarretta S, Zhai P, Shao D, et al. Activation of Nox4 in the endoplasmic reticulum promotes cardiomyocyte autophagy and survival during energy stress through the PERK/eIF-2α/ATF4 pathway. Circ Res. 2013;113:1253–64. doi: 10.1161/CIRCRESAHA.113.301787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sung B, Prasad S, Ravindran J, et al. Capsazepine, a TRPV1 antagonist, sensitizes colorectal cancer cells to apoptosis by TRAIL through ROS-JNK-CHOP-mediated upregulation of death receptors. Free Radic Biol Med. 2012;53:1977–87. doi: 10.1016/j.freeradbiomed.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Quercetin enhances TRAIL induced apoptosis in human ovarian cancer cells.
Fig. S2 Quercetin enhances TRAIL induced mitochondrial dysfunction and downregulation of some cell survival proteins in human ovarian cancer cells.