Abstract
The interactions of tumor cells with platelets contribute to the progression of tumor malignancy, and the expression levels of platelet aggregation-inducing factors positively correlate with the metastatic potential of osteosarcoma cells. However, it is unclear how tumor-platelet interaction contributes to the proliferation of osteosarcomas. We report here that osteosarcoma-platelet interactions induce the release of platelet-derived growth factor (PDGF) from platelets, which promotes the proliferation of osteosarcomas. Co-culture of platelets with MG63 or HOS osteosarcoma cells, which could induce platelet aggregation, enhanced the proliferation of each cell line in vitro. Analysis of phospho-antibody arrays revealed that co-culture of MG63 cells with platelets induced the phosphorylation of platelet derived growth factor receptor (PDGFR) and Akt. The addition of supernatants of osteosarcoma-platelet reactants also increased the growth of MG63 and HOS cells as well as the level of phosphorylated-PDGFR and -Akt. Sunitinib or LY294002, but not erlotinib, significantly inhibited the platelet-induced proliferation of osteosarcoma cells, indicating that PDGF released from platelets plays an important role in the proliferation of osteosarcomas by activating the PDGFR and then Akt. Our results suggest that inhibitors that specifically target osteosarcoma-platelet interactions may eradicate osteosarcomas.
Keywords: Akt, osteosarcoma, platelet aggregation, platelet derived growth factor receptor, tumor-platelet interaction
Osteosarcoma is the most common primary malignant bone tumor of children and adolescents and is derived from primitive mesenchymal cells.(1) This disease is highly aggressive, and distant metastasis develop in approximately 45% of patients despite treatment with a potent neoadjuvant, which consists of high doses of multiple chemotherapeutic agents.(2) Approximately 20% of patients have metastatic sites in the lungs or bones at diagnosis (3) and have a poor prognosis despite aggressive surgery and chemotherapy.(4) Thus, more effective therapeutic approaches are required for treating these patients.
The interactions of tumor cells with platelets play a critical role in the progression of tumor malignancy. Tumor cell-induced platelet aggregation enhances the rate of tumor embolization in the microvasculature and protects tumor cells from immunological assault and blood-shear stress.(5) Moreover, several factors such as transforming growth factor-β, vascular endothelial growth factor, and platelet-derived growth factor (PDGF) are stored in platelet granules and are released during platelet aggregation.(6,7) Such platelet-derived factors promote the epithelial-mesenchymal transition, tumor vascular angiogenesis, and vascular permeability.(8,9) Further, experimentally induced thrombocytopenia and antiplatelet agents decrease the rate of lung metastasis in mouse models, indicating the requirement for platelets in the formation of hematogenous metastasis.(10)– (12)
Osteosarcoma cells possess the potential to induce platelet aggregation, and there is positive correlation between the expression level of platelet aggregation-inducing factors and the potential of osteosarcomas to metastasize to the lungs.(13) However, the effect of osteosarcoma-platelet interactions on the proliferation of osteosarcoma cells is unknown. We report here that osteosarcoma-platelet interactions induce the release of PDGF from platelets and enhance the proliferation of osteosarcomas. Co-culture of osteosarcoma cells with platelets promoted the proliferation of osteosarcoma cells. Analysis of phospho-antibody arrays revealed that the osteosarcoma-platelet interaction increased the phosphorylation of PDGFR and Akt. Using kinase inhibitors, phosphorylation of PDGFR and Akt were shown to be important for the platelet-dependent proliferation of osteosarcoma cells. These results suggest that osteosarcoma-platelet interactions initiate platelet aggregation, release PDGF from activated platelets, and activate the PDGFR-Akt signaling pathway to increase the growth of osteosarcoma cells.
Materials and Methods
Plasmid construction
The open reading frame (ORF) of a human codon-optimized variant of wild-type Zoanthus sp. green fluorescence protein (ZsGreen) was subcloned from the pZsGreen-N1 vector (Takara Bio, Shiga, Japan) into the pQCXIN retroviral vector (Takara Bio), and the resulting construct was designated pQCXIN-ZsGreen. Retroviral infection was performed according to the manufacturer's protocols.
Cell lines
The human osteosarcoma cell lines, MG63 and HOS, were purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA) and cultured in Dulbecco's modified Eagle's medium (DMEM, Sigma-Aldrich, St. Louis, MO, USA) containing 10% FBS (DMEM growth medium). MG63 and HOS cells that had stably transfected with ZsGreen gene (MG63/ZsGreen and HOS/ZsGreen, respectively) were cultured in DMEM growth medium containing 400 μg/mL of G418 (Life Technologies, Carlsbad, CA, USA).
Immunoblot analysis
Sample preparation was performed as described previously.(14) Briefly, cells were lysed in TENSV buffer (50 mM Tris–HCl (pH 7.5), 2 mM ethylenediaminetetraacetic acid (EDTA), 100 mM NaCl, 1 mM Na3VO4, 1% NP-40, 0.1% aprotinin, and 2 mM phenylmethylsulfonyl fluoride), and electrophoresed in sodium dodecyl sulfate (SDS)-polyacrylamide gel. The proteins were transferred to a membrane and immunoblotted with an anti-Akt (pan) monoclonal antibody (mAb) (clone C67E7, Cell Signaling Technology, Danvers, MA, USA), anti-phospho-Akt (Ser473) mAb (clone D9E, Cell Signaling Technology), anti-PDGFRβ polyclonal antibody (P-20, Santa Cruz Biotechnology, Santa Cruz, CA, USA), anti-phospho-PDGFRβ mAb (clone 42F9, Cell Signaling Technology), and anti-α-tubulin mAb (clone YL1/2, AbD Serotec, Kidlington, UK). The LAS-3000 mini system (Fujifilm, Tokyo, Japan) was used for visualization and quantification of signals.
Human phospho-RTK and human phospho-kinase arrays
Phosphorylation of signaling molecules was estimated using the Human Phospho-RTK Array Kit (ARY001B, R&D Systems, Minneapolis, MN, USA) and Human Phospho-Kinase Array Kit (ARY003B, R&D Systems) according to the manufacturer's protocols. Briefly, MG63 cells were co-cultured with buffer or platelets for 2 h. Three hundred micrograms of total cell lysates were incubated with each array. Proteins were detected using horse radish peroxidase (HRP)-conjugated mouse anti-phospho-tyrosine antibody or streptavidin-HRP. Data were acquired using the LAS-3000 mini system. Image quantification was performed using Multi Gauge ver.3.0 software (Fujifilm). The signal intensities of duplicate spots were quantified.
Platelet preparation and aggregation assay
Whole blood was drawn by cardiac puncture from Jcl: ICR mice terminally anesthetized with chloroform and taken with 0.38% sodium citrate solution or 10 units/mL of heparin. The blood was centrifuged at 150 g for 8 min to obtain platelet-rich plasma (PRP) from the supernatant. Washed platelets were prepared from pellets of PRP by centrifugation at 500 g for 10 min following washing with modified Tyrode's buffer (137 mM NaCl, 11.9 mM NaHCO3, 0.4 mM Na2HPO4, 2.7 mM KCl, 1.1 mM MgCl2, and 5.6 mM glucose). Washed platelets were resuspended in modified Tyrode's buffer containing 1–2% murine platelet-poor plasma (PPP), and 200 or 250 μM CaCl2 (each concentration used are shown in figure legends) was added to the platelet suspensions before starting the experiments. Platelet suspensions (200 μL) in the reaction tubes were stirred at 37°C and preincubated for 2 min before the addition of osteosarcoma cells. The platelet aggregation assay was performed using a platelet aggregometer (MCM HEMA TRACER 313M; SSR Engineering, Kanagawa, Japan) as previously described.(15)
Cell viability assay
MG63/ZsGreen and HOS/ZsGreen cells were suspended in DMEM medium containing 0.5% FBS (0.5 × 104 and 2.0 × 104 cells/mL, respectively) and seeded 0.1 mL in a 96-well plate. After overnight incubation, cells were co-cultured with washed platelets resuspended in modified Tyrode's buffer containing 200 μM CaCl2. At the appropriate times, supernatants were removed, and TENSV buffer was added to the cultured cells. The fluorescence of ZsGreen in cell lysates was measured using a TriStar LB941 Multimode Microplate Reader (Berthold Technologies, Bad Wildbad, Germany). Buffer alone indicates the treatment of the cells with modified Tyrode's buffer containing 200 μM CaCl2. In some experiments, the supernatant harvested from osteosarcoma-platelet reactants was added to the cultured osteosarcoma cells instead of platelets.
Preparation of supernatants of osteosarcoma cell-platelet reactants
Washed mouse platelets were prepared using 0.38% sodium citrate as described in the platelet preparation. Platelets (2.0 × 108 platelets/mL) were resuspended in modified Tyrode's buffer containing 1% murine PPP and 200 μM CaCl2 and then incubated with phosphate-buffered saline (PBS) or osteosarcoma cells (2.5 × 105 cells/mL) for 30 min, at 37°C. After centrifuging twice at 10 000 g for 10 min, the supernatants of the reaction mixtures were designated PBS-platelet reactant and osteosarcoma-platelet reactant, respectively.
Animals
Jcl:ICR mice were purchased from Clea Japan (Tokyo, Japan). The animal procedures followed protocols approved by the Japanese Foundation for Cancer Research Animal Care and Use Committee.
Statistical analysis
The Student's t-test was performed to determine the statistical significance of the results of the proliferation assays. Significant P-values are defined as **P < 0.01, *P < 0.05. NS indicates a value that is not significant. All statistical tests were two-sided.
Results
Osteosarcoma-platelet interaction promotes platelet aggregation and osteosarcoma cell growth
Osteosarcomas form pulmonary metastasis by inducing platelet aggregation.(16,17) To assess the role of osteosarcoma-platelet interactions in determining the malignant phenotype of osteosarcomas, we first measured the abilities of the human osteosarcoma cell lines MG63 and HOS to induce platelet aggregation. We found that each osteosarcoma cell line induced platelet aggregation to an extent that is consistent with published studies (Fig. 1a). We next examined the influence of platelets on the growth of the osteosarcoma cell lines. Because of the high concentration of adenosine triphosphate (ATP) in platelets, we were unable to determine the growth of osteosarcoma cells using proliferation assays that measure ATP. Therefore, we generated stable transfectants of MG63 and HOS cells that expressed ZsGreen (MG63/ZsGreen and HOS/ZsGreen, respectively) and measured ZsGreen fluorescence to determine the number of viable cells. Although the growth rates of MG63/ZsGreen and HOS/ZsGreen cells were slow in the presence of 0.5% FBS, the growth rate of each cell line was significantly enhanced in proportion to the number of washed platelets added to the cultured cells (Fig. 1b). We found that the addition of the supernatant of an osteosarcoma-platelet reactant, but not that of the PBS-platelet reactant, significantly enhanced the growth of MG63/ZsGreen and HOS/ZsGreen cells (Fig. 1c). These results indicate that the proliferation of osteosarcoma cells is increased in the presence of platelets as well as by supernatants of osteosarcoma-platelet reactant.
Fig. 1.
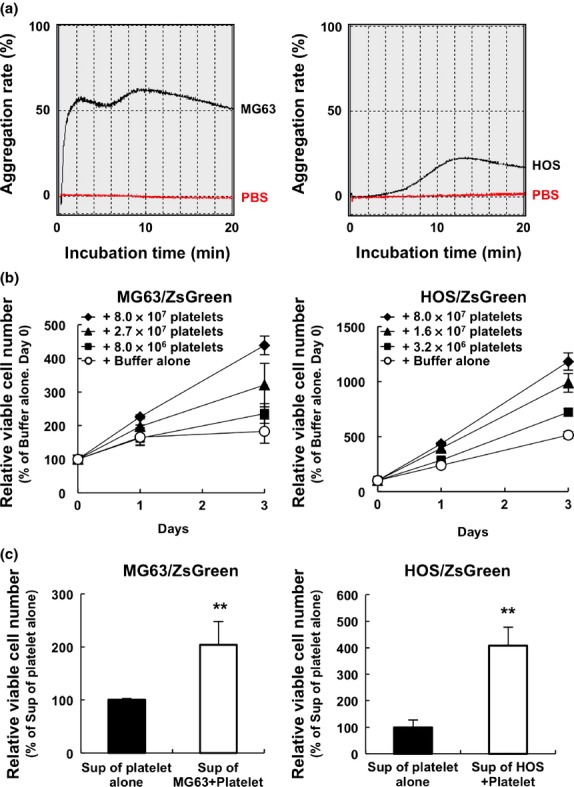
Co-culture with platelets promotes platelet aggregation and the proliferation of osteosarcoma cells. (a) Platelets (4.0 × 107) prepared with heparin from the whole blood of Jcl:ICR mice were resuspended in modified Tyrode's buffer containing 2% PPP and 250 μM CaCl2, followed by incubation with PBS (red lines) or osteosarcoma MG63 (left panel, black line) and HOS (right panel, black line) cells (1.0 × 108 cells). The transmission of light by the samples was measured using an aggregometer to determine the aggregation rate. (b) MG63 and HOS cells stably transfected with ZsGreen gene, MG63/ZsGreen (left panel) and HOS/ZsGreen (right panel), respectively, were co-cultured with the indicated number of washed mouse platelets in medium containing 0.5% FBS. TENSV buffer was added to each well at the times indicated, and the fluorescence of ZsGreen was used to determine the number of viable osteosarcoma cells. The error bars indicate the mean ± standard deviation (SD) of triplicate experiments. (c) Washed mouse platelets (2.0 × 107 platelets) were resuspended in modified Tyrode's buffer containing 1% PPP and 200 μM CaCl2 followed by incubation with PBS or osteosarcoma cells (2.5 × 104 cells) for 30 min. After centrifugation twice at 10 000 g for 10 min, the supernatant of osteosarcoma-platelet reactants or the PBS-platelet reactant was added to the cultures of MG63/ZsGreen (left panel) and HOS/ZsGreen (right panel) cells. After 24 h, TENSV buffer was added to each well and the fluorescence of ZsGreen was used to determine the relative number of viable cells. The error bars indicate the mean ± SD of triplicate experiments. **P < 0.01 by the Student t-test.
Activation of the PDGFR-Akt axis in platelet-induced osteosarcoma proliferation
Platelets contain many growth factors and cytokines, including transforming growth factor-β, vascular endothelial growth factors, and PDGFs,(6,7) which are stored in platelet granules and released on platelet aggregation. Such platelet-derived factors promote the epithelial-mesenchymal transition, tumor vascular angiogenesis, and tumor growth.(8) To identify the mechanism that mediated the effects of platelets and supernatants of osteosarcoma-platelet reactants on osteosarcoma cell proliferation, we used arrays comprising a panel of antibodies specific for cytokines, growth factor receptors, or downstream signaling components. Incubation of the array with cell lysates prepared from reactants of MG63 cells and platelets increased the intensity of the spots corresponding to the positions of antibodies against phosphorylated PDGFRα, phosphorylated PDGFRβ, phosphorylated epidermal growth factor receptor (EGFR), and phosphorylated Akt1/2/3 antibodies (Figs. 2a–d). To exclude the possibility that the addition of platelets altered the respective protein expression levels in MG63 cells, we performed western blot analysis. Consistent with the array data, we confirmed the increase in phospho-PDGFRβ in MG63 cells that were co-cultured with platelets (Fig. 2e). Because PDGFRβ was not detected in the platelet lysate, indicating that co-culture induced PDGFRβ phosphorylation in MG63 cells. An increase in the level of phospho-Akt induced by co-culture was also detected using western blotting (Fig. 2e). Because the elecrophoretic mobility of mouse Akt in platelets appeared higher compared with human Akt in MG63 cells, the intensity of the phospho-Akt signal may represent phosphorylation of human but not mouse Akt. We obtained similar results using another HOS cell line (Fig. 2f). These data suggest that osteosarcoma-platelet interactions activate the PDGFR-Akt signaling pathway.
Fig. 2.
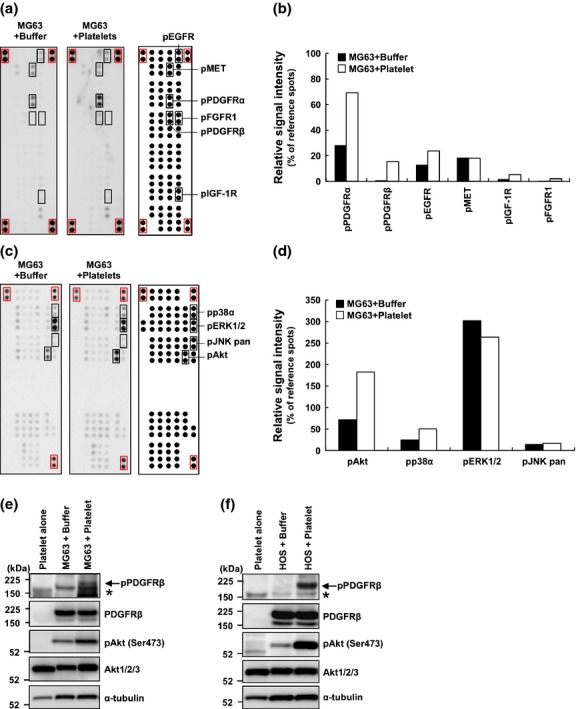
Activation of the platelet derived growth factor receptor (PDGFR)-Akt signaling axis in MG63 cells co-cultured with platelets. (a, b) Platelets prepared with heparin from the whole blood of Jcl:ICR mice were resuspended in modified Tyrode's buffer containing 200 μM CaCl2. MG63/ZsGreen cells were co-cultured with buffer alone (MG63 + Buffer) or platelets (MG63 + Platelet) for 2 h. The preparation of cell lysates and incubation with the human phospho-RTK array were performed according to the manufacturer's protocol. Representative images of the probed arrays are shown (a). The signal intensity of each spot was determined using an LAS-3000 mini and quantified using Multi Gauge ver.3.0 software. The signal intensities of eight reference spots (within red squares) in each membrane were measured and defined as 100%. The relative intensities of duplicate spots are shown (b). (c, d) Analysis of the human phospho-kinase array using lysates prepared from MG63 cells co-cultured with platelets in modified Tyrode's buffer containing 200 μM CaCl2. MG63/ZsGreen cells were cultured with buffer alone (MG63 + Buffer) or with platelets (MG63 + Platelet) for 2 h. The preparation of cell lysates and incubation with the human phospho-kinase array were performed according to the manufacturer's protocol. Representative images of reacted membranes are shown (c). The signal intensity of each spot was measured using a LAS-3000 mini and quantified using Multi Gauge ver.3.0 software. The signal intensities of six reference spots (red squares) in each membrane were defined as 100%. The relative intensities of duplicate spots are shown (d). (e, f) Platelets prepared with 0.38% sodium citrate were resuspended Tyrode's buffer containing 200 μM CaCl2. MG63/ZsGreen (e) or HOS/ZsGreen (f) cells were incubated with buffer alone or platelets (2.0 × 107/24-well) for 2 h. Platelets alone, osteosarcoma cells alone (+Buffer) or co-cultures of osteosarcoma cells with platelets (+Platelet) were lysed and immunoblotted using the antibodies to phospho-PDGFRβ, PDGFRβ, phospho-Akt (S473), Akt, or α-tubulin.
PDGFs released on the osteosarcoma cell-induced platelet aggregation contribute to the activation of the PDGFR-Akt signaling axis
Activation of PDGFRα and PDGFRβ is mediated by PDGFs, and only PDGF-BB can activate both PDGFRs. To determine whether PDGF-BB was released during the osteosarcoma cell-mediated platelet aggregation, we measured the amount of PDGF-BB in the supernatants of the osteosarcoma-platelet reactants using an enzyme linked immunosorbent assay (ELISA). The level of PDGF-BB was increased in supernatants of osteosarcoma-platelet aggregates compared with platelets alone (Fig. 3a). To assess the contribution of PDGFs to the activation of PDGFR-Akt axis, we treated MG63/ZsGreen and HOS/ZsGreen cells with the supernatant of the osteosarcoma-platelet reactant in the absence or presence of PDGFRs inhibitor, sunitinib. We found that the levels of phospho-PDGFRβ and phospho-Akt increased in osteosarcoma cell lines in the absence of, but not in the presence of sunitinib (Figs 3b,c). These results indicate that PDGFs released from activated platelets by the initiation of osteosarcoma-platelet interactions activated the PDGFR-Akt signaling axis.
Fig. 3.
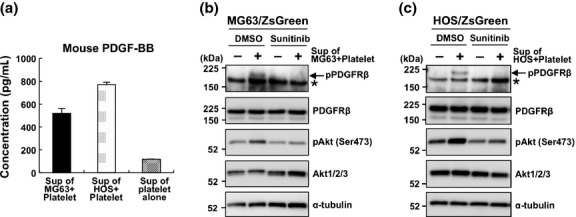
Platelet-derived growth factors (PDGFs) released on the osteosarcoma-induced platelet aggregation contribute to the activation of the PDGFR-Akt signaling axis. (a–c) Washed mouse platelets (2.0 × 107 platelets) were resuspended in modified Tyrode's buffer containing 1% PPP and 200 μM CaCl2 followed by incubation with PBS or with the indicated osteosarcoma cells (2.5 × 104 cells) for 30 min. After centrifuging twice at 10 000 g for 10 min, the concentration of PDGF-BB in the supernatants was measured using an enzyme linked immunosorbent assay (ELISA) (a). The cultured MG63/ZsGreen (b) and HOS/ZsGreen (c) cells were treated with (+) or without (−) the supernatant of osteosarcoma-platelet aggregates in the presence of DMSO or 1 μM sunitinib. After a 30-min incubation, cells were lysed with TENSV buffer and immunoblotted with the indicated antibodies.
Activation of the PDGFR-Akt signaling axis contributes to the platelet-dependent proliferation of osteosarcoma cell lines
To assess the role of the activation of the PDGFR-Akt axis in the growth of MG63 cells, we determined the effects of sunitinib, LY294002, or erlotinib, which inhibit the activity of the PDGFRs, phosphatidylinositol-4,5-bisphosphate 3-kinase (PI3K), or EGFR, respectively. Sunitinib and LY294002, but not erlotinib, inhibited the growth of MG63 and HOS cells when they were co-cultured with platelets (Figs 4a,b). These results indicate that activation of the PDGFR-Akt axis contributes to the platelet-dependent proliferation of osteosarcoma cells.
Fig. 4.
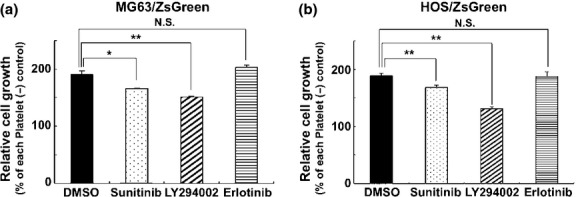
Involvement of the platelet-derived growth factor receptor (PDGFR) -Akt signaling axis in the platelet-induced growth of osteosarcoma cells. (a, b) MG63/ZsGreen (a) or HOS/ZsGreen (b) cells in 24-well were co-cultured with platelets (2 × 107 in Tyrode's buffer containing 200 μM CaCl2) in the presence of DMSO, the epidermal growth factor receptor (EGFR) inhibitor erlotinib (1 μM), the PDGFR inhibitor sunitinib (1 μM), or the PI3K inhibitor LY294002 (20 μM). After a 48-h incubation, cells were lysed and the fluorescence of ZsGreen was measured to determine relative cell growth. The error bars indicate the mean ± SD of triplicate experiments. **P < 0.01 and *P < 0.05 (Student's t-test). NS, not significant.
Discussion
Osteosarcoma is highly aggressive with distant metastasis, and approximately 20% of patients have metastases in lung or bone at diagnosis.(18) Although the frequency of 5-year disease-free survival of patients with nonmetastatic osteosarcoma is approximately 70%,(19) the average survival rate after recurrence in distant organs is only 1 year.(20) Therefore, metastasis is the most common cause of death of patients with osteosarcomas as well as other cancers. There is a positive correlation between the expression level of platelet aggregation-inducing factors and the potential of osteosarcomas to metastasize to the lung.(13) Mehta et al.(21) suggest that stimulation of platelets by osteosarcoma cells correlates with their potential to metastasize to the lung.
The platelet receptor glycoprotein Ibα, sialyl Lewisx/sialyl Lewisa, integrins, thrombospondin-1 (TSP-1), and Aggrus/podoplanin has been reported to induce platelet aggregation.(16,22)– (26) Among them, TSP-1 and Aggrus are key molecules that mediate osteosarcoma-induced platelet aggregation.(17,18) TSP-1 and Aggrus are expressed on cell surface, indicating that they may serve as targets of therapeutic antibodies against osteosarcoma. In fact, we confirmed Aggrus expression in the used two human osteosarcoma cell lines (Fig. S1a) and the addition of our established neutralizing anti-Aggrus antibody (MS-1)(14) suppressed the MG63-dependent platelet aggregation and the PDGF release from platelets at a significant level (Fig. S1b,c). These results suggest that Aggrus expression on osteosarcomas contributes to the interaction with platelets and that Aggrus could be a therapeutic target of osteosarcoma. We also observed the MG63-induced platelet aggregation was suppressed by the addition of antibody against von Willebrand factor, which is one of the platelet aggregation-mediating molecules and known to be overexpressed in metastatic osteosarcoma (Fig. S2).(13) Thus, von Willebrand factor also plays some roles in osteosarcoma-mediated platelet aggregation.
Platelet derived growth factor (PDGF) and the PDGFR are implicated in the pathogenesis of sarcomas such as Ewing sarcoma, chondrosarcoma, rhabdomyosarcoma, intimal sarcoma, and osteosarcoma.(27)– (30) Immunohistochemical analysis revealed that PDGFRα and PDGFRβ are frequently expressed in osteosarcomas (79.6% and 86%, respectively, n = 54), and the prognosis of patients with co-expression of PDGFRα and PDGF-AA is significantly poorer.(19) In the present study, osteosarcoma-platelet interactions promoted the proliferation of osteosarcoma cell lines through the activation of the PDGFR-Akt signaling axis (Fig. 2). However, sunitinib treatment partially suppressed the platelet-dependent proliferation of osteosarcoma cells (Fig. 4). Moreover, the inhibitory effects of a PI3K inhibitor LY294002, which functions downstream in the signaling pathways of certain receptor tyrosine kinases, were increased compared with sunitinib (Fig. 4). These results suggest the participation of other signaling pathways in the platelet-dependent proliferation of osteosarcoma cells. For example, insulin-like growth factor-1 (IGF-1), which is released from the α-granules of activated platelets, increases the growth of MG63 cells.(31) Although we did not detect other phosphorylated proteins, including the IGF-1 receptor, using antibody arrays, other proliferative signals may contribute to the platelet-dependent proliferation of osteosarcomas. Therefore, specific inhibitors that block osteosarcoma-platelet interactions may be useful for the suppression of platelet-dependent proliferation of osteosarcoma.
The use of chemotherapeutic agents is essential for the treatment of osteosarcoma patients; however, the efficacy of the current treatment regimen including adriamycin, which was originally developed in the mid-1980s, is limited.(19) Apoptosis induced by adriamycin was attenuated by co-culture with platelets in osteosarcomas (Fig. S3). Moreover, their invasiveness was promoted by co-culture with platelets (Fig. S4). Because prior administration of neutralizing anti-Aggrus antibodies has been reported to prevent hematogenous metastasis of Aggrus-positive tumor cells in mouse models (8,14,32) and to attenuate PDGF release from platelets (Fig. S1c), the combination therapy of anti-Aggrus antibodies with standard chemotherapeutic agents may be effective for inhibiting the proliferation of osteosarcomas and for preventing metastasis.
Acknowledgments
We thank Dr R. Katayama for valuable suggestions. This study was supported in part by the Advanced Research for Medical Products Mining Programme of the National Institute of Biomedical Innovation (NIBIO) (to NF), by a Grant-in-Aid for Scientific Research on Innovative Areas “Integrative Research on Cancer Microenvironment Network” from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (to NF), and by a Grant-in-Aid for Young Scientists (B) (to ST).
Disclosure Statement
The authors have no conflict of interest.
Supporting Information
Additional supporting information may be found in the online version of this article:
Fig. S1. Involvement of Aggrus/podoplanin in platelet aggregation and PDGF release during osteosarcoma cell-induced platelet aggregation.
Fig. S2. Attenuation of MG63-dependent platelet aggregation by an anti-von Willebrand factor (vWF) antibody.
Fig. S3. Co-culture with platelets contributes to the resistance to apoptosis induced by adriamycin in osteosarcoma cells.
Fig. S4. Platelets promote invasiveness of osteosarcomas.
References
- 1.Ritter J, Bielack SS. Osteosarcoma. Ann Oncol. 2010;21(Suppl 7):vii320–5. doi: 10.1093/annonc/mdq276. [DOI] [PubMed] [Google Scholar]
- 2.Kuijjer ML, Hogendoorn PC, Cleton-Jansen AM. Genome-wide analyses on high-grade osteosarcoma: making sense of a genomically most unstable tumor. Int J Cancer. 2013;133:2512–21. doi: 10.1002/ijc.28124. [DOI] [PubMed] [Google Scholar]
- 3.Meyers PA, Heller G, Healey JH, et al. Osteogenic sarcoma with clinically detectable metastasis at initial presentation. J Clin Oncol. 1993;11:449–53. doi: 10.1200/JCO.1993.11.3.449. [DOI] [PubMed] [Google Scholar]
- 4.Bacci G, Briccoli A, Ferrari S, et al. Neoadjuvant chemotherapy for osteosarcoma of the extremity: long-term results of the Rizzoli's 4th protocol. Eur J Cancer. 2001;37:2030–9. doi: 10.1016/s0959-8049(01)00229-5. [DOI] [PubMed] [Google Scholar]
- 5.Gay LJ, Felding-Habermann B. Contribution of platelets to tumour metastasis. Nat Rev Cancer. 2011;11:123–34. doi: 10.1038/nrc3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bambace NM, Holmes CE. The platelet contribution to cancer progression. J Thromb Haemost. 2011;9:237–49. doi: 10.1111/j.1538-7836.2010.04131.x. [DOI] [PubMed] [Google Scholar]
- 7.Sierko E, Wojtukiewicz MZ. Platelets and angiogenesis in malignancy. Semin Thromb Hemost. 2004;30:95–108. doi: 10.1055/s-2004-822974. [DOI] [PubMed] [Google Scholar]
- 8.Fujita N, Takagi S. The impact of Aggrus/podoplanin on platelet aggregation and tumour metastasis. J Biochem. 2012;152:407–13. doi: 10.1093/jb/mvs108. [DOI] [PubMed] [Google Scholar]
- 9.Schumacher D, Strilic B, Sivaraj KK, Wettschureck N, Offermanns S. Platelet-derived nucleotides promote tumor-cell transendothelial migration and metastasis via P2Y receptor. Cancer Cell. 2013;24:130–7. doi: 10.1016/j.ccr.2013.05.008. [DOI] [PubMed] [Google Scholar]
- 10.Gasic GJ, Gasic TB, Stewart CC. Antimetastatic effects associated with platelet reduction. Proc Natl Acad Sci USA. 1968;1:46–52. doi: 10.1073/pnas.61.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karpatkin S, Pearlstein E, Salk PL, Yogeeswaran G. Role of platelets in tumor cell metastasis. Ann N Y Acad Sci. 1981;370:101–18. doi: 10.1111/j.1749-6632.1981.tb29726.x. [DOI] [PubMed] [Google Scholar]
- 12.Kunita A, Kashima TG, Morishita Y, et al. The platelet aggregation-inducing factor aggrus/podoplanin promotes pulmonary metastasis. Am J Pathol. 2007;170:1337–47. doi: 10.2353/ajpath.2007.060790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eppert K, Wunder JS, Aneliunas V, Kandel R, Andrulis IL. von Willebrand factor expression in osteosarcoma metastasis. Mod Pathol. 2005;18:388–97. doi: 10.1038/modpathol.3800265. [DOI] [PubMed] [Google Scholar]
- 14.Takagi S, Sato S, Oh-Hara T, et al. Platelets promote tumor growth and metastasis via direct interaction between Aggrus/podoplanin and CLEC-2. PLoS ONE. 2013;8:e73609. doi: 10.1371/journal.pone.0073609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nakazawa Y, Takagi S, Sato S, et al. Prevention of hematogenous metastasis by neutralizing mice and its chimeric anti-Aggrus/podoplanin antibodies. Cancer Sci. 2011;102:2051–7. doi: 10.1111/j.1349-7006.2011.02058.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clezardin P, Serre CM, Trzeciak MC, Drouin J, Delmas PD. Thrombospondin binds to the surface of human osteosarcoma cells and mediates platelet-osteosarcoma cell interaction. Cancer Res. 1991;51:2621–7. [PubMed] [Google Scholar]
- 17.Kunita A, Kashima TG, Ohazama A, Grigoriadis AE, Fukayama M. Podoplanin is regulated by AP-1 and promotes platelet aggregation and cell migration in osteosarcoma. Am J Pathol. 2011;179:1041–9. doi: 10.1016/j.ajpath.2011.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Voland C, Serre CM, Delmas P, Clézardin P. Platelet-osteosarcoma cell interaction is mediated through a specific fibrinogen-binding sequence located within the N-terminal domain of thrombospondin 1. J Bone Miner Res. 2000;15:361–8. doi: 10.1359/jbmr.2000.15.2.361. [DOI] [PubMed] [Google Scholar]
- 19.Kubo T, Piperdi S, Rosenblum J, et al. Platelet-derived growth factor receptor as a prognostic marker and a therapeutic target for imatinib mesylate therapy in osteosarcoma. Cancer. 2008;112:2119–29. doi: 10.1002/cncr.23437. [DOI] [PubMed] [Google Scholar]
- 20.Kempf-Bielack B, Bielack SS, Jürgens H, et al. Osteosarcoma relapse after combined modality therapy: an analysis of unselected patients in the Cooperative Osteosarcoma Study Group (COSS) J Clin Oncol. 2005;23:559–68. doi: 10.1200/JCO.2005.04.063. [DOI] [PubMed] [Google Scholar]
- 21.Mehta P, Lawson D, Ward MB, Kimura A, Gee A. Effect of human tumor cells on platelet aggregation: potential relevance to pattern of metast. Cancer Res. 1987;47:3115–7. [PubMed] [Google Scholar]
- 22.Oleksowicz L, Mrowiec Z, Schwartz E, Khorshidi M, Dutcher JP, Puszkin E. Characterization of tumor-induced platelet aggregation: the role of immunorelated GPIb and GPIIb/IIIa expression by MCF-7 breast cancer cells. Thromb Res. 1995;79:261–74. doi: 10.1016/0049-3848(95)00113-6. [DOI] [PubMed] [Google Scholar]
- 23.Nakamori S, Kameyama M, Imaoka S, et al. Increased expression of sialyl Lewisx antigen correlates with poor survival in patients with colorectal carcinoma: clinicopathological and immunohistochemical study. Cancer Res. 1993;53:3632–7. [PubMed] [Google Scholar]
- 24.Mannori G, Crottet P, Cecconi O, et al. Differential colon cancer cell adhesion to E-, P-, and L-selectin: role of mucin-type glycoproteins. Cancer Res. 1995;55:4425–31. [PubMed] [Google Scholar]
- 25.Felding-Habermann B, Habermann R, Saldívar E, Ruggeri ZM. Role of beta3 integrins in melanoma cell adhesion to activated platelets under flow. J Biol Chem. 1996;271:5892–900. doi: 10.1074/jbc.271.10.5892. [DOI] [PubMed] [Google Scholar]
- 26.Kato Y, Fujita N, Kunita A, et al. Molecular identification of Aggrus/T1alpha as a platelet aggregation-inducing factor expressed in colorectal tumors. J Biol Chem. 2003;278:51599–605. doi: 10.1074/jbc.M309935200. [DOI] [PubMed] [Google Scholar]
- 27.Uren A, Merchant MS, Sun CJ, et al. Beta-platelet-derived growth factor receptor mediates motility and growth of Ewing's sarcoma cells. Oncogene. 2003;22:2334–42. doi: 10.1038/sj.onc.1206330. [DOI] [PubMed] [Google Scholar]
- 28.Sulzbacher I, Birner P, Trieb K, Mühlbauer M, Lang S, Chott A. Platelet-derived growth factor-alpha receptor expression supports the growth of conventional chondrosarcoma and is associated with adverse outcome. Am J Surg Pathol. 2001;25:1520–7. doi: 10.1097/00000478-200112000-00008. [DOI] [PubMed] [Google Scholar]
- 29.McDermott U, Ames RY, Iafrate AJ, et al. Ligand-dependent platelet-derived growth factor receptor (PDGFR)-alpha activation sensitizes rare lung cancer and sarcoma cells to PDGFR kinase inhibitors. Cancer Res. 2009;69:3937–46. doi: 10.1158/0008-5472.CAN-08-4327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dewaele B, Floris G, Finalet-Ferreiro J, et al. Coactivated platelet-derived growth factor receptor α and epidermal growth factor receptor are potential therapeutic targets in intimal sarcoma. Cancer Res. 2010;70:7304–14. doi: 10.1158/0008-5472.CAN-10-1543. [DOI] [PubMed] [Google Scholar]
- 31.Raile K, Hille R, Laue S, et al. Insulin-like growth factor I (IGF-I) stimulates proliferation but also increases caspase-3 activity, Annexin-V binding, and DNA-fragmentation in human MG63 osteosarcoma cells: co-activation of pro- and anti-apoptotic pathways by IGF-I. Horm Metab Res. 2003;35:786–93. doi: 10.1055/s-2004-814140. [DOI] [PubMed] [Google Scholar]
- 32.Takagi S, Oh-Hara T, Sato S, Gong B, Takami M, Fujita N. Expression of Aggrus/podoplanin in bladder cancer and its role in pulmonary metastasis. Int J Cancer. 2014;134:2605–14. doi: 10.1002/ijc.28602. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Involvement of Aggrus/podoplanin in platelet aggregation and PDGF release during osteosarcoma cell-induced platelet aggregation.
Fig. S2. Attenuation of MG63-dependent platelet aggregation by an anti-von Willebrand factor (vWF) antibody.
Fig. S3. Co-culture with platelets contributes to the resistance to apoptosis induced by adriamycin in osteosarcoma cells.
Fig. S4. Platelets promote invasiveness of osteosarcomas.


