Abstract
In multiple myeloma (MM), the hypoxic environment is an important factor causing tumor angiogenesis, which is strongly correlated to disease progression and unfavorable outcome by activating the key transcription factor, hypoxia-inducible factor-1α (HIF-1α). Gambogic acid (GA) is the major active ingredient of gamboge, which has been shown to possess antitumor effect by in vitro and in vivo study. However, the underlying molecular mechanism of whether GA inhibits tumor angiogenesis remains poorly understood. In this study, we investigated the effects of GA on expression of HIF-1α, and its downstream target gene vascular endothelial growth factor (VEGF) in human MM U266 cells. We found that hypoxia induced increase in the level of HIF-1α subunit protein and activated the phosphatidylinositol 3-kinase (PI3K)/Akt/mammalian target protein of rapamycin (mTOR) pathway. Moreover, the treatment with GA markedly decreased HIF-1α and VEGF expression under hypoxic conditions. Mechanistic studies exhibited that GA inhibited the production of HIF-1α by reducing phosphorylation of Akt and mTOR in U266 cells. Furthermore, in vivo study revealed that intravenous injection of GA once every other day for 2 weeks could suppress tumor volumes by antiangiogenesis activity. Taken together, our results identify that GA suppresses hypoxia-activated pathways that are linked to MM progression, at least partly, by the inhibition of the PI3K/Akt/mTOR signaling pathway. Therefore, GA may be a new potent therapeutic agent against human MM cells.
Keywords: Angiogenesis, gambogic acid, hypoxia-inducible factor 1 alpha subunit, multiple myeloma, vascular endothelial growth factor A
Multiple myeloma (MM) is the second most common (10–15% of all) hematological cancers characterized by the accumulation of malignant plasma cells within the bone marrow.(1,2) It is responsible for 15–20% of deaths from hematological cancer and about 2% of all deaths from cancer.(2) Angiogenesis, supporting the growth and survival of plasma cells, plays a critical role in the pathophysiology and progression of MM.(3,4) Hypoxia, a key feature of the tumor microenvironment, has been shown to be a leading cause of angiogenesis. In the tumor cells, hypoxia-inducible factor-1α (HIF-1α) has been regarded as the most important transcriptional factor promoting angiogenesis by upregulating pro-angiogenic genes such as vascular endothelial growth factor (VEGF),(5) which can enhance the microvascular density of bone marrow and accounts for the abnormal structure of myeloma tumor vessels.(6)
Under hypoxia, HIF-1α can escape the Von Hippel-Lindau tumor suppressor protein (VHL) binding and proteasomal degradation, translocate to the nucleus, heterodimerize with HIF-1β, and induce transcription of numerous target genes related to cell migration, vascular remodeling and angiogenesis for adaptation to hypoxia.(7,8) Increased HIF-1α levels are also associated with increased risk of mortality in many human cancers.(9) The previous studies have demonstrated the bone marrow microenvironment is hypoxic in MM patients(10) and determined the role of hypoxia in progression and dissemination of MM mouse models.(11) These findings suggest HIF-1α could be a potential therapeutic target.
Recent studies have shown many mechanisms regulate the HIF-1α expression at the level of transcription, translation and protein stability, such as nuclear factor-κ B (NF-κB), phosphatidylinositol 3-kinase (PI3K)/Akt signaling pathways, and prolyl hydroxylase (PHD)-pVHL-dependent mechanisms.(12) Among these mechanisms, PI3K/AKT pathway is an important element in response to hypoxia.(13,14) Hypoxia can stimulate the activation of PI3K/Akt pathway.(15,16) It has become increasingly evident that the increase of HIF-1α synthesis is associated with activated PI3K/Akt signaling that stimulates mammalian target protein of rapamycin (mTOR) activity, result in augmenting translation of HIF-1α mRNA into protein.(17) The suppression of PI3K and mTOR can inhibit HIF-1α expression in non-hypoxic and hypoxic cells. Therefore, pharmacological inhibition of HIF-1α activity may represent a useful treatment strategy.(17,18)
Gambogic acid (GA), a main active ingredient of gamboge, is a brownish to orange dry resin secreted from Garcinia hanburyi, which is a plant distributed widely in nature (Fig. 1). Recently, in vitro and in vivo studies have demonstrated that GA exerts potent anti-tumor effects on solid tumors and hematological malignancies.(19) In addition, GA can inhibit angiogenesis through suppressing secretion of VEGF.(20)
Fig. 1.
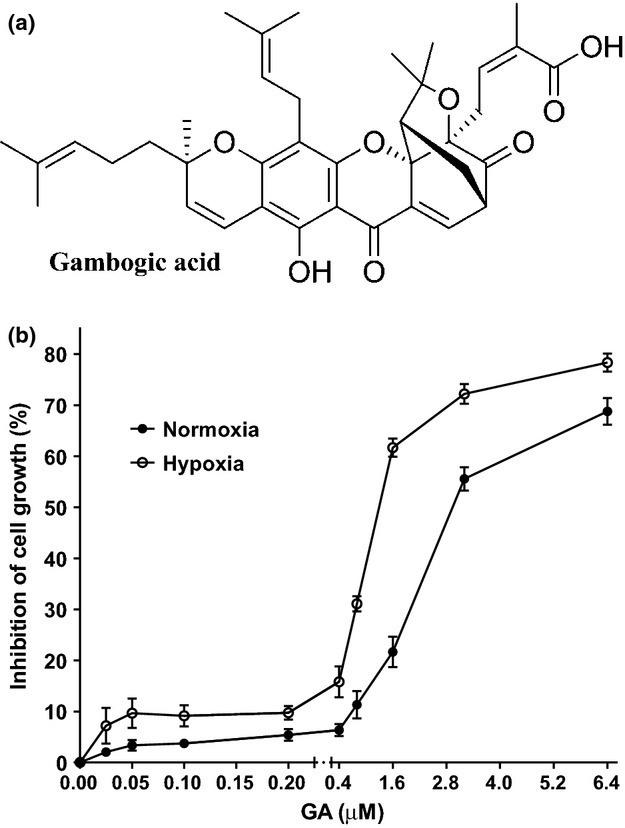
Effect of gambogic acid (GA) on the cytotoxicity against myeloma U266 cells. (a) The chemical structure of GA. (b) U266 cells were treated with various concentrations of GA for 8 h under normoxia and hypoxia condition. Cell viability was detected by CCK8 assay. Bars are the mean ± SD (n = 3).
However, whether GA has an ability to suppress HIF-1α/VEGF expression via inhibition of PI3K/Akt/mTOR signaling pathway under hypoxia in human multiple myeloma cells still remains unclear. Thus, in our study, we investigated the effect of GA on HIF-1α/VEGF induction under hypoxia in human myeloma cell line, U266, and its underlying molecular mechanism.
Materials and Methods
Cell culture and induction of hypoxia
The human myeloma cell line, U266 was obtained from Shanghai Cell Bank of Chinese Academy of Sciences (Shanghai, China). The cells were maintained in RPMI-1640 medium (Gibco, Grand Island, NY, USA) supplemented with 15% heat-inactivated fetal bovine serum (Sijiqing, Hangzhou, China), 100 U/mL penicillin and 100 μg/mL streptomycin (Sigma-Aldrich, St. Louis, MO, USA) in a humidified atmosphere of 5% CO2 at 37°C. For hypoxia induction, cells were incubated in a sealed hypoxic chamber flushed with a gas mixture of 94% N2, 5% CO2 and 1% O2.
Cytotoxicity assay
Gambogic acid (Key Laboratory of Carcinogenesis and Intervention, China Pharmaceutical University, China) was dissolved in DMSO (Sigma-Aldrich), stored at −20°C, and diluted as needed in RPMI-1640. Cytotoxic effect of GA on proliferating cells was assayed by CCK8 (Dojindo, Kumamoto, Japan). Briefly, cells were seeded onto 96-well plates at a density of 3 × 104 cells/well and treated with various concentrations of GA for 8 h. The CCK-8 solution (10 μL) was added to each well and incubated for 3 h. The absorbance was measured at 450 nm by Multiskan MK3 (Thermo Scientific, Shanghai, China). Cell viability was calculated as a percentage of viable cells in the GA-treated group versus the untreated control.
Quantitative real-time PCR assay
Total RNA was isolated from cells by using RNAiso Plus (TaKaRa, Dalian, China). Reverse transcription was performed with PrimeScript RT reagent kit with gDNA Eraser (TaKaRa), and real time PCR was carried out using SYBR Premix Ex Taq (TaKaRa). The real time PCR reaction contained: 10 μL of SYBR Premix Ex Taq, 0.4 μL of forward primer, 0.4 μL of reverse primer, 0.4 μL ROX Reference Dye, 1 μL of cDNA template, and 7.8 μL of dH2O. The program was 95°C for 30 s, followed by 40 cycles of 95°C for 30 s, 60°C for 30 s, and 72°C for 30 s. The relative expression level of mRNAs was normalized to that of internal control β-actin using the 2−ΔΔCt cycle threshold method. The primer sequences were as follows:
VEGFA (forward) 5′- GAGCCTTGCCTTGCTGCTCTAC-3′;
VEGFA (reverse) 5′-CACCAGGGTCTCGATTGGATG-3′;
HIF-1α (forward) 5′-TGAGGAAATGAGAGAAATGCTTACA-3′;
HIF-1α (reverse) 5′-ACACTGAGGTTGGTTACTGTTGGT-3′;
β-actin (forward) 5′-AAAGACCTGTACGCCAACAC-3′;
β-actin (reverse) 5′- GTCATACTCCTGCTTGCTGAT-3′.
ELISA
3 × 105 cells were seeded onto 6-well plates in serum-free medium containing various concentrations of GA (0, 0.05, 0.1 and 0.2 μM) and incubated for 8 h at hypoxia or normoxia. The conditioned medium was collected, and VEGF levels were determined by VEGF ELISA kit (Neobioscience, Shenzhen, China) according to the manufacturer's protocols. VEGF was expressed as a picogram of VEGF protein per milliliter medium and per 105 cells.
Immunofluorescent staining
Cells were dripped on glass slides and fixed with 4% paraformaldehyde for 30 min at room temperature, treated with 0.2% Triton X-100 and blocking solution (5% BSA and 0.2% Triton X-100 in PBS) for 30 min, respectively. The cells were incubated with mouse anti-human HIF-1α (1:50, Santa Cruz Biotechnology, Santa Cruz, CA, USA) overnight at 4°C and then incubated with FITC-conjugated goat anti-mouse IgG (1:100, Santa Cruz Biotechnology) for 2 h at room temperature. Then the cell nucleus was stained with DAPI (Beyotime, Haimen, China) for 5 min. Between each step, the cells were washed with PBS three times. The results of staining were photographed with a fluorescence confocal microscope (FV-1000, Olympus, Tokyo, Japan).
Western blot analysis
Rapamycin and LY294002 were purchased from Sigma. Protein extraction from cells and western blot analysis were performed as described previously.(21) Blots were incubated respectively with various appropriately diluted primary antibodies for HIF-1α and VEGF (Santa Cruz Biotechnology), Akt, phospho-Akt, mTOR and phospho-mTOR (Cell Signaling, Beverly, MA, USA) overnight at 4°C, and then followed by horseradish peroxidase-conjugated goat anti-rabbit or mouse secondary antibody (Santa Cruz Biotechnology) for 2 h at room temperature. The β-actin (Santa Cruz Biotechnology) was used as the internal control. For quantity, images were analyzed using Image J software from the NIH (Bethesda, MD, USA).
Mouse xenograft model
Six-week-old male BALB/c nude mice, with body weights of 18–22 g, were procured from Shanghai National Center for Laboratory Animals (Shanghai, China) and maintained in a specific pathogen-free environment. Studies were performed in adherence with the Guidelines established by the National Science Council, Republic of China.
The mice were subcutaneously injected with 1 × 107 cells. Tumor volume was measured every other day with caliper and calculated according to the formula: V = a2b/2, where a is the smallest superficial diameter and b is the largest superficial diameter. When the tumor volume reached approximately 50 mm3, the mice (n = 6/group) were randomly assigned to three groups: solvent vehicle control groupL 2 mg/kg per 2 days GA group; 4 mg/kg per 2 days GA group. The intravenous treatments were done once every other day for 14 days. After 2 weeks, the mice were killed, and the tumors were removed and measured.
Immunohistochemistry
Immunohistochemical staining was performed using UltraSensitive S-P IHC Kit (Maixin, Fuzhou, China) according to the manufacturer's protocols. The sections were incubated with anti-CD31, anti-VEGF or anti-HIF-1α (1:100, Santa Cruz Biotechnology) at 4°C overnight. Then, sections were stained with a streptavidin-peroxidase system, the signal was visualized using diaminobenzidine substrate and counterstaining was done with hematoxylin. For quantity, the microvessel density and the levels of VEGF and HIF-1α were measured using Image-Pro Plus 6.0 (Media Cybernetics, Silver Spring, MD, USA).
Statistical analysis
All values were expressed as mean ± standard deviation (SD) from triplicate experiments performed in a parallel manner unless otherwise indicated. Data were analyzed using an unpaired, two-tailed Student's t-test. The level of significance was indicated as *P < 0.05 and **P < 0.01.
Results
Effects of GA on growth of U266 cells in vitro
To determine potential cytotoxic and anti-proliferative effects of GA, U266 cells were exposed to GA at various concentrations (0.025–6.4 μM) under normoxia and hypoxia for 8 h. The results showed that treatment with GA at concentrations above 0.4 μM led to a significant dose-dependent inhibition of U266 cell growth under normoxia and hypoxia (Fig. 1). Considering low cytotoxicity, 0.2 μM GA or less were chosen in our study.
GA suppresses hypoxia-induced VEGF expression and secretion in U266 cells
We examined VEGF expression and secretion from the U266 cells under normoxia and hypoxia and the effect of GA on that. The results showed that VEGF was more secreted under hypoxia and GA decreased VEGF secretion compared to the control group under hypoxia much more significantly than under normoxia (Fig. 2a).
Fig. 2.
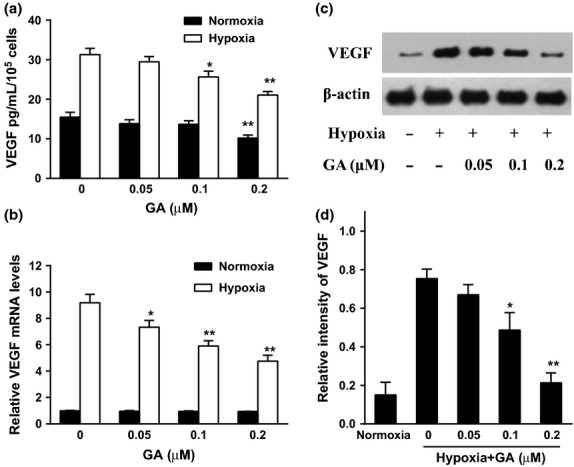
Effect of gambogic acid (GA) on the secretion and expression of vascular endothelial growth factor (VEGF) in U266 cells under normoxia and hypoxia. (a) U266 cells were treated by GA (0, 0.05, 0.1, and 0.2 μM) for 8 h under normoxia and hypoxia. VEGF secretion was detected by ELISA assay. Bars were the mean ± SD (n = 3). The comparisons were made relative to untreated controls, and the different levels of significance were indicated as *P < 0.05 and **P < 0.01. (b) Effect of GA on VEGF mRNA level under normoxia and hypoxia. U266 cells were treated with various concentrations of GA (0, 0.05, 0.1, and 0.2 μM) for 4 h under normoxic and hypoxic conditions. VEGF mRNA was detected by real-time polymerase chain reaction (PCR) and analyzed by the ΔΔCt method. Bars were shown as mean ± SD (n = 3) and represent VEGF/β-actin fold relative to the untreated group. The different levels of significance were indicated as *P < 0.05 and **P < 0.01. (c) Effect of GA on VEGF protein expression under normoxia and hypoxia. U266 cells were treated with various concentrations of GA (0, 0.05, 0.1, and 0.2 μM) for 4 h under normoxic and hypoxic conditions. VEGF protein expression was analyzed by western blots. (d) For quantity of (c), images were analyzed using Image J. Bars are the mean ± SD (n = 3). The comparisons were made relative to hypoxia alone group, and the different levels of significance were indicated as *P < 0.05 and **P < 0.01.
Real time-PCR and western blots were used to measure the expression of VEGF, the results showed hypoxia induced upregulation of VEGF mRNA (Fig. 2b) and protein (Fig. 2c,d). The increases of VEGF caused by hypoxia were dramatically inhibited by GA in a dose-dependent manner.
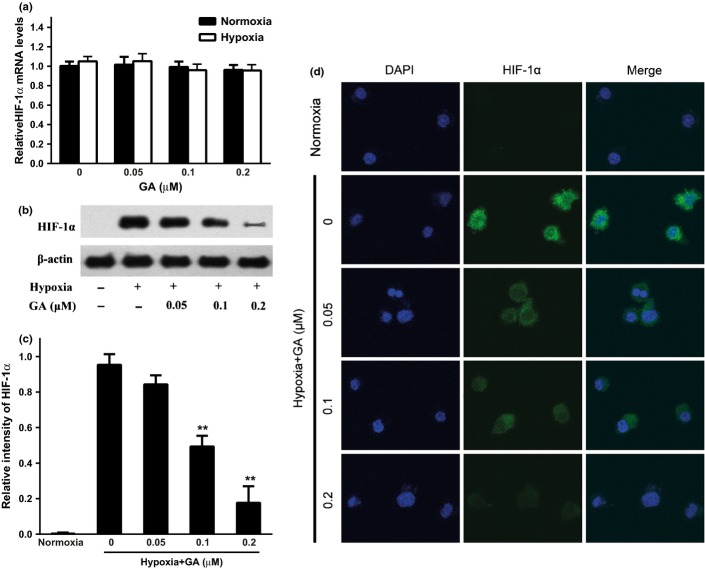
GA downregulates HIF-1α expression in hypoxia
To investigate why GA exhibited more marked effect on VEGF secretion and expression of U266 cells under hypoxia, real time-PCR, western blots and immunofluorescence were used to evaluate the expression of HIF-1α, the key regulator of hypoxia. The results showed that in both of normoxia and hypoxia, the levels of HIF-1α mRNA were unchanged with or without GA treatment on U266 cells (Fig. 3a), but compared with little expression under normoxia, hypoxic condition elicited a robust increase of HIF-1α protein level, which can be reduced by GA in a concentration-dependent manner (Fig. 3b,c). This observation was confirmed by an immunofluorescence assay in vitro that the intracellular expression of HIF-1α was relatively sparser and weaker compared to those before GA treatment (Fig. 3d). Taken together, these findings suggested that GA might downregulate HIF-1α through decreasing translation.
Fig. 3.
Gambogic acid (GA) attenuates the hypoxia-induced HIF-1α activation in U266 cells. (a) Effect of GA on hypoxia-inducible factor-1α (HIF-1α) mRNA level under normoxia and hypoxia. U266 cells were treated with various concentrations of GA (0, 0.05, 0.1, and 0.2 μM) for 4 h under normoxia and hypoxia, and HIF-1α mRNA level was detected by real-time PCR and analyzed by the ΔΔCt method. Bars are shown as mean ± SD (n = 3) and represent HIF-1α/β-actin fold relative to the untreated group. (b) Effect of GA on HIF-1α protein expression under normoxia and hypoxia. U266 cells were treated with various concentrations of GA (0, 0.05, 0.1, and 0.2 μM) for 4 h under normoxia and hypoxia. HIF-1α protein expression was analyzed by western blots. (c) For quantity of (b), images were analyzed using Image J. Bars are the mean ± SD (n = 3). The comparisons were made relative to the hypoxia alone group, and the different levels of significance was indicated as **P < 0.01. (d) Immunofluorescent staining analysis of the effect of GA on intracellular HIF-1α expression in normoxic and hypoxic U266 cells. Cells were treated with various concentrations of GA (0, 0.05, 0.1, and 0.2 μM) for 4 h under normoxia and hypoxia. Green color was detected for HIF-1α, while nuclei were counterstained with blue color using DAPI (4′6′-diamidino-2-phenylindole dihydrochloride).
The PI3K/Akt/mTOR pathway involves hypoxia-induced HIF-1α protein accumulation
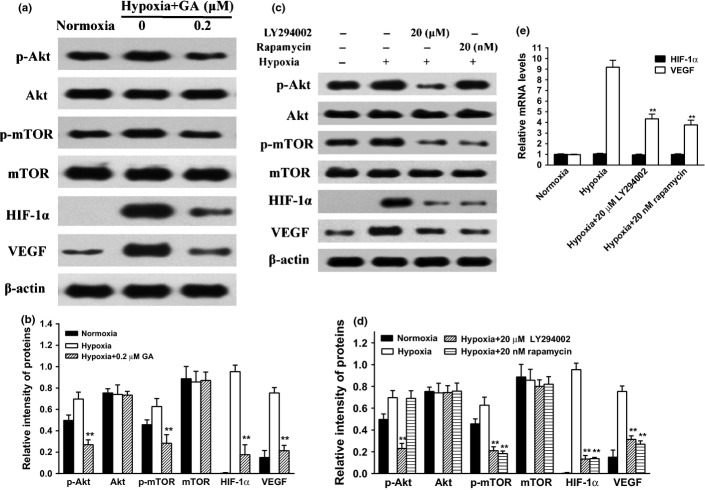
The PI3K/Akt/mTOR pathway is a crucial regulator of cell proliferation and angiogenesis. It has been reported that PI3K/Akt signaling pathway may be involved in hypoxia-induced HIF-1α protein accumulation and its downstream target gene expression.(14,15) To explore whether GA can inhibit hypoxia-mediated activation of PI3K/Akt/mTOR, U266 cells were treated with 0.2 μM GA for 4 h under hypoxia. Our data showed hypoxia augmented phosphorylation of Akt and mTOR in U266 cells, which were all attenuated by GA (Fig. 4a,b). Interestingly, GA did not affect the total protein levels of these kinases, suggesting that the action of GA was specific to the protein activation.
Fig. 4.
Role of PI3K/Akt/mTOR in the accumulation of in U266 cells. (a) U266 cells were exposed to normoxia or hypoxia in the presence or absence of different concentrations of GA (0 and 0.2 μM) for 4 h. Then, the protein expression involved PI3K/Akt/mTOR pathway as analyzed by western blots. β-actin was used as a loading control. (b) For quantity of (a), images were analyzed using Image J. Bars were the mean ± SD (n = 3). The comparisons were made relative to hypoxia alone group, and the different levels of significance was indicated as **P < 0.01. (c) U266 cells were exposed to normoxia or hypoxia in the presence or absence of LY294002 (20 μM) or rapamycin (20 nM) for 4 h. Then, the protein expression involved PI3K/Akt/mTOR pathway was analyzed by western blots. β-actin was used as a loading control. (d) For quantity of (c), images were analyzed using Image J. Bars are the mean ± SD (n = 3). The comparisons were made relative to hypoxia alone group, and the different levels of significance was indicated as **P < 0.01. (e) U266 cells were exposed to normoxia or hypoxia in the presence or absence of LY294002 (20 μM) or rapamycin (20 nM) for 4 h. vascular endothelial growth factor (VEGF) and hypoxia-inducible factor-1α (HIF-1α) mRNA were detected by real-time polymerase chain reaction (PCR) and analyzed by the ΔΔCt method. Bars are shown as mean ± SD (n = 3). The comparisons were made relative to hypoxia alone group, and the different levels of significance was indicated as **P < 0.01.
To further check the role of the PI3K/Akt/mTOR signaling pathway in the HIF-1α/VEGF cascade, U266 cells were treated respectively with 20 μM LY294002, a commonly used inhibitor of PI3K, or 20 nM rapamycin, an inhibitor of mTOR. The results showed that blocking PI3K/Akt activation by LY294002 significantly blunted the elevation of hypoxia-induced HIF-1α and VEGF via suppressing p-Akt and p-mTOR protein levels, and inhibiting mTOR activation by rapamycin had the same effect excepting unchanged p-Akt protein level (Fig. 4c,d). Correspondingly, the increases of VEGF mRNA caused by hypoxia were dramatically inhibited after blocking PI3K/Akt/mTOR pathway, while the levels of HIF-1α mRNA were unchanged (Fig. 4e), suggesting that blocking PI3K/Akt/mTOR pathway might downregulate HIF-1α through decreasing translation. In view of these facts, our results indicated that GA decreased the hypoxia-induced HIF-1α/VEGF protein levels in U266 cells by downregulation of PI3K/Akt/mTOR pathway.
GA inhibits the growth of transplantable tumors
Tumor xenografts transplanted by U266 cells were used to evaluate the antitumor effect of GA in BALB/c nude mice in vivo. After 14-day treatments, the tumors were moved and photographed (Fig. 5a). The average tumor size of control group was 615.5 ± 69.8 mm3 while those of GA treated groups were 323.3 ± 53.5 mm3 (2 mg/kg per 2 days) and 163.3 ± 30.1 mm3 (4 mg/kg per 2 days), respectively (Fig. 5b). The average tumor weight of the control group was 0.590 ± 0.099 g, while those of GA treated groups were 0.431 ± 0.074 g (2 mg/kg per 2 days) and 0.223 ± 0.064 g (4 mg/kg per 2 days), respectively (Fig. 5c). The results indicated that GA significantly inhibited tumor growth in a dosage-dependent manner. Moreover, administration of GA did not affect the body weight of mice (Fig. 5d), suggesting the maximal dose of GA (4 mg/kg per 2 days) is not toxic or at least a low toxicity for mice.
Fig. 5.
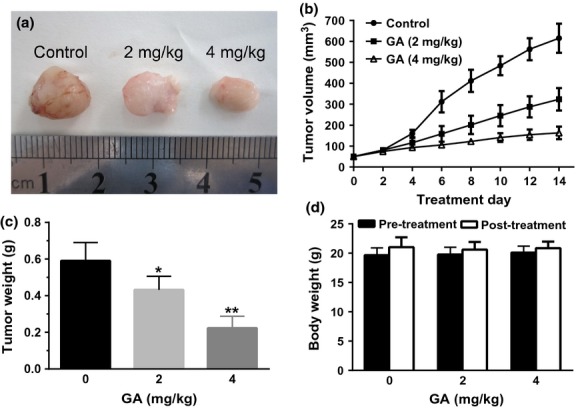
Gambogic acid (GA) inhibited tumor growth in a xenograft mouse model. The BALB/c nude mice were injected with U266 cells for some days followed by treatment with solvent or various doses of GA for 14 days. Then, the mice were killed, tumor removed and photographed (a). In addition, the tumor size (b), weight (c) and body weight (d) were measured. Bars are shown as mean ± SD (n = 6). The comparisons were made relative to untreated controls, and the different levels of significance were indicated as *P < 0.05 and **P < 0.01.
GA suppresses tumor angiogenesis via inhibition of HIF-1α/VEGF in vivo
To confirm the macroscopic observations and address the potential effect of GA in vivo, immunohistochemistry was performed. The results showed that the presence of endothelial-specific antibody CD31-stained capillaries in xenografts was dose-dependently reduced by GA treatment (Fig. 6a,b), suggesting GA attenuated the tumor angiogenesis. To investigate whether the anti-angiogenesis effect of GA was due to the decreased level of VEGF and HIF-1α, immunohistochemistry for VEGF and HIF-1α was performed. The same as CD31, the tumor sections treated with GA showed significantly lower levels of VEGF and HIF-1α than that of control tumor tissue (Fig. 6a,c). These results further supported that GA, a HIF-1α inhibitor, is a potential compound for MM therapy.
Fig. 6.
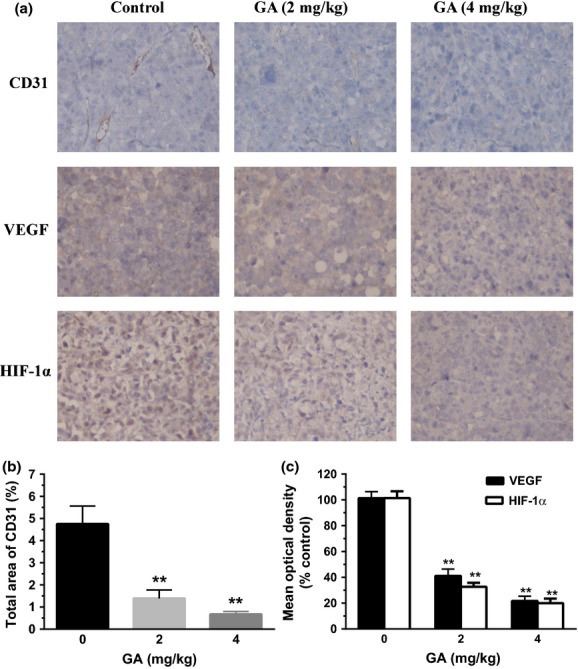
Gambogic acid (GA) inhibited tumor angiogenesis in U266 xenograft mouse model. (a) Immunohistochemistry was performed in tumor sections with antibodies of hypoxia-inducible factor-1α (HIF-1α), vascular endothelial growth factor (VEGF) and CD31. The result showed a remarkable decrease in expression of HIF-1α, VEGF and CD31 in the treated groups with 2 and 4 mg/kg GA compared with untreated control groups. (b) To identify the tumor angiogenesis, the stained area of CD31 in 10 fields was quantified by using Image Pro Plus. Bars are the mean ± SD (n = 10). The comparisons were made relative to untreated controls, and the different levels of significance was indicated as **P < 0.01. (c) The images were quantified using Image Pro Plus and mean optical densities (of control) of VEGF and HIF-1α were shown. Bars are the mean ± SD (n = 10). The comparisons were made relative to untreated controls, and the different levels of significance was indicated as **P < 0.01.
Discussion
Hypoxia-inducible factor-1α plays a critical role in tumor progression and dissemination. It is well established that many hematological malignancies, such as chronic lymphocytic leukemia,(22) diffuse large B cell and follicular non-Hodgkin lymphomas,(23) Hodgkin lymphoma,(24) and MM,(7,25) express HIF-1α protein in tumor cells. The bone marrow microenvironment is also hypoxic in MM patients.(10) The HIF-1α protein is even more constitutively expressed and activated in bone marrow endothelial cells of 45% of MM relapsed/refractory patients.(26) The hypoxic myeloma cells can induce the secretion of cytokines and growth factors, including interleukin-6, VEGF, insulin-like growth factor 1, members of the superfamily of tumor necrosis factor, transforming growth factor β1, and interleukin-10.(3) Previous studies have shown that selective HIF-1α inhibition can block angiogenesis by suppressing the production of angiogenic cytokines, resulting in a potent anti-MM effect.(27) Here, our results suggested that the U266 cells augmented the HIF-1α protein accumulation, and the expression and secretion of VEGF under hypoxia. Therefore, HIF-1α may be a logical target to control MM cell-derived angiogenesis.
As a promising anti-cancer agent with multiple protein targets, GA mediates a wide variety of functional antitumor effects including the induction of cell apoptosis, inhibition of proliferation and prevention of cancer metastasis and angiogenesis.(28,29)
In this experiment, we demonstrated that GA dramatically inhibited the expression of HIF-1α protein in a dose-dependent manner in U266 cells under hypoxia, but did not affect HIF-1α at the mRNA level, suggesting the suppression of HIF-1α expression by GA through decreased protein translation. Furthermore, there was no obvious chance in the cell viability of U266 after treatment with GA (0.05–0.2 μM) in the presence of hypoxia, showing that the effect of GA was not due to cytotoxicity.
Hypoxia is a potent inducer of HIF-1α and VEGF expression, and this induction can be augmented by PI3K/Akt activation. Previous studies have proposed that hypoxia can initiate a PI3K/Akt signaling cascade through ligand-independent activation of growth factor receptors.(15,16,30,31) In patients with colorectal cancer and mantle cell lymphoma, hypoxia-induced HIF-1α protein accumulation is also in close interaction with an active PI3K/Akt/mTOR pathway.(32,33) In addition, the PI3K inhibitor, LY294002, can significantly blunt HIF-1α induction under both hypoxia and the hypoxia mimetic CoCl2 in glioblastoma cells and attenuate VEGF mRNA expression.(34) In some other studies, the mTOR kinase inhibitor impairs production of HIF-1α in kidney tumor cell lines under hypoxic condition.(35) Moreover, rapamycin treatment effectively blocks the HIF-1α activation in gastric cancer cell lines under hypoxia and reduced the size of the CD-31-positive vessel area, leading to decreased tumor growth in a subcutaneous implantation model.(36) In our study, hypoxic conditions can also induce activation of PI3K/Akt/mTOR pathway in U266 cells. Furthermore, treatment with LY294002, a specific inhibitor of PI3K/Akt, or rapamycin, a specific inhibitor of mTOR, inhibited hypoxia-stimulated expression of HIF-1α; however, the levels of HIF-1α mRNA were unchanged. These results support the notion that PI3K/Akt/mTOR pathway is important for hypoxia mediated HIF-1α protein synthesis.
In order to assess whether inhibition of HIF-1α by GA in hypoxia is involved in PI3K/AKT/mTOR pathway, the signaling proteins were examined in the presence or absence of GA in hypoxia. The phosphorylation of AKT/mTOR cascade was significantly inhibited by GA in hypoxic U266 cells, suggesting that inhibition of PI3K/AKT/mTOR signaling pathway may, at least partly, contribute to the attenuated effect of GA on HIF-1α expression. The inhibition of HIF-1 activity involves HIF-1 mRNA expression, HIF-1 protein translation, HIF-1 protein degradation. In our study, The suppression of HIF-1α expression by GA was due to decreased translation. The question arises whether GA might be effective on protein degradation pathways that have been tried to date.
A xenograft myeloma tumor model in nude mice was generated by subcutaneous inoculation of U266 cells to evaluate the anti-tumor and anti-angiogenesis activity of GA in vivo. As expected, consecutive administration of GA for 14 days significantly reduced the tumor size and tumor vascularization evidenced by decreased CD31 expression in tumors. Furthermore, accompanied by reduced vascular density, immunohistochemical staining of the sections revealed decrease of HIF-1α and VEGF protein, which was consistent with the findings in vitro. These results demonstrate that GA can prevent the development of intratumoral hypoxia that may be attributed to inhibition of tumor angiogenesis and growth, possibly by attenuating HIF-1α and VEGF expression inside the tumors.
In summary, we conclude that GA downregulates HIF-1α protein levels of U266 cells in hypoxia. The inhibition of HIF-1α/VEGF protein expression is associated with the suppression of PI3K/Akt/mTOR pathway, suggesting that GA may suppress human multiple myeloma progression and angiogenesis. Taken together, these results not only provide a novel mechanism to explain the antiangiogenic and anticancer effects of GA, but also imply that GA might be a new potential drug for human multiple myeloma therapy.
Disclosure Statement
The authors have no conflict of interest.
References
- 1.Palumbo A, Anderson K. Multiple myeloma. N Engl J Med. 2011;364:1046–60. doi: 10.1056/NEJMra1011442. [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J, Shin HR, Bray F, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 3.Vacca A, Ria R, Reale A, et al. Angiogenesis in multiple myeloma. Chem Immunol Allergy. 2014;99:180–96. doi: 10.1159/000353312. [DOI] [PubMed] [Google Scholar]
- 4.Bhutani M, Turkbey B, Tan E, et al. Bone marrow angiogenesis in myeloma and its precursor disease: a prospective clinical trial. Leukemia. 2014;28:413–6. doi: 10.1038/leu.2013.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sato Y. Persistent vascular normalization as an alternative goal of anti-angiogenic cancer therapy. Cancer Sci. 2011;102:1253–6. doi: 10.1111/j.1349-7006.2011.01929.x. [DOI] [PubMed] [Google Scholar]
- 6.Hideshima T, Mitsiades C, Tonon G, et al. Understanding multiple myeloma pathogenesis in the bone marrow to identify new therapeutic targets. Nat Rev Cancer. 2007;7:585–98. doi: 10.1038/nrc2189. [DOI] [PubMed] [Google Scholar]
- 7.Martin SK, Diamond P, Gronthos S, et al. The emerging role of hypoxia, HIF-1 and HIF-2 in multiple myeloma. Leukemia. 2011;25:1533–42. doi: 10.1038/leu.2011.122. [DOI] [PubMed] [Google Scholar]
- 8.Huang J, Yao X, Zhang J, et al. Hypoxia-induced downregulation of miR-30c promotes epithelial-mesenchymal transition in human renal cell carcinoma. Cancer Sci. 2013;104:1609–17. doi: 10.1111/cas.12291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Semenza GL. Defining the role of hypoxia-inducible factor 1 in cancer biology and therapeutics. Oncogene. 2010;29:625–34. doi: 10.1038/onc.2009.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Colla S, Storti P, Donofrio G, et al. Low bone marrow oxygen tension and hypoxia-inducible factor-1alpha overexpression characterize patients with multiple myeloma: role on the transcriptional and proangiogenic profiles of CD138(+) cells. Leukemia. 2010;24:1967–70. doi: 10.1038/leu.2010.193. [DOI] [PubMed] [Google Scholar]
- 11.Azab AK, Hu J, Quang P, et al. Hypoxia promotes dissemination of multiple myeloma through acquisition of epithelial to mesenchymal transition-like features. Blood. 2012;119:5782–94. doi: 10.1182/blood-2011-09-380410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Keith B, Johnson RS, Simon MC. HIF1alpha and HIF2alpha: sibling rivalry in hypoxic tumour growth and progression. Nat Rev Cancer. 2012;12:9–22. doi: 10.1038/nrc3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dai T, Zheng H, Fu GS. Hypoxia confers protection against apoptosis via the PI3K/Akt pathway in endothelial progenitor cells. Acta Pharmacol Sin. 2008;29:1425–31. doi: 10.1111/j.1745-7254.2008.00904.x. [DOI] [PubMed] [Google Scholar]
- 14.Hong SW, Jung KH, Lee HS, et al. SB365 inhibits angiogenesis and induces apoptosis of hepatocellular carcinoma through modulation of PI3K/Akt/mTOR signaling pathway. Cancer Sci. 2012;103:1929–37. doi: 10.1111/j.1349-7006.2012.02409.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mirzoeva S, Kim ND, Chiu K, et al. Inhibition of HIF-1 alpha and VEGF expression by the chemopreventive bioflavonoid apigenin is accompanied by Akt inhibition in human prostate carcinoma PC3-M cells. Mol Carcinog. 2008;47:686–700. doi: 10.1002/mc.20421. [DOI] [PubMed] [Google Scholar]
- 16.Kim DH, Hossain MA, Kim MY, et al. A novel resveratrol analogue, HS-1793, inhibits hypoxia-induced HIF-1alpha and VEGF expression, and migration in human prostate cancer cells. Int J Oncol. 2013;43:1915–24. doi: 10.3892/ijo.2013.2116. [DOI] [PubMed] [Google Scholar]
- 17.Zhong H, Chiles K, Feldser D, et al. Modulation of hypoxia-inducible factor 1 alpha expression by the epidermal growth factor/phosphatidylinositol 3-kinase/PTEN/AKT/FRAP pathway in human prostate cancer cells: implications for tumor angiogenesis and therapeutics. Cancer Res. 2000;60:1541–5. [PubMed] [Google Scholar]
- 18.Karar J, Cerniglia GJ, Lindsten T, et al. Dual PI3K/mTOR inhibitor NVP-BEZ235 suppresses hypoxia-inducible factor (HIF)-1alpha expression by blocking protein translation and increases cell death under hypoxia. Cancer Biol Ther. 2012;13:1102–11. doi: 10.4161/cbt.21144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang LJ, Chen Y. New targets for the antitumor activity of gambogic acid in hematologic malignancies. Acta Pharmacol Sin. 2013;34:191–8. doi: 10.1038/aps.2012.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lu N, Hui H, Yang H, et al. Gambogic acid inhibits angiogenesis through inhibiting PHD2-VHL-HIF-1alpha pathway. Eur J Pharm Sci. 2013;49:220–6. doi: 10.1016/j.ejps.2013.02.018. [DOI] [PubMed] [Google Scholar]
- 21.Zhang W, Chen BA, Jin JF, et al. Involvement of c-Jun N-terminal kinase in reversal of multidrug resistance of human leukemia cells in hypoxia by 5-bromotetrandrine. Leuk Lymphoma. 2013;54:2506–16. doi: 10.3109/10428194.2013.776681. [DOI] [PubMed] [Google Scholar]
- 22.Ghosh AK, Shanafelt TD, Cimmino A, et al. Aberrant regulation of pVHL levels by microRNA promotes the HIF/VEGF axis in CLL B cells. Blood. 2009;113:5568–74. doi: 10.1182/blood-2008-10-185686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Minoia C, Quero C, Asselti M, et al. Changes in angiogenesis and hypoxia-inducible factor-1alpha protein expression in relapsed/refractory indolent non-Hodgkin lymphomas. Br J Haematol. 2013;163:640–5. doi: 10.1111/bjh.12560. [DOI] [PubMed] [Google Scholar]
- 24.Passam FH, Alexandrakis MG, Kafousi M, et al. Histological expression of angiogenic factors: VEGF, PDGFR alpha, and HIF-1alpha in Hodgkin lymphoma. Pathol Res Pract. 2009;205:11–20. doi: 10.1016/j.prp.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 25.Bhaskar A, Gupta R, Vishnubhatla S, et al. Angiopoietins as biomarker of disease activity and response to therapy in multiple myeloma. Leuk Lymphoma. 2013;54:1473–8. doi: 10.3109/10428194.2012.745523. [DOI] [PubMed] [Google Scholar]
- 26.Ria R, Catacchio I, Berardi S, et al. HIF-1 alpha of bone marrow endothelial cells implies relapse and drug resistance in patients with multiple myeloma and may act as a therapeutic target. Clin Cancer Res. 2014;20:847–58. doi: 10.1158/1078-0432.CCR-13-1950. [DOI] [PubMed] [Google Scholar]
- 27.Storti P, Bolzoni M, Donofrio G, et al. Hypoxia-inducible factor (HIF)-1 alpha suppression in myeloma cells blocks tumoral growth in vivo inhibiting angiogenesis and bone destruction. Leukemia. 2013;27:1697–706. doi: 10.1038/leu.2013.24. [DOI] [PubMed] [Google Scholar]
- 28.Wang X, Chen W. Gambogic acid is a novel anti-cancer agent that inhibits cell proliferation, angiogenesis and metastasis. Anticancer Agents Med Chem. 2012;12:994–1000. doi: 10.2174/187152012802650066. [DOI] [PubMed] [Google Scholar]
- 29.Wang Y, Xiang W, Wang M, et al. Methyl jasmonate sensitizes human bladder cancer cells to gambogic acid-induced apoptosis through down-regulation of EZH2 expression by miR-101. Br J Pharmacol. 2014;171:618–35. doi: 10.1111/bph.12501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mazure NM, Chen EY, Laderoute KR, et al. Induction of vascular endothelial growth factor by hypoxia is modulated by a phosphatidylinositol 3-kinase/Akt signaling pathway in Ha-ras-transformed cells through a hypoxia inducible factor-1 transcriptional element. Blood. 1997;90:3322–31. [PubMed] [Google Scholar]
- 31.Chen EY, Mazure NM, Cooper JA, et al. Hypoxia activates a platelet-derived growth factor receptor/phosphatidylinositol 3-kinase/Akt pathway that results in glycogen synthase kinase-3 inactivation. Cancer Res. 2001;61:2429–33. [PubMed] [Google Scholar]
- 32.Argyriou P, Papageorgiou SG, Panteleon V, et al. Hypoxia-inducible factors in mantle cell lymphoma: implication for an activated mTORC1–>HIF-1alpha pathway. Ann Hematol. 2011;90:315–22. doi: 10.1007/s00277-010-1070-6. [DOI] [PubMed] [Google Scholar]
- 33.Greijer AE, Delis-Van Diemen PM, Fijneman RJ, et al. Presence of HIF-1 and related genes in normal mucosa, adenomas and carcinomas of the colorectum. Virchows Arch. 2008;452:535–44. doi: 10.1007/s00428-008-0578-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pore N, Jiang Z, Shu HK, et al. Akt1 activation can augment hypoxia-inducible factor-1 alpha expression by increasing protein translation through a mammalian target of rapamycin-independent pathway. Mol Cancer Res. 2006;4:471–9. doi: 10.1158/1541-7786.MCR-05-0234. [DOI] [PubMed] [Google Scholar]
- 35.Thomas GV, Tran C, Mellinghoff IK, et al. Hypoxia-inducible factor determines sensitivity to inhibitors of mTOR in kidney cancer. Nat Med. 2006;12:122–7. doi: 10.1038/nm1337. [DOI] [PubMed] [Google Scholar]
- 36.Lang SA, Gaumann A, Koehl GE, et al. Mammalian target of rapamycin is activated in human gastric cancer and serves as a target for therapy in an experimental model. Int J Cancer. 2007;120:1803–10. doi: 10.1002/ijc.22442. [DOI] [PubMed] [Google Scholar]