Abstract
The aim of this open-label, multicenter, randomized phase II trial was to evaluate the efficacy and safety of zoledronic acid in combination with docetaxel in previously treated patients with non-small-cell lung cancer (NSCLC) and bone metastases. In this study, patients randomly received docetaxel (60 mg/m2) with (group DZ) or without (group D) zoledronic acid every 21 days. There were 50 patients in each group, and the primary endpoint was progression-free survival. In an efficacy analysis of 94 patients (DZ, 48; D, 46), the median progression-free survival was 2.7 months (95% confidence interval [CI], 1.5–3.5 months) for the DZ group and 2.6 months (95% CI, 1.5–3.4 months) for the D group (stratified log-rank test, P = 0.89). The median overall survival was 10.4 months (95% CI, 7.0–15.8 months) for the DZ group and 9.7 months (95% CI, 6.1–12.5 months) for the D group (stratified log-rank test, P = 0.62). There were no clinically relevant differences in the frequencies of grade 3 or 4 adverse events between the two groups. No treatment-related deaths occurred in the DZ group. Zoledronic acid combined with docetaxel was well tolerated but did not meet the primary endpoint of demonstrating a longer progression-free survival in advanced NSCLC patients with bone metastases compared with docetaxel alone. This trial was registered with the University Hospital Medical Information Network (UMIN000001098).
Keywords: Chemotherapy, docetaxel, non–small-cell lung cancer, phase II, zoledronic acid
Lung cancer is the leading cause of cancer death worldwide and non–small-cell lung cancer (NSCLC) accounts for more than 80% of all cases of lung cancer.(1) For individuals with advanced NSCLC, first-line treatment with platinum-based chemotherapy offers only a moderate improvement in survival and quality of life over best supportive care (BSC) alone.(2,3) Second-line treatment with docetaxel, despite a low tumor response rate, is a standard treatment option on the basis of phase III studies comparing docetaxel with ifosfamide, vinorelbine or BSC alone.(4,5) Thus, there is a need for new treatment options to prolong the survival of patients with advanced NSCLC. Approximately 30–40% of patients with NSCLC develop bone metastases, which often cause skeletal-related events (SRE) such as pathologic fracture, spinal cord compression, or the need for palliative radiation or surgery to the bone.(6) SRE are associated with decreased quality of life, increased health-care costs and poor survival; therefore, it is clinically imperative to prevent SRE during the treatment of advanced NSCLC.(7)– (10)
Zoledronic acid, a nitrogen-containing bisphosphonate, significantly delays the appearance of SRE and reduces the incidence of SRE compared with a placebo in patients with cancer and bone metastases, including those with NSCLC.(11,12) Furthermore, several preclinical and clinical studies provide evidence supporting the use of zoledronic acid for the treatment of patients with advanced NSCLC.(13)– (16) The inclusion of zoledronic acid in chemotherapy regimens has an additive and/or synergistic antitumor effect on NSCLC cell lines and may prolong survival and delay disease progression in patients with advanced NSCLC.(17)– (19) However, whether the inclusion of zoledronic acid in such regimens has clinically meaningful survival benefits in patients with NSCLC and bone metastases is uncertain. Therefore, we conducted this study to evaluate the efficacy and safety of zoledronic acid in combination with docetaxel in previously treated patients with NSCLC and bone metastases.
Patients and Methods
Study design
We conducted an open-label, multicenter, randomized phase II study in Japan. The study protocol was approved by the West Japan Oncology Group (WJOG) Protocol Review Committee and the institutional review board of each participating institution. This trial was registered with the University Hospital Medical Information Network (UMIN000001098).
Eligibility criteria
Patients were required to be histologically or cytologically diagnosed with NSCLC and bone metastases (at least one bone metastasis that had not been treated with radiation therapy) and to have had previous treatment with one or two chemotherapy regimens. Other inclusion criteria included an age of ≥20 years, Eastern Cooperative Oncology Group performance status of 0–2, measurable disease, no history of chemotherapy with docetaxel, no history of prior treatment with zoledronic acid, adequate baseline organ function (leukocyte count ≥3500/mm3; absolute neutrophil count ≥2000/mm3; hemoglobin ≥9.0 g/dL; platelet count ≥100 000/mm3; total bilirubin ≤2.0 mg/dL; aspartate aminotransferase and alanine aminotransferase [ALT] levels ≤100 IU/L; creatinine clearance, ≥30 mL/min; and SpO2 under room air, ≥90%). Written informed consent was obtained from all patients. Patients were ineligible if they had active concomitant malignancy, third-space fluid collection requiring drainage, radiographic signs of interstitial pneumonia or pulmonary fibrosis, active SRE at the time of registration, hypercalcemia requiring prompt treatment, active periodontal disease or severe comorbidities (active infectious disease, severe heart disease, uncontrolled diabetes mellitus, gastrointestinal bleeding, intestinal paralysis, bowel obstruction or psychiatric disease), or a history of drug allergy. Patients receiving systemic steroid medication and pregnant or breast-feeding women were also excluded.
Treatment
Equal numbers of patients randomly received 60 mg/m2 docetaxel intravenously for 1 h with (DZ group) or without (D group) intravenous zoledronic acid for 15 min. Random assignment was stratified by institution, gender and performance status (0–1 or 2). The dose of zoledronic acid for each patient was based on his or her creatinine clearance (>60 mL/min, 4 mg; 50–60 mL/min, 3.5 mg; 40–49 mL/min, 3.3 mg; 30–39 mL/min, 3.0 mg). Zoledronic acid was administered to patients in the DZ group immediately after docetaxel administration. Patients were treated every 3 weeks until their disease progressed, toxicity became intolerable or they refused additional treatment. The dose of docetaxel was decreased to 50 mg/m2 if any of the following was observed: leukocyte count <1000/mm3, platelet count <25 000/mm3, grade 3 febrile neutropenia or grade 3 nonhematological toxicity (with the exception of hyponatremia, hypocalcaemia and alopecia). In cases of grade 4 nonhematological toxicity or continued toxicity requiring a second dose reduction, the protocol treatment was terminated. Other criteria for protocol treatment termination included use of excluded concomitant therapy and physician recommendation.
Patients received full supportive care as required, including transfusion of blood products. Granulocyte colony-stimulating factor was administered as needed. There was no restriction on subsequent chemotherapy after disease progression in this study.
Evaluation
Patient assessment, including physical examination, complete blood count and biochemistry, was performed every 1–2 weeks. Bone markers and levels of urinary N-terminal telopeptide of type I collagen (NTX) and serum C-terminal telopeptide of type I collagen (I-CTP) were evaluated every 4 weeks. SRE included pathologic fracture, spinal cord compression and need for palliative radiation or surgery to the bone, and were assessed every 6 weeks.
Patients who received one or more protocol treatment were evaluated for safety during treatment. Adverse events were recorded and graded using the Common Terminology Criteria for Adverse Events, Version 3.0. The Response Evaluation Criteria in Solid Tumors guideline version 1.0 was used to evaluate tumor response.(20) Computed tomography was performed at baseline and every 6 weeks. A complete response (CR) or a partial response (PR) was confirmed at least 4 weeks after the first documentation of the response. Stable disease (SD) was defined as either sufficient tumor shrinkage to qualify as a CR or a PR or sufficient increase in tumor mass to qualify as progressive disease (PD) after at least 6 weeks. Progression-free survival (PFS) was defined as the time from patient registration to objective tumor progression or patient death. Patients whose disease had not progressed at the time of termination of protocol treatment were assessed until progression or death was documented. SRE-free survival was defined as the time from patient registration to the appearance of SRE or the death of the patient. Patients who had not experienced SRE at the time of termination of protocol treatment were assessed until SRE or death was documented. Overall survival (OS) was defined as the time from patient registration to death from any cause. All patients were followed up for 1 year after the last patient had enrolled.
Study endpoints and statistical analyses
The primary endpoint in this study was PFS. The secondary endpoints included OS, overall response rate (ORR), SRE rate, SRE-free survival and safety. This randomized phase II study was designed to detect a 1-month improvement in PFS, with an assumed PFS of 2 months in the D group and 3 months in the DZ group, with a two-sided alpha error of 20% and a power of approximately 80%. A total of 100 patients were registered over 2 years with a 1-year follow-up period after the last enrollment. Survival curves were estimated using the Kaplan–Meier method and compared by log-rank test. Fisher's exact test was used for categorical data. All analyses were performed using SAS version 9.1.3 (SAS Institute, Cary, NC, USA).
Results
Patient characteristics
From May 2007 to March 2010, 100 patients from 15 Japanese institutions were enrolled in this study: 50 patients were randomly assigned to the DZ group and 50 to the D group (Fig. 1). Patient demographics and baseline disease characteristics were well-balanced between the two treatment groups (Table 1). While one patient in the DZ group did not receive any protocol treatment, 99 patients (49 for DZ and 50 for D) were assessable for safety. In the DZ group 1 patient and in the D group 4 patients were ineligible, and 94 patients (48 for DZ and 46 for D) were included in the efficacy analysis (Fig. 1). The median number of treatment cycles was three for the DZ group (range, 1–19 cycles) and three for the D group (range, 1–17 cycles). The median number of administered doses of zoledronic acid was 3 (range, 1–19), with a median drug exposure of 12.0 mg (range, 3.5–76.0 mg). Reasons for going off protocol included disease progression (37 for DZ and 33 for D), patient refusal (eight for DZ and eight for D), unacceptable toxicity (two for DZ and five for D) and others (two for DZ and four for D).
Fig. 1.
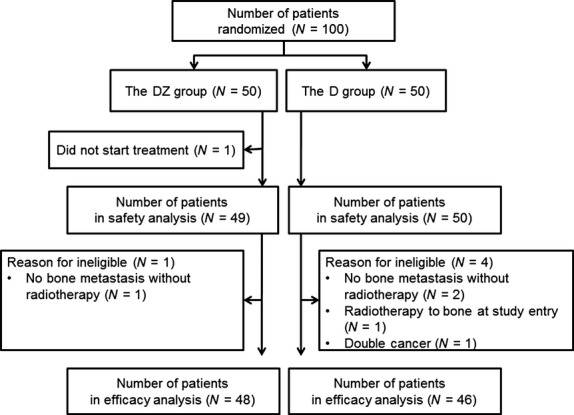
Patient disposition. D, docetaxel alone; DZ, docetaxel with zoledronic acid.
Table 1.
Patient demographics and baseline disease characteristics
| Characteristic | DZ group (N = 50) |
D group (N = 50) |
||
|---|---|---|---|---|
| Number | % | Number | % | |
| Age, years | ||||
| Median | 62 | 63 | ||
| Range | 34–77 | 45–79 | ||
| Sex | ||||
| Female | 19 | 38 | 18 | 36 |
| Male | 31 | 62 | 32 | 64 |
| ECOG performance status | ||||
| 0–1 | 47 | 94 | 47 | 94 |
| 2 | 3 | 6 | 3 | 6 |
| Smoking status | ||||
| Smoker | 19 | 38 | 15 | 30 |
| Never smoked | 31 | 62 | 35 | 70 |
| Histological subtype | ||||
| Adenocarcinoma | 39 | 78 | 38 | 76 |
| Squamous cell carcinoma | 5 | 10 | 7 | 14 |
| Others | 6 | 12 | 5 | 10 |
| Number of prior chemotherapies | ||||
| 1 | 34 | 68 | 39 | 78 |
| 2 | 15 | 30 | 11 | 22 |
| No data | 1 | 2 | 0 | 0 |
| Number of bone metastases | ||||
| Single | 11 | 22 | 12 | 24 |
| Multiple | 39 | 78 | 38 | 76 |
| Prior SRE | ||||
| No | 41 | 82 | 42 | 84 |
| Yes | 8 | 16 | 8 | 16 |
| No data | 1 | 2 | 0 | 0 |
| Urinary NTX | ||||
| High level (≥64 nmol/mmol creatinine) | 20 | 40 | 22 | 44 |
| Normal level (<64 nmol/mmol creatinine) | 23 | 46 | 22 | 44 |
| No data | 7 | 14 | 6 | 12 |
| Serum I-CTP | ||||
| High level (≥4.5 ng/mL) | 35 | 70 | 35 | 70 |
| Normal level (<4.5 ng/mL) | 8 | 16 | 9 | 18 |
| No data | 7 | 14 | 6 | 12 |
D, docetaxel alone; DZ, docetaxel with zoledronic acid; ECOG, Eastern Cooperative Oncology Group; I-CTP, C-terminal telopeptide of type I collagen; NTX, N-terminal telopeptide of type I collagen; SRE, skeletal-related event.
Safety
Adverse events for the 99 patients included in the safety analysis are summarized in Table 2. The occurrence of adverse events was similar in the two groups, with the exception of any grade of hypocalcemia (76% vs 30%) and pyrexia (39% vs 10%), which were more frequent in the DZ group compared with the D group. One patient in the DZ group experienced periodontal disease, but no cases of osteonecrosis of the jaw (ONJ) were observed in either group. The most common adverse events worse than grade 3 were leukopenia (63% and 56% for DZ and D, respectively), neutropenia (78% and 80% for DZ and D, respectively), febrile neutropenia (4% and 12% for DZ and D, respectively) and elevated ALT level (27% and 30% for DZ and D, respectively). There were no clinically relevant differences in the frequencies of adverse events of grade 3 or higher between the two groups. The protocol treatment was terminated in seven patients because of unacceptable toxicity levels, including grade 3 nail change (N = 1) and grade 2 periodontal disease (N = 1) in the DZ group, and required a second dose reduction because of grade 4 leukopenia (N = 1) or grade 3 febrile neutropenia (N = 1), grade 4 infection (N = 1), grade 3 allergic reaction (N = 1) and grade 1 pneumonitis (N = 1) in the D group. No treatment-related deaths were observed in the DZ group, while two treatment-related deaths were observed in the D group (infection, N = 1; gastrointestinal perforation, N = 1).
Table 2.
Summary of adverse events (CTCAE)
| Adverse event | DZ group (N = 49) |
D group (N = 50) |
||||||
|---|---|---|---|---|---|---|---|---|
| All |
≥Grade 3 |
All |
≥Grade 3 |
|||||
| Number | % | Number | % | Number | % | Number | % | |
| Leukopenia | 45 | 92 | 31 | 63 | 47 | 94 | 28 | 56 |
| Neutropenia | 45 | 92 | 38 | 78 | 46 | 92 | 40 | 80 |
| Anemia | 33 | 67 | 3 | 6 | 31 | 62 | 3 | 6 |
| Thrombocytopenia | 2 | 4 | 0 | 0 | 5 | 10 | 0 | 0 |
| Elevated ALT level | 24 | 49 | 13 | 27 | 21 | 42 | 15 | 30 |
| Elevated AST level | 19 | 39 | 4 | 8 | 16 | 32 | 3 | 6 |
| Elevated creatinine level | 7 | 14 | 1 | 2 | 13 | 26 | 2 | 4 |
| Hypercalcemia | 2 | 4 | 0 | 0 | 8 | 16 | 1 | 2 |
| Hypocalcemia | 37 | 76 | 3 | 6 | 15 | 30 | 0 | 0 |
| Febrile neutropenia | 2 | 4 | 2 | 4 | 6 | 12 | 6 | 12 |
| Infection | 13 | 27 | 5 | 10 | 5 | 10 | 3 | 6 |
| Sensory neuropathy | 12 | 24 | 2 | 4 | 11 | 22 | 1 | 2 |
| Fatigue | 33 | 67 | 2 | 4 | 33 | 66 | 2 | 4 |
| Anorexia | 30 | 61 | 2 | 4 | 30 | 60 | 1 | 2 |
| Nausea | 20 | 41 | 1 | 2 | 23 | 46 | 0 | 0 |
| Vomiting | 8 | 16 | 1 | 2 | 8 | 16 | 0 | 0 |
| Allergic reaction | 3 | 6 | 0 | 0 | 2 | 4 | 1 | 2 |
| Gastrointestinal perforation | 0 | 0 | 0 | 0 | 1 | 2 | 1 | 2 |
| Pyrexia | 19 | 39 | 0 | 0 | 5 | 10 | 0 | 0 |
| Periodontal disease | 1 | 2 | 0 | 0 | 0 | 0 | 0 | 0 |
ALT, alanine transaminase; AST, aspartate aminotransferase; CTCAE, Common Terminology Criteria for Adverse Events, version 3.0; D, docetaxel alone; DZ, docetaxel with zoledronic acid.
Efficacy
For the 94 patients included in the efficacy analysis, the ORR was 8% for the DZ group (CR, N = 0; PR, N = 4; SD, N = 18; PD, N = 25; not evaluable, N = 1) and 4% for the D group (CR, N = 0; PR, N = 2; SD, N = 20; PD, N = 23; not evaluable, N = 1). The difference in ORR between the two groups was not statistically significant (P = 0.88). Median PFS was 2.7 (95% CI, 1.5–3.5) months for the DZ group and 2.6 (95% CI, 1.5–3.4) months for the D group (stratified log-rank test, P = 0.89; Fig. 2a). Median OS was 10.4 (95% CI, 7.0–15.8) months for the DZ group and 9.7 (95% CI, 6.1–12.5) months for the D group (stratified log-rank test, P = 0.62; Fig. 2b). No remarkable difference in PFS (Fig. 3a) or OS (Fig. 3b) was observed according to demographic characteristics (number of bone metastases, prior SRE, baseline urinary NTX and baseline serum I-CTP).
Fig. 2.
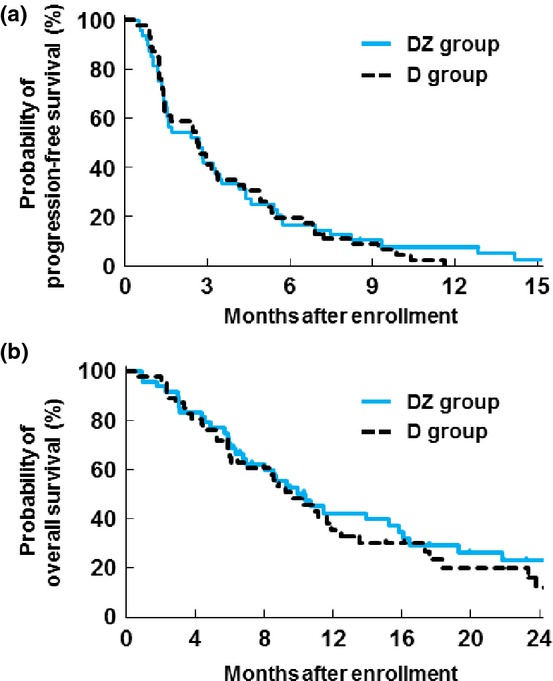
(a) Progression-free survival and (b) overall survival in the DZ and D groups. D, docetaxel alone; DZ, docetaxel with zoledronic acid.
Fig. 3.
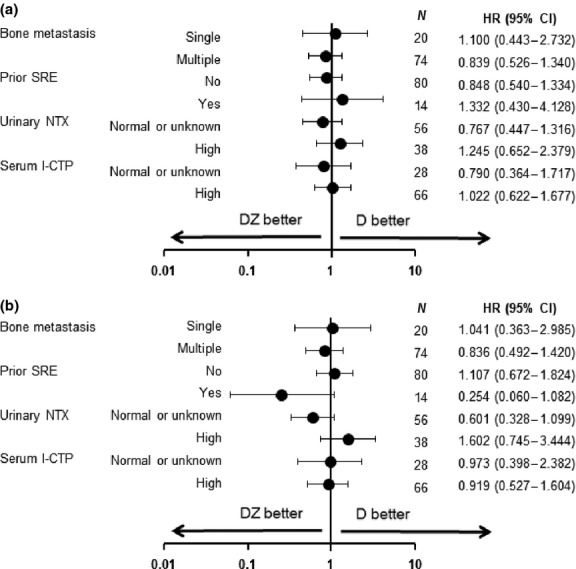
(a) Subgroup analyses of hazard ratio for progression-free survival and (b) overall survival in the DZ and D groups. D, docetaxel alone; DZ, docetaxel with zoledronic acid; I-CTP, C-terminal telopeptide of type I collagen; NTX, N-terminal telopeptide of type I collagen; SRE, skeletal-related event.
For the 94 patients included in the efficacy analysis, the cumulative incidence rates of an SRE at 3, 6, 9 and 12 months were 17%, 20%, 27% and 30%, respectively, for the DZ group, and 16%, 27%, 39% and 39%, respectively, for the D group (Fig. 4a). Median SRE-free survival was 7.2 (95% CI, 4.9–10.7) months for the DZ group and 6.0 (95% CI, 4.4–8.5) months for the D group (stratified log-rank test, P = 0.84). In subset analyses of the SRE rate according to baseline bone marker levels (Fig. 4b), the cumulative incidence rates of SRE at 12 months were 44% for the DZ group (N = 19) and 48% for the D group (N = 19) in patients with high baseline urinary NTX levels, 24% for the DZ group (N = 29) and 30% for the D group (N = 27) in patients with normal or unknown baseline urinary NTX levels, 43% for the DZ group (N = 34) and 38% for the D group (N = 32) in patients with high baseline serum I-CTP levels, and 7% for the DZ group (N = 14) and 37% for the D group (N = 14) in patients with normal or unknown baseline serum I-CTP levels.
Fig. 4.
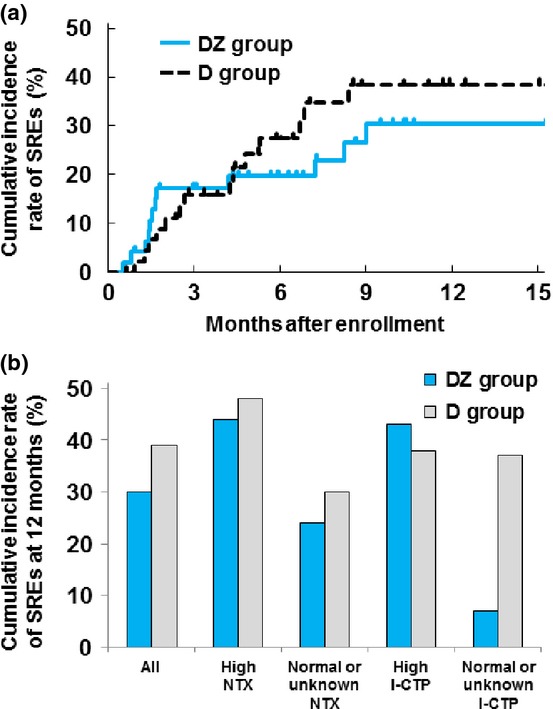
(a) Cumulative incidence rate of SRE in the DZ and D groups. (b) Subgroup analyses of SRE rate according to baseline bone marker levels in the DZ and D groups. D, docetaxel alone; DZ, docetaxel with zoledronic acid; I-CTP, C-terminal telopeptide of type I collagen; NTX, N-terminal telopeptide of type I collagen; SRE, skeletal-related event.
Discussion
This is the first prospective, randomized, phase II study to evaluate the efficacy and safety of zoledronic acid in combination with docetaxel in previously treated advanced NSCLC patients with bone metastases. The similarity in the median PFS and OS of patients in the DZ and D groups suggests that the combination of zoledronic acid and docetaxel might not provide survival benefits to patients with NSCLC and bone metastases compared with docetaxel alone. In a previous randomized phase III study, a subgroup analysis of patients with NSCLC (N = 382) revealed that zoledronic acid significantly reduced the risk of a first on-study SRE compared with a placebo. However, there was no significant difference in OS between the two groups (median 187 days for zoledronic acid vs 157 days for placebo; P = 0.539).(11,12,14) Two randomized studies in which zoledronic acid was combined with standard treatment also showed no survival benefits for patients with NSCLC who had no bone involvement.(21,22) These results are consistent with our observation that zoledronic acid failed to prolong the survival of NSCLC patients with bone metastases. In a recent subgroup analysis of a randomized phase III study, denosumab significantly improved OS, whereas zoledronic acid did not. This analysis was conducted on a group of 811 patients with lung cancer and bone metastases (median 8.9 vs 7.7 months for denosumab and zoledronic acid, respectively; hazard ratio for death, 0.80; 95% CI, 0.67–0.95; P = 0.01) and 702 patients with NSCLC and bone metastases (median 9.5 vs 8.0 months for denosumab and zoledronic acid, respectively; hazard ratio for death, 0.78; 95% CI, 0.65–0.94; P = 0.01).(23,24) Denosumab, a human anti-RANKL monoclonal antibody, is a potential anticancer therapy for patients with NSCLC and bone metastases and should be evaluated further in future studies.
For patients with NSCLC and bone metastases, increased SRE risk correlated with a history of SREs, multiple bone metastases, and bone turnover markers.(25)– (27) Significantly high levels of urinary NTX, a sensitive bone resorption marker, were also associated with increased SRE risk and poor survival prognosis.(27) In agreement, the cumulative incidence rates of SRE were high in patients with high baseline urinary NTX levels in our study. A retrospective analysis of a phase III study revealed that zoledronic acid significantly reduces the risk of death compared with a placebo in 144 patients with NSCLC and high baseline NTX levels (hazard ratio for death, 0.65; 95% CI, 0.45–0.95; P = 0.025).(15) In our study, for 38 patients (19 for DZ and 19 for D) with NSCLC and high baseline NTX levels, the median OS was 8.6 months for the DZ group and 11.2 months for the D group (hazard ratio for death, 1.60; 95% CI, 0.75–3.44). Therefore, combination treatment with zoledronic acid and docetaxel did not improve OS in previously treated patients with NSCLC and bone metastases in addition to high baseline NTX levels. However, the number of patients in our study was small; as such, this study was not powered to detect differences in secondary variables, and statistical testing was performed for exploratory purposes.
The most common severe toxicities in the present study were leukopenia, neutropenia, febrile neutropenia and elevated ALT levels, which were similar in the two groups. No treatment-related deaths were observed in the DZ group. Although hypocalcemia and pyrexia were more frequent in the DZ group than in the D group, they were mild and manageable in most cases. A possible reason for the high incidence of hypocalcemia in this study was underuse of calcium supplements and vitamin D. Prophylactic oral administration of daily calcium supplements and vitamin D should be considered during treatment with zoledronic acid. No patient experienced ONJ in this study, although it may be argued that the duration of zoledronic acid treatment was too short for this to occur. No additional adverse events were observed in the present study compared with previous studies.(11,12,23,24)
The present study demonstrated the safety and tolerability of the combination of zoledronic acid and docetaxel but did not meet the primary endpoint of PFS in advanced NSCLC patients with bone metastasis. Based on these results, we abandoned assessment of the survival benefits of adding zoledronic acid to docetaxel treatment in a larger phase III study. There are potential limitations to our study. First, we used an open-label study design despite the use of PFS as the primary endpoint. Second, the sample size of the present study was relatively small. Third, we did not collect data regarding post-study treatment with zoledronic acid. New treatment options are still needed to prolong the survival of advanced NSCLC patients with bone metastasis.
Acknowledgments
The authors would like to thank Ms Kaori Mori and Mr Koichi Hosoda for data management and Dr Shinichiro Nakamura (WJOG Data Center) for oversight and management of the present study. The authors are also grateful to Dr Keisuke Tomii (Kobe City Medical Center General Hospital, Hyogo), Dr Hideo Saka (National Hospital Organization Nagoya Medical Center, Aichi), Dr Yasuo Iwamoto (Hiroshima City Hospital, Hiroshima), Dr Norihiko Ikeda (Tokyo Medical University Hospital, Tokyo), Dr Sunao Ushijima (Kumamoto Chuo Hospital, Kumamoto), Dr Masaaki Kawahara (Otemae Hospital, Osaka), Dr Takashi Kijima (Osaka University Hospital, Osaka) and Dr Shigeki Sato (Nagoya City University Hospital, Aichi) for their contributions to this study.
Disclosure Statement
Haruyasu Murakami received research funding from Sanofi K.K. and Novartis Pharma K.K. Takashi Seto received research funding from Novartis Pharma K.K. Yoichi Nakanishi received research funding from Novartis Pharma K.K. and others from Novartis Pharma K.K.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Non-small Cell Lung Cancer Collaborative Group. Chemotherapy in non-small cell lung cancer: a meta-analysis using updated data on individual patients from 52 randomised clinical trials. Non-small Cell Lung Cancer Collaborative Group. BMJ. 1995;311:899–909. [PMC free article] [PubMed] [Google Scholar]
- 3.Azzoli CG, Baker S, Jr, Temin S, et al. American Society of Clinical Oncology Clinical Practice Guideline update on chemotherapy for stage IV non-small-cell lung cancer. J Clin Oncol. 2009;27:6251–66. doi: 10.1200/JCO.2009.23.5622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shepherd FA, Dancey J, Ramlau R, et al. Prospective randomized trial of docetaxel versus best supportive care in patients with non-small-cell lung cancer previously treated with platinum-based chemotherapy. J Clin Oncol. 2000;18:2095–103. doi: 10.1200/JCO.2000.18.10.2095. [DOI] [PubMed] [Google Scholar]
- 5.Fossella FV, DeVore R, Kerr RN, et al. Randomized phase III trial of docetaxel versus vinorelbine or ifosfamide in patients with advanced non-small-cell lung cancer previously treated with platinum-containing chemotherapy regimens. The TAX 320 Non-Small Cell Lung Cancer Study Group. J Clin Oncol. 2000;18:2354–62. doi: 10.1200/JCO.2000.18.12.2354. [DOI] [PubMed] [Google Scholar]
- 6.Coleman RE. Metastatic bone disease: clinical features, pathophysiology and treatment strategies. Cancer Treat Rev. 2001;27:165–76. doi: 10.1053/ctrv.2000.0210. [DOI] [PubMed] [Google Scholar]
- 7.Weinfurt KP, Li Y, Castel LD, et al. The significance of skeletal-related events for the health-related quality of life of patients with metastatic prostate cancer. Ann Oncol. 2005;16:579–84. doi: 10.1093/annonc/mdi122. [DOI] [PubMed] [Google Scholar]
- 8.Delea T, Langer C, McKiernan J, et al. The cost of treatment of skeletal-related events in patients with bone metastases from lung cancer. Oncology. 2004;67:390–6. doi: 10.1159/000082923. [DOI] [PubMed] [Google Scholar]
- 9.Tsuya A, Kurata T, Tamura K, Fukuoka M. Skeletal metastases in non-small cell lung cancer: a retrospective study. Lung Cancer. 2007;57:229–32. doi: 10.1016/j.lungcan.2007.03.013. [DOI] [PubMed] [Google Scholar]
- 10.Brodowicz T, O'Byrne K, Manegold C. Bone matters in lung cancer. Ann Oncol. 2012;23:2215–22. doi: 10.1093/annonc/mds009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rosen LS, Gordon D, Tchekmedyian S, et al. Zoledronic acid versus placebo in the treatment of skeletal metastases in patients with lung cancer and other solid tumors: a phase III, double-blind, randomized trial–The Zoledronic Acid Lung Cancer and Other Solid Tumors Study Group. J Clin Oncol. 2003;21:3150–7. doi: 10.1200/JCO.2003.04.105. [DOI] [PubMed] [Google Scholar]
- 12.Rosen LS, Gordon D, Tchekmedyian NS, et al. Long-term efficacy and safety of zoledronic acid in the treatment of skeletal metastases in patients with nonsmall cell lung carcinoma and other solid tumors: a randomized, Phase III, double-blind, placebo-controlled trial. Cancer. 2004;100:2613–21. doi: 10.1002/cncr.20308. [DOI] [PubMed] [Google Scholar]
- 13.Mahtani R, Khan R, Jahanzeb M. The potential application of zoledronic acid as anticancer therapy in patients with non-small-cell lung cancer. Clin Lung Cancer. 2011;12:26–32. doi: 10.3816/CLC.2011.n.003. [DOI] [PubMed] [Google Scholar]
- 14.Lipton A, Cook R, Saad F, et al. Normalization of bone markers is associated with improved survival in patients with bone metastases from solid tumors and elevated bone resorption receiving zoledronic acid. Cancer. 2008;113:193–201. doi: 10.1002/cncr.23529. [DOI] [PubMed] [Google Scholar]
- 15.Hirsh V, Major PP, Lipton A, et al. Zoledronic acid and survival in patients with metastatic bone disease from lung cancer and elevated markers of osteoclast activity. J Thorac Oncol. 2008;3:228–36. doi: 10.1097/JTO.0b013e3181651c0e. [DOI] [PubMed] [Google Scholar]
- 16.Zarogoulidis K, Boutsikou E, Zarogoulidis P, et al. The impact of zoledronic acid therapy in survival of lung cancer patients with bone metastasis. Int J Cancer. 2009;125:1705–9. doi: 10.1002/ijc.24470. [DOI] [PubMed] [Google Scholar]
- 17.Ozturk OH, Bozcuk H, Burgucu D, et al. Cisplatin cytotoxicity is enhanced with zoledronic acid in A549 lung cancer cell line: preliminary results of an in vitro study. Cell Biol Int. 2007;31:1069–71. doi: 10.1016/j.cellbi.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 18.Lu S, Zhang J, Zhou Z, et al. Synergistic inhibitory activity of zoledronate and paclitaxel on bone metastasis in nude mice. Oncol Rep. 2008;20:581–7. [PubMed] [Google Scholar]
- 19.Chang JW, Hsieh JJ, Shen YC, et al. Bisphosphonate zoledronic acid enhances the inhibitory effects of gefitinib on EGFR-mutated non-small cell lung carcinoma cells. Cancer Lett. 2009;278:17–26. doi: 10.1016/j.canlet.2008.12.019. [DOI] [PubMed] [Google Scholar]
- 20.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–16. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 21.Pandya KJ, Gajra A, Warsi GM, et al. Multicenter, randomized, phase 2 study of zoledronic acid in combination with docetaxel and carboplatin in patients with unresectable stage IIIB or stage IV non-small cell lung cancer. Lung Cancer. 2010;67:330–8. doi: 10.1016/j.lungcan.2009.04.020. [DOI] [PubMed] [Google Scholar]
- 22.Scagliotti GV, Kosmidis P, de Marinis F, et al. Zoledronic acid in patients with stage IIIA/B NSCLC: results of a randomized, phase III study. Ann Oncol. 2012;23:2082–7. doi: 10.1093/annonc/mds128. [DOI] [PubMed] [Google Scholar]
- 23.Henry DH, Costa L, Goldwasser F, et al. Randomized, double-blind study of denosumab versus zoledronic acid in the treatment of bone metastases in patients with advanced cancer (excluding breast and prostate cancer) or multiple myeloma. J Clin Oncol. 2011;29:1125–32. doi: 10.1200/JCO.2010.31.3304. [DOI] [PubMed] [Google Scholar]
- 24.Scagliotti GV, Hirsh V, Siena S, et al. Overall survival improvement in patients with lung cancer and bone metastases treated with denosumab versus zoledronic acid: subgroup analysis from a randomized phase 3 study. J Thorac Oncol. 2012;7:1823–9. doi: 10.1097/JTO.0b013e31826aec2b. [DOI] [PubMed] [Google Scholar]
- 25.Hirsh V, Tchekmedyian NS, Rosen LS, et al. Clinical benefit of zoledronic acid in patients with lung cancer and other solid tumors: analysis based on history of skeletal complications. Clin Lung Cancer. 2004;6:170–4. doi: 10.3816/CLC.2004.n.030. [DOI] [PubMed] [Google Scholar]
- 26.Sekine I, Nokihara H, Yamamoto N, et al. Risk factors for skeletal-related events in patients with non-small cell lung cancer treated by chemotherapy. Lung Cancer. 2009;65:219–22. doi: 10.1016/j.lungcan.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 27.Brown JE, Cook RJ, Major P, et al. Bone turnover markers as predictors of skeletal complications in prostate cancer, lung cancer, and other solid tumors. J Natl Cancer Inst. 2005;97:59–69. doi: 10.1093/jnci/dji002. [DOI] [PubMed] [Google Scholar]


