Abstract
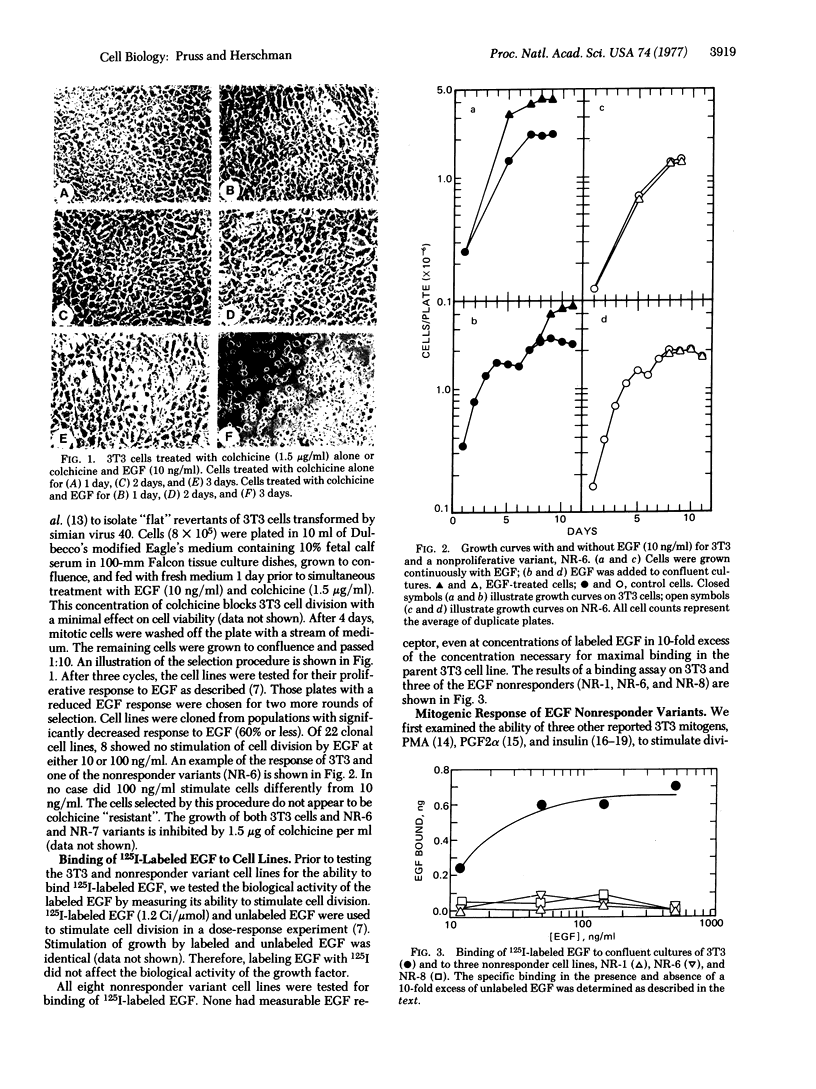
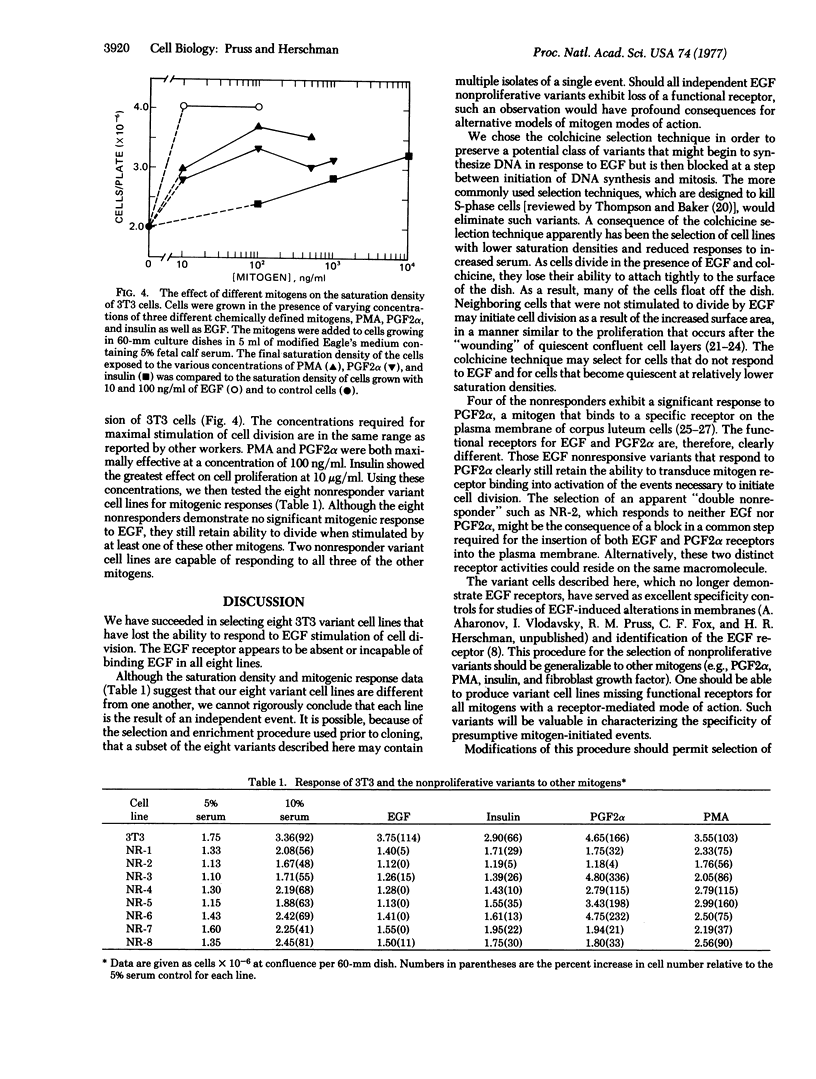
We have previously demonstrated that epidermal growth factor (EGF) can serve as a potent mitogen for 3T3 cells. We have now selected variant 3T3 cell lines unable to respond to EGF, in order to define cellular events unique to the EGF response and to distinguish which of these events are necessary and which are merely correlative to mitogenesis. By simultaneously treating cells with EGF and colchicine, we eliminated those cells stimulated by EGF to enter mitosis. Of the eight clonal EGF nonresponder variants selected by this procedure, none retains a functional EGF receptor. The EGF nonresponsive va-riant lines still retain the ability to respond to other mitogens.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carpenter G., Lembach K. J., Morrison M. M., Cohen S. Characterization of the binding of 125-I-labeled epidermal growth factor to human fibroblasts. J Biol Chem. 1975 Jun 10;250(11):4297–4304. [PubMed] [Google Scholar]
- Cohen S. The stimulation of epidermal proliferation by a specific protein (EGF). Dev Biol. 1965 Dec;12(3):394–407. doi: 10.1016/0012-1606(65)90005-9. [DOI] [PubMed] [Google Scholar]
- Das M., Miyakawa T., Fox C. F., Pruss R. M., Aharonov A., Herschman H. R. Specific radiolabeling of a cell surface receptor for epidermal growth factor. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2790–2794. doi: 10.1073/pnas.74.7.2790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Asua L. J., Clingan D., Rudland P. S. Initiation of cell proliferation in cultured mouse fibroblasts by prostaglandin F2alpha. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2724–2728. doi: 10.1073/pnas.72.7.2724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulbecco R., Stoker M. G. Conditions determining initiation of DNA synthesis in 3T3 cells. Proc Natl Acad Sci U S A. 1970 May;66(1):204–210. doi: 10.1073/pnas.66.1.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulbecco R. Topoinhibition and serum requirement of transformed and untransformed cells. Nature. 1970 Aug 22;227(5260):802–806. doi: 10.1038/227802a0. [DOI] [PubMed] [Google Scholar]
- HUNTER W. M., GREENWOOD F. C. Preparation of iodine-131 labelled human growth hormone of high specific activity. Nature. 1962 May 5;194:495–496. doi: 10.1038/194495a0. [DOI] [PubMed] [Google Scholar]
- Hershko A., Mamont P., Shields R., Tomkins G. M. "Pleiotypic response". Nat New Biol. 1971 Aug;232(33):206–211. [PubMed] [Google Scholar]
- Hollenberg M. D., Cuatrecasas P. Epidermal growth factor: receptors in human fibroblasts and modulation of action by cholera toxin. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2964–2968. doi: 10.1073/pnas.70.10.2964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollenberg M. D., Cuatrecasas P. Insulin and epidermal growth factor. Human fibroblast receptors related to deoxyribonucleic acid synthesis and amino acid uptake. J Biol Chem. 1975 May 25;250(10):3845–3853. [PubMed] [Google Scholar]
- Hollwy R. W., Kiernan J. A. Control of the initiation of DNA synthesis in 3T3 cells: serum factors. Proc Natl Acad Sci U S A. 1974 Jul;71(7):2908–2911. doi: 10.1073/pnas.71.7.2908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton A., Klinger I., Paul D., Holley R. W. Migration of mouse 3T3 fibroblasts in response to a serum factor. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2799–2801. doi: 10.1073/pnas.68.11.2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PUCK T. T., CIECIURA S. J., FISHER H. W. Clonal growth in vitro of human cells with fibroblastic morphology; comparison of growth and genetic characteristics of single epithelioid and fibroblast-like cells from a variety of human organs. J Exp Med. 1957 Jul 1;106(1):145–158. doi: 10.1084/jem.106.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell W. S., Hammerström S., Samuelsson B. Localization of a prostaglandin F2alpha receptor in bovine Corpus luteum plasma membranes. Eur J Biochem. 1976 Jan 15;61(2):605–611. doi: 10.1111/j.1432-1033.1976.tb10056.x. [DOI] [PubMed] [Google Scholar]
- Rao C. V. Cationic dependency of high affinity prostaglandin F2 alpha receptors in bovine corpus luteum cell membranes. Biochem Biophys Res Commun. 1975 Dec 1;67(3):1242–1250. doi: 10.1016/0006-291x(75)90806-2. [DOI] [PubMed] [Google Scholar]
- Rao C. V. Prostaglandin F2alpha receptors in bovine corpus luteum cell membranes. Effect of enzymes and protein reagents. Biochim Biophys Acta. 1976 Jun 4;436(1):170–182. doi: 10.1016/0005-2736(76)90228-5. [DOI] [PubMed] [Google Scholar]
- Rheinwald J. G., Green H. Epidermal growth factor and the multiplication of cultured human epidermal keratinocytes. Nature. 1977 Feb 3;265(5593):421–424. doi: 10.1038/265421a0. [DOI] [PubMed] [Google Scholar]
- Richman R. A., Claus T. H., Pilkis S. J., Friedman D. L. Hormonal stimulation of DNA synthesis in primary cultures of adult rat hepatocytes. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3589–3593. doi: 10.1073/pnas.73.10.3589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose S. P., Pruss R. M., Herschman H. R. Initiation of 3T3 fibroblast cell division by epidermal growth factor. J Cell Physiol. 1975 Dec;86 (Suppl 2)(3 Pt 2):593–598. doi: 10.1002/jcp.1040860504. [DOI] [PubMed] [Google Scholar]
- Savage C. R., Jr, Cohen S. Epidermal growth factor and a new derivative. Rapid isolation procedures and biological and chemical characterization. J Biol Chem. 1972 Dec 10;247(23):7609–7611. [PubMed] [Google Scholar]
- Sivak A. Induction of cell division: role of cell membrane sites. J Cell Physiol. 1972 Oct;80(2):167–173. doi: 10.1002/jcp.1040800203. [DOI] [PubMed] [Google Scholar]
- Stoker M. G., Pigott D., Taylor-Papadimitriou J. Response to epidermal growth factors of cultured human mammary epithelial cells from benign tumours. Nature. 1976 Dec 23;264(5588):764–767. doi: 10.1038/264764a0. [DOI] [PubMed] [Google Scholar]
- Stoker M. G., Rubin H. Density dependent inhibition of cell growth in culture. Nature. 1967 Jul 8;215(5097):171–172. doi: 10.1038/215171a0. [DOI] [PubMed] [Google Scholar]
- Thompson L. H., Baker R. M. Isolation of mutants of cultured mammalian cells. Methods Cell Biol. 1973;6:209–281. doi: 10.1016/s0091-679x(08)60052-7. [DOI] [PubMed] [Google Scholar]
- Todaro G. J., Lazar G. K., Green H. The initiation of cell division in a contact-inhibited mammalian cell line. J Cell Physiol. 1965 Dec;66(3):325–333. doi: 10.1002/jcp.1030660310. [DOI] [PubMed] [Google Scholar]
- Vogel A., Risser R., Pollack R. Isolation and characterization of revertant cell lines. 3. Isolation of density-revertants of SV40-transformed 3T3 cells using colchicine. J Cell Physiol. 1973 Oct;82(2):181–188. doi: 10.1002/jcp.1040820206. [DOI] [PubMed] [Google Scholar]
- Yarnell M. M., Schnebli H. P. Release from density-dependent inhibition of growth in the absence of cell locomotion. J Cell Sci. 1974 Oct;16(1):181–188. doi: 10.1242/jcs.16.1.181. [DOI] [PubMed] [Google Scholar]




