Abstract
Indoleamine 2,3-dioxygenase (IDO) is a tryptophan-catabolizing enzyme that has immunoregulatory functions. Our prior study showed that tumoral IDO overexpression is involved in disease progression and impaired patient survival in human ovarian cancer, although its mechanism remains unclear. The purpose of the present study is to clarify the role of IDO during the process of peritoneal dissemination of ovarian cancer. Indoleamine 2,3-dioxygenase cDNA was transfected into the murine ovarian carcinoma cell line OV2944-HM-1, establishing stable clones of IDO-overexpressing cells (HM-1-IDO). Then HM-1-IDO or control vector-transfected cells (HM-1-mock) were i.p. transplanted into syngeneic immunocompetent mice. The HM-1-IDO-transplanted mice showed significantly shortened survival compared with HM-1-mock-transplanted (control) mice. On days 11 and 14 following transplantation, the tumor weight of peritoneal dissemination and ascites volume were significantly increased in HM-1-IDO-transplanted mice compared with those of control mice. This tumor-progressive effect was coincident with significantly reduced numbers of CD8+ T cells and natural killer cells within tumors as well as increased levels of transforming growth factor-β and interleukin-10 in ascites. Finally, treatment with the IDO inhibitor 1-methyl-tryptophan significantly suppressed tumor dissemination and ascites with reduced transforming growth factor-β secretion. These findings showed that tumor-derived IDO promotes the peritoneal dissemination of ovarian cancer through suppression of tumor-infiltrating effector T cell and natural killer cell recruitment and reciprocal enhancement of immunosuppressive cytokines in ascites, creating an immunotolerogenic environment within the peritoneal cavity. Therefore, IDO may be a promising molecular target for the therapeutic strategy of ovarian cancer.
Keywords: Immunosuppression; indoleamine 2,3-dioxygenase; ovarian cancer; peritoneal dissemination; tolerance
Ovarian cancer is usually diagnosed at an advanced stage with metastasis, and is the leading cause of death from gynecologic malignancy. Despite the development of cytoreductive surgery and chemotherapy, the 5-year survival rate of these advanced-stage patients remains only 25–30%.(1) Peritoneal dissemination is the most common metastatic process of ovarian cancer and is found in over 75% of all cases. Therefore, it is important to understand the mechanism for this specific metastatic pathway and to develop novel therapeutic strategies targeting molecules involved in the step of peritoneal metastasis of ovarian cancer.
Immune escape and acquisition of tolerance by tumor cells are essential to cancer growth and progression. Tumors are known to be able to escape the host immune surveillance by multiple mechanisms:(2)– (5) the downregulation of human leukocyte antigen class I, loss of tumor antigens, production of immunosuppressive cytokines such as TGF-β and IL-10, and expression of immunosuppressive molecules such as Fas ligand and programmed cell death 1 ligand. These tumor-induced immunosuppressive mechanisms are supposed to be involved in the progression and metastasis of ovarian cancer,(6)– (8) although detailed mechanisms are still unclear.
Indoleamine 2,3-dioxygenase is an intracellular enzyme that catalyzes the initial and rate-limiting step in the metabolism of the essential amino acid tryptophan.(9) Evidence for an immunosuppressive function of IDO was first documented in the mouse placenta, where IDO prevents rejection of the allogeneic fetus during pregnancy.(10) In malignancy, IDO is expressed by tumor cells and/or tumor-draining lymph nodes, and depletes tryptophan locally and produces a toxic tryptophan catabolite kynurenine, which causes growth arrest and the apoptosis of cytotoxic T cells or NK cells.(11,12) In addition, IDO induces immunosuppressive host Tregs.(13,14)
In human cancer, IDO is expressed in various types of human tumor, and correlated with impaired patient survival.(15)– (17) Our prior studies indicated that high IDO expression was associated with poor clinical outcome in endometrial and ovarian cancers.(18)– (20) Actually, high IDO expressing tumors showed significantly lower numbers of TIL than IDO non-expressing tumors.(19,20) In line with these clinical data, our subsequent study has shown that IDO overexpression in human endometrial cancer cells enhances tumor growth in nude mouse xenograft models, through suppressing host NK cell numbers and function.(21) However, these data were insufficient to clarify the immunological implications of IDO because host T cell function is absent in nude mice. Furthermore, there have been no reports on the immunological functions of IDO in peritoneal metastasis and progression of ovarian cancer. Thus, in the present study, we attempt to clarify the role of IDO in the process of peritoneal dissemination of ovarian cancer and its impact on the tumor microenvironment using syngeneic mouse models under host immunocompetent conditions.
Materials and Methods
Antibodies and reagents
The following mAbs were used for immunohistochemical and Western blot analyses: rat anti-mouse IDO mAb (Novus, Littleton, CO, USA), rabbit anti-mouse CD8 mAb (Epitomics, Burlingame, CA, USA), rat anti-mouse pan-NK mAb (Becton Dickinson, Franklin Lakes, NJ, USA), and α-SMA mAb (DakoCytomation, Copenhagen, Denmark). 1-Methyl-D-tryptophan was purchased from Sigma-Aldrich (St. Louis, MO, USA). Recombinant mouse IFN-γ, TGF-β1, and IL-10 were purchased from R&D Systems (Minneapolis, MN, USA).
Cell lines and culture
A mouse ovarian carcinoma cell line, OV2944-HM-1, derived from B6C3F1 mice (pairings of C57BL/6× C3H/HeN), was purchased from Riken BRC Cell Bank (Tsukuba, Japan). HM-1 cells were cultured in MEM-alpha (Life Technologies, Carlsbad, CA, USA) supplemented with 10% FCS (Sigma-Aldrich) and penicillin–streptomycin–amphotericin B (Life Technologies).
Establishment of stable IDO-overexpressing transfectants
The expression vector for mouse IDO was constructed by inserting full-size mouse IDO cDNA(22) into pcDNA3.1 (Invitrogen, Carlsbad, CA, USA). Transfection into HM-1 cells was carried out as described previously.(21) More than 20 stable clones were selected as IDO-overexpressing cells (HM-1-IDO). For a negative control, the control vector was transfected into HM-1 cells (HM-1-mock). HM-1-IDO cells and IDO-mock cells were cultured using G418 (Sigma-Aldrich) at 200 μg/mL in MEM-alpha supplemented with 10% FCS.
Western blot analysis
The cells were lysed in lysis buffer followed by centrifugation at 15 000 g for 20 min, and the supernatant was obtained. The protein extracts (30 μg) were separated by SDS-PAGE (12.5%), transferred onto a nitrocellulose membrane, and immunoblotted with anti-IDO mAb, followed by chemiluminescence detection (EZ West lumi; ATTO, Tokyo, Japan).
High-performance liquid chromatography
Indoleamine 2,3-dioxygenase enzyme activity was evaluated by measuring the concentrations of tryptophan and kynurenine in the conditioned medium of cells cultured for 48 h using a Shimadzu Prominence HPLC system (GL Sciences, Tokyo, Japan).
Cell proliferation assay
Cells (4 × 103 cells/well) were cultured in 96-well microplates for 24–72 h. Cell viability was assayed using a Cell Counting Kit-8 (WST-8; Dojindo Laboratories, Kumamoto, Japan). In another series, effects of TGF-β or IL-10 on cell proliferation were examined using the WST-8 assay.
Wound healing assay for cell migration
Cells were grown in 10-cm culture dishes. When they became confluent, confluent monolayers of cells were wounded with a uniform scratch using a sterile pipette tip, rinsed to remove debris, and then incubated in culture medium containing 10% FCS for 12, 24, and 48 h. The wound healing was measured quantitatively using 20 randomly chosen distances of cell migration across the wound. In the next experiments, confluent monolayers of cells were wounded and incubated in culture medium containing 10% FCS alone or 10% FCS with 1 ng/mL TGF-β or IL-10 for 24 h, and the wound healing was measured similarly.
In vivo studies using a syngeneic mouse model
An in vivo model of peritoneal carcinomatosis of mouse ovarian cancer using HM-1 cells was established as described previously,(23) which was widely used as a good model mimicking peritoneal dissemination of human ovarian cancer.(8,24) Six-week-old female B6C3F1 mice were purchased from Clea Japan (Tokyo, Japan). Mice were i.p. injected with HM-1-IDO or HM-1-mock cells (1 × 106 cells/mouse). The mice were killed on day 11 or day 14 after inoculation, and tumor dissemination and ascites volume were evaluated. The survival time of each mouse was also analyzed. In another series, HM-1-IDO-transplanted mice were treated with i.p. injection of the IDO inhibitor 1-MT (4.0 mg/mouse) three times a week. All procedures were carried out in accordance with the Regulations for Animal Experiments of the Laboratory Animal Center, Wakayama Medical University (Wakayama, Japan).
Enzyme-linked immunosorbent assay
Ascites was collected 11 or 14 days after tumor cell inoculation, and the levels of TGF-β, IL-10, VEGF, IL-6, IFN-γ, TNF-α, and IL-1α were measured using commercial ELISA kits (R&D Systems). The detection limits in each method were: TGF-β > 4.6 pg/mL; IL-10 > 4.0 pg/mL; VEGF > 3 pg/mL; IL-6 > 1.6 pg/mL; IFN-γ > 2 pg/mL; TNF-α > 1.88 pg/mL; and IL-1α > 2.5 pg/mL.
Immunohistochemistry
The tumor specimens were fixed in 4% paraformaldehyde solution and embedded in paraffin, after which sections were made (4-μm thick). For histological evaluation, the sections were stained with H&E. For immunostaining, the sections were incubated with anti-CD8 mAb, anti-pan-NK mAb, anti-IDO mAb, or anti-α-SMA mAb as the primary antibodies. After the incubation of biotinylated secondary antibodies, the immune complex was visualized using the avidin–biotin immunoperoxidase method. Tumor-infiltrating CD8+ T cells and NKs were counted in a microscopic field at ×400 in the 20 areas with the most abundant cell infiltration.
Statistical analyses
The means and SD were calculated for all parameters determined. Statistical significance was evaluated using Student's t-test. Survival curves were drawn by the Kaplan–Meier method, and analyzed by the log–rank test. P < 0.05 was accepted as statistically significant.
Results
Establishment of stable clones of IDO-overexpressing mouse ovarian cancer cells
Under steady-state conditions, HM-1 cells slightly expressed IDO protein, but it was markedly enhanced by the IFN-γ treatment (Fig. 1a), which is consistent with a previous study in other tumor cell lines.(25) In order to establish stable clones of IDO-overexpressing mouse ovarian cancer cells, we transfected mouse IDO cDNA into HM-1 cells and selected clones with high IDO expression (Fig. 1b). In the next step, we evaluated IDO enzymatic activity in these clones by measuring the concentrations of tryptophan and its main catabolite kynurenine in the conditioned medium. In clone 8, tryptophan was almost completely depleted, and reciprocally kynurenine was strongly produced after 48 h (Fig. 1c), suggesting that this clone had high tryptophan catabolizing activity. Thus, we used clone 8 as the IDO-overexpressing cells (HM-1-IDO) in the following experiments. Clones transfected with control vector (HM-1-mock) scarcely expressed IDO protein, and showed no enzymatic activity (Fig. 1b,c).
Fig. 1.
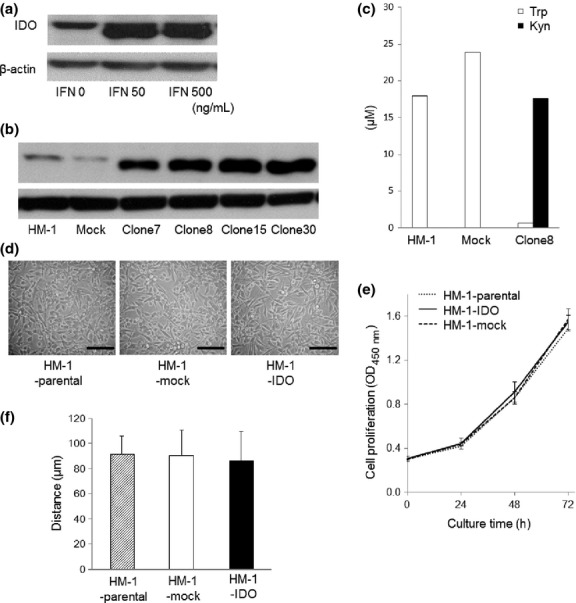
(a) Western blot analyses of indoleamine 2,3-dioxygenase (IDO) expression in interferon-γ (IFN-γ)-treated mouse ovarian carcinoma OV2944-HM-1 (HM-1) cells. Treatment with IFN-γ enhanced IDO protein expression. (b) Establishment of IDO-overexpressing HM-1 cells. Western blot analyses of IDO expression in stable clones of HM-1 cells transfected with mouse IDO cDNA (HM-1-IDO). (c) Evaluation of IDO enzymatic activity by measuring the concentrations of tryptophan (Trp) and its main catabolite, kynurenine (Kyn), in the conditioned medium using HPLC. Representative results from three independent experiments are shown. (d) Morphological views of HM-1-parental, control vector-transfected (HM-1-mock), and HM-1-IDO cells. Bar = 50 μm. (e) In vitro cell proliferative activity of HM-1-parental, HM-1-mock, and HM-1-IDO cells. Mean ± SD from three independent experiments are shown. O.D., optical density. (f) In vitro cell migratory potential of HM-1-parental, HM-1-mock, and HM-1-IDO cells evaluated by wound healing assay after 24 h. Mean ± SD from three independent experiments are shown.
Effect of IDO overexpression on in vitro behavior of mouse ovarian cancer cells
There were no significant differences in morphology (Fig. 1d), proliferative activity (Fig. 1e), or migratory potential (Fig. 1f) among HM-1-parental, HM-1-mock, and HM-1-IDO cells.
Effect of IDO overexpression on tumor progression in a syngeneic mouse model
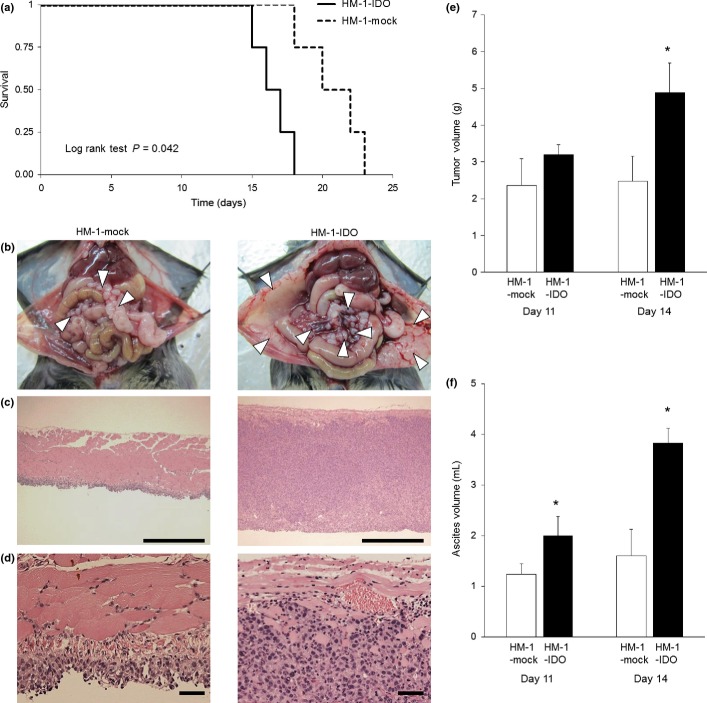
In order to explore the pathogenic roles of IDO in the tumor progression of ovarian cancer in vivo, HM-1-IDO or HM-1-mock cells were i.p. transplanted into B6C3F1 syngeneic mice, and used as a model of peritoneal dissemination of ovarian cancer. In HM-1-IDO-transplanted mice (HM-1-IDO group), the survival rate was significantly impaired compared with that of HM-1-mock-transplanted mice (HM-1-mock group; Fig. 2a). Actually, on day 14 after tumor cell injection, macroscopic findings showed that the tumor peritoneal dissemination was more evident and widespread in the HM-1-IDO group than in the HM-1-mock group (Fig. 2b). In parallel, histological findings indicated deep invasion of tumor cells into the peritoneum, resulting in much thicker peritoneum in the HM-1-IDO group (Fig. 2c,d). Consistently, the total weight of disseminated tumors in the HM-1-IDO group was significantly increased on day 14 compared with that in the HM-1-mock group (Fig. 2e). Moreover, on days 11 and 14 after tumor cell injection, the ascites volume was significantly larger in the HM-1-IDO group than in the HM-1-mock group (Fig. 2f). These observations implied that tumor-derived IDO could promote the peritoneal dissemination and progression of ovarian cancer.
Fig. 2.
Pathogenic roles of indoleamine 2,3-dioxygenase (IDO) in the tumor progression of ovarian cancer in vivo. Stable clones of IDO-overexpressing (HM-1-IDO) or control vector-transfected (HM-1-mock) OV2944-HM-1 cells were i.p. transplanted into syngeneic B6C3F1 mice as a model of peritoneal dissemination of ovarian cancer. (a) Survival rate of mice transplanted with HM-1-IDO or HM-1-mock cells. There was a significant difference between the two groups by log–rank test (P = 0.042). (b) Macroscopic views of peritoneal dissemination in mice transplanted with HM-1-mock and HM-1-IDO cells on day 14. Arrows indicate disseminated tumor nodules. Representative results from six independent mice in each group are shown. (c, d) Histopathological findings of disseminated tumors on the peritoneum in mice transplanted with HM-1-mock and HM-1-IDO cells on day 14. Original magnification, ×100 (c) and ×400 (d). Bar = 500 μm (c) and 50 μm (d). (e, f) Total tumor weight and ascites volume were evaluated in mice transplanted with HM-1-mock and HM-1-IDO cells on days 11 and 14. All values represent mean ± SD. *P < 0.05, HM-1-mock versus HM-1-IDO.
Reduced recruitment of CD8+ TILs and NK cells in tumor microenvironment
In the next series, we immunohistochemically examined the recruitment of CD8+ TILs and NK cells that were essentially involved in tumor immunity. In HM-1-IDO-transplanted mice, the IDO-positive signals in tumor cells were confirmed within the disseminated tumors, whereas they were not observed in the HM-1-mock group (Fig. 3a). The number of CD8+ TILs and NK cells within the tumors was significantly reduced in the HM-1-IDO group, compared with that in the HM-1-mock group (Fig. 3b,c,e,f). These observations suggested that IDO overexpression could suppress the recruitment of CD8+ TILs and NK cells into the tumor microenvironment. In contrast, the number of α-SMA+ myofibroblasts in the tumor stroma was significantly increased in the HM-1-IDO group, compared to that in the HM-1-mock group (Fig. 3d,g).
Fig. 3.
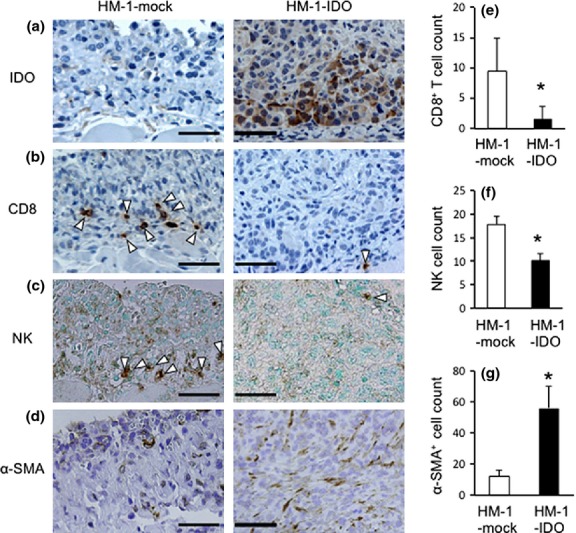
Immunohistochemical detection of indoleamine 2,3-dioxygenase (IDO)+ cells (a), CD8+ cells (b), natural killer (NK) cells (c), and α-smooth muscle actin (α-SMA)+ myofibroblasts (d) in the tumor microenvironment of mice transplanted with control vector-transfected (HM-1-mock) and IDO-overexpressing (HM-1-IDO) OV2944-HM-1 cells (on day 14). Representative results from six independent experiments are shown. Arrowheads indicate positive cells. Original magnification, ×400. Bar = 50 μm. (e–g) Enumeration of tumor-infiltrating CD8+ T cells (e), NK cells (f) and α-SMA+ myofibroblasts (g) on day 14. All values represent mean ± SD. *P < 0.05, HM-1-mock versus HM-1-IDO.
Cytokine levels in ascites of mice transplanted with IDO-overexpressing tumor cells
Several cytokines in ascites, such as VEGF, IL-6, TGF-β, and IL-10, have been suggested to promote tumor progression in ovarian cancer patients.(26)– (29) Thus, we examined the effect of tumoral IDO overexpression on cytokine profiles in ascites during the development of peritoneal carcinomatosis using our mouse models. The protein levels of TGF-β and IL-10, both of which are key molecules for immunosuppression, were significantly higher in the ascites from HM-1-IDO-transplanted mice than in those from HM-1-mock-transplanted ones (TGF-β on day 11 and IL-10 on day 14; Fig. 4a,b). An angiogenic and immunosuppressive factor, VEGF, was higher (albeit not significantly) in HM-1-IDO-transplanted mice than in HM-1-mock-transplanted ones (Fig. 4c). In contrast, IL-6 tended to be lower (but not significantly) in the HM-1-IDO group (Fig. 4d). Additionally, we examined the levels of other inflammatory/proinflammatory cytokines such as IFN-γ, TNF-α, and IL-1α in the ascites. There were no significant differences in the levels of IFN-γ or TNF-α between HM-1-IDO-transplanted mice and HM-1-mock-transplanted mice (data not shown). Interleukin-1α could not be detected in the ascites from either group.
Fig. 4.
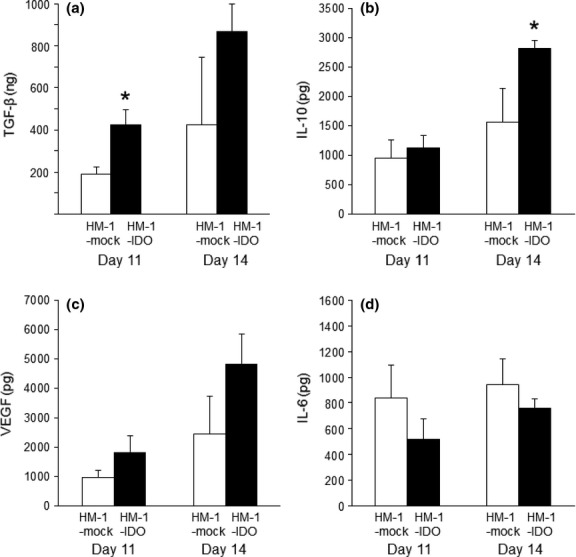
Levels of transforming growth factor-β (TGF-β) (a), interleukin-10 (IL-10) (b), vascular endothelial growth factor (VEGF) (c), and IL-6 (d) in ascites obtained from mice transplanted with control vector-transfected (HM-1-mock) and indoleamine 2,3-dioxygenase-overexpressing (HM-1-IDO) murine ovarian carcinoma OV2944-HM-1 cells. Each cytokine was measured by ELISA. All values represent mean ± SD. *P < 0.05, HM-1-mock versus HM-1-IDO.
Effects of TGF-β and IL-10 on cell proliferation and migratory potential in vitro
Because the levels of TGF-β and IL-10 in the ascites were significantly higher in HM-1-IDO-transplanted mice than in HM-1-mock-transplanted ones, we examined whether TGF-β and IL-10 could affect the proliferation and migration of HM-1 cells in vitro. Transforming growth factor-β enhanced the cell migratory activity in HM-1-parental, HM-1-mock, and HM-1-IDO cells to similar extents, whereas IL-10 had no significant effects on cell migration (Fig. 5). Neither TGF-β nor IL-10 showed significant effects on the proliferative activity of these cells (data not shown).
Fig. 5.
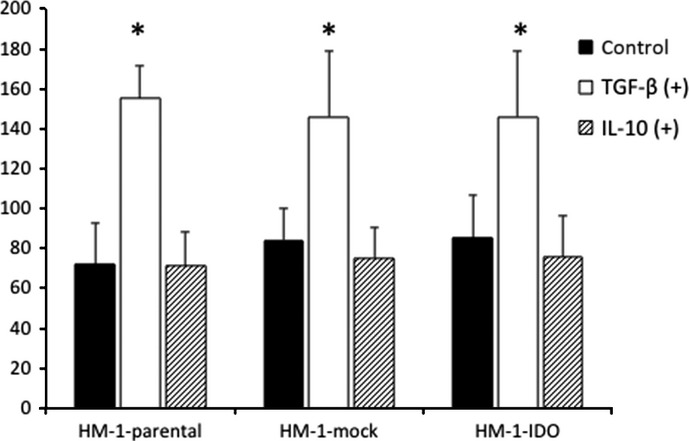
Effects of transforming growth factor-β (TGF-β) and interleukin-10 (IL-10) on in vitro cell migratory potential of HM-1-parental, control vector-transfected (HM-1-mock), and indoleamine 2,3-dioxygenase-overexpressing (HM-1-IDO) murine ovarian carcinoma OV2944-HM-1 cells. The cell migratory activity in the presence or absence of 1 ng/mL TGF-β or IL-10 was evaluated by wound healing assay after 24 h. Mean ± SD from three independent experiments are shown. *P < 0.05, TGF-β (+) versus control.
Effects of IDO inhibitor on tumor progression of ovarian cancer
Finally, we investigated the therapeutic potential of 1-MT, a specific IDO inhibitor, using the same mouse model of peritoneal carcinomatosis. On day 14 after tumor injection, the 1-MT treatment significantly suppressed the tumor volume and ascites accumulation compared with those in the untreated (vehicle alone) group (Fig. 6a,b). In parallel with decreased tumor dissemination, the TGF-β level in ascites was significantly reduced in the 1-MT treatment group compared with that in the untreated group (Fig. 6c). There was no significant difference in IL-10 levels between the two groups (data not shown).
Fig. 6.
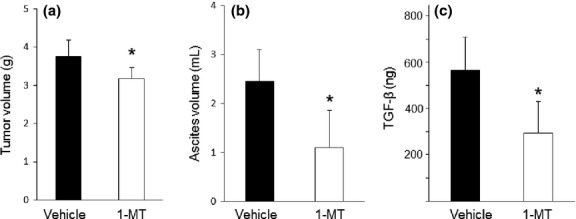
Therapeutic effects of 1-methyl-tryptophan 1-MT on tumor progression in mice. Mice transplanted with indoleamine 2,3-dioxygenase-overexpressing murine ovarian carcinoma OV2944-HM-1 cells (HM-1-IDO) were treated with 1-MT three times weekly. On day 14, tumor volume (a), ascites volume (b), and the level of transforming growth factor-β (TGF-β) in ascites (c) were evaluated. All values represent mean ± SD. *P < 0.05, vehicle versus 1-MT.
Discussion
In human ovarian cancer, IDO was overexpressed in paclitaxel-resistant ovarian cancer tissues, and patients with diffuse IDO expression had poor clinical outcomes in stage III–IV serous ovarian carcinoma.(16) Similarly, our recent immunohistochemical study using a larger number of samples showed that high IDO expression was found in over 70% of cases with stage II–IV advanced ovarian cancer, and was significantly correlated with impaired patient survival.(20) These results indicate that IDO is associated with disease progression, and may be a prognostic indicator for ovarian cancer, as in other types of human cancer.(17) Furthermore, we and others showed that IDO overexpression of human endometrial and ovarian cancer cells promotes tumor progression partly through the inhibition of NK cell function in nude mouse models.(21,30,31) However, these studies had considerable limitations in immunological aspects because T-cell-mediated immune systems are deficient in nude mice. Therefore, in the present study, we established IDO-overexpressing mouse ovarian cancer cells and examined the effect of IDO on ovarian cancer progression under immunocompetent host conditions.
Initially, we selected a stable clone of IDO-overexpressing HM-1 cells with the capacity to catabolize tryptophan and strongly produce kynurenine, indicating that this cell line not only expressed abundant IDO protein, but also had high IDO enzymatic activity. Our in vitro study showed that overexpression of IDO had no significant impact on cell proliferation or migratory potential. However, our in vivo study indicated that IDO-overexpressing HM-1-transplanted mice had significantly shortened survival time. Actually, tumors disseminating to the peritoneum and ascites in these mice were markedly increased compared with those in mock-transplanted mice. More interestingly, the intratumoral recruitment of CD8+ T cells and NK cells was significantly suppressed in IDO-overexpressing tumor-transplanted mice. These observations strongly suggest that tumor-derived IDO enhances the peritoneal dissemination of ovarian cancer through inhibiting both effector T and NK cells, and inducing an immunotolerogenic tumor microenvironment. Recent studies have shown that IDO may promote cancer progression not only by suppressing cytotoxic T and NK cells, but also by enhancing immunosuppressive host Tregs.(14) Curiel et al.(32) reported that high intratumoral Treg accumulation correlates with poor survival in ovarian cancer patients. Further studies are needed to clarify the interaction between IDO and host immunosuppressor cells in the pathway of peritoneal dissemination of ovarian cancer.
The development of peritoneal metastasis of ovarian cancer is accompanied by the accumulation of ascites. Recent studies have shown that specific cytokines in ascites, such as TGF-β, IL-10, VEGF, and IL-6, have immunosuppressive functions and play key roles in ovarian cancer progression.(26)– (29) Thus, it is of interest to clarify the relationship between IDO expression and the cytokine profiles in ascites during the peritoneal dissemination of ovarian cancer in vivo. Our results showed that IDO-overexpressing tumor-transplanted mice showed significantly higher intra-ascitic levels of TGF-β and IL-10, but not other inflammatory/proinflammatory cytokines including IL-6, IFN-γ, TNF-α, and IL-1. Furthermore, our additional data showed that the migratory activity of HM-1 cells was significantly enhanced by TGF-β. These findings suggested that tumoral IDO upregulated the secretion of immunosuppressive cytokines, TGF-β and IL-10, and could create an immunotolerogenic environment within the peritoneal cavity, in parallel with enhancement of tumor cell migration, which may contribute to peritoneal dissemination of ovarian cancer. The source of increased TGF-β in ascites remains to be clarified. Our immunohistochemical study indicated that the IDO-overexpressing tumors showed increased expression of α-SMA+ tumor stromal fibroblasts, which may produce TGF-β and exert tumor-promoting effects as cancer-associated fibroblasts,(33) although host immune cells such as macrophages or tumor cells themselves might produce TGF-β.
Indoleamine 2,3-dioxygenase-targeted therapy to restore host antitumor immunity may have clinical potential. Our results showed that 1-MT treatment significantly suppressed tumor dissemination in parallel with reducing TGF-β level in ascites. These findings suggest that IDO inhibition by 1-MT may have therapeutic efficacy against ovarian cancer, possibly, at least in part, through changing the immunological conditions in the peritoneal cavity. Indoleamine 2,3-dioxygenase is expressed not only by tumor cells, but also by antigen-presenting cells such as plasmacytoid dendritic cells.(11) However, it is still unclear which is the molecular target for cancer therapy: tumor cell-derived IDO, or host cell-derived IDO. In the present study, we clearly showed that IDO was specifically expressed in tumor cells, but not in tumor stromal cells or immune cells, suggesting tumor-derived IDO plays a pivotal role in ovarian cancer progression, which is consistent with a prior report on other types of tumor.(15) In contrast, recent studies using IDO-knockout mice have shown the importance of the role of host-derived IDO. Holmgaard et al.(34) showed that IDO deficiency in the host resulted in the reduction of tumor burden with a concomitant increase of effector T-cell recruitment. Similarly, Smith et al.(35) showed that host IDO deficiency led to reduced lung tumor burden/metastasis and improved survival. In addition, Wainwright et al.(14) showed that both tumoral IDO and host-derived IDO are involved in poor survival in brain glioblastoma. These observations suggest that not only tumor cell-derived, but also host cell-derived IDO could contribute to cancer progression; thus, either or both could become a molecular target with IDO inhibitors, depending on the type of tumors.
In summary, the present study revealed that tumor cell-derived IDO could enhance ovarian cancer progression through the development of immunotolerogenic conditions within the tumor microenvironment. Thus, targeting IDO may be a promising novel therapeutic strategy for ovarian cancer. Although single use of the IDO inhibitor 1-MT is currently being evaluated in a phase I study in patients with refractory solid tumors,(36) further clinical studies on the effect of combination therapy of IDO inhibition with chemotherapeutic agents or other immunotherapies may be needed.
Acknowledgments
We sincerely thank Prof. Toshikazu Kondo for his critical reading and helpful comments in the preparation of this manuscript. This work was supported in part by a Grant-in-Aid for Scientific Research (Grant No. 23592459 to K.I.) from the Ministry of Education, Culture, Sports, Science and Technology, Japan.
Glossary
- α-SMA
α-smooth muscle actin
- IFN-γ
interferon-γ
- HM-1
mouse ovarian carcinoma cell line OV2944-HM-1
- IDO
indoleamine 2,3-dioxygenase
- IL
interleukin
- 1-MT
1-methyl-tryptophan
- NK
natural killer
- TGF-β
transforming growth factor-β
- TIL
tumor-infiltrating lymphocyte
- TNF-α
tumor necrosis factor-α
- Treg
regulatory T cell
- VEGF
vascular endothelial growth factor
Disclosure Statement
The authors have no conflict of interest.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol. 2002;3:991–8. doi: 10.1038/ni1102-991. [DOI] [PubMed] [Google Scholar]
- 3.Gajewski TF, Meng Y, Blank C, et al. Immune resistance orchestrated by the tumor microenvironment. Immunol Rev. 2006;213:131–45. doi: 10.1111/j.1600-065X.2006.00442.x. [DOI] [PubMed] [Google Scholar]
- 4.Zitvogel L, Tesniere A, Kroemer G. Cancer despite immunosurveillance: immunoselection and immunosubversion. Nat Rev Immunol. 2006;6:715–27. doi: 10.1038/nri1936. [DOI] [PubMed] [Google Scholar]
- 5.Zou W. Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nat Rev Cancer. 2005;5:263–74. doi: 10.1038/nrc1586. [DOI] [PubMed] [Google Scholar]
- 6.Yigit R, Massuger LF, Figdor CG, Torensma R. Ovarian cancer creates a suppressive microenvironment to escape immune elimination. Gynecol Oncol. 2010;117:366–72. doi: 10.1016/j.ygyno.2010.01.019. [DOI] [PubMed] [Google Scholar]
- 7.Kandalaft LE, Motz GT, Duraiswamy J, Coukos G. Tumor immune surveillance and ovarian cancer: lessons on immune mediated tumor rejection or tolerance. Cancer Metastasis Rev. 2011;30:141–51. doi: 10.1007/s10555-011-9289-9. [DOI] [PubMed] [Google Scholar]
- 8.Abiko K, Mandai M, Hamanishi J, et al. PD-L1 on tumor cells is induced in ascites and promotes peritoneal dissemination of ovarian cancer through CTL dysfunction. Clin Cancer Res. 2013;19:1363–74. doi: 10.1158/1078-0432.CCR-12-2199. [DOI] [PubMed] [Google Scholar]
- 9.Takikawa O. Biochemical and medical aspects of the indoleamine 2,3-dioxygenase-initiated L-tryptophan metabolism. Biochem Biophys Res Commun. 2005;338:12–9. doi: 10.1016/j.bbrc.2005.09.032. [DOI] [PubMed] [Google Scholar]
- 10.Munn DH, Zhou M, Attwood JT, et al. Prevention of allogeneic fetal rejection by tryptophan catabolism. Science. 1998;281:1191–3. doi: 10.1126/science.281.5380.1191. [DOI] [PubMed] [Google Scholar]
- 11.Munn DH, Sharma MD, Hou D, et al. Expression of indoleamine 2,3-dioxygenase by plasmacytoid dendritic cells in tumor-draining lymph nodes. J Clin Invest. 2004;114:280–90. doi: 10.1172/JCI21583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frumento G, Rotondo R, Tonetti M, Damonte G, Benatti U, Ferrara GB. Tryptophan-derived catabolites are responsible for inhibition of T and natural killer cell proliferation induced by indoleamine 2,3-dioxygenase. J Exp Med. 2002;196:459–68. doi: 10.1084/jem.20020121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen W, Liang X, Peterson AJ, Munn DH, Blazar BR. The indoleamine 2,3-dioxygenase pathway is essential for human plasmacytoid dendritic cell-induced adaptive T regulatory cell generation. J Immunol. 2008;181:5396–404. doi: 10.4049/jimmunol.181.8.5396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wainwright DA, Balyasnikova IV, Chang AL, et al. IDO expression in brain tumors increases the recruitment of regulatory T cells and negatively impacts survival. Clin Cancer Res. 2012;18:6110–21. doi: 10.1158/1078-0432.CCR-12-2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Uyttenhove C, Pilotte L, Theate I, et al. Evidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2,3-dioxygenase. Nat Med. 2003;9:1269–74. doi: 10.1038/nm934. [DOI] [PubMed] [Google Scholar]
- 16.Okamoto A, Nikaido T, Ochiai K, et al. Indoleamine 2,3-dioxygenase serves as a marker of poor prognosis in gene expression profiles of serous ovarian cancer cells. Clin Cancer Res. 2005;11:6030–9. doi: 10.1158/1078-0432.CCR-04-2671. [DOI] [PubMed] [Google Scholar]
- 17.Godin-Ethier J, Hanafi LA, Piccirillo CA, Lapointe R. Indoleamine 2,3-dioxygenase expression in human cancers: clinical and immunologic perspectives. Clin Cancer Res. 2011;17:6985–91. doi: 10.1158/1078-0432.CCR-11-1331. [DOI] [PubMed] [Google Scholar]
- 18.Ino K, Yoshida N, Kajiyama H, et al. Indoleamine 2,3-dioxygenase is a novel prognostic indicator for endometrial cancer. Br J Cancer. 2006;95:1555–61. doi: 10.1038/sj.bjc.6603477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ino K, Yamamoto E, Shibata K, et al. Inverse correlation between tumoral indoleamine 2,3-dioxygenase expression and tumor-infiltrating lymphocytes in endometrial cancer: its association with disease progression and survival. Clin Cancer Res. 2008;14:2310–7. doi: 10.1158/1078-0432.CCR-07-4144. [DOI] [PubMed] [Google Scholar]
- 20.Inaba T, Ino K, Kajiyama H, et al. Role of the immunosuppressive enzyme indoleamine 2,3-dioxygenase in the progression of ovarian carcinoma. Gynecol Oncol. 2009;115:185–92. doi: 10.1016/j.ygyno.2009.07.015. [DOI] [PubMed] [Google Scholar]
- 21.Yoshida N, Ino K, Ishida Y, et al. Overexpression of indoleamine 2,3-dioxygenase in human endometrial carcinoma cells induces rapid tumor growth in a mouse xenograft model. Clin Cancer Res. 2008;14:7251–9. doi: 10.1158/1078-0432.CCR-08-0991. [DOI] [PubMed] [Google Scholar]
- 22.Habara-Ohkubo A, Takikawa O, Yoshida R. Cloning and expression of a cDNA encoding mouse indoleamine 2,3-dioxygenase. Gene. 1991;105:221–7. doi: 10.1016/0378-1119(91)90154-4. [DOI] [PubMed] [Google Scholar]
- 23.Toyoshima M, Tanaka Y, Matumoto M, et al. Generation of a syngeneic mouse model to study the intraperitoneal dissemination of ovarian cancer with in vivo luciferase imaging. Luminescence. 2009;24:324–31. doi: 10.1002/bio.1112. [DOI] [PubMed] [Google Scholar]
- 24.Yamamura S, Matsumura N, Mandai M, et al. The activated transforming growth factor-beta signaling pathway in peritoneal metastases is a potential therapeutic target in ovarian cancer. Int J Cancer. 2012;130:20–8. doi: 10.1002/ijc.25961. [DOI] [PubMed] [Google Scholar]
- 25.Takikawa O, Kuroiwa T, Yamazaki F, Kido R. Mechanism of interferon-gamma action. Characterization of indoleamine 2,3-dioxygenase in cultured human cells induced by interferon-gamma and evaluation of the enzyme-mediated tryptophan degradation in its anticellular activity. J Biol Chem. 1988;263:2041–8. [PubMed] [Google Scholar]
- 26.Plante M, Rubin SC, Wong GY, Federici MG, Finstad CL, Gastl GA. Interleukin-6 level in serum and ascites as a prognostic factor in patients with epithelial ovarian cancer. Cancer. 1994;73:1882–8. doi: 10.1002/1097-0142(19940401)73:7<1882::aid-cncr2820730718>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 27.Mustea A, Konsgen D, Braicu EI, et al. Expression of IL-10 in patients with ovarian carcinoma. Anticancer Res. 2006;26:1715–8. [PubMed] [Google Scholar]
- 28.Chen YL, Chang MC, Chen CA, Lin HW, Cheng WF, Chien CL. Depletion of regulatory T lymphocytes reverses the imbalance between pro- and anti-tumor immunities via enhancing antigen-specific T cell immune responses. PLoS ONE. 2012;7:e47190. doi: 10.1371/journal.pone.0047190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu CZ, Zhang L, Chang XH, et al. Overexpression and immunosuppressive functions of transforming growth factor 1, vascular endothelial growth factor and interleukin-10 in epithelial ovarian cancer. Chin J Cancer Res. 2012;24:130–7. doi: 10.1007/s11670-012-0130-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nonaka H, Saga Y, Fujiwara H, et al. Indoleamine 2,3-dioxygenase promotes peritoneal dissemination of ovarian cancer through inhibition of natural killer cell function and angiogenesis promotion. Int J Oncol. 2011;38:113–20. [PubMed] [Google Scholar]
- 31.Wang D, Saga Y, Mizukami H, et al. Indoleamine-2,3-dioxygenase, an immunosuppressive enzyme that inhibits natural killer cell function, as a useful target for ovarian cancer therapy. Int J Oncol. 2012;40:929–34. doi: 10.3892/ijo.2011.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Curiel TJ, Coukos G, Zou L, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–9. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 33.Räsänen K, Vaheri A. Activation of fibroblasts in cancer stroma. Exp Cell Res. 2010;316:2713–22. doi: 10.1016/j.yexcr.2010.04.032. [DOI] [PubMed] [Google Scholar]
- 34.Holmgaard RB, Zamarin D, Munn DH, Wolchok JD, Allison JP. Indoleamine 2,3-dioxygenase is a critical resistance mechanism in antitumor T cell immunotherapy targeting CTLA-4. J Exp Med. 2013;210:1389–402. doi: 10.1084/jem.20130066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smith C, Chang MY, Parker KH, et al. IDO is a nodal pathogenic driver of lung cancer and metastasis development. Cancer Discov. 2012;2:722–35. doi: 10.1158/2159-8290.CD-12-0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. 1-Methyl-D-Tryptophan in treating patients with metastatic or refractory solid tumors that cannot be removed by surgery. Information provided by National Cancer Institute (NCI). http://clinicaltrials.gov/ct2/show/study/NCT00567931.