Abstract
The purpose of this meta-analysis was to compare higher dose (≥30 Gy) and lower dose (<30 Gy) radiotherapy (RT) on palliation of symptoms and survival in patients with locally advanced lung cancer. A search of PubMed and Google Scholar was conducted on 10 June 2013 using combinations of the search terms: radiotherapy, non-small-cell lung carcinoma, palliative, supportive, symptom relief. Inclusion criteria were: (i) palliative thoracic RT; (ii) randomized controlled trial; (iii) English language; and (iv) compared outcomes between higher dose (≥30 Gy) and lower dose (<30 Gy) RT. The primary outcome was palliation of symptoms (cough, chest pain, hemoptysis), and 1- and 2-year overall survival. Tests of heterogeneity, sensitivity, and publication bias were performed. Five randomized controlled trials with a total of 1730 patients with lung cancer were included in the meta-analysis. There were 925 patients treated with a higher RT dose (≥30 Gy) and 805 treated with a lower RT dose (<30 Gy). The combined odds ratios (ORs) indicated no significant difference in palliation of cough, chest pain, and hemoptysis between the higher dose and lower dose RT groups (combined ORs = 0.88, 1.83, 1.39, respectively). The 1- and 2-year OS rates were similar between the high and low dose RT groups (combined ORs = 1.09 and 1.38, respectively). This meta-analysis indicates that high dose (≥30 Gy) and lower dose (<30 Gy) RT provide similar symptom palliation and 1- and 2-year OS in patients with locally advanced lung cancer.
Keywords: Inoperable, locally advanced, lung cancer, palliation, radiotherapy
Most patients who present with inoperable, locally advanced lung cancer are treated with palliative intent. The goals of therapy are to relieve pain and other symptoms such as cough, dyspnea, and hemoptysis, and to improve or maintain quality of life (QoL). Radiotherapy (RT) has been clearly shown to improve symptoms such as hemoptysis, cough, chest pain, dyspnea, and airway obstruction in patients with lung cancer.(1)– (3) However, the optimal dose of RT required to palliate symptoms has not been well-defined, and trials comparing different regimens have reported the conflicting results with respect to symptom palliation, survival, and QoL.(1)– (7) Specifically, it is not clear if short protracted courses of RT provide similar results to longer courses, and if lower dose RT can provide palliation of symptoms and survival similar to higher dose RT.
Prior systematic reviews and meta-analyses have found no difference in symptom relief with high dose vs. lower dose regimens; however, a higher dose of RT has been associated with a small increase in survival, especially in patients with a better performance status.(8)– (10) While higher dose regimens may result in an improvement in survival, they are associated with greater toxicity, especially esophagitis.(9,10) Interestingly, a Cochrane review by Lester et al.(9) indicated that because of the heterogeneity among studies no formal meta-analysis could be conducted.
Recent evidence-based clinical practice guidelines from the American Society for Radiation Oncology for palliative thoracic RT in patients with lung cancer indicated that higher dose/fractionation external beam RT (EBRT) (e.g., 30 Gy/10 fraction equivalent or greater) was associated with modest improvements in symptom scores and survival, particularly in patients with a good performance status; however, the higher doses were associated with greater esophageal toxicity.(11) Because of the increased toxicity, the report suggested that shorter EBRT dose/fractionation schedules (e.g., 20 Gy in five fractions), which provide adequate symptom relief with fewer side effects should be used for patients who desire a shorter treatment course or those with a poor performance status.
Based on the 30 Gy dosage defined in the aforementioned guidelines, the purpose of this meta-analysis was to compare the effect of higher dose (≥30 Gy) and lower dose (<30 Gy) EBRT on palliation of symptoms and survival in patients with locally advanced lung cancer.
Materials and Methods
Literature search strategy
A search was conducted of PubMed and Google Scholar using combinations of the search terms: radiotherapy, non-small-cell lung carcinoma, palliative, supportive, symptom relief. The search date was 10 June 2013. Each publication was carefully examined, including the names of all authors, to avoid duplication of data.
Selection criteria
Studies were selected for inclusion in this analysis based on the following criteria: (i) palliative thoracic RT, which was defined as RT given to the chest by an external beam with a palliative intent (i.e., with the intent of controlling symptoms, not cure); (ii) randomized controlled trial; (iii) English language; and (iv) compared outcomes between higher dose (≥30 Gy) and lower dose (<30 Gy) RT. Exclusion criteria for this analysis were as follows: (i) endobronchial brachytherapy, also known as internal radiotherapy (the radiation source is placed inside the bronchus); (ii) prospective non-randomized study; (iii) retrospective study; (iv) one arm study; and (v) letters, comments, editorials, case reports.
Data extraction
Two independent reviewers extracted the data from eligible studies. A third reviewer was consulted for resolution of disagreement. Data extracted included author, year of publication, study type, stage of lung cancer, RT regimen, number of cases, age and gender of the patients, palliation of cough, chest pain, and hemoptysis, 1- and 2-year overall survival, and treatment related toxicities.
Quality assessment and data analysis
The Delphi list was used for the quality assessment of randomized clinical trials.(12)
Two outcome measures were used to evaluate the clinical effectiveness of two different doses of RT. The primary outcome was palliation of symptoms (cough, chest pain, and hemoptysis), and the secondary outcomes were 1- and 2-year overall survival (OS) rates. Odds ratios (OR) with 95% confidence intervals (CIs) were calculated for binary outcomes and compared between patients treated with higher dose (≥30 Gy) and those treated with lower dose (<30 Gy) RT; an OR >1 indicates that the higher RT dose is favored. Heterogeneity among the studies was assessed by calculating Cochran Q and the I2 statistic. For the Q statistic, P < 0.10 was considered to indicate statistically significant heterogeneity. The I2 statistic indicates the percentage of the observed between-study variability caused by heterogeneity. Heterogeneity determined using the I2 statistic was defined as follows: 0–24% = no heterogeneity; 25–49% = moderate heterogeneity; 50–74% = large heterogeneity; and 75–100% = extreme heterogeneity. If either the Q statistic (P < 0.1) or I2 statistic (>50%) indicated heterogeneity existed between studies, the random-effects model (DerSimonian-Laird method) was used. Otherwise, the fixed-effects model was used (Mantel-Haenszel method). Pooled ORs for all binary outcomes were calculated and a two-sided P-value < 0.05 was considered to indicate statistical significance. Sensitivity analysis was performed for the primary outcome based on the leave-one-out approach. All statistical analyses were performed using the Comprehensive Meta-Analysis statistical software, version 2.0 (Biostat, Englewood, NJ, USA).
Results
Literature search and study characteristics
A total of five studies(13)– (17) met the inclusion criteria, and were included in this analysis. A flow diagram of the study selection is shown in Figure 1. The basic characteristics of the studies included in the meta-analysis are summarized in Table 1. Among the five studies included, there were two equivalent and three non-inferior randomized controlled trials. A total 1730 patients with non-small cell lung cancer were enrolled in the five studies, consisting of 925 treated with a higher RT dose (≥30 Gy) and 805 treated with a lower RT dose (<30 Gy). The total number of patients in each of the studies ranged from 148 to 509. For patients treated with a higher RT dose, palliation of cough ranged from 49% to 58%, palliation of chest pain from 64% to 86%, and palliation of hemoptysis from 80% to 97%; for patients treated with a lower RT dose, palliation of cough ranged from 47% to 65%, palliation of chest pain from 50% to 81%, and palliation of hemoptysis from 75% to 95%. For patients treated with higher dose RT, 1-year OS ranged from 11% to 36%, and 2-year OS ranged from 8% to 12%; for patients treated with lower dose RT, 1- and 2-year OS ranged from 19% to 29% and 4% to 9%, respectively.
Fig. 1.
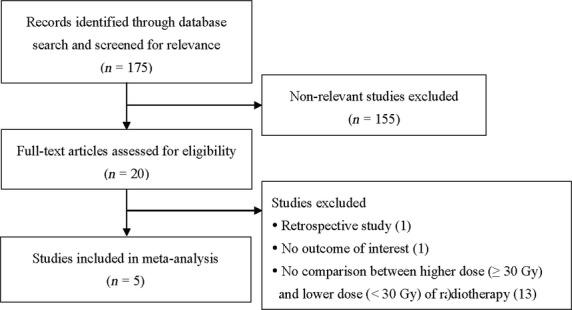
Flow diagram of study selection.
Table 1.
Characteristics of studies included in the meta-analysis
| Reference number | First author (year) | Study type | Type of lung cancer | RT regimen |
Number of cases (higher versus lower dose) | Age (years), median | Gender, male (%) | Palliation of symptoms (%) |
Overall survival rate (%) |
Treatment-related toxicity | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Higher dose group (RT dose ≥30 Gy) | Lower dose group (RT dose <30 Gy) | Cough | Chest pain | Hemoptysis | 1-year | 2-year | ||||||||
| 13 | Kramer (2005) | RCT, non-inferior | Inoperable, stage IIIA/B, IV | 30 Gy in 10 fractions | 16 Gy in two fractions | 148 vs. 149 | 68.9 vs. 69.3 | 79.7 vs. 79.9 | NA | NA | NA | 11 vs. 20 | NA | NA |
| 14 | Erridge (2005) | RCT, non-inferior | NA | 30 Gy in 10 fractions | 10 Gy single fraction | 74 vs. 74 | Mean: 67.7 vs. 66.2 | 73.0 vs. 62.2 | 58 vs. 51 | 84 vs. 50 | 97 vs. 88 | 28 vs. 19 | 8 vs. 4 | Moderate or severe fatigue 49%, nausea and vomiting 34% vs. moderate or severe fatigue 41%, nausea and vomiting 30% |
| 15 | Sundstrøm (2004) | RCT, equivalent | Locally advanced, stage III, IV | 42 Gy in 15 fractions; 50 Gy in 25 fractions | 17 Gy in two fractions | 264 vs. 143 | 68.5 vs. 68 | 74.3 vs. 77.0 | 49 vs. 47 | NA | 91.5 vs. 82 | 30 vs. 29 | 12 vs. 8 | NA |
| 16 | Macbeth (1996) | RCT, non-inferior | Inoperable | 39 Gy in 13 fractions | 17 Gy in two fractions | 254 vs. 255 | NA | 79 vs. 79 | 49 vs. 55 | 64 vs. 65 | 89 vs. 95 | 36 vs. 31 | 12 vs. 9 | Nausea (20%), anorexia (34%), dysphagia (41%) versus nausea (24%), anorexia (37%), dysphagia (29%) |
| 17 | Medical Research Council (1991) | RCT, equivalent | Inoperable | 30 Gy in 10 fractions | 17 Gy in 2 fractions | 185 vs. 184 | NA | NA | 56 vs. 65 | 86 vs. 81 | 80 vs. 75 | 23 vs. 20 | 5 vs. 5 | NA |
Data for number of cases, age, gender, palliation of individual symptoms, survival rates and treatment-related toxicity are presented as higher versus lower dose of RT. NA, non-available; RCT, randomized controlled trial; RT, radiotherapy.
Primary outcome: palliation of symptoms
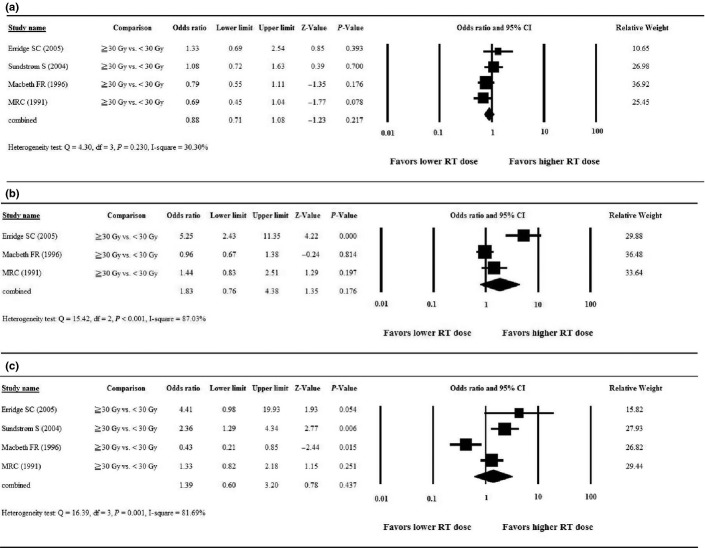
One study(13) did not report relevant data with respect to the primary outcome of palliation of cough; thus, four studies were included in the analysis of palliation of cough. After pooling of data, no significant heterogeneity among the studies was found (Q = 4.30, df = 3, P = 0.230; I2 = 30.30%); therefore, a fixed-effects model was used for the analysis. The combined OR revealed no significant difference in palliation of cough between patients treated with a higher RT dose compared to those treated with a lower RT dose. Among the four studies, ORs ranged from 0.69 to 1.33, with the combined OR = 0.88 (95% CI = 0.71–1.08, P = 0.217, Fig. 2a).
Fig. 2.
Forest plots of the meta-analysis comparing higher dose (≥30 Gy) versus lower dose (<30 Gy) radiotherapy for palliation of symptoms. (a) Cough; (b) chest pain; (c) hemoptysis. CI, confidence interval; RT, radiotherapy.
Two studies(13,15) did not report relevant data with respect to palliation of chest pain; thus, three studies were included in the analysis of palliation of chest pain. After pooling of the data, significant heterogeneity among the studies was found (Q = 15.42, df = 2, P < 0.001; I2 = 87.03%); therefore, a random-effects model was used for the analysis. The combined OR revealed no significant difference in palliation of chest pain between patients treated with a higher RT dose compared to those treated with a lower RT dose. Among the three studies, the ORs ranged from 0.96 to 1.44, with the combined OR = 1.83 (95% CI = 0.76–4.38, P = 0.176, Fig. 2b).
One study(13) did not report relevant data with respect to palliation of hemoptysis; thus, four studies were included in the analysis of palliation of hemoptysis. After pooling of the data, significant heterogeneity among the studies was found (Q = 16.39, df = 3, P = 0.001; I2 = 81.69%); therefore, a random-effects model was used for the analysis. The combined OR revealed no significant difference in palliation of hemoptysis between patients treated with a higher RT dose compared to those with a lower RT dose. Among the four studies, ORs ranged from 0.43 to 4.41, with the combined OR = 1.39 (95% CI = 0.60–3.20, P = 0.437, Fig. 2c).
Secondary outcome: OS rate
The Forest plot of the meta-analysis for the 1-year OS rate is presented in Figure 3(a). After pooling of the data, no significant heterogeneity among the studies was found (Q = 7.21, d.f. = 4, P = 0.125; I2 = 44.50%); therefore, a fixed-effects model was used for the meta-analysis of the 1-year OS rate. The combined OR revealed no significant difference in 1-year OS between patients treated with a higher RT dose compared to those with a lower RT dose. Among the five studies, ORs ranged from 0.50 to 1.66, with the combined OR = 1.09 (95% CI = 0.88–1.37, P = 0.425, Fig. 3a).
Fig. 3.
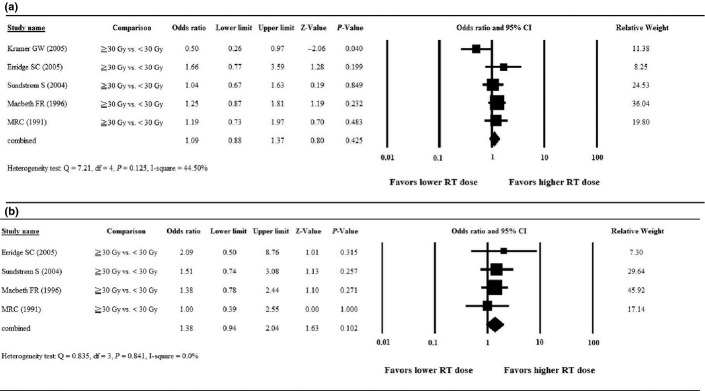
Forest plots of the meta-analysis comparing higher dose (≥30 Gy) versus lower dose (<30 Gy) radiotherapy for overall survival. (a) 1-year overall survival; (b) 2-year overall survival. CI, confidence interval; RT, radiotherapy.
One study(13) did not report data regarding the 2-year OS rate; thus, four studies were included in the analysis of 2-year OS. After pooling of the data, no significant heterogeneity among the studies was found (Q = 0.835, d.f. = 3, P = 0.841; I2 = 0.0%); therefore, a fixed-effects model was used for the analysis. The combined OR revealed no significant difference in 2-year OS between patients treated with a higher RT dose compared to those with a lower RT dose. Among the four studies, ORs ranged from 1.00 to 2.09, with the combined OR = 1.38 (95% CI = 0.94–2.04, P = 0.102, Fig. 3b).
Sensitivity analysis
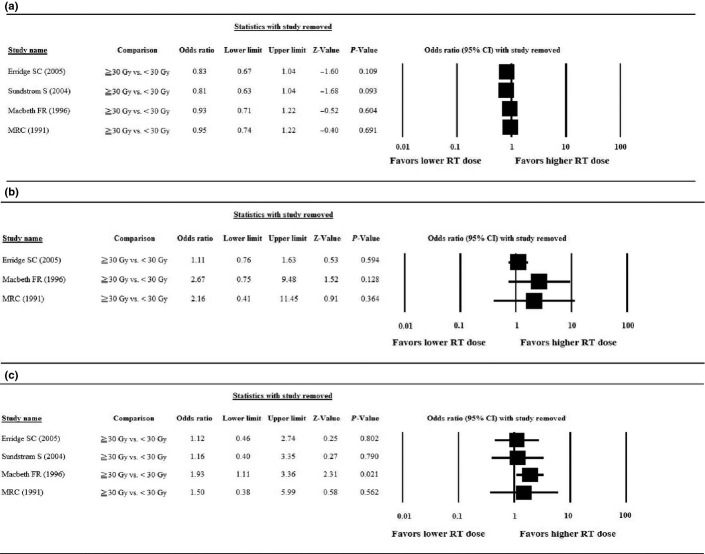
The results of the sensitivity analyses, in which the studies were omitted one-by-one, are summarized in Figure 4. For palliation of cough (Fig. 4a) and chest pain (Fig. 4b), the direction and magnitude of the pooled estimate did not vary markedly with the removal of any study, which indicates good reliability in this meta-analysis. However, for palliation of hemoptysis (Fig. 4c), the removal of the study by Macbeth et al.(16) caused the pooled OR to change from non-significant (OR = 1.39, 95% CI = 0.60–3.20, P = 0.437) to significant (OR = 1.93, 95% CI = 1.11–3.36, P = 0.021).
Fig. 4.
Results of the sensitivity analysis to examine the influence of individual studies on pooled estimates for the primary outcome of palliation of symptoms as determined using the leave-one-out approach. (a) Cough; (b) chest pain; (c) hemoptysis. CI, confidence interval.
Quality assessment and publication bias
Dephi assessment of the included studies indicated they were of high quality (Table 2). Due to the small number of selected studies, it was inappropriate to assess for publication bias using a Funnel plot. It has been previously shown that five or fewer studies are insufficient to detect Funnel plot asymmetry.(18)
Table 2.
Quality assessment of the included studies based on the Delphi list
| First author | Kramer(13) | Erridge(14) | Sundstrøm(15) | Macbeth(16) | Medical Research Council(17) |
|---|---|---|---|---|---|
| Year of publication | 2005 | 2005 | 2004 | 1996 | 1991 |
| Treatment allocation | |||||
| Was a method of randomization performed? | Y | Y | Y | Y | Y |
| Was the treatment allocation concealed? | D | Y | D | D | D |
| Were the groups similar at baseline regarding the most important prognostic indicators? | Y | Y | Y | Y | Y |
| Were the eligibility criteria specified? | Y | Y | Y | Y | Y |
| Was the outcome assessor blinded? | D | D | D | D | D |
| Was the care provider blinded? | D | D | D | D | D |
| Was the patient blinded? | D | D | D | D | D |
| Were point estimates and measures of variability presented for the primary outcome measures? | Y | Y | N | Y | Y |
| Did the analysis include an intention-to-treat analysis? | Y | Y | Y | Y | Y |
D, don't know; N, no; Y, yes.
Treatment-related toxicity
Among the five studies included in the analysis, only two(14,16) reported treatment-related toxicities. For this reason, a formal analysis could not be performed. Treatment-related toxicities were not dissimilar in the high and low dose RT groups, and the data are summarized in Table 1.
Discussion
The results of this meta-analysis comparing high dose (≥30 Gy) and a lower EBRT dose (<30 Gy) in patients with locally advanced lung cancer showed that both dosages provided equal symptom relief, and 1- and 2-year OS were similar with the two dosages.
There were five studies included in the meta-analysis, two equivalent and three non-inferior randomized controlled trials. The Dutch National Study(13) included 297 patients with stage III and IV non-small cell lung cancer (NSCLC). The patients were randomly assigned to receive radiotherapy in 10 fractions of 3 Gy (10 × 3 Gy) or in two fractions of 8 Gy (2 × 8 Gy). No difference in symptom control between groups was noted during the first 9 months; however, the patterns of control over time suggested that the higher dose group had less worsening of symptoms. One-year survival was significantly greater in the higher dose group (19.6% vs. 10.9%, P = 0.03).
Erridge et al.(14) randomly assigned 149 patients with incurable lung cancer of any type to receive EBRT of either 30 Gy in 10 fractions or 10 Gy in a single fraction. The total symptom score (TSS) was improved in 77% of patients who received the single fraction and in 92% that received 10 fractions (difference of 15%, 95% confidence interval [CI] 3–28%). The median survival was 22.7 weeks in the 10 Gy single fraction group and 28.3 weeks in the 30 Gy 10 fraction group (P = 0.197). No differences were observed in toxicity. It should be noted that this study was conducted in May 1988 and July 1993, and was underpowered with only 148 patients. In addition, symptoms were scored by the attending physicians and not the patients.
Sundstrøm et al.(15) randomly assigned 421 patients with stage III or IV NSCLC to receive either 17 Gy in two fractions (group A), 42 Gy in 15 fractions (group B), or 50 Gy in 25 fractions (group C) and the European Organization for Research and Treatment of Cancer (EORTC) Quality of Life Questionnaire (QLQ)-C30 and EORTC QLQ-lung cancer-specific module (LC13) were used to evaluate outcomes. Health-related quality-of-life (HRQoL) and symptom relief were equivalent between the three groups, and no difference in survival was noted (median survival of group A, B, and C 8.2, 7.0, and 6.8 months, respectively). The data, however, suggested that patients with stage III disease with a good performance status may achieve greater long-term survival with protracted higher dose schedules.
The Medical Research Council (MRC) Lung Cancer Working Party study of 1996 prepared by Macbeth et al.(16) randomly assigned 509 patients with inoperable locally advanced NSCLC to RT with either 17 Gy in two fractions 1 week apart or 39 Gy in 13 fractions 5 days per week. Overall symptom palliation was greater in the group that received 17 Gy in two fractions, whereas survival was greater in the group that received 39 Gy in 13 fractions, with survival rates of 31% and 36% at 1 year and 9% and 12% at 2 years in the 17 Gy and 39 Gy groups, respectively. Treatment-related dysphagia, was greater in the higher dose group (41% vs. 29%).
An earlier MRC study (1991)(17) randomly assigned 369 patients with inoperable, symptomatic NSCLC to receive RT of 17 Gy in two fractions 1 week apart or a conventional multifractionated regime of either 30 Gy in 10 fractions or 27 Gy in six fractions (a biologically equivalent dose [BED]), given daily except on weekends. The percentage of patients that achieved palliation and duration of palliation was similar between the two groups, as was median survival (17 Gy group, 179 days; 30 Gy group, 177 days).
While the meta-analysis of the studies described above indicated that there was no difference in the palliation of hemoptysis with the higher or lower dosage of RT, when the study by Macbeth et al.(16) was excluded, the combined OR became significant in favor of higher dose RT. This finding may be due to different dosages and frequencies of RT administration used in the various studies, or due to different criteria for defining hemoptysis and its palliation. Erridge et al.(14) used symptom scores to grade hemoptysis with a score of 0 indicating no hemoptysis and 4 indicating copious hemoptysis with an improvement of one grade or more considered a palliative response. Sundstrøm et al.(15) used EORTC QLQ-lung cancer-specific module (LC13) for evaluating hemoptysis and other airway symptoms and used a difference in mean score ≥10 points to indicate a clinically significant change. The 1991 MCR study(17) simply graded hemoptysis as none, mild, moderate, and severe and considered an improvement of at least one grade as a palliative response. The 1996 MRC study prepared by Macbeth et al.(16) on the other hand, used the Rotterdam Symptom Checklist and defined palliation as improvement of at least one category during the first 3 months of treatment.
Other systematic reviews have been performed examining the effects of RT regimens in patients with inoperable lung cancer. Sirzén et al.(8) performed a systematic review in 2003 that included a total of 18 301 patients, and concluded that RT for patients with inoperable disease or patients refusing surgery with stage I/II NSCLC prolongs survival and that there is some evidence that two large RT fractions may be as effective as 10–13 smaller fractions with respect to palliation of symptoms. Though no formal meta-analysis could be conducted because of heterogeneity among studies, a Cochrane review by Lester et al.(9) in 2006 concluded that no strong evidence exists that any regimen gives greater palliation, and that survival is greater in patients with a better performance status. Fairchild et al.(10) performed a systematic review in 2008, which included 13 randomized controlled trials and 3473 patients and converted RT dosages to the BED Gy10. The results showed that symptom control was similar between patients that received lower dose and higher dose RT, though a greater likelihood of symptom improvement was seen in patients who received schedules of 35 Gy10 versus a lower BED. At 1 year after treatment, survival in the higher dose and lower dose groups was 26.5% vs. 21.7%, respectively (P = 0.002). Though RT doses were converted to a BED for analysis, “lower doses” in some studies could be “higher doses” in other studies, thus heterogeneity of doses may still be present in the analysis. Of note, the study by Fairrchild et al.(10) differs methodologically from the Cochrane analysis in two ways: doses in each treatment regimen were converted to the BED, and dose outcome relationships were compared among all trials even though there was heterogeneity in the patients.
In this meta-analysis, 30 Gy was used as the cut-off value for the definition of higher or lower dose RT. For more specific evaluation, BED should be used to quantify the effects caused by long-course fractionation or hypofractionation. For this reason we calculated the BED of the higher and lower dose groups of each included study based on the formula of Fairchild et al.(10) (Table 3). As can be seen in Table 3, although the total dose is quite different between the higher dose RT groups and the lower dose RT groups, the calculated BED is only slightly larger for the higher dose RT groups than the lower dose RT groups. This may explain why our results show that both groups exhibit similar symptom control and overall survival. More rigorous comparison may be needed to differentiate the effects caused by higher dose RT versus lower dose RT, and whether long-course fractionated RT or hypofractionated RT is more clinically effective.
Table 3.
Calculated biologically equivalent dose (BED) (Gy10) of the higher and lower dose RT groups of the included studies
| First author | Year | RT regimen |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Higher dose group (≥30 Gy) |
Lower dose group (<30 Gy) |
||||||||
| Gy | Number of fractions | Duration (weeks) | BED (Gy10) | Gy | Number of fractions | Duration (days) | BED (Gy10) | ||
| Kramer(13) | 2005 | 30 | 10 | 2 | 33.5 | 16 | 2 | 8 | 28.0 |
| Erridge(14) | 2005 | 30 | 10 | 2 | 33.5 | 10 | 1 | 1 | 24.8 |
| Sundstrøm(15) | 2004 | 42 | 15 | 3 | 42.7 | 17 | 2 | 8 | 30.7 |
| 50 | 25 | 5 | 37.8 | ||||||
| Macbeth(16) | 1996 | 39 | 13 | 2.5 | 42.4 | 17 | 2 | 8 | 30.7 |
| Medical Research Council(17) | 1991 | 30 | 10 | 2 | 33.5 | 17 | 2 | 8 | 30.7 |
BED was calculated based on the formula of Fairchild et al.(10): BED (Gy10) = n d [1 + d/(α/β)] − Ln 2 (T − Tko)/(A) (Tp), where: α/β ratio = 10; T, overall treatment time; Tko (kickoff time for accelerated repopulation) = 7; A = 0.35 (as a measure of intrinsic radiosensitivity); Tp (effective doubling time) = 2.5 days; d, dose per fraction; n, number of fractions; RT, radiotherapy.
There are limitations to the current analysis that should be considered. Only five studies were included; however, all were randomized controlled trials and the total number of patients was large. There was heterogeneity of the dose regimen between studies, fractionation schedule was not taken into consideration in the analysis, and QoL life was not assessed. The use of concurrent or adjuvant chemotherapy was not examined.(19,20) Lastly, cost considerations between different RT schedules were not examined.(21)
In conclusion, the results of this meta-analysis comparing high dose (≥30 Gy) and a lower EBRT dose (<30 Gy) in patients with locally advanced lung cancer showed that both dosages provided equal symptom relief and similar 1- and 2-year OS rates. While prior studies have suggested a survival advantage with higher dose RT, particularly in patients with a better performance status, this must be weighed against greater toxicity, especially esophagitis and dysphasia associated with a higher dose of RT.
Acknowledgments
None.
Disclosure Statement
None.
References
- 1.Kepka L, Olszyna-Serementa M. Palliative thoracic radiotherapy for lung cancer. Expert Rev Anticancer Ther. 2010;10:559–69. doi: 10.1586/era.10.22. [DOI] [PubMed] [Google Scholar]
- 2.Bezjak A. Palliative therapy for lung cancer. Semin Surg Oncol. 2003;21:138–47. doi: 10.1002/ssu.10031. [DOI] [PubMed] [Google Scholar]
- 3.Brundage MD, Bezjak A, Dixon P, et al. The role of palliative thoracic radiotherapy in non-small cell lung cancer. Can J Oncol. 1996;6(Suppl. 1):25–32. [PubMed] [Google Scholar]
- 4.Abratt RP, Shepherd LJ, Mameena Salton DG. Palliative radiation for stage 3 non-small cell lung cancer: a prospective study of two moderately high dose regimens. Lung Cancer. 1995;13:137–43. doi: 10.1016/0169-5002(95)00487-4. [DOI] [PubMed] [Google Scholar]
- 5.Bezjak A, Dixon P, Brundage M, et al. Randomized phase III trial of single versus fractionated thoracic radiation in the palliation of patients with lung cancer (NCIC CTG SC. 15) Int J Radiat Oncol Biol Phys. 2002;54:719–28. doi: 10.1016/s0360-3016(02)02989-9. [DOI] [PubMed] [Google Scholar]
- 6.Senkus-Konefka E, Dziadziuszko R, Bednaruk-Młyński E, et al. A prospective, randomized study to compare two palliative radiotherapy schedules for non-small-cell lung cancer (NSCLC) Br J Cancer. 2005;92:1038–45. doi: 10.1038/sj.bjc.6602477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Simpson JR, Francis ME, Perez-Tamayo R, Marks RD, Rao DV. Palliative radiotherapy for inoperable carcinoma of the lung: final report of a RTOG multi-institutional trial. Int J Radiat Oncol Biol Phys. 1985;11:751–8. doi: 10.1016/0360-3016(85)90307-4. [DOI] [PubMed] [Google Scholar]
- 8.Sirzén F, Kjellén E, Sörenson S, Cavallin-Ståhl E. A systematic overview of radiation therapy effects in non-small cell lung cancer. Acta Oncol. 2003;42:493–515. doi: 10.1080/02841860310014453. [DOI] [PubMed] [Google Scholar]
- 9.Lester JF, Macbeth FR, Toy E, Coles B. Palliative radiotherapy regimens for non-small cell lung cancer. Cochrane Database Syst Rev. 2006;(4):CD002143. doi: 10.1002/14651858.CD002143.pub2. [DOI] [PubMed] [Google Scholar]
- 10.Fairchild A, Harris K, Barnes E, et al. Palliative thoracic radiotherapy for lung cancer: a systematic review. J Clin Oncol. 2008;26:4001–11. doi: 10.1200/JCO.2007.15.3312. [DOI] [PubMed] [Google Scholar]
- 11.Rodrigues G, Videtic GM, Sur R, et al. Palliative thoracic radiotherapy in lung cancer: an American Society for Radiation Oncology evidence-based clinical practice guideline. Pract Radiat Oncol. 2011;1:60–71. doi: 10.1016/j.prro.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Verhagen AP, de Vet HC, de Bie RA, et al. The Delphi list: a criteria list for quality assessment of randomized clinical trials for conducting systematic reviews developed by Delphi consensus. J Clin Epidemiol. 1998;51:1235–41. doi: 10.1016/s0895-4356(98)00131-0. [DOI] [PubMed] [Google Scholar]
- 13.Kramer GW, Wanders SL, Noordijk EM, et al. Results of the Dutch National study of the palliative effect of irradiation using two different treatment schemes for non-small-cell lung cancer. J Clin Oncol. 2005;23:2962–70. doi: 10.1200/JCO.2005.01.685. [DOI] [PubMed] [Google Scholar]
- 14.Erridge SC, Gaze MN, Price A, et al. Symptom control and quality of life in people with lung cancer: a randomised trial of two palliative radiotherapy fractionation schedules. Clin Oncol (R Coll Radiol) 2005;17:61–7. doi: 10.1016/j.clon.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 15.Sundstrøm S, Bremnes R, Aasebø U, et al. Hypofractionated palliative radiotherapy (17 Gy per two fractions) in advanced non-small-cell lung carcinoma is comparable to standard fractionation for symptom control and survival: a national phase III trial. J Clin Oncol. 2004;22:801–10. doi: 10.1200/JCO.2004.06.123. [DOI] [PubMed] [Google Scholar]
- 16.Macbeth FR, Bolger JJ, Hopwood P, et al. Randomized trial of palliative two-fraction versus more intensive 13-fraction radiotherapy for patients with inoperable non-small cell lung cancer and good performance status. Medical Research Council Lung Cancer Working Party. Clin Oncol (R Coll Radiol) 1996;8:167–75. doi: 10.1016/s0936-6555(96)80041-0. [DOI] [PubMed] [Google Scholar]
- 17.Inoperable non-small-cell lung cancer (NSCLC): a Medical Research Council randomised trial of palliative radiotherapy with two fractions or ten fractions. Report to the Medical Research Council by its Lung Cancer Working Party. Br J Cancer. 1991;63:265–70. doi: 10.1038/bjc.1991.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sutton AJ, Duval SJ, Tweedie RL, Abrams KR, Jones DR. Empirical assessment of effect of publication bias on meta-analyses. BMJ. 2000;320:1574–7. doi: 10.1136/bmj.320.7249.1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nawrocki S, Krzakowski M, Wasilewska-Tesluk E, et al. Concurrent chemotherapy and short course radiotherapy in patients with stage IIIA to IIIB non-small cell lung cancer not eligible for radical treatment: results of a randomized phase II study. J Thorac Oncol. 2010;5:1255–62. doi: 10.1097/JTO.0b013e3181e15d33. [DOI] [PubMed] [Google Scholar]
- 20.Ball D, Smith J, Bishop J, et al. A phase III study of radiotherapy with and without continuous-infusion fluorouracil as palliation for non-small-cell lung cancer. Br J Cancer. 1997;75:690–7. doi: 10.1038/bjc.1997.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van den Hout WB, Kramer GW, Noordijk EM, Leer JW. Cost-utility analysis of short- versus long-course palliative radiotherapy in patients with non-small-cell lung cancer. J Natl Cancer Inst. 2006;98:1786–94. doi: 10.1093/jnci/djj496. [DOI] [PubMed] [Google Scholar]