Abstract
Transforming growth factor (TGF)-β exhibits both pro-apoptotic and anti-apoptotic effects on epithelial cells in a context-dependent manner. The anti-apoptotic function of TGF-β is mediated by several downstream regulatory mechanisms, and has been implicated in the tumor-progressive phenotype of breast cancer cells. We conducted RNA sequencing of mouse mammary gland epithelial (NMuMG) cells and identified a long non-coding RNA, termed lncRNA-Smad7, which has anti-apoptotic functions, as a target of TGF-β. lncRNA-Smad7 was located adjacent to the mouse Smad7 gene, and its expression was induced by TGF-β in all of the mouse mammary gland epithelial cell lines and breast cancer cell lines that we evaluated. Suppression of lncRNA-Smad7 expression cancelled the anti-apoptotic function of TGF-β. In contrast, forced expression of lncRNA-Smad7 rescued apoptosis induced by a TGF-β type I receptor kinase inhibitor in the mouse breast cancer cell line JygMC(A). The anti-apoptotic effect of lncRNA-Smad7 appeared to occur independently of the transcriptional regulation by TGF-β of anti-apoptotic DEC1 and pro-apoptotic Bim proteins. Small interfering RNA for lncRNA-Smad7 did not alter the process of TGF-β-induced epithelial–mesenchymal transition, phosphorylation of Smad2 or expression of the Smad7 gene, suggesting that the contribution of this lncRNA to TGF-β functions may be restricted to apoptosis. Our findings suggest a complex mechanism for regulating the anti-apoptotic and tumor-progressive aspects of TGF-β signaling.
Keywords: Apoptosis, breast cancer, long non-coding RNA, Smad7, transforming growth factor-β
Cancer cells make use of the tumor-promoting aspects of certain cytokines, while inhibiting their tumor-suppressive functions. Inhibition of the apoptotic program is one of the strategies used by cancer cells to continue dysregulated proliferation and tumor formation. The anti-apoptotic Bcl-2 family proteins are often upregulated, while several pro-apoptotic BH3-only proteins are inhibited, either transcriptionally or functionally, through interference with the upstream signaling pathways.(1)
Transforming growth factor (TGF)-β family proteins are representative proteins that possess bidirectional roles during tumor progression.(2) TGF-β exhibits cytostatic effects on various epithelial cells; many cancer cells are refractory to the cytostatic signals induced by TGF-β. Instead, they undergo an epithelial–mesenchymal transition (EMT) in response to TGF-β through induction of several factors, leading to stimulation of tumor migration and invasion.(3) TGF-β also displays both pro-apoptotic and anti-apoptotic effects on epithelial cells in a context-dependent manner. We previously showed that endogenous TGF-β produced by cancer cells plays a protective role in starvation-induced apoptosis of mouse breast cancer JygMC(A) and 4T1 cells through induction of the basic helix-loop-helix protein DEC1 and suppression of the BH3-only protein Bim.(4,5) TGF-β also suppresses rapamycin-induced apoptosis of serum-starved human basal-type breast cancer cells (MDA-MB-231 cell line).(6)
High throughput analyses have revealed that only approximately one-quarter of the transcripts are protein coding and that there are many non-coding RNA (ncRNA) in mice.(7) ncRNA are categorized based on their size and function, and long non-coding RNA (lncRNA) form a family of long transcripts with more than 200 nucleotides.(8) A limited number of lncRNA have been found to have essential biological functions,(9)– (11) and little is known about whether the majority of lncRNA play a role in cancer cells; this is in contrast to the wealth of information currently available about the roles of microRNA (miRNA) family transcripts in cancer.(12)
In the present study, we identified a lncRNA, designated lncRNA-Smad7 during a search for TGF-β-regulated transcripts in mouse mammary gland epithelial cells (NMuMG cell line). Interestingly, we found that lncRNA-Smad7 exhibited an anti-apoptotic effect upon stimulation by TGF-β in the mouse breast cancer cell line JygMC(A).
Materials and Methods
Cell culture
The mouse breast cancer cell lines JygMC(A) and 4T1 were cultured in DMEM containing 10% FBS, 50 units/mL penicillin and 50 μg/mL streptomycin. The mouse mammary epithelial cell line NMuMG was cultured in the above medium supplemented with 10 μg/mL insulin. Cells were grown in a 5% CO2 atmosphere at 37°C.
Reagents and antibodies
Recombinant TGF-β (TGF-β3) and the TGF-β type I receptor inhibitor SB431542 were purchased from R&D systems (Ixonia, WI, USA) and Sigma-Aldrich (St. Louis, MO, USA), respectively. The following antibodies were used: rabbit anti-phospho-Smad2 (Cell Signaling Technology, Danvers, MA, USA.), mouse anti-α-tubulin (DM1A [Sigma-Aldrich]), mouse anti-Smad2/3 (BD Biosciences, Franklin Lakes, NJ, USA.), rabbit anti-PARP (Cell Signaling Technology), mouse anti-E-cadherin (BD Biosciences), mouse anti-N-cadherin (BD Biosciences) and rabbit anti-fibronectin (Sigma-Aldrich).
Northern blotting
A specific probe to detect lncRNA-Smad7 was made with the DIG T7 RNA Labeling Kit (Roche Diagnostics, Rotkreuz, Risch, Switzerland.) using an EST sequence (CX230377), which was cloned into pBlueScript SK vector as a template. Northern blotting was performed with the DIG Northern Starter Kit (Roche Diagnostics). The probed membrane was washed twice with 2 × SSC (300 mM NaCl, 30 mM sodium citrate, pH 7.0) with 0.1% SDS for 5 min at room temperature and twice with 0.1 × SSC with 0.1% SDS for 15 min at 68°C. HRP-conjugated anti-digoxigenin was used to detect the hybridized probe by chemiluminescent imaging with a lumino-image analyzer (LAS-4000 [GE Health Care, Hino, Tokyo, Japan]).
Rapid amplification of cDNA ends
5′ and 3′ rapid amplification of cDNA ends (RACE) were performed using the SMARTer RACE cDNA Amplification Kit (TakaraBio, Otsu, Shiga, Japan.) in accordance with the manufacturer's protocol. Gene-specific primers used for 5′ and 3′ RACE were CAGTGCCGTGGAGACCAGAAGAATCCAG and CTGGATTCTTCTGGTCTCCACGGCACTG, respectively. Following the identification of the 5′ or 3′ ends of the RACE transcripts, we obtained clones of the expected size for lncRNA-Smad7 by PCR using a set of primers designed to hybridize to each end of the transcript.
Immunoprecipitation and immunoblotting
Lysis buffer (1% NP-40, 150 mM NaCl, 50 mM Tris-HCl pH 8.0, 5 mM EDTA and cOmplete EDTA-free protease inhibitor cocktail [Roche Diagnostics]) was used for cell lysis. SDS-gel electrophoresis and immunoblotting were performed as described previously,(13) using the LAS-4000. Quantification of the immunoblotting images was performed using Image-J software (National Institute of Health, Bethesda, MD, USA).
Adenoviral expression vectors
Ad-LacZ and Ad-lncRNA-Smad7 (variant 1) were prepared using the pAd-CMV-V5 vector (TakaraBio). Crude viral lysate was purified using ViraKit Adeno 4 (VIRAPUR, San Diego, CA, USA). Titration was performed with the Adeno-X Rapid Titer Kit (BD Biosciences).
RNA interference
We used two different siRNA against lncRNA-Smad7 (1: 5′-AAGAGUUGGAGUCCGCCAAACUAGG-3′ and 2: 5′-GGGAAGAAGACAGCAGUCAAGAAGA-3′) designed with the BLOCK-iT RNAi Designer (Life Technologies, Carlsbad, CA, USA). Control siRNA was purchased from Life Technologies (Cat. 12935–112, sequence not available). siRNA were introduced into JygMC(A) cells with Lipofectamine 2000 reagent (Life Technologies) according to the manufacturer's instructions. The final concentration of siRNA in the culture medium was 60 nM.
RNA sequencing
Total RNA (10 μg) was purified from NMuMG cells either stimulated with TGF-β or left untreated for 24 h and then polyA selected with Dynabeads Oligo(dT)25 (Life Technologies). Libraries were made and directionally sequenced with the Illumina Genome Analyzer IIx. One lane of the Illumina flow cell was used for each library to obtain sequencing data. Sequenced read tags were directionally aligned to either mm9 transcripts or to the reported genomic loci of lncRNA (mm9)(14) by TopHat2,(15) and differential gene expression calls were performed with the Cuffdiff function of Cufflinks.(16) Note that the reported lncRNA formed multi-exonic structures that were not fully defined. Therefore, we counted all of the mapped tags in the correct direction on each gene locus for the calculation above. The resultant “fragments per kilobase of exon per million mapped fragments sequenced” (FPKM) values thus potentially contain the sequence tags mapped to introns.
Apoptosis assay
Cells were treated as described previously.(4,5) The method is outlined in detail in the online Supporting Information.
Phalloidin staining
Phalloidin staining was performed as described with modification (available in online Supporting Information).(17)
Tumor xenograft assay
Four-week-old female Balb/c nude mice were inoculated subcutaneously with 1.0 × 107 siRNA-transfected JygMC(A) cells. Tumor volume was measured every other day and calculated using the following formula: ([major axis] × [minor axis]2)/2. All animal experiments were performed in accordance with the policies of the animal ethics committee of the University of Tokyo.
Statistical analysis
Repeated measures ANOVA was used for tumor xenograft assay. The Tukey–Kramer test was used for multiple data comparisons.
Accession numbers
Full nucleotide sequences of lncRNA-Smad7 are available from the DNA Data Bank of Japan (AB904933 and AB904934).
Method for RNA isolation, quantitative real-time PCR and primer sequences for quantitative RT-PCR (qRT-PCR) are available from the online Supporting Online.
Results
Identification of lncRNA-Smad7 as a target gene of transforming growth factor-β
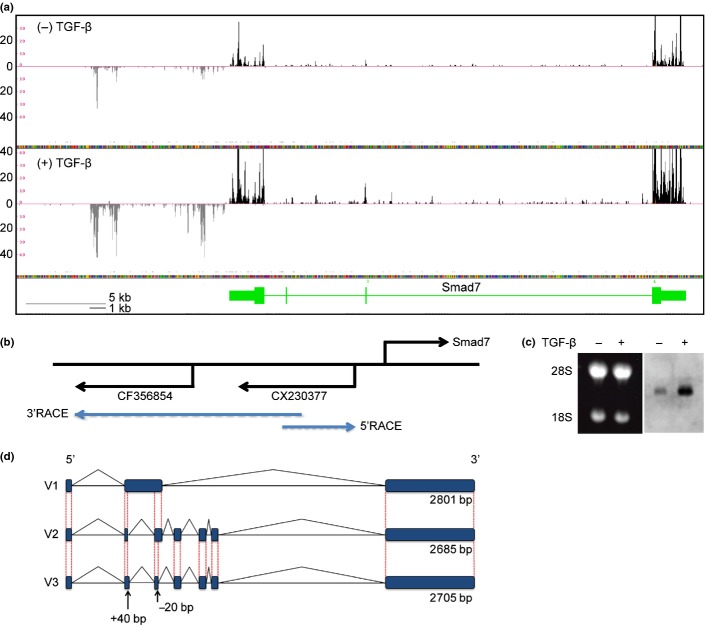
We performed RNA sequencing to quantitatively screen for lncRNA downstream of TGF-β signaling in NMuMG cells. To this end, we calculated changes in the expression of the reported lncRNA after the cells were treated with TGF-β (Table S1).(14) We focused on a TGF-β-induced and highly expressed lncRNA transcribed from an upstream and antisense strand of the Smad7 gene, which encodes a known target and inhibitor of TGF-β signaling (Fig. 1a). Distribution of the sequenced tags fell within two spliced EST, CX230377 and CF356854, and did not overlap with Smad7 (Fig. 1b). Northern blotting using CX230377 as a probe resulted in the detection of an approximately 3-kb transcript that was strongly induced by TGF-β treatment (Fig. 1c). We then cloned the transcript by RACE using gene-specific primers designed from CX230377 sequences and identified two transcripts, which we designated lncRNA-Smad7 V1 (2685 bp) and V2 (2801 bp) (Fig. 1d). In addition, variant 3 of lncRNA-Smad7 (V3) was identified through RT-PCR analysis of JygMC(A) cells (data not shown). There were only short open reading frames (ORF) encoding <97 amino acids in lncRNA-Smad7. These ORF did not have a canonical Kozak sequence (data not shown).
Fig. 1.
Cloning of lncRNA-Smad7. (a) RNA-sequencing data from NMuMG cells at the mouse Smad7 gene locus. Black and grey tags are aligned to the sense and antisense genomic sequences, respectively. Vertical axis: mapped tag numbers. Smad7 gene is shown in green, and its exons are shown as boxes (thin boxes, untranslated regions; thick boxes, coding regions) connected by horizontal lines representing introns. (b) Relative positions of the Smad7 gene and EST clones with direction of transcription. Positions of the gene-specific primers used for RACE are shown at the back ends of the blue arrows. (c) Northern blotting of lncRNA-Smad7. NMuMG cells were treated with TGF-β or left untreated for 24 h. Left panel: Ethidium bromide staining of the gel to show the positions of 28s/18s rRNA and equal loading of the samples. Right panel: Northern blotting with an EST sequence (CX230377) as a probe. (d) Schematic representation of the identified lncRNA-Smad7 transcripts. V3 transcript was identified by quantitative RT-PCR (qRT-PCR) analysis of JygMC(A) and confirmed by sequencing of the PCR product. V3 had a longer exon 2 and a shorter exon 3 than V2 transcript.
We next analyzed the expression of lncRNA-Smad7 in 4T1 and JygMC(A) mouse breast cancer cells by qRT-PCR. Expression of lncRNA-Smad7 was induced early after stimulation with TGF-β and continued for 48 h (Fig. 2a). Upregulation of lncRNA-Smad7 by TGF-β was observed in two other mouse mammary gland cell lines, EpH4 and EpRas (Fig. S1). Both 4T1 and JygMC(A) cells secrete endogenous TGF-β,(5) and basal expression of lncRNA-Smad7 was inhibited by the TGF-β type I receptor kinase inhibitor SB431542 (Fig. 2b). These results suggest that lncRNA-Smad7 is expressed in several mouse mammary gland epithelial cell lines and that TGF-β upregulates its expression in breast cancer cells.
Fig. 2.
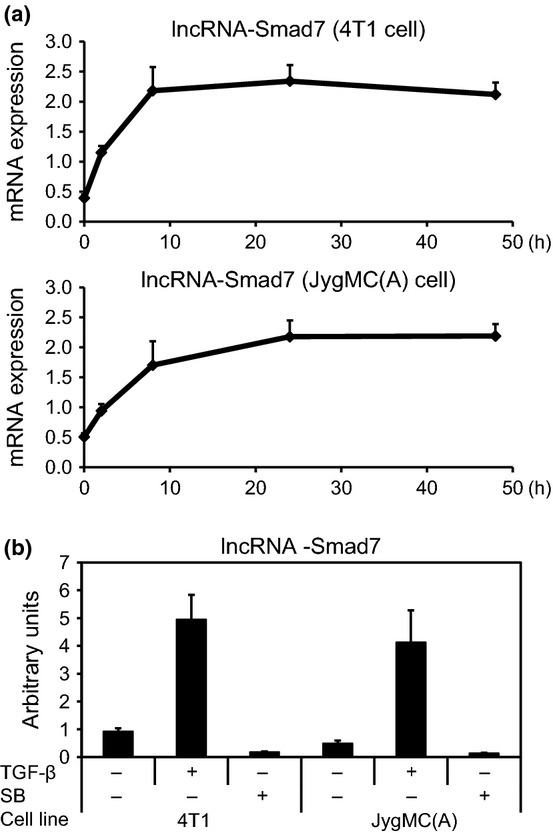
Upregulation of expression of lncRNA-Smad7 by TGF-β. (a) Effect of TGF-β on expression of lncRNA-Smad7 in mouse JygMC(A) and 4T1 breast cancer cells. Cells were treated with 1 ng/mL TGF-β for the indicated periods, and expression of lncRNA-Smad7 relative to that of TATA box binding protein (Tbp) was determined by quantitative RT-PCR (qRT-PCR). (b) Effect of TGF-β and the TGF-β type I receptor kinase inhibitor SB431542 (SB) on lncRNA-Smad7 expression. Cells were treated with 1 ng/mL TGF-β or 10 μM SB431542 for 48 h or left untreated. Relative expression of lncRNA-Smad7 was determined as in (a). Error bars: standard deviations.
lncRNA-Smad7 inhibits apoptosis of breast cancer cells induced by transforming growth factor-β
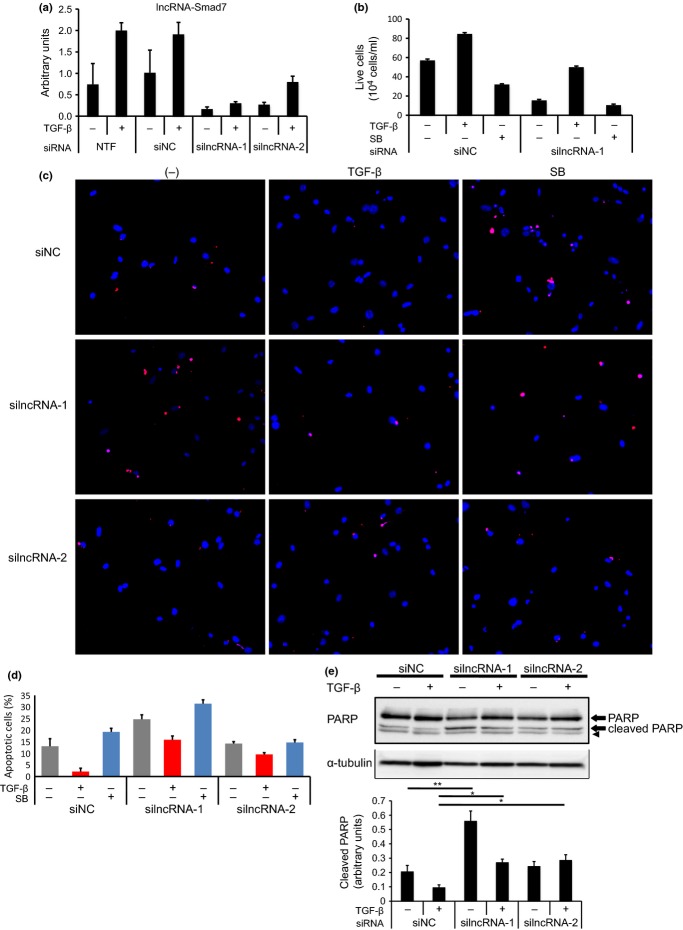
We next knocked down the expression of lncRNA-Smad7 by two different siRNA; that is, silncRNA-1 and silncRNA-2 (Fig. 3a). silncRNA-1 was designed to knock down all three variants, while silncRNA-2 was designed to knock down only V1. As shown in Figure 3a, expression of lncRNA-Smad7 was repressed efficiently by silncRNA-1 and moderately by silncRNA-2 in JygMC(A) cells. We found that live cell counts were decreased by silncRNA-1 in vitro (Fig. 3b). JygMC(A) cells underwent apoptosis upon serum starvation, and the apoptosis was induced by SB431542 and inhibited by TGF-β.(4) The role of lncRNA-Smad7 in TGF-β-induced anti-apoptosis was then evaluated. Inhibition of apoptosis by TGF-β was partially cancelled by suppression of lncRNA-Smad7 expression, as determined by TUNEL staining (Fig. 3c,d). Consistent with the silencing efficiencies, the effects of silncRNA-2 were less marked than those of silncRNA-1 (Fig. 3c,d). Cleaved PARP levels in TGF-β-stimulated cells were also increased following treatment with silncRNA-1 and silncRNA-2 (Fig. 3e).
Fig. 3.
Knockdown of lncRNA-Smad7 induces apoptosis of JygMC(A) cells. (a) Effect of siRNA for lncRNA-Smad7 in JygMC(A) cells. Cells were transfected with the indicated siRNA and stimulated with 1 ng/mL TGF-β for 48 h after transfection. silncRNA-1 was used for knockdown of all variants of lncRNA-Smad7, while silncRNA-2 was designed for knockdown of V1 only. Relative expression of lncRNA was determined as in Figure 2a. NTF, no transfection control; siNC, negative control siRNA. (b) Live-cell counting of JygMC(A) cells treated with siRNA for lncRNA-Smad7. Cells were transfected with siRNA as indicated and treated with 1 ng/mL TGF-β or 10 μM SB431542 with serum starvation 48 h after transfection. Live cells were counted using TC20 (BioRad, Hercules, California, USA.) after 48 h. (c) TUNEL staining of JygMC(A) cells treated with siRNA for lncRNA-Smad7. Cells were treated as in (b). TUNEL, red; DAPI, blue. (d) The percentage of TUNEL-positive cells among DAPI-positive cells was determined. (e) Immunoblot analysis of cleaved PARP. Cells were treated as in (b) and lysed for SDS-PAGE. An arrow head shows nonspecific band. The graph in the bottom shows quantification of cleaved PARP normalized to α-tubulin. Error bars: standard deviations. *P < 0.05, **P < 0.001.
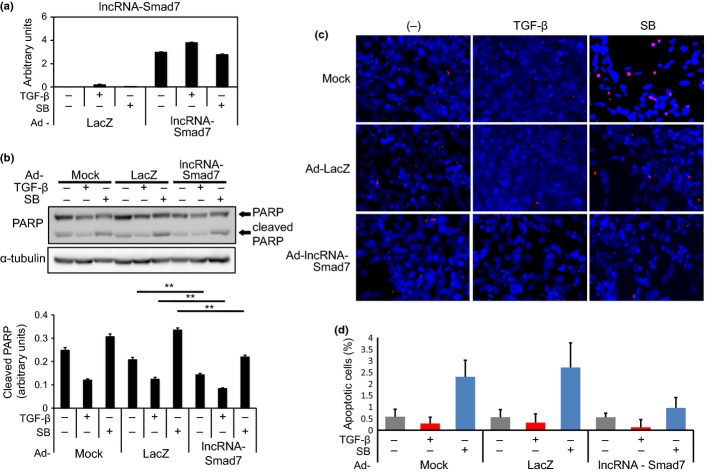
The adenoviral lncRNA-Smad7 expression vector (Ad-lncRNA-Smad7) was then constructed to examine its effect on apoptosis. Expression of exogenous lncRNA-Smad7 was confirmed by qRT-PCR and compared with endogenous expression levels and with a sample without reverse transcription (Fig. 4a and data not shown). Partial inhibition by Ad-lncRNA-Smad7 of cleaved PARP expression was observed in both TGF-β-stimulated and TGF-β-unstimulated cells (Fig. 4b). The numbers of TUNEL-positive cells induced by SB431542 treatment were reduced by forced expression of lncRNA-Smad7 (Fig. 4c,d), which suggests that lncRNA-Smad7 acts as an anti-apoptotic factor downstream of TGF-β signaling.
Fig. 4.
Forced expression of lncRNA-Smad7 partially inhibits apoptosis of JygMC(A) cells. (a) Cells were infected with either adenoviral lncRNA-Smad7 expression vector (Ad-lncRNA-Smad7) or Ad-LacZ as a control. Twelve hours after infection, cells were serum starved and treated with 1 ng/mL TGF-β or 10 μM SB431542 (SB) for 48 h. Expression of lncRNA-Smad7 was determined by quantitative RT-PCR (qRT-PCR). (b) Effect of Ad-lncRNA-Smad7 on cleaved PARP level was determined by immunoblotting. Cells were treated as in (a) and lysed for SDS-PAGE. The bottom graph shows quantification of cleaved PARP normalized to α-tubulin. **P < 0.001. (c) TUNEL staining of JygMC(A) cells. Cells were treated as in (a). TUNEL, red; DAPI, blue. (d) The percentage of TUNEL-positive cells among DAPI-positive cells was determined. Error bars: standard deviations.
Effect of lncRNA-Smad7 on transforming growth factor-β-Smad signaling pathway and transforming growth factor-β-induced epithelial–mesenchymal transition
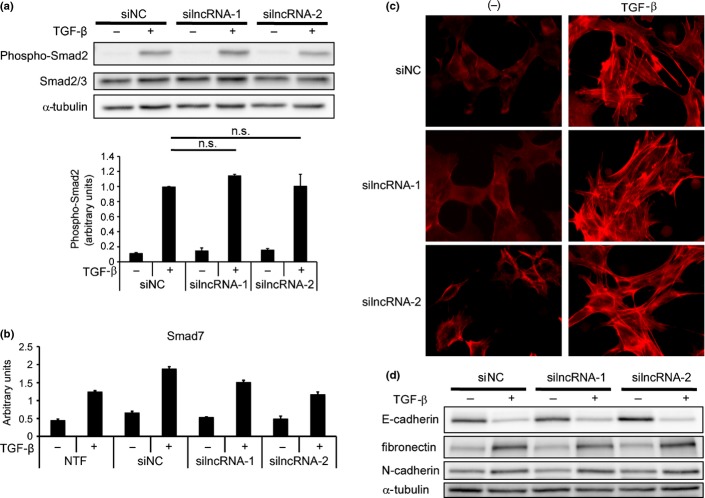
lncRNA have several modes of function, including neutralizing miRNA and inhibiting transcription of target genomic regions.(8) Therefore, we next evaluated the effect of siRNA for lncRNA-Smad7 on the TGF-β-Smad signaling pathway. Knockdown of lncRNA-Smad7 did not change phospho-Smad2 expression in JygMC(A) cells (Fig. 5a). Moreover, expression of Smad7 by TGF-β stimulation was minimally inhibited by the siRNA for lncRNA-Smad7 (Fig. 5b). We then evaluated the effect of silncRNA on TGF-β-induced EMT in NMuMG cells. Actin stress fiber formation by TGF-β stimulation was not inhibited by the silncRNA, and silncRNA-2 showed a mild F-actin-inducing effect in the absence of TGF-β (Fig. 5c). Both downregulation of E-cadherin and induction of fibronectin and N-cadherin by TGF-β were not affected by the silncRNA (Fig. 5d). These results suggest that lncRNA-Smad7 does not have a marked effect on TGF-β signaling in general but does regulate anti-apoptosis-specific effects. Upregulation of Bhlhe40 (encoding the anti-apoptotic DEC1 protein) and inhibition of Bcl2l11 (encoding the pro-apoptotic BH3-only protein Bim) through downregulation of Foxc1 have been demonstrated as mechanisms of TGF-β-induced anti-apoptosis in breast cancer cells.(4,5) We examined the effect of lncRNA-Smad7 on the mRNA levels of these molecules, but the changes in their expression profiles could not explain the anti-apoptotic function of lncRNA-Smad7 (Fig. S2). We also searched for other pro-apoptotic and anti-apoptotic factors whose mRNA and protein expression levels were regulated by lncRNA-Smad7, but we were not able to identify any definite targets (data not shown).
Fig. 5.
lncRNA-Smad7 does not regulate phospho-Smad2 levels or TGF-β-induced EMT. (a) JygMC(A) cells were transfected with siRNA as indicated and stimulated with TGF-β for 48 h. Phospho-Smad2 and total Smad2/3 levels were determined by immunoblotting (top panel). The bottom graph shows quantification of phospho-Smad2 normalized to total Smad2/3. n.s.: not significant. (b) Effect of siRNA for lncRNA-Smad7 on Smad7 expression evaluated by quantitative RT-PCR (qRT-PCR). Transfected cells were stimulated with TGF-β for 48 h. NTF, no transfection; error bars, standard deviations. (c) NMuMG cells were reverse-transfected with siRNA as indicated. Twelve hours later, cells were stimulated with TGF-β for 24 h and fixed. F-actin formation was evaluated by phalloidin staining. (d) NMuMG cells were treated as in (c) and expression of EMT marker proteins was determined by immunoblotting.
Role of lncRNA-Smad7 in xenografted tumor formation
Finally, we examined whether lncRNA-Smad7 affects the tumor-forming ability of JygMC(A) cells. Tumor size was periodically measured after subcutaneous inoculation of siRNA-transfected JygMC(A) cells into mice. As shown in Figure 6, knockdown of lncRNA-Smad7 resulted in reduced tumor size, suggesting impaired graft survival in the absence of lncRNA-Smad7 (Fig. 6a,b).
Fig. 6.
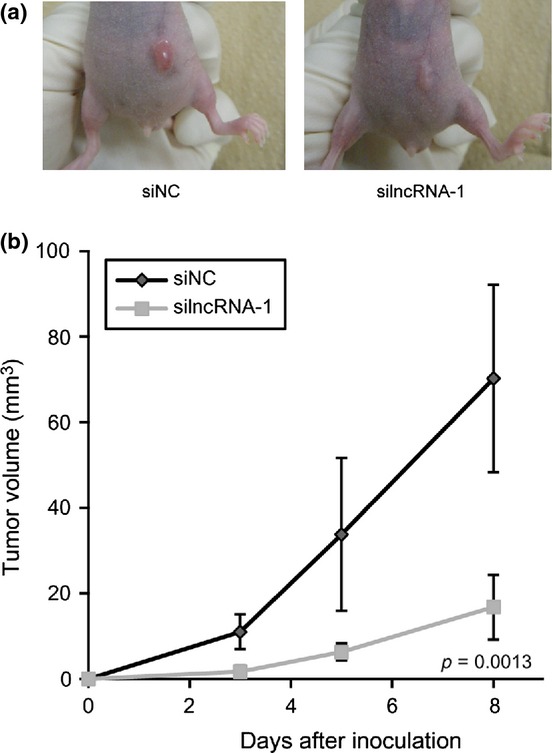
Knockdown of lncRNA-Smad7 represses tumor-forming ability of JygMc(A) cells. (a) Effect of siRNA for lncRNA-Smad7 (silncRNA-1) on the size of xenografted tumors (n = 6). Photos were taken 8 d after tumor inoculation. Representative images are shown in this figure. (b) Sequential tumor volume measurement after inoculation. Error bars: standard deviations.
Discussion
In the present study, we identified lncRNA-Smad7 through a search for TGF-β-regulated transcripts in mouse mammary NMuMG cells. lncRNA-Smad7 is located adjacent to the mouse Smad7 gene and is strongly upregulated by TGF-β in the mouse mammary gland epithelial cells and mouse breast cancer cells. lncRNA-Smad7 appears to function as a downstream anti-apoptotic factor of TGF-β in the mouse breast cancer cell line JygMC(A) without affecting activation of Smad2 and induction of EMT.
lncRNA regulate gene expression through several mechanisms in cancer.(18) The lncRNA HOTAIR was identified in breast tumors as a poor prognosis marker that induces alteration of Polycomb-regulated histone H3K27 modification and gene expression.(10,19) Subsequently, the presence of HOTAIR was reported to be associated with the prognosis of nasopharyngeal carcinoma and esophageal cancer.(20,21) Zhang et al.(22) reported that the lncRNA-RoR inhibited translation of p53 and apoptosis in MCF-7 breast cancer cells. Importantly, lncRNA-RoR also functions as a miRNA sponge to inhibit a set of miRNA in human ES cells, suggesting that even a single type of lncRNA has several modes of action.(23) Other lncRNA are related to the pathogenesis of breast cancers. The ATM target gene lncRNA-JADE protects MCF7 cells from several DNA-damaging drugs to maintain tumor formation.(24) In addition, Hu et al.(25) analyzed lncRNA expression signatures during the twist-induced EMT of MCF10A cells.
We identified lncRNA-Smad7 as a new anti-apoptosis factor in mouse breast cancer cells, although we could not identify the regulatory mechanism of apoptosis induced by lncRNA-Smad7. In principle, siRNA-based loss-of-function analyses of lncRNA may not be an ideal means of evaluating their transcriptional or epigenomic regulatory mechanisms. Therefore, we cannot conclude that lncRNA-Smad7 does not regulate transcription of the adjacent Smad7 and other genes. In contrast, the anti-apoptotic activity of lncRNA-Smad7 observed in our siRNA experiments could instead be executed through post-transcriptional regulatory mechanisms. Determining the effect of lncRNA-Smad7 on Smad7 expression will be an important topic for future analysis, because Smad7 sensitizes MCF7 cells to IRF1-induced apoptosis.(26)
Our present analysis revealed that lncRNA-Smad7 was widely expressed in mouse mammary epithelial cells and mouse breast cancer cells. This transcript was previously found in a list yielded by transcriptome analyses of mouse ES cells,(14) suggesting that it is broadly expressed in many mouse cell types and tissues. Transcriptional upregulation of lncRNA-Smad7 by TGF-β would also be a general mechanism in mice, because the transcriptional start site of lncRNA-Smad7 is close to Smad7, a well-known target of TGF-β/Smad signaling. Guttman et al. included this lncRNA in their loss-of-function screening based on the good conservation among species, and lncRNA-Smad7 was designated as linc1316 in their list (table 1).(14) However, our efforts to find a human ortholog by using our unpublished RNA-sequencing data from several TGF-β-treated human epithelial cell lines and cancer cell lines were unsuccessful (data not shown). Moreover, a BLAST-based search for lncRNA-Smad7-like genomic sequences showed that only approximately 400 nucleotides out of the 3-kb mouse lncRNA-Smad7 are conserved upstream of SMAD7 in humans, which was in contrast to the high conservation between mice and rats (data not shown). Therefore, lncRNA-Smad7 appears to be a rodent-specific transcript. However, there is still a possibility that the identified 400-bp region in humans might be transcribed in a limited condition and has functions similar to that of mouse lncRNA-Smad7 in human breast cancers.
Comprehensive analysis of transcriptional regulation of the many target genes of TGF-β through ChIP-sequencing or ChIP-chip analyses have been performed in several cancer cell lines,(27)– (31) and large-scale transcriptome data from several cancer types are available. In the case of lncRNA, Zhou et al.(32) reported a list of TGF-β-Smad3 target lncRNA expressed during renal inflammation and fibrosis. However, the lack of knowledge about the function of non-coding transcripts often limits the utility of these types of data. Our findings, together with the findings of low conservation of lncRNA among species, suggest that determination of the roles of each non-coding transcript is required to understand their relevance to cancer biology.
Acknowledgments
The authors are grateful to Kaori Shiina and Keiko Yuki for technical assistance, as well as to members of the Miyazono Laboratory for discussion and advice. This research was supported by KAKENHI (grants-in-aid for scientific research on Innovative Areas [Integrative Research on Cancer Microenvironment Network; 22112002, K.M.], Scientific Research [S] [20221009, H.A.], [C] [24501311, D.K.] and [B] [24390070, K.M.]) from the Ministry of Education, Culture, Sports, Science and Technology of Japan (MEXT) and a grant from the Ministry of Health, Labor, and Welfare of Japan (D.K.). This study was performed in part as a research program of the Project for Development of Innovative Research on Cancer Therapeutics (P-Direct) from MEXT, and a grant from the Swedish Cancer Foundation (No. 100452, K.M.). D.K. is supported by a grant from the Mochida Memorial Foundation for Medical and Pharmaceutical Research. M.A. is supported by grants from the Ishidsu Shun Memorial Scholarship and the Japan Society for the Promotion of Science. M.M. is supported by the Kanae Foundation for Research Abroad and the ITO Genboku and SAGARA Chian Memorial Scholarship.
Disclosure Statement
The authors have no conflict of interest to declare.
Supporting Information
Additional supporting information may be found in the online version of this article:
Table S1. lncRNA regulated by transforming growth factor (TGF)-β
Fig. S1 Upregulation of lncRNA-Smad7 in mouse mammary gland cell lines.
Fig. S2. Effects of siRNA for lncRNA-Smad7 on expression of DEC1 and Bim.
References
- 1.Happo L, Strasser A, Cory S. BH3-only proteins in apoptosis at a glance. J Cell Sci. 2012;125:1081–7. doi: 10.1242/jcs.090514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roberts AB, Wakefield LM. The two faces of transforming growth factor-β in carcinogenesis. Proc Natl Acad Sci USA. 2003;100:8621–3. doi: 10.1073/pnas.1633291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miyazono K, Ehata S, Koinuma D. Tumor-promoting functions of transforming growth factor-β in progression of cancer. Ups J Med Sci. 2012;117:143–52. doi: 10.3109/03009734.2011.638729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ehata S, Hanyu A, Hayashi M, et al. Transforming growth factor-β promotes survival of mammary carcinoma cells through induction of antiapoptotic transcription factor DEC1. Cancer Res. 2007;67:9694–703. doi: 10.1158/0008-5472.CAN-07-1522. [DOI] [PubMed] [Google Scholar]
- 5.Hoshino Y, Katsuno Y, Ehata S, Miyazono K. Autocrine TGF-β protects breast cancer cells from apoptosis through reduction of BH3-only protein, Bim. J Biochem. 2011;149:55–65. doi: 10.1093/jb/mvq114. [DOI] [PubMed] [Google Scholar]
- 6.Gadir N, Jackson DN, Lee E, Foster DA. Defective TGF-β signaling sensitizes human cancer cells to rapamycin. Oncogene. 2008;27:1055–62. doi: 10.1038/sj.onc.1210721. [DOI] [PubMed] [Google Scholar]
- 7.Okazaki Y, Furuno M, Kasukawa T, et al. Analysis of the mouse transcriptome based on functional annotation of 60,770 full-length cDNAs. Nature. 2002;420:563–73. doi: 10.1038/nature01266. [DOI] [PubMed] [Google Scholar]
- 8.Chen LL, Carmichael GG. Decoding the function of nuclear long non-coding RNAs. Curr Opin Cell Biol. 2010;22:357–64. doi: 10.1016/j.ceb.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bartolomei MS, Zemel S, Tilghman SM. Parental imprinting of the mouse H19 gene. Nature. 1991;351:153–5. doi: 10.1038/351153a0. [DOI] [PubMed] [Google Scholar]
- 10.Gupta RA, Shah N, Wang KC, et al. Long non-coding RNA HOTAIR reprograms chromatin state to promote cancer metastasis. Nature. 2010;464:1071–6. doi: 10.1038/nature08975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yan B, Wang Z. Long noncoding RNA: its physiological and pathological roles. DNA Cell Biol. 2012;31(Suppl. 1):S34–41. doi: 10.1089/dna.2011.1544. [DOI] [PubMed] [Google Scholar]
- 12.Serpico D, Molino L, Di Cosimo S. MicroRNAs in breast cancer development and treatment. Cancer Treat Rev. 2014;40:595–604. doi: 10.1016/j.ctrv.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 13.Mizutani A, Saitoh M, Imamura T, Miyazawa K, Miyazono K. Arkadia complexes with clathrin adaptor AP2 and regulates EGF signalling. J Biochem. 2010;148:733–41. doi: 10.1093/jb/mvq127. [DOI] [PubMed] [Google Scholar]
- 14.Guttman M, Donaghey J, Carey BW, et al. lincRNAs act in the circuitry controlling pluripotency and differentiation. Nature. 2011;477:295–300. doi: 10.1038/nature10398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim D, Pertea G, Trapnell C, Pimentel H, Kelley R, Salzberg SL. TopHat2: accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biol. 2013;14:R36. doi: 10.1186/gb-2013-14-4-r36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Trapnell C, Roberts A, Goff L, et al. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat Protoc. 2012;7:562–78. doi: 10.1038/nprot.2012.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shirakihara T, Saitoh M, Miyazono K. Differential regulation of epithelial and mesenchymal markers by δEF1 proteins in epithelial mesenchymal transition induced by TGF-β. Mol Biol Cell. 2007;18:3533–44. doi: 10.1091/mbc.E07-03-0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gutschner T, Diederichs S. The hallmarks of cancer: a long non-coding RNA point of view. RNA Biol. 2012;9:703–19. doi: 10.4161/rna.20481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chu C, Qu K, Zhong FL, Artandi SE, Chang HY. Genomic maps of long noncoding RNA occupancy reveal principles of RNA-chromatin interactions. Mol Cell. 2011;44:667–78. doi: 10.1016/j.molcel.2011.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nie Y, Liu X, Qu S, Song E, Zou H, Gong C. Long non-coding RNA HOTAIR is an independent prognostic marker for nasopharyngeal carcinoma progression and survival. Cancer Sci. 2013;104:458–64. doi: 10.1111/cas.12092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ge XS, Ma HJ, Zheng XH, et al. HOTAIR, a prognostic factor in esophageal squamous cell carcinoma, inhibits WIF-1 expression and activates Wnt pathway. Cancer Sci. 2013;104:1675–82. doi: 10.1111/cas.12296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang A, Zhou N, Huang J, et al. The human long non-coding RNA-RoR is a p53 repressor in response to DNA damage. Cell Res. 2013;23:340–50. doi: 10.1038/cr.2012.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang Y, Xu Z, Jiang J, et al. Endogenous miRNA sponge lincRNA-RoR regulates Oct4, Nanog, and Sox2 in human embryonic stem cell self-renewal. Dev Cell. 2013;25:69–80. doi: 10.1016/j.devcel.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 24.Wan G, Hu X, Liu Y, et al. A novel non-coding RNA lncRNA-JADE connects DNA damage signalling to histone H4 acetylation. EMBO J. 2013;32:2833–47. doi: 10.1038/emboj.2013.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hu P, Yang J, Hou Y, et al. LncRNA expression signatures of twist-induced epithelial-to-mesenchymal transition in MCF10A cells. Cell Signal. 2014;26:83–93. doi: 10.1016/j.cellsig.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 26.Hong S, Kim HY, Kim J, et al. Smad7 protein induces interferon regulatory factor 1-dependent transcriptional activation of caspase 8 to restore tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-mediated apoptosis. J Biol Chem. 2013;288:3560–70. doi: 10.1074/jbc.M112.400408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang Y, Handley D, Kaplan T, et al. High throughput determination of TGF-β1/SMAD3 targets in A549 lung epithelial cells. PLoS ONE. 2011;6:e20319. doi: 10.1371/journal.pone.0020319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mizutani A, Koinuma D, Tsutsumi S, et al. Cell type-specific target selection by combinatorial binding of Smad2/3 proteins and hepatocyte nuclear factor 4α in HepG2 cells. J Biol Chem. 2011;286:29848–60. doi: 10.1074/jbc.M110.217745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kennedy BA, Deatherage DE, Gu F, et al. ChIP-seq defined genome-wide map of TGF-β/SMAD4 targets: implications with clinical outcome of ovarian cancer. PLoS ONE. 2011;6:e22606. doi: 10.1371/journal.pone.0022606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Qin H, Chan MW, Liyanarachchi S, et al. An integrative ChIP-chip and gene expression profiling to model SMAD regulatory modules. BMC Syst Biol. 2009;3:73. doi: 10.1186/1752-0509-3-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morikawa M, Koinuma D, Miyazono K, Heldin CH. Genome-wide mechanisms of Smad binding. Oncogene. 2013;32:1609–15. doi: 10.1038/onc.2012.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhou Q, Chung AC, Huang XR, Dong Y, Yu X, Lan HY. Identification of novel long noncoding RNAs associated with TGF-β/Smad3-mediated renal inflammation and fibrosis by RNA sequencing. Am J Pathol. 2014;184:409–17. doi: 10.1016/j.ajpath.2013.10.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. lncRNA regulated by transforming growth factor (TGF)-β
Fig. S1 Upregulation of lncRNA-Smad7 in mouse mammary gland cell lines.
Fig. S2. Effects of siRNA for lncRNA-Smad7 on expression of DEC1 and Bim.