Abstract
Whether S-1 could replace 5-Fluorouracil (5-Fu) or not in the treatment of advanced gastrointestinal (GI) cancer (including advanced gastric cancer [AGS] and metastatic colorectal cancer [mCRC]) in Asian patients has been controversial. This meta-analysis was performed to compare the activity, efficacy and toxicity of S-1-based versus 5-Fu-based chemotherapy in those Asian patients. Randomized controlled trials (RCTs) were identified by electronic search of Pubmed. Relevant abstracts were manually searched to identify relevant trials. A total of 2182 patients from eight RCTs were included, and our results demonstrated that S-1-based chemotherapy significantly improved overall survival (OS) (hazard ratio [HR], 0.87; 95% confidence interval [CI], 0.77–1.00) and overall response rate (ORR) (odds ratio [OR], 1.72; 95% CI, 1.09–2.70), but no significant progression-free survival (PFS) benefit was found between arms (HR, 0.87; 95% CI, 0.72–1.06). Subgroup analyses revealed that S-1-based chemotherapy significantly improved OS and ORR in subgroups of patients with non-platinum containing regimens (P = 0.041; P = 0.034) and patients with no prior chemotherapy history (P = 0.025; P = 0.016). Statistically significant improvements of PFS and ORR in the S-1-based chemotherapy were observed in the subgroup of patients with AGC (P < 0.001; P = 0.005). S-1-based chemotherapy was characterized by significantly higher incidences of diarrhea, fatigue and thrombocytopenia, and a lower incidence of nausea. This analysis provided strong evidence for survival benefits of S-1, and S-1-based chemotherapy could be considered to replace 5-Fu-based therapy for the treatment of advanced GI cancer in Asian patients.
Keywords: 5-Fluorouracil, chemotherapy, gastrointestinal cancer, meta-analysis, S-1
Along with recent improvements in diagnostic and therapeutic modalities, the multidisciplinary management of cancer treatments has been explored to improve outcomes.(1)– (3) However, distant metastasis or recurrences require effective chemotherapies.(4) 5-Fu has been a core anticancer agent for malignancies since it was introduced by Heidelberger et al.(5) in 1957, and it has been widely used in international standard regimens for GI malignancies except etoposide, doxorubicin and cisplatin (EAP) therapy.
Gastric cancer and colorectal cancer, the two mainly discussed GI cancers in this paper, are the second and fourth highest causes of cancer-related death in the world.(6) For advanced gastric cancer (AGS), there has been no standard palliative chemotherapy regimen worldwide, while palliative chemotherapy including 5-Fu, platin compounds, docetaxel and epirubicin has been proved to prolong survival time and improve quality of life compared with best support care.(7) For mCRC, regimens of FOLFOX (5-Fu, leucovorin, and oxaliplatin) or FOLFIRI (5-Fu, leucovorin, and irinotecan) plus bevacizumab are being currently used as first line treatment.(8,9) Although the regimens of AGS and mCRC are different, 5-Fu has been an important drug in both cancer chemotherapy regimens for decades.
5-Fu is usually administered by i.v. bolus or by continuous i.v. infusion. Although the latter route is the most efficient and least toxic, it is costly and inconvenient, and most importantly, catheter-related safety concerns emerge.(10) Oral administration could avoid such iatrogenic issues, and the balance of cost and benefit has been discussed.(11,12) S-1 is a fourth generation oral fluoropyrimidine containing tegafur, 5-chloro-2, 4-dihydroxypyridine (CDHP), and potassium oxonate, in which tegafur is a pro-drug of fluorouracil, CDHP is a dihydropyrimidine dehydrogenase (DPD) inhibitor maintaining the serum concentration of fluorouracil, and potassium oxonate is an inhibitor of orotate phosphoribosyltransferase (OPRT), reducing GI toxicities.(13) Whether S-1 could replace 5-Fu has been hotly explored and discussed in recent years. The results from recent randomized phase II/III studies for AGS or mCRC have demonstrated that S-1 in combination with chemotherapies such as cisplatin, oxaliplatin, or irinotecan were at least not inferior to conventional 5-Fu-based regimens, with the benefit of convenience and reduced toxicity.(14)– (18) Some trials even reported significant overall survival (OS) or progression-free survival (PFS) benefits in favor of S-1-based chemotherapy.(14,16,19) The results of these studies, whether S-1 was more or less effective than 5-Fu, were not completely consistent. Accordingly, we undertook this meta-analysis to compare the effects of S-1-based chemotherapy with that of 5-Fu-based chemotherapy on OS, PFS, overall response rate (ORR), and toxicity in patients with advanced GI cancer.
Materials and Methods
Search strategy
We searched all published (as English-language full paper or abstract) and unpublished trials that compared S-1 with 5-Fu in the treatment of advanced GI cancer. The search was performed using PubMed and Proceedings of American Society of Clinical Oncology (ASCO) (1983 to December 2013), with various combinations of different terms: “S-1”, “5-Fluorouracil”, “randomized controlled trial”, “gastrointestinal cancer”, “gastric cancer”, “colorectal cancer”,. “colon cancer”, and “rectal cancer”. References of selected articles and previous systematic reviews were checked for any other relevant trials.
Selection of trials
Trials had to fulfill the following inclusion criteria: (i) Asian patients with AGC or mCRC at baseline; (ii) prospective phase II and III RCTs; (iii) S-1 and 5-Fu were compared without confounding by additional agents or interventions (i.e., in the combination chemotherapy, the control and experimental arms had to differ only by S-1 or 5-Fu component).
Two independent reviewers (C.C. and X.Z.) assessed the eligibility of abstracts identified by the search. The full-text article of any trial that appeared to meet the inclusion criteria was retrieved for closer examination. If multiple publications of the same trial were retrieved, or if there were data inconsistencies between publications of the same trial, all publications were included, but only the most recent and the most informative data were used.
Quality assessment
The quantitative 5-point Jadad score was used to assess the quality of included trials based on the report of the methods and results of the studies.(20)
Data extraction
To avoid bias in the data extraction process, the same two reviewers (C.C. and X.Z.) independently extracted the data from the trials and compared results. The following information was extracted from each article: (i) publication details such as type of cancer, first author, year of publication, country, phase of study, and form of publication (full/abstract); (ii) information of treatment such as chemotherapy regimens, treatment line, median OS, median PFS, ORR and toxicity; (iii) characteristics of patients such as number of patients, age, gender rate, prior chemotherapy history and ECOG (Eastern Cooperative Oncology Group) performance status (PS). Before performing the analyses, data of each published study were carefully double-checked by another reviewer (M.K.), and any disagreements were resolved by discussion. Whenever possible, we tried to obtain the updated results from the researchers via email, particularly for trials published only in abstract form.
Statistical analysis
The primary outcome measure was OS, which was defined as time from random assignment to death. Secondary outcome measures were PFS, defined as the time between date of random assignment and date of progression, or date of death for patients dead without progression, or last date of follow-up for censored patients; ORR, defined as the sum of partial and complete response rates; and toxicity, which was graded according to NCI Common Toxicity Criteria (CTC)(14,19) or on the basis of the Common Terminology Criteria for Adverse Events (CTCAE).(21)
A hazard ration (HR) was calculated to assess the survival advantage of the S-1-based chemotherapy as compared with the 5-Fu-based chemotherapy. Odds ratios (ORs) were calculated to assess objective response rate and toxic events. For toxic events, not all publications reported all grades adverse events, so severe (grade 3–4) adverse events data was extracted.
Between-study heterogeneity was estimated using the χ2-based Q statistic.(22) Heterogeneity was considered statistically significant when P heterogeneity ≤ 0.1 or I2 >50%. Primary analyses were done with a fixed effects model; secondary confirmatory analyses were done with a random effects model if there was significant heterogeneity. The presence of publication bias was evaluated by using the Begg's and Egger's tests.(23,24)
All statistical analyses were conducted with STATA version 10.0 software (Stata Corporation, College Station, TX, USA). A statistical test with a P-value < 0.05 was considered significant. All P-values were two-sided. All CIs had a two-sided probability coverage of 95%.
Subgroup analyses were done to establish whether therapeutic efficacy was affected by histological type, prior chemotherapy history and combinations with or without platinum.
Results
Characteristics of included trials
Eight eligible trials(14)– (19,25,26) were identified, including four trials for mCRC and four trials for AGC. The flow diagram is shown in Figure 1. The analysis was conducted on the data of 2182 patients and randomly assigned to receive chemotherapy with S-1 or with 5-Fu, respectively. Of the eight trials, five trials were conducted in Japan,(15,17,19,25,26) and three in China.(14,16,18) The characteristics of the eight included trials are summarized in Table 1. More than 66.9% of patients have no history of chemotherapy. At the time of analysis, four trials were fully published journal articles,(14,19,21,25) while the rest of the trials were published only in abstract form. Finally, all trials used doublet or triplet combination chemotherapy except the Japan Clinical Oncology Group (JCOG) 9912 study(19) that used S-1 as the single agent.
Fig. 1.
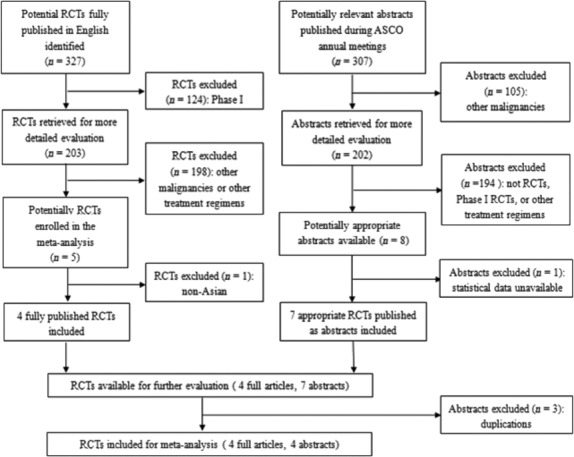
Trials flow diagram.
Table 1.
Baseline characteristics of eight randomized clinical trials in the meta-analysis
| Author, year | Country | Type | Phase | Line | Treatment regimen | Number | Male (%) | Median age (years) | Median OS (months) | Median PFS (months) | CR+PR (%) | ECOG PS | Jadad score |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Andoh et al. 2011 | Japan | mCRC | — | — | S-1/CPT-11/BV | 30 | — | — | — | 11.5 | 72.0 | — | 1 |
| 5-Fu/l-LV/CPT-11/BV | 30 | — | — | — | 10.8 | 61.5 | |||||||
| Otsuji et al. 2012 | Japan | mCRC | II | 1st | S-1/LV/L-OHP | 56 | — | — | 28.5 | 9.6 | — | 0–1 | 2 |
| 5-Fu/l-LV/L-OHP | 49 | — | — | 25.9 | 6.9 | — | |||||||
| Baba et al. 2011 | Japan | mCRC | II/III | 2nd | S-1/CPT-11 | 213 | 56.3 | 61.0 | 18.0 | 5.8 | 18.8 | 0–1 | 3† |
| 5-Fu/l-LV/CPT-11 | 213 | 57.7 | 63.0 | 17.5 | 5.1 | 16.7 | |||||||
| Yamada et al. 2013 | Japan | mCRC | III | 1st | S-1/L-OHP/BV | 256 | 66.0 | 63.0 | 29.6 | 11.7 | 62.0 | 0–1 | 3† |
| 5-Fu/l-LV/L-OHP/BV | 255 | 62.0 | 63.0 | 30.9 | 11.5 | 63.0 | |||||||
| Xu et al. 2013 | China | AGC | III | — | S-1/DDP | 120 | — | — | 10.0 | — | 22.5 | — | 1 |
| 5-Fu/DDP | 116 | — | — | 10.5 | — | 21.5 | |||||||
| Jin et al. 2008 | China | AGC | III | 1st | S-1/DDP | 74 | — | 56.5 | — | — | 37.8 | — | 1 |
| 5-Fu/DDP | 73 | — | 58.0 | — | — | 19.2 | |||||||
| Boku et al. 2009 | Japan | AGC | III | 1st | S-1 | 234 | 74.8 | 64.0 | 11.4 | 4.2 | 28.0 | 0–2‡ | 3† |
| 5-Fu | 234 | 75.2 | 63.5 | 10.8 | 2.9 | 9.0 | |||||||
| Huang et al. 2013 | China | AGC | II | 1st | S-1/paclitaxel | 119 | 74.8 | 56.0 | — | 5.1 | 42.0 | — | 2† |
| 5-Fu/paclitaxel | 110 | 69.1 | 54.0 | — | 4.3 | 24.8 |
AGC, advanced gastric cancer; BV, bevacizumab; CPT-11, irinotecan; DDP, cisplatin; ECOG PS, Eastern Cooperative Oncology Group performance status; L-OHP, oxaliplatin; LV, leucovorin; mCRC, metastatic colorectal cancer; ORR, overall response rate; OS, overall survival; PFS, progression-free survival. — not reported;
full published;
Only three persons had an ECOG performance status of two in each group, all the other individuals had an ECOG performance status of 0 or 1.
Efficacy
Data on OS were available for four trials (1235 patients; Table 2). S-1-based chemotherapy was associated with a statistically significant 13% reduction in the hazard for death as compared with 5-Fu-based chemotherapy (HR, 0.87; 95% CI, 0.77–1.00; P = 0.043; Fig. 2). Data on PFS were available for five trials (1739 patients; Table 2). S-1-based chemotherapy was also associated with a clinically 13% reduction in the hazard for death as compared with 5-Fu-based chemotherapy, but this difference was not significant (HR, 0.87; 95% CI, 0.72–1.06; P = 0.160; Fig. 3). Response rate was stated in seven trials, which included 2077 patients (Table 2). S-1-based regimens was characterized by a significant 72% increase in the OR for response in comparison with 5-Fu-based chemotherapy (OR, 1.72; 95% CI, 1.09–2.70; P = 0.019; Fig. 4).
Table 2.
Hazard ratios, P-value, and heterogeneity for progression-free survival (PFS), overall survival (OS) and overall response rate (ORR) in the stratified analyses
| Efficacy | OS |
PFS |
ORR |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | HR | P | PH | I2/% | HW | n | HR | P | PH | I2/% | HW | n | OR | P | PH | I2/% | HW | |
| All | 4 | 0.87 (0.77, 1.00) | 0.043 | 0.687 | 0.0 | 100 | 5 | 0.87 (0.72, 1.06) | 0.160 | 0.016 | 67.4 | 100 | 7 | 1.72 (1.09, 2.70) | 0.019 | 0.001 | 73.7 | 100 |
| Type | ||||||||||||||||||
| mCRC | 2 | 0.88 (0.72, 1.07) | 0.206 | 0.562 | 0.0 | 43.2 | 3 | 1.03 (0.91, 1.18) | 0.635 | 0.695 | 0.0 | 57.6 | 3 | 1.05 (0.78, 1.42) | 0.736 | 0.663 | 0.0 | 42.3 |
| AGC | 2 | 0.87 (0.73, 1.03) | 0.113 | 0.287 | 11.9 | 56.8 | 2 | 0.73 (0.62, 0.86) | <0.001 | 0.313 | 1.6 | 42.4 | 4 | 2.31 (1.29, 4.13) | 0.005 | 0.020 | 69.5 | 57.7 |
| PCH | ||||||||||||||||||
| No | 2 | 0.82 (0.69, 0.99) | 0.034 | 0.761 | 0.0 | 51.4 | 4 | 0.82 (0.65, 1.02) | 0.079 | 0.037 | 64.7 | 76.0 | 4 | 2.20 (1.06, 4.55) | 0.034 | <0.001 | 84.7 | 60.8 |
| Yes/not clear | 2 | 0.93 (0.77, 1.13) | 0.471 | 0.497 | 0.0 | 48.6 | 1 | 1.06 (0.87, 1.29) | — | — | — | 24.0 | 3 | 1.16 (0.79, 1.70) | 0.459 | 0.826 | 0.0 | 39.2 |
| Platinum | ||||||||||||||||||
| With | 2 | 0.94 (0.69, 1.29) | 0.696 | 0.337 | 0.0 | 17.2 | 2 | 1.01 (0.84, 1.21) | 0.899 | 0.430 | 0.0 | 33.6 | 3 | 1.28 (0.75, 2.19) | 0.363 | 0.069 | 62.5 | 45.8 |
| Without | 2 | 0.86 (0.75, 0.99) | 0.041 | 0.576 | 0.0 | 82.8 | 3 | 0.82 (0.62, 1.08) | 0.153 | 0.010 | 78.1 | 66.4 | 4 | 2.15 (1.16, 4.00) | 0.016 | 0.020 | 69.6 | 54.2 |
AGC, advanced gastric cancer; HR, hazard ratio; mCRC, metastatic colorectal cancer; PCH, prior chemotherapy history; PH, heterogeneity P.
—, cannot be calculated.
Fig. 2.
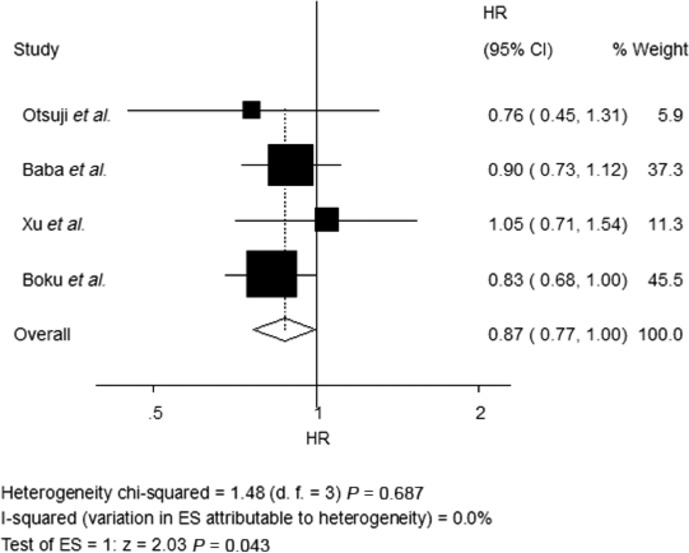
Fixed-effects model of hazard ratio (95% CI) of overall survival (OS) associated with S-1-based therapy compared with 5-Fu-based therapy.
Fig. 3.
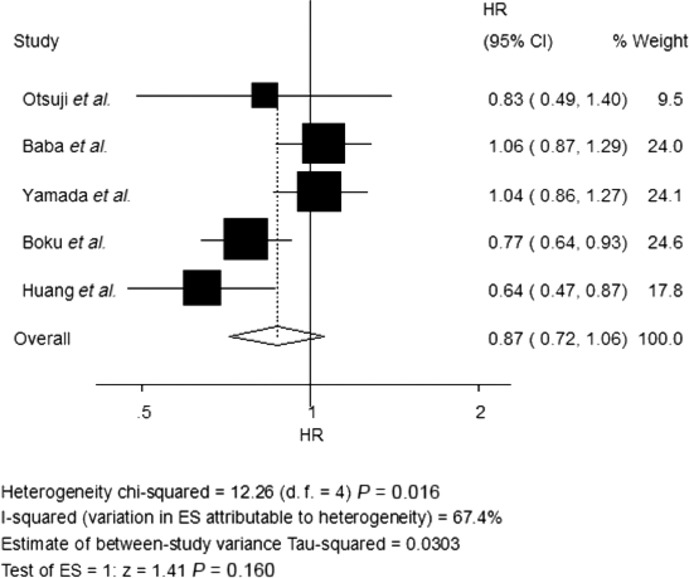
Random-effects model of hazard ratio (95% CI) of progression-free survival (PFS) associated with S-1-based therapy compared with 5-Fu-based therapy.
Fig. 4.
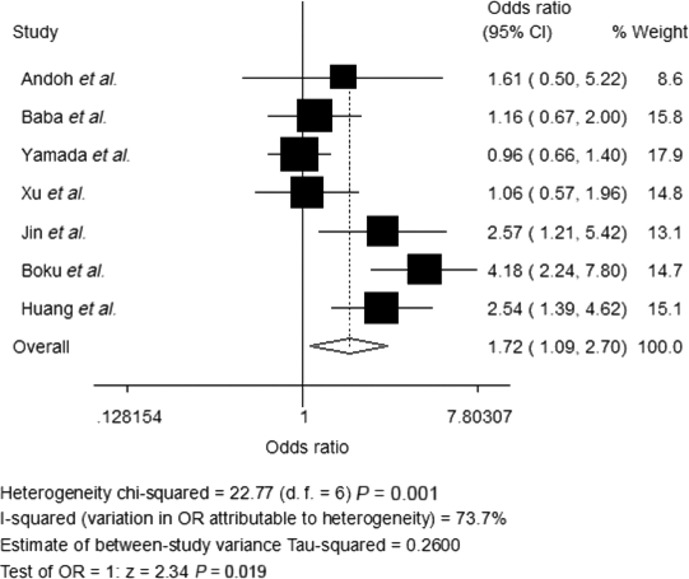
Random-effects model of hazard ratio (95% CI) of overall response rate (ORR) associated with S-1-based therapy compared with 5-Fu -based therapy.
There was no statistically significant heterogeneity in the HR for OS from the trials, and a fixed-effects model was used. Nevertheless, there was statistically significant heterogeneity both in the HR for PFS and the OR for ORR, so random-effects models were undertaken (Table 2).
Toxicity
A summary of grade 3–4 adverse effects are reported in Table 3. More than 10% of the total patients suffered neutropenia, leucopenia, anemia, or anorexia, but no significant difference of each adverse event was observed between S-1-based and 5-Fu-based chemotherapy. In contrast, S-1-based chemotherapy was characterized by a significantly higher incidence of diarrhea, fatigue or thrombocytopenia (OR: 3.18, 2.67, 2.30, respectively), and a lower incidence of nausea (OR: 0.69). Nevertheless, the incidence of each mentioned significant adverse effect was much lower than 10%. In addition, no significant difference was observed with regard to treatment-related death. Heterogeneity existed for some adverse effects among studies, possibly due to the different combinations and doses used.
Table 3.
Summary of grade 3–4 adverse events (AEs)
| Grade 3–4 AEs | No. trials | S-1 n (%) | Adverse reactions 5-Fu n (%) | OR (95% CI) | P |
|---|---|---|---|---|---|
| Neutropenia | 7 | 269 (24.77) | 302 (28.28) | 1.01 (0.43,2.35) | 0.987 |
| Anorexia | 7 | 122 (11.23) | 102 (9.55) | 1.23 (0.91,1.65) | 0.177 |
| Diarrhea | 7 | 104 (9.58) | 29 (2.72) | 3.18 (1.54,6.57) | 0.002 |
| Leukopenia | 6 | 184 (16.94) | 147 (13.76) | 1.36 (0.68,2.73) | 0.387 |
| Nausea | 6 | 85 (7.83) | 104 (9.74) | 0.69 (0.48,1.00) | 0.049 |
| Fatigue | 5 | 45 (4.14) | 17 (1.59) | 2.67 (1.52,4.68) | 0.001 |
| Anemia | 5 | 143 (13.17) | 131 (12.27) | 1.15 (0.81,1.62) | 0.427 |
| Stomatitis | 4 | 14 (1.29) | 9 (0.84) | 1.66 (0.33,8.37) | 0.542 |
| Treatment-related deaths | 4 | 6 (0.55) | 5 (0.47) | 1.15 (0.39,3.45) | 0.798 |
| Vomiting | 3 | 56 (5.16) | 55 (5.15) | 0.97 (0.60,1.56) | 0.904 |
| Thrombocytopenia | 3 | 63 (5.80) | 34 (3.18) | 2.30 (1.40,3.77) | 0.001 |
| Sensory neuropathy | 2 | 36 (3.31) | 36 (3.37) | 2.37 (0.14,41.28) | 0.554 |
Subgroup analysis
Subgroup analyses, which were based on tumor type (mCRC vs AGC), prior chemotherapy history (with no prior chemotherapy history vs with prior chemotherapy history or not clear), and combinations (with vs without platinum), were performed for OS, PFS and ORR (Table 2). The results showed that, non-platinum containing regimens resulted in a modest but significant OS benefit (HR, 0.86; 95% CI, 0.75–0.99; P = 0.041) and a 115% increase in ORR (OR, 2.15; 95% CI, 1.16–4.00; P = 0.016) in favor of S-1-based regimens. Moreover, S-1-based regimens also significantly improved OS (HR, 0.82; 95% CI, 0.69–0.99; P = 0.034) and ORR (OR, 2.20; 95% CI, 1.06–4.55; P = 0.034) in patients with no prior chemotherapy. In the subgroup of patients with AGC, statistically significant improvements of PFS (HR, 0.73; 95% CI, 0.62–0.86; P < 0.001) and ORR (OR, 2.31; 95% CI, 1.29–4.13; P = 0.005) were observed in the S-1-based regimens.
Publication bias
We performed Begg's funnel plot and Egger's test to assess the publication bias of literature. The shapes of the funnel plots (Figures not shown) indicated the absence of publication bias. Furthermore, Egger's test was used to statistically confirm the funnel plot symmetry (P = 0.814 for OS;P = 0.554 for PFS). The results still did not suggest any evidence of publication bias.
Discussion
The final results of this meta-analysis showed that S-1-based chemotherapy significantly improved OS in comparison with 5-Fu-based chemotherapy. Our data on ORR reinforces further the survival result because there was a higher response rate in the S-1 arm than that in the 5-Fu arm. However, meta-analysis showed that S-1-based therapy was not better than 5-Fu-based therapy with respect to PFS. With regard to safety profile, there was no significant difference between the two groups with respect to all grade 3–4 adverse events except for diarrhea, fatigue thrombocytopenia and nausea. However, the four mentioned adverse events appeared uncommonly in both arms. Accordingly, S-1-based therapy was associated with longer OS and higher response rate and almost equivalent safety compared with 5-Fu-based therapy.
Four of the eight trials provided data on OS (Table 2).(15,17)– (19) The results in all four trials showed that S-1-based chemotherapy did not prolong OS of patients with GI cancer. Regardless of there being no significant difference on OS in each trial, the median OS in patients assigned S-1 was much longer than that in patients assigned 5-Fu in all trials except the Xu et al. trial (Table 1). Meanwhile, the forest plot of OS (Fig. 2) showed favorable results for S-1 compared with 5-Fu for all included trials except the same trial. All the trials indicated the potential benefit of S-1 except the Xu et al. trial. Xu reported that median OS was 10.00 months (95% CI, 8.59–14.52) in the S-1 group compared with 10.46 months (95% CI, 8.92–13.84) in the 5-Fu group (HR, 1.05; 95% CI, 0.71–1.54). In spite of this, statistically significant difference in OS in favor of S-1 was still observed in this meta-analysis, and in view of the convenience of an oral administration, S-1 could be considered to replace 5-Fu for treatment of AGC or mCRC.
Seven trials provided data on response rate directly or indirectly (Table 2).(14)– (16,18,19,25,26) All of the trials reported that the response rate in the S-1 group was higher than that in the 5-Fu group except the SOFT study(25) (62% vs 63% in each group). The overall response rates were 38.6% in the S-1 arm and 30.5% in the 5-Fu arm in this analysis, which demonstrated a 72% increase in the OR for response in the S-1 arm than that in the 5-Fu arm, and the difference was significant (P = 0.019).
Data on PFS were available in five trials,(14,15,17,19,25) and no significant difference was observed on PFS in our meta-analysis (Table 2). However, two of the five trials,(14,19) which investigated the benefit of S-1 in AGC patients, both demonstrated a significant difference in favor of S-1-based therapy. Subgroup analysis based on tumor type confirmed the significant improvement on PFS in AGC patients with S-1-based regimens. The PFS benefit of S-1 should be further investigated in more trials.
In addition, the following issues may confound the assessment of survival and response rate and are worthy of further discussion. First, the inconsistency of systemic therapy before and after the study among the eight trials may affect the end points. Subgroup analysis based on prior chemotherapy history indicated that S-1-based therapy could prolong OS and increase ORR of patients with no history of prior chemotherapy in comparison with 5-Fu-based therapy. Second, platinum is toxic and is not well-tolerated for some patients, which may also potentially affect the results. In the subgroup of non-platinum containing regimens, OS and ORR were significantly improved in the S-1-based-therapy. Third, tumor type may be the influencing factor. As for AGC patients, S-1-based regimens were associated with statistically significant improvements of PFS and ORR in comparison with 5-Fu-based regimens.
The findings of our study showed that almost equivalent tolerance was observed between the two groups except for significant increases in grade 3–4 diarrhea, fatigue, thrombocytopenia and a decrease in grade 3–4 nausea in S-1-based group. However, these mentioned significant adverse effects were reported in a few patients in each group, and the incidence of each adverse event was much lower than 10% (Table 3). All the toxicities were tolerable, predictable, and manageable. As for treatment-related death, which was an important toxic indicator, was reported only in four trials.(14,19,21,25) Three of the four trials(14,19,21) reported not more than one person died in each group, and the SOFT study(25) reported four and three persons died in each group, respectively. In total, the difference on treatment-related death was not statistically significant in this meta-analysis.
The pharmacokinetic data demonstrated that the appropriate dose of S-1 is dependent on ethnic differences as well as differences in toxicity profile.(27) The FLAGS trial(28) has been the only non-Asian trial that compared S-1 with 5-Fu in advanced GI cancer until now, and the dose of S-1 was lower than that in these included Asian trials. This trial showed that cisplatin/S-1 did not prolong OS of patients with advanced gastric cancers compared with cisplatin/5-Fu, but it did result in a significantly improved safety profile.(28) With these differences, our results in this meta-analysis cannot be simply extrapolated to Western patients. And the survival benefits of S-1-based therapy should be further investigated in European and North American populations in the near future.
The limitations of these studies also need attention. First, as we all know, the results of any meta-analysis were affected by the quality of the individual studies. All of the trials were RCTs, while four of them were published only in abstract form, and an insufficient amount of data might potentially limit detection of S-1-based therapy effects. Furthermore, no updated or confirmed results could be obtained from the authors. Therefore, our results should be interpreted with care. Second, our meta-analysis was based on abstracted data and not on individual patient data (IPD). Meta-analyses based on IPD tend to give a more robust estimation for the association compared with published data analyses. Third, the difference in treatment schedules among the trials (data not shown) might contribute to increase the clinical heterogeneity of the meta-analysis. Fourth, the line of therapy was inconsistent among the eight trials that might confound the assessment of OS if the drugs of its use differed before or after the study.(29) Finally, lack of blinding, which could be inevitable in all these included studies, might have resulted in an overestimate of the effects. Because the two treatment methods studied were quite different (tablet vs injection), the treatment allocation could not be masked from the investigators or patients.
Combination therapy is now a predominant approach in cancer chemotherapy. Most recent combination studies of S-1 with cisplatin, irinotecan, and oxaliplatin and other anticancer agents indicate the crucial importance of exploring the combination between the best partner drug and S-1. While, S-1 plus molecular-targeted agents are promising.(13,27) Furthermore, some experts reported that “S-1 and low-dose CDDP therapy” and “alternate-day S-1 regimen” might be considered as the most patient-friendly therapies available to date.(13)
In summary, S-1-based chemotherapy was not only superior to 5-Fu-based chemotherapy in terms of OS, but also lead to increased responses, especially in subgroups of patients with no history of prior chemotherapy and with non-platinum containing regimens. Although non-significant difference on PFS between the two arms was obtained in this meta-analysis, significant PFS and ORR benefits of S-1 in comparison with 5-Fu were indicated in the subgroup of AGC patients. All of these results confirmed that oral S-1 could replace infusional 5-Fu in the treatment of advanced GI cancer with almost equivalent tolerance and much more convenience. The superiority of S-1 to 5-Fu needed to be further evaluated and confirmed through larger studies with longer observation periods in both Asian and Western countries.
Acknowledgments
This study was supported by National Basic Science Research Development Program of China (2013CB911302); the Project of Plans for the Development of Science and Technology of Nanjing, China (201208020);;the National Natural Science Foundation of China (81272469); the clinical special project for Natural Science Foundation of Jiangsu Province (BL2012016); and Nanjing 12th Five-Year key Scientific Project of medicine to Dr. Jinfei Chen.
Disclosure Statement
The authors declare no conflict of interest.
References
- 1.Hidalgo M. Pancreatic cancer. N Engl J Med. 2010;362:1605–17. doi: 10.1056/NEJMra0901557. [DOI] [PubMed] [Google Scholar]
- 2.Spazzapan S, Crivellari D, Bedard P, et al. Therapeutic management of breast cancer in the elderly. Expert Opin Pharmacother. 2011;12:945–60. doi: 10.1517/14656566.2011.540570. [DOI] [PubMed] [Google Scholar]
- 3.Casanova M, Ferrari A. Pharmacotherapy for pediatric soft-tissue sarcomas. Expert Opin Pharmacother. 2011;12:517–31. doi: 10.1517/14656566.2011.524926. [DOI] [PubMed] [Google Scholar]
- 4.Schoffski P. The modulated oral fluoropyrimidine prodrug S-1, and its use in gastrointestinal cancer and other solid tumors. Anticancer Drugs. 2004;15:85–106. doi: 10.1097/00001813-200402000-00001. [DOI] [PubMed] [Google Scholar]
- 5.Heidelberger C, Chaudhuri NK, Danneberg P, et al. Fluorinated pyrimidines, a new class of tumour-inhibitory compounds. Nature. 1957;179:663–6. doi: 10.1038/179663a0. [DOI] [PubMed] [Google Scholar]
- 6.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 7.Bilici A. Treatment options in patients with metastatic gastric cancer: current status and future perspectives. World J Gastroenterol. 2014;20:3905–15. doi: 10.3748/wjg.v20.i14.3905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goldberg RM, Sargent DJ, Morton RF, et al. A randomized controlled trial of fluorouracil plus leucovorin, irinotecan, and oxaliplatin combinations in patients with previously untreated metastatic colorectal cancer. J Clin Oncol. 2004;22:23–30. doi: 10.1200/JCO.2004.09.046. [DOI] [PubMed] [Google Scholar]
- 9.Tournigand C, Andre T, Achille E, et al. FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: a randomized GERCOR study. J Clin Oncol. 2004;22:229–37. doi: 10.1200/JCO.2004.05.113. [DOI] [PubMed] [Google Scholar]
- 10.Malet-Martino M, Martino R. Clinical studies of three oral prodrugs of 5-fluorouracil (capecitabine, UFT, S-1): a review. Oncologist. 2002;7:288–323. doi: 10.1634/theoncologist.7-4-288. [DOI] [PubMed] [Google Scholar]
- 11.Maroun J, Asche C, Romeyer F, et al. A cost comparison of oral tegafur plus uracil/folinic acid and parenteral fluorouracil for colorectal cancer in Canada. Pharmacoeconomics. 2003;21:1039–51. doi: 10.2165/00019053-200321140-00004. [DOI] [PubMed] [Google Scholar]
- 12.Ward S, Kaltenthaler E, Cowan J, Brewer N. Clinical and cost-effectiveness of capecitabine and tegafur with uracil for the treatment of metastatic colorectal cancer: systematic review and economic evaluation. Health Technol Assess. 2003;7:1–93. doi: 10.3310/hta7320. [DOI] [PubMed] [Google Scholar]
- 13.Shirasaka T. Development history and concept of an oral anticancer agent S-1 (TS-1): its clinical usefulness and future vistas. Jpn J Clin Oncol. 2009;39:2–15. doi: 10.1093/jjco/hyn127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang D, Ba Y, Xiong J, et al. A multicentre randomised trial comparing weekly paclitaxel+S-1 with weekly paclitaxel+5-fluorouracil for patients with advanced gastric cancer. Eur J Cancer. 2013;49:2995–3002. doi: 10.1016/j.ejca.2013.05.021. [DOI] [PubMed] [Google Scholar]
- 15.Baba H, Muro K, Yasui H, et al. Updated results of the FIRIS study: a phase II/III trial of 5-FU/l-leucovorin/irinotecan (FOLFIRI) versus irinotecan/S-1 (IRIS) as second-line chemotherapy for metastatic colorectal cancer (mCRC) [Abstract] J Clin Oncol. 2011;29:3562. [Google Scholar]
- 16.Jin M, Lu H, Li J, et al. Randomized 3-armed phase III study of S-1 monotherapy versus S-1/CDDP (SP) versus 5-FU/CDDP (FP) in patients (pts) with advanced gastric cancer (AGC): SC-101 study [Abstract] J Clin Oncol. 2008;26:4533. [Google Scholar]
- 17.Otsuji T, Yamazaki K, Ojima H, et al. Updated survival results of the randomized phase II study of S-1, oral leucovorin, and oxaliplatin combination therapy (SOL) versus mFOLFOX6 in patients with untreated metastatic colorectal cancer (mCRC) [Abstract] J Clin Oncol. 2012;30:586. [Google Scholar]
- 18.Xu R, Sun G, Lu H, et al. A phase III study of S-1 plus cisplatin versus fluorouracil plus cisplatin in patients with advanced gastric or gastroesophageal junction adenocarcinoma [Abstract] J Clin Oncol. 2013;31:4025. [Google Scholar]
- 19.Boku N, Yamamoto S, Fukuda H, et al. Fluorouracil versus combination of irinotecan plus cisplatin versus S-1 in metastatic gastric cancer: a randomised phase 3 study. Lancet Oncol. 2009;10:1063–9. doi: 10.1016/S1470-2045(09)70259-1. [DOI] [PubMed] [Google Scholar]
- 20.Moher D, Pham B, Jones A, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta-analyses? Lancet. 1998;352:609–13. doi: 10.1016/S0140-6736(98)01085-X. [DOI] [PubMed] [Google Scholar]
- 21.Muro K, Boku N, Shimada Y, et al. Irinotecan plus S-1 (IRIS) versus fluorouracil and folinic acid plus irinotecan (FOLFIRI) as second-line chemotherapy for metastatic colorectal cancer: a randomised phase 2/3 non-inferiority study (FIRIS study) Lancet Oncol. 2010;11:853–60. doi: 10.1016/S1470-2045(10)70181-9. [DOI] [PubMed] [Google Scholar]
- 22.Zintzaras E, Ioannidis JP. Heterogeneity testing in meta-analysis of genome searches. Genet Epidemiol. 2005;28:123–37. doi: 10.1002/gepi.20048. [DOI] [PubMed] [Google Scholar]
- 23.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–101. [PubMed] [Google Scholar]
- 24.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yamada Y, Takahari D, Matsumoto H, et al. Leucovorin, fluorouracil, and oxaliplatin plus bevacizumab versus S-1 and oxaliplatin plus bevacizumab in patients with metastatic colorectal cancer (SOFT): an open-label, non-inferiority, randomised phase 3 trial. Lancet Oncol. 2013;14:1278–86. doi: 10.1016/S1470-2045(13)70490-X. [DOI] [PubMed] [Google Scholar]
- 26.Andoh H, Kato S, Gamoh M, et al. A randomized pilot study comparing safety and efficacy of irinotecan plus S-1 plus bevacizumab (IRIS+BV) and modified FOLFIRI plus BV (mFOLFIRI+BV) in patients (pts) with metastatic colorectal cancer (mCRC): the result of efficacy report of T-CORE0702 [Abstract] J Clin Oncol. 2011;29:e14001. [Google Scholar]
- 27.Satoh T, Sakata Y. S-1 for the treatment of gastrointestinal cancer. Expert Opin Pharmacother. 2012;13:1943–59. doi: 10.1517/14656566.2012.709234. [DOI] [PubMed] [Google Scholar]
- 28.Ajani JA, Rodriguez W, Bodoky G, et al. Multicenter phase III comparison of cisplatin/S-1 with cisplatin/infusional fluorouracil in advanced gastric or gastroesophageal adenocarcinoma study: the FLAGS trial. J Clin Oncol. 2010;28:1547–53. doi: 10.1200/JCO.2009.25.4706. [DOI] [PubMed] [Google Scholar]
- 29.Liu L, Cao Y, Tan A, Liao C, Gao F. Cetuximab-based therapy versus non-cetuximab therapy for advanced cancer: a meta-analysis of 17 randomized controlled trials. Cancer Chemother Pharmacol. 2010;65:849–61. doi: 10.1007/s00280-009-1090-x. [DOI] [PubMed] [Google Scholar]


