Abstract
Although Th17 cells play crucial roles in the pathogenesis of many autoimmune and inflammatory disorders, their roles in malignancies are currently under debate. The role and mechanism of Th17 cells in patients with acute myeloid leukemia (AML) remain poorly understood. Here we demonstrated that the frequency of Th17 cells was significantly increased in peripheral blood mononuclear cells (PBMCs) and bone marrow mononuclear cells from AML patients compared with healthy donors. Plasma levels of interleukin (IL)-17, IL-22, IL-23, IL-1β, IL-6, and transforming growth factor (TGF)-β1 were significantly increased in blood and bone marrow in AML patients compared with healthy donors. The in vitro experiments demonstrated that IL-1β, IL-6, IL-23, but not TGF-β1 promoted the generation and differentiation of Th17 cells from naive CD4+ T cells in humans. IL-17A, a signature cytokine secreted by Th17 cells, induced the proliferation of IL-17 receptor (IL-17R)-positive AML cells via IL-17R, in which activation of PI3K/Akt and Jak/Stat3 signaling pathway may play important roles. In addition, combination of IL-17A and IL-22 significantly reduced the generation of Th1 cells and the production of interferon (IFN)-γ from healthy donor or AML patient peripheral blood mononuclear cells. Patients with high Th17 cell frequency had poor prognosis, whereas patients with high Th1 cell frequency had prolonged survival. Combined analysis of Th1 and Th17 cell frequencies improved the ability to predict patient outcomes. In conclusion, Th17 cells play a crucial role in the pathogenesis of AML and may be an important therapeutic target and prognostic predictor.
Keywords: Acute myeloid leukemia, interleukin-17, prognostic factors, Th1 cells, Th17 cells
A marked impairment of T-cell function is observed in patients with acute myeloid leukemia (AML).(1) Although other immunologic abnormalities also exist in AML patients, accumulating evidence suggests that CD4+ T cells play a central role in initiating and maintaining immune responses against leukemic cells.(2) CD4+ T cells participate in many facets of the adaptive immune system and provide regulating signals to help other immune effector cells to kill tumor cells.
T helper type 17 (Th17) cells, a newly identified CD4+ T cell subset, exhibit effector functions distinct from Th1 and Th2 cells and play a crucial role in inflammation, autoimmune diseases, and acute graft-versus-host disease.(3)– (6) Although Th17 cells have been examined in the tumor microenvironment in many cancers, the role of Th17 cells in protection or progression of tumors is currently under debate.(7,8) Interleukin-17 (IL-17) is the signature effector cytokine produced by Th17 cells. The biological function of Th17 cells mainly depends on its secreted IL-17. Interleukin-17 has pleiotropic functions with multiple targets, in which it has been reported to regulate cellular proliferation, angiogenesis and metastasis in many cancer cells.(9,10) Th17 cells as well as IL-17 have been reported as a poor prognostic indicator in many cancers and a favorable indicator in other cancers.(9,11)– (14)
The differentiation of Th1 and Th17 cells were initially thought to be distinct and possibly antagonistic.(15) Interferon-γ (IFN-γ) inhibited Th17 cell differentiation while IL-17 inhibited Th1 cell differentiation in murine models of malignant pleural effusion.(16) Th17 cells were able to induce Th1-type chemokines and recruit Th1 cells into the tumor microenvironment of ovarian cancer patients.(17) Recently, a substantial fraction of CD4+ T cells in the tissue of patients with autoimmune disease or cancer have been found to coexpress IFN-γ and IL-17.(9,18) Therefore, the interplay of Th1 and Th17 cells in tumor microenvironment plays a crucial role in tumor development, but little is known in hematological malignancies.
Acute myeloid leukemia represents an ideal model to evaluate the impact of tumor on the host immune system as the disease is usually widely disseminated, so that immune cells in the peripheral blood (PB) and bone marrow (BM) are in close proximity to the tumor cells. The immune system fails to recognize and eradicate leukemia cells, which plays significant roles in the development of AML. Despite there being few studies focusing on Th17 cells in AML, the nature, regulation, role and prognostic predictor of Th17 cells is poorly understood in the context of tumor immunity in AML patients as their results are divergent.(19)– (22)
In the present study, we demonstrate that Th17 cell frequencies and IL-17 are elevated in PB and BM of AML patients; IL-17A promotes the proliferation of U937 cells as well as IL-17 receptor (IL-17R)-positive primary AML cells via IL-17R; patients with high Th17 cell frequencies have poor prognosis whereas patients with high Th1 frequencies have prolonged survival.
Materials and Methods
Patients
Between January 2009 and September 2013, 98 newly diagnosed patients with AML (age range, 16–79 years; median, 46 years) were enrolled from the First Affiliated Hospital of Wenzhou Medical University. Our study was approved by the First Affiliated Hospital of Wenzhou Medical University institutional review board and written consents were obtained from all subjects participating in this study in accordance with the Declaration of Helsinki protocol. All patients met World Health Organization (WHO) 2008 classification criteria for AML. Thirty-two age-matched healthy donors for control were simultaneously enrolled in this study.
Flow cytometric analysis
To evaluate Th17 cell frequencies, peripheral blood mononuclear cells (PBMCs) and bone marrow mononuclear cells (BMMCs) isolated from AML patients and healthy donors were stimulated with phorbol 12-myristate13-acetate (PMA) and ionomycin for 5 h in the presence of brefeldin A (all from Sigma-Aldrich, St. Louis, MO, USA). After incubation, cell surface staining was performed with PerCP-conjugated anti-CD3 and FITC-conjugated anti-CD8 monoclonal antibodies (mAbs) at room temperature in the dark for 20 min. The cells were subsequently fixed and permeabilized and stained with PE-conjugated anti-IL-17A and APC-conjugated anti-IFN-γ mAbs (BD Biosciences, San Jose, CA, USA). Stained cells were acquired and analyzed using a FACSCalibur instrument and CellQuest software (BD Biosciences).
ELISA for cytokine measurements
Plasma samples were collected from PB and BM after centrifugation and stored at −80°C until use. Plasma levels of IL-17, IL-22, IL-23, IL-6, IL-1β, and IFN-γ were measured using commercial ELISA kits (R & D Systems, Minneapolis, MN, USA) according to the manufacturer's instructions.
Quantitative PCR for gene expression analysis
Total RNA was extracted with Trizol (Invitrogen, Carlsbad, CA, USA) and reverse transcribed into cDNA, and then cDNAs were amplified and quantified by ABI Prism 7500 using SYBR Green PCR master mix (TaKaRa, Dalian, China) with primer pairs (for primers sequences, see Table S1), respectively.
MTT assay
The effects of IL-17A on AML cell lines HL-60, U937, and Dami as well as primary AML cells were assessed using the 3-(4,5-dlmethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) (Sigma-Aldrich). Cells were seeded in 96-well plates with or without IL-17A (50 ng/mL; Peprotech, Rocky Hill, NJ, USA) for 7 days. The absorbance was read at 490 nm using an ELISA reader (ELx800; Bio-Tek Instruments, Winooski, VT, USA).
Differentiation assays
Naive CD4+ T cells were isolated by negative selection with Naive CD4+ T cell Isolation Kit II (Miltenyi Biotec, Bergisch Gladbach, Germany) according to the manufacturer's instructions. The purity of naive CD4+ T cells measured by flow cytometry was approximately 95%. Naive CD4+ T cells were stimulated with plate-bound anti-CD3 (1.5 μg/mL; eBiosciences, San Diego, CA, USA) and soluble CD28 mAbs (1 μg/mL; BD Biosciences) for 7 days in 1 mL complete medium containing human IL-2 (2 ng/mL) with or without the indicated cytokines (IL-1β, 10 ng/mL; IL-6, 100 ng/mL; IL-23, 10 ng/mL; TGF-β1, 5 ng/mL). All exogenous cytokines used were purchased from R & D Systems.
Western blot assays
After treatment with IL-17A, U937 cells and primary AML cells were collected, lysed, and further subjected to western blot analysis with antibodies against phospho-Akt (Ser473), Akt, GAPDH (Cell Signaling Technology, Beverly, MA, USA), phospho-Stat3 (Tyr705), and Stat3 (Bioworld Technology, St. Louis Park, MN, USA), respectively, according to our previously described method.(23)
IL-12-induced IFN-γ-producing Th1 cells assays
To determine induced Th1 cells, PBMCs isolated from healthy donors or AML patients were stimulated with Th1-polarizing cocktail including IL-12 (10 ng/mL; R & D Systems) and anti-CD3 antibodies for 6 days in RPMI 1640 medium with 10% FBS. Cells were further expanded with IL-2 for additional 6 days prior to restimulation with PMA and ionomycin in the presence of brefeldin A. After stimulation, cells were stained for CD3, CD8, and intracellular anti-IFN-γ.
Statistical analysis
Data are expressed as mean ± SEM. Statistical analyses were performed using a one-way analysis of variance (anova) as well as Mann–Whitney test. P-values less than 0.05 was considered statistically significant.
Results
Elevated frequencies of Th17 cells and reduced frequencies of Th1 cells in PBMCs and BMMCs from untreated patients with AML
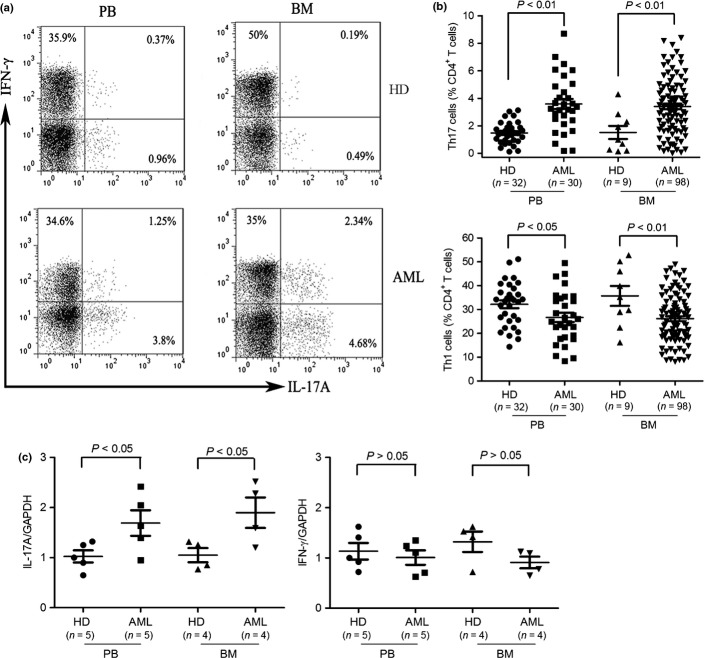
We evaluated the frequencies of Th17 cells in freshly isolated PBMCs and BMMCs from AML patients and compared them with those in PBMCs and BMMCs from healthy donors. After gating on CD3+CD8- T cells, intracellular production of IL-17A and IFN-γ were analyzed. The frequencies of Th17 cells were 3.59 ± 0.37% in AML PBMCs compared with 1.47 ± 0.14% in healthy donor PBMCs (P < 0.01) and 3.40 ± 0.21% in AML BMMCs compared with 1.51 ± 0.48% in healthy donor BMMCs (P < 0.01) (Fig. 1b). The frequencies of Th17 cells were significantly increased in PBMCs and BMMCs from AML patients compared with those in healthy donor PBMCs and BMMCs, whereas the frequencies of Th1 cells were significantly decreased in AML PBMCs and BMMCs compared to healthy donor PBMCs and BMMCs (Fig. 1a,b). We further confirmed elevated frequencies of IL-17A-producing cells in CD4+ cells from AML patients compared to healthy donors by qPCR, while IFN-γ-producing cells, although high, is not statistically significant by qPCR (Fig. 1c).
Fig. 1.
Elevated frequencies of Th17 cells and reduced frequencies of Th1 cells in freshly isolated peripheral blood mononuclear cells (PBMCs) and bone marrow mononuclear cells (BMMCs) from acute myeloid leukemia (AML) patients. (a) PBMCs and BMMCs were isolated from AML patients and healthy donors (HDs) and stimulated for 5 h with phorbol 12-myristate13-acetate (PMA) and ionomycin in the presence of brefeldin A and then stained for CD3, CD8, intracellular interleukin (IL)-17A and interferon (IFN)-γ. Frequencies of Th17 cells and Th1 cells were determined by flow cytometry. Representative dot plots using matching peripheral blood (PB) and bone marrow (BM) samples from AML patients and HDs were shown. (b) Collective results presented for Th17 and Th1 cells within CD4+ T population. (c) Total RNA was isolated from CD4+ T cells obtained from AML patients and HDs and reverse transcribed into cDNA and subsequently real time polymerase chain reaction (PCR) for IL-17A and IFN-γ. Results were expressed as mean ± SEM.
Phenotypic characteristics of Th17 cells in AML
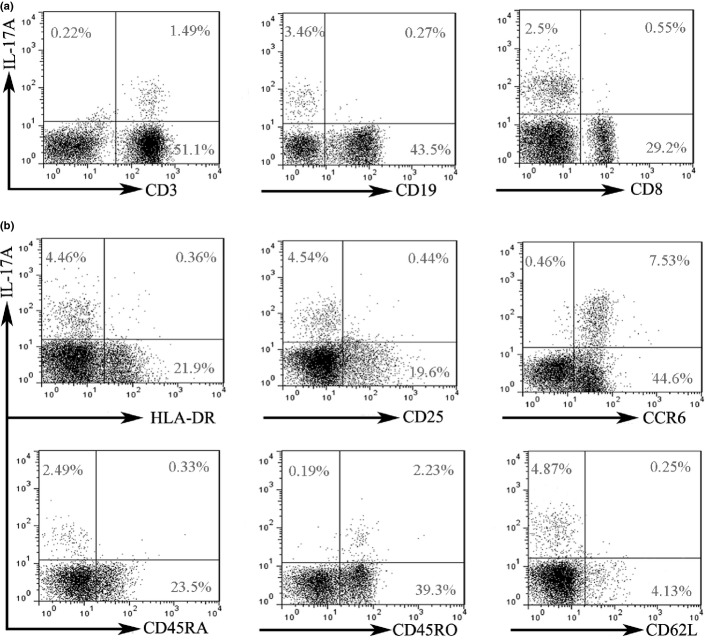
Higher Th17 cell frequencies in AML patients compared with those in healthy donors were shown, which provoked our interests to examine the phenotype of Th17 cells in BM, a tumor microenvironment. As shown in Figure 2(a), we found that IL-17A was mainly produced by T cells rather than B cells. The majority of tumor-infiltrating IL-17A+ T cells were IL-17A+CD4+ (Th17) cells but not IL-17A+CD8+ cells. Tumor-infiltrating Th17 cells express high levels of CCR6 and negligible levels of HLA-DR, CD25, and CD62L (Fig. 2b). CCR6 is a surface receptor of Th17 cells and Th17 cells can be migrated towards tumor in a CCR6/CCL20 dependent manner, which leads to an enrichment of Th17 cells in the tumor microenvironment.(24) We also observed that Tumor-infiltrating Th17 cells were mainly CD4+CD45RO+ memory T cells, but not CD4+CD45RA+ naive T cells.
Fig. 2.
Phenotype of tumor-infiltrating Th17 cells. After stimulation with phorbol 12-myristate13-acetate (PMA) and ionomycin for 5 h, freshly isolated bone marrow mononuclear cells (BMMCs) were subjected to membrane and intracellular staining and analyzed by flow cytometry. Representative data were shown from 21 untreated AML patients. (a) Interleukin (IL)-17A expression in T and B cells. IL-17A expression was analyzed in BMMCs. (b) The expression of HLA-DR, CD25, CCR6, CD45RA, CD45RO, and CD62L in tumor-infiltrating Th17 cells.
Generation and differentiation of Th17 cells in AML
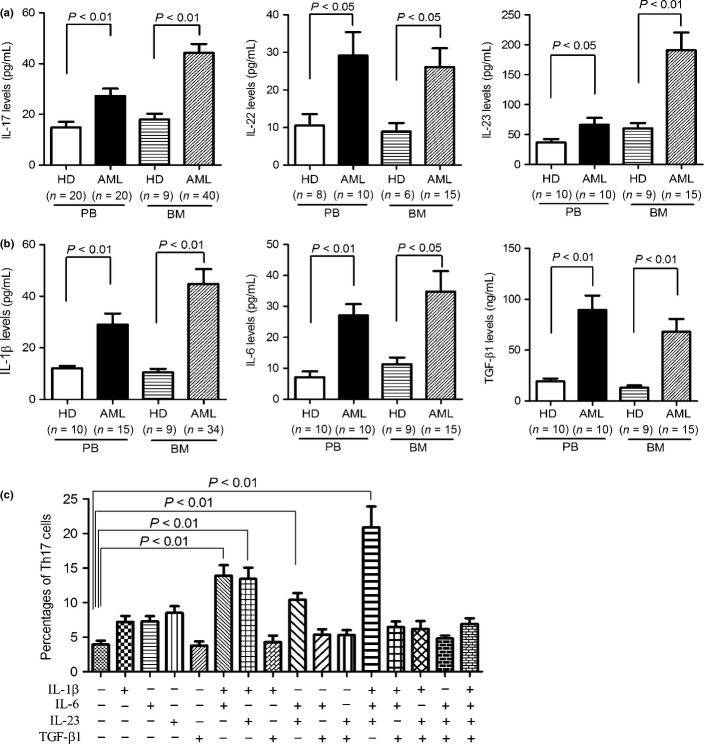
We evaluated the levels of Th17-producing cytokines to further confirm increased existence of Th17 cells in AML patients. Significant elevation of IL-17A, IL-22, and IL-23, three cytokines secreted by Th17 cells, were observed in both PB and BM from untreated AML patients compared with those from healthy donors as measured by ELISA (Fig. 3a). We next evaluated other cytokines that had been reported to associate with the generation and differentiation of human Th17 cells. As shown in Figure 3(b), higher levels of IL-1β, IL-6, and TGF-β1 were observed in PB and BM from AML patients compared with those from healthy donors. These results suggested that these proinflammatory cytokines present in AML BM microenvironment might modulate the generation and differentiation of Th17 cells.
Fig. 3.
Generation and differentiation of Th17 cells from peripheral blood (PB) and bone marrow (BM) regulated by interleukin (IL)-1β, IL-6, and IL-23. Elevated levels of Th17-producing cytokines (a) and Th17-associated proinflammatory cytokines (b) in PB and BM samples from acute myeloid leukemia (AML) patients were determined by ELISA. (c) Th17 cells detected in naive CD4+ T cells purified from AML patients after stimulation with indicated cytokines. The naive CD4+ T cells were isolated from PB of AML patients and subsequently stimulated by plate-bound anti-CD3 and soluble anti-CD28 mAbs with or without the indicated cytokines. After 7 days, the cells were restimulated with phorbol 12-myristate13-acetate (PMA) and ionomycin in the presence of brefeldin A and analyzed for intercellular IL-17A expression. The data were expressed as mean ± SEM representing four independent experiments from different AML patients.
To determine whether these cytokines contributed to the generation and differentiation of Th17 cells in AML patients, naive T cells were purified from PB samples of AML patients and cultured in the presence of one or more of IL-1β, IL-6, IL-23, and TGF-β1. None of the cytokines alone promoted the generation and differentiation of Th17 cells from naive T cells. Th17 cells were significantly increased in the presence of IL-1β plus IL-6, IL-1β plus IL-23, IL-6 plus IL-23, or IL-1β plus IL-6 plus IL-23 (Fig. 3c). Surprisingly, TGF-β1 reduced the increased percentage of Th17 cells induced by the above cytokines. These findings indicated that the tumor microenvironment of AML patients might promote the generation and differentiation of Th17 cells.
IL-17A exhibits pro-tumor effects on IL-17R+ AML cells
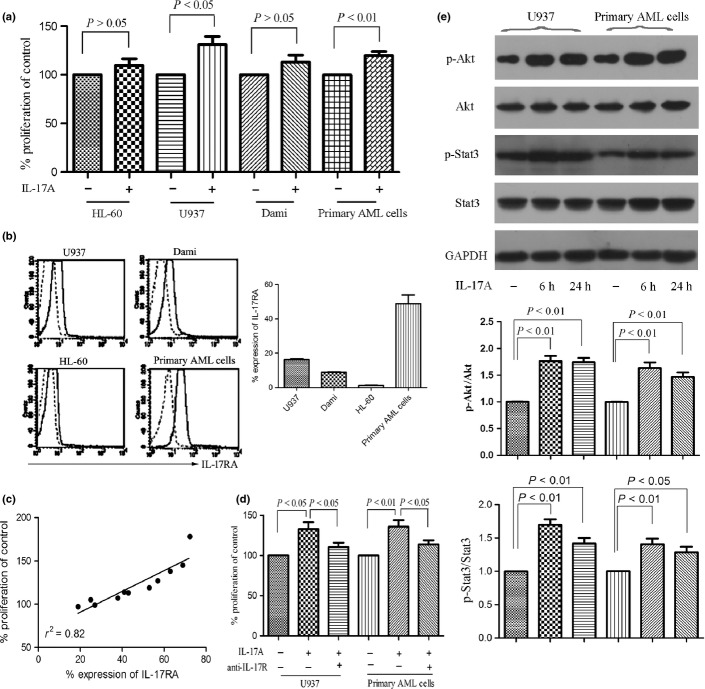
After showing elevated existence of Th17 cells in BM from AML patients, we next investigated whether IL-17A, a signature cytokine secreted by Th17 cells, has any effects on AML cells. First, we assessed the proliferation of AML cells in the presence or absence of IL-17A. As shown in Figure 4(a), 50 ng/mL IL-17A only exhibited an increase in the proliferation of U937 as well as primary AML cells, but not in HL-60 and Dami cells. After treatment with IL-17A for 7 days, S phase cells of U937 and primary AML cells, although increased, were not statistically significant compared with control (P > 0.05, Fig. S1). IL-17A had also no significant effect on the induction of apoptosis (Fig. S2). IL-17R has been found to be ubiquitously expressed in many tissues and cell types.(25) We further investigated whether the IL-17R expression associates with IL-17-induced proliferation. Abundant expression of IL-17RA was present in U937 cells as well as most primary AML cells, but only weak expression in HL-60 and Dami cells (Fig. 4b), which was further confirmed by measuring the relative expression of IL-17R mRNA (Fig. S3). A positive correlation between IL-17A-induced proliferation and the IL-17RA expression was observed in primary AML cells (Fig. 4c). Therefore, we determined the role of IL-17R in IL-17A-induced AML cell proliferation using anti-IL-17R antibody. As shown in Figure 4(d), anti-IL-17R antibody significantly inhibited AML cell proliferation in the presence of IL-17A.
Fig. 4.
Interleukin (IL)-17A promotes the proliferation of AML cells via IL-17 receptor (IL-17R). (a) Acute myeloid leukemia (AML) cell lines HL-60, U937, Dami, and primary AML cells isolated from AML patients (n = 23) were incubated with or without IL-17A (50 ng/mL) for 7 days and proliferation was assayed by MTT. Data are showed in proliferation in the presence of IL-17A compared with control and expressed as mean ± SEM representing at least three independent experiments. (b) The IL-17RA expression of AML cells was measured using anti-CD217 PE antibody (solid line) or mouse IgG1 PE antibody (dotted line) by flow cytometry. Representative histograms (left panel) and statistical data (right panel) were shown. (c) A correlation was observed between the IL-17RA expression and the IL-17A-inducing proliferation. (d) The effects of IL-17R on U937 and primary AML cell proliferation were determined by incubating the cells with IL-17A (50 ng/mL) in the presence or absence of anti-IL-17R antibody (3 μg/mL) for 7 days. (e) Western blotting showed the phosphorylation of Akt and Stat3 were significantly increased after IL-17A stimulation for 6 h and lasted for 24 h in IL-17R+ AML cells. Representatives and statistical data were shown for four independent experiments.
Interleukin-17A exhibited a stronger pro-proliferation effect on IL-17R+ AML cells in vitro, we further investigated the corresponding mechanisms. PI3K/Akt and Jak2/Stat3 signaling pathway activation have been found to regulate downstream components likely promoting survival and proliferation in many tumors.(26) Therefore, we detected the phosphorylation of Akt and Stat3, two key markers of these two signaling pathways. As shown in Figure 4(e), Akt and Stat3 phosphorylation are constitutively activated in U937 cells as well as primary AML cells, and IL-17A stimulation increases the phosphorylation of Akt and Stat3. The findings indicate that Th17 cells exhibit a pro-tumor effect on AML cells through secreting IL-17A.
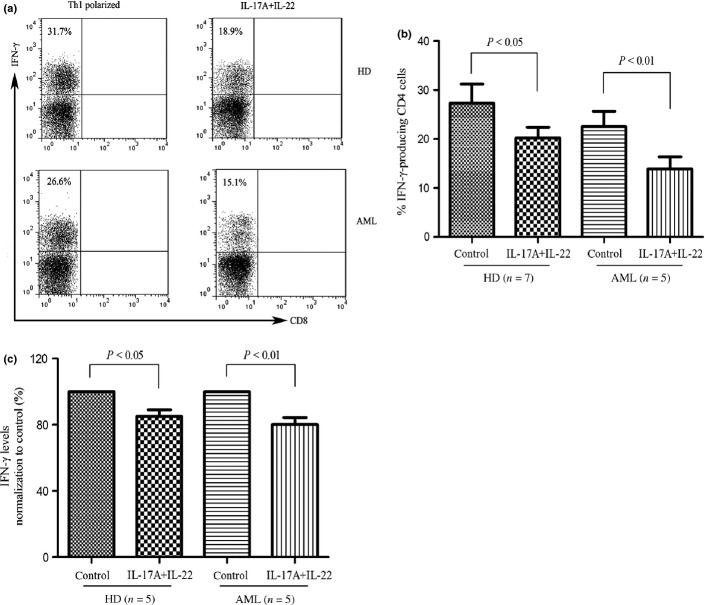
IL-17A and IL-22 downregulate Th1 cell responses in AML
Interleukin-17 has been reported to reduce the production of IFN-γ in PBMCs stimulated with IL-12.(27) We therefore determined whether IL-17A and IL-22, two cytokines secreted by Th17 cells,(28) affect the generation and differentiation of Th1 cells in AML. Interleukin-17A and IL-22 significantly inhibited the IL-12-induced IFN-γ-producing cells in PBMCs isolated from healthy donors or AML patients (Fig. 5a,b). Furthermore, the production of IFN-γ was reduced in the presence of combination of IL-17A and IL-22 (Fig. 5c). The findings suggest that Th17 cells-associated cytokines down-regulate Th1 cell responses in AML patients.
Fig. 5.
Interleukin (IL)-17A and IL-22 inhibited IL-12-inducing interferon (IFN)-γ-producing cells in peripheral blood mononuclear cells (PBMCs). (a) & (b) PBMCs from healthy donors or AML patients were activated with Th1 polarizing cytokines (IL-12, 10 ng/mL; anti-CD3 antibody, 1.5 μg/mL) with or without IL-17A (10 ng/mL) and IL-22 (10 ng/mL) for 12 days. Cells were subsequently stimulated with phorbol 12-myristate13-acetate (PMA) and ionomycin in the presence of brefeldin A, stained for intercellular IFN-γ, and analyzed by flow cytometry. A representative dot plot analysis showing percentage of IFN-γ-positive cells within gated CD3+CD4+ (CD3+CD8-) population. Results are expressed as mean ± SEM representing five independent experiments from different healthy donors or AML patients. (c) PBMCs from healthy donors or AML patients were stimulated with Th1 polarizing cytokines with or without IL-17A and IL-22 for 6 days, and supernatants were determined for IFN-γ by ELISA.
Increased Th17 cell frequency in combination with reduced Th1 cell frequency predicts poor patient survival
Interleukin-17A secreted by Th17 cells promotes the proliferation of AML cells and inhibits the differentiation of Th1 cells in vitro as well as elevated Th17 cell frequencies are present in BM, which suggest that Th17 cells may contribute to tumor immune escape in AML patients. We analyzed the impact of Th17 cell frequency and Th1 cell frequency in BM on patient survival. Regardless of the treatments each patient received, the patients were classified into two groups based on the median frequencies of Th17 (3.41%) or Th1 (26.16%) cells, respectively. The median overall survival in high Th17 group was significantly shorter than those in low Th17 group (P = 0.033, Fig. 6a), which suggested that increased Th17 cell frequency might be an unfavorable prognostic marker for AML patients. We also found that increased Th1 cell frequency is a favorable prognostic marker for AML patients by comparing the median survival in the high Th1 group and the low Th1 group (P = 0.025, Fig. 6b). Furthermore, patients were divided into four groups based on both the median frequencies of Th17 and Th1 cells. Irrespective of being in the low Th17 group or high Th17 group, patients with high frequencies of Th1 cells had a better survival than patients with low frequencies of Th1 cells (Fig. 6c). Thus the best combination associated with overall survival was low Th17 and high Th1 group, whereas the worst combination was the high Th17 and low Th1 group (P < 0.01). No difference was found in the high Th17 and high Th1 group and the low Th17 and low Th1 group. These data suggest that the combination of Th17 cell frequency and Th1 cell frequency is a potential marker to judge patient prognosis.
Fig. 6.
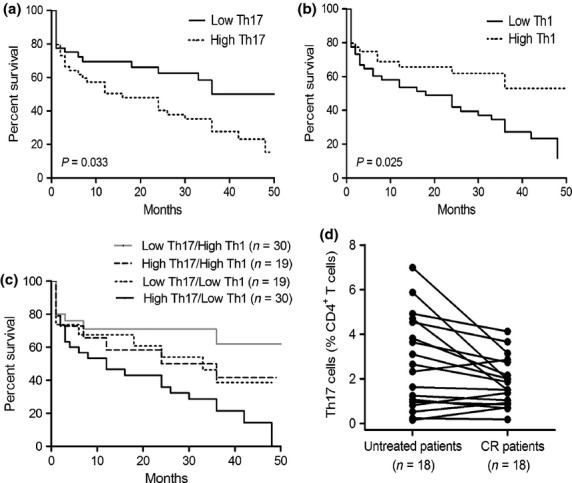
Increased frequencies of Th17 cells and reduced frequencies of Th1 cells predict poor survival in acute myeloid leukemia (AML) patients. Kaplan–Meier curves for overall survival were assessed in combination with frequencies of Th17 cells (a), frequencies of Th1 cells (b), or frequencies of Th1 together with Th17 cells (c), in bone marrow (BM) microenvironment of 98 AML patients. (d) The frequencies of Th17 cells decreased in BM when patients achieved complete remission (CR).
In addition, we also determined the frequencies of Th17 cells in BM from AML patients who achieved complete remission (CR) and found that the frequencies of Th17 cells obviously decreased in CR patients compared with previously untreated patients (P < 0.05) (Fig. 6d).
Discussion
Although Th17 cells have been intensively studied in the pathogenesis of autoimmune and inflammatory diseases, there are very few studies seeking to explore the role of Th17 cells in AML.(19)– (22) In the present study, we have shown that elevated Th17 cells and decreased Th1 cells present in AML patients. IL-17A, mainly secreted by Th17 cells, can promote the proliferation of IL-17R+ AML cells and inhibit the generation and differentiation of Th1 cells. To our knowledge, this study identified for the first time that monitoring Th17 cell frequency in combination with Th1 cell frequency may predict survival in AML patients.
Numerous immune abnormalities are present in PB and BM of AML patients.(29,30) Interactions between AML cells and the BM microenvironment result in the production of multiple cytokines and chemokines with immuno-modulatory activity that may preferentially skew Th subsets toward Th17 cells. Consistent with elevated frequencies of Th17 cells, we also found that Th17-associated cytokines were increased in PB and BM of AML patients compared with those of healthy donors. We have further demonstrated whether these cytokines lead to the development of Th17 cells. IL-1β, IL-6, and IL-23, but not TGF-β1, are crucial for Th17 cell induction in vitro. Although high level of TGF-β1 is detected in BM microenvironment of AML patients, TGF-β1 plays a negative effect on Th17 cell development, inconsistent with previous reports.(31,32) It is evident that TGF-β1 is responsible for the development of naive T cells into Th17 cells in mice.(33) However, the role of TGF-β1 in human Th17 cell development remains controversial.(31,32,34) In this study, Th17 cell frequencies and IL-17 are not quantitatively associated with TGF-β1. Therefore, TGF-β1 may not be crucial for Th17 cell development in the AML tumor microenvironment.
It has been reported that IL-17 can promote the in vitro proliferation of myeloma cells and BM-derived mesenchymal stem cells.(9,35) We therefore investigated whether IL-17A has any effects on AML cell proliferation. We found that IL-17A only promotes the proliferation of U937 cells and IL-17R+ primary AML cells, but not all AML cell lines as well as primary AML cells, consistent with a previous report.(20) A positive correlation was observed between the expression of IL-17RA and the proliferation of AML cells induced by IL-17, suggesting that IL-17 directly promotes the proliferation of AML cells through IL-17R. As predicted, blocking IL-17R by antibody inhibits IL-17-induced proliferation of IL-17R+ AML cells. This provides a conceptual basis for IL-17 and IL-17R blockade as a potential therapy in AML.
Interleukin-17 and IL-22, two cytokines secreted by Th17 cells, have been found to inhibit the differentiation and expansion of Th1 cells.(9) We also demonstrated here that IL-17A and IL-22 can significantly inhibit the production of Th1 cells and IFN-γ, a signature cytokine secreted by Th1 cells. IFN-γ secreted by Th1 cells plays a pivotal role in promoting innate and adaptive immune responses in the host defense against tumors.(36) Therefore, IL-17 may play a pro-tumor effect on IL-17R-negative AML cells via inhibiting the production of IFN-γ.
Activation of Stat3 is a pivotal pathway implicated in promoting tumorigenesis, which also incites tumor immune suppression by promoting accumulation of tumor-infiltrating myeloid-derived suppressor cells and regulatory T cells.(37) In our study, IL-17A (50 ng/mL) significantly increased the phosphorylation of Stat3 in U937 cells and IL-17R+ primary AML cells, which lasted for at least 24 h, further confirming a previous report that IL-17 (0.5 ng/mL) gradually but steadily increases the phosphorylation of Stat3 of U937 cells within 10 min.(38)
Although Th17 cell frequency has been reported to be associated with patient survival in many tumors, the prognostic predictive of Th17 cell frequency remains controversial.(11,17,39) In our study, Th17 and Th1 clusters may yield opposite effects on patient survival in AML. Patients with high Th17 cell frequencies had a poor prognosis, whereas patients with high Th1 cell frequencies had a prolonged survival. The combined analysis of Th1 and Th17 cell frequencies gave a better discrimination for survival. Plasma levels of IL-17A have been reported to be not associated with overall survival of AML patients.(40) These findings suggest that Th17 cell frequencies may be a better indicator to predict patient survival than levels of IL-17 in AML patients.
In summary, we observed significantly elevated frequencies of Th17 cells in AML along with elevated levels of IL-17 and other pro-inflammatory cytokines supporting the differentiation and expansion of Th17 cells as well as suppressing immune responses. These findings suggest Th17 cells and IL-17 as a potential therapeutic target in AML for both anti-AML responses as well as to improve immune function.
Acknowledgments
This work was supported by National Natural Science of Foundation of China (No. 81100355, 81172613, 81300430), and Zhejiang Provincial Natural Science Foundation of China (No. LQ12H08002).
Conflict of Interest Statement
The authors have no conflict of interest.
Supporting Information
Additional supporting information may be found in the online version of this article:
Fig S1. The effects of IL-17A on cell cycle of AML cells.
Fig. S2. IL-17A treatment does not induce apoptosis in AML cells.
Fig. S3. The relative expression of IL-17R mRNA of AML cells.
Table S1. The sequences of the primers used for real-time qPCR.
References
- 1.Le Dieu R, Taussig DC, Ramsay AG, et al. Peripheral blood T cells in acute myeloid leukemia (AML) patients at diagnosis have abnormal phenotype and genotype and form defective immune synapses with AML blasts. Blood. 2009;114:3909–16. doi: 10.1182/blood-2009-02-206946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ustun C, Miller JS, Munn DH, Weisdorf DJ, Blazar BR. Regulatory T cells in acute myelogenous leukemia: is it time for immunomodulation? Blood. 2011;118:5084–95. doi: 10.1182/blood-2011-07-365817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annu Rev Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 4.Ouyang W, Kolls JK, Zheng Y. The biological functions of T helper 17 cell effector cytokines in inflammation. Immunity. 2008;28:454–67. doi: 10.1016/j.immuni.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huber M, Heink S, Pagenstecher A, et al. IL-17A secretion by CD8+ T cells supports Th17-mediated autoimmune encephalomyelitis. J Clin Invest. 2013;123:247–60. doi: 10.1172/JCI63681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Couturier M, Lamarthee B, Arbez J, et al. IL-22 deficiency in donor T cells attenuates murine acute graft-versus-host disease mortality while sparing the graft-versus-leukemia effect. Leukemia. 2013;27:1527–37. doi: 10.1038/leu.2013.39. [DOI] [PubMed] [Google Scholar]
- 7.Wilke CM, Kryczek I, Wei S, et al. Th17 cells in cancer: help or hindrance? Carcinogenesis. 2011;32:643–9. doi: 10.1093/carcin/bgr019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Muranski P, Restifo NP. Does IL-17 promote tumor growth? Blood. 2009;114:231–2. doi: 10.1182/blood-2009-04-215541. [DOI] [PubMed] [Google Scholar]
- 9.Prabhala RH, Pelluru D, Fulciniti M, et al. Elevated IL-17 produced by TH17 cells promotes myeloma cell growth and inhibits immune function in multiple myeloma. Blood. 2010;115:5385–92. doi: 10.1182/blood-2009-10-246660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Numasaki M, Fukushi J, Ono M, et al. Interleukin-17 promotes angiogenesis and tumor growth. Blood. 2003;101:2620–7. doi: 10.1182/blood-2002-05-1461. [DOI] [PubMed] [Google Scholar]
- 11.Tosolini M, Kirilovsky A, Mlecnik B, et al. Clinical impact of different classes of infiltrating T cytotoxic and helper cells (Th1, th2, treg, th17) in patients with colorectal cancer. Cancer Res. 2011;71:1263–71. doi: 10.1158/0008-5472.CAN-10-2907. [DOI] [PubMed] [Google Scholar]
- 12.Amedei A, Niccolai E, Benagiano M, et al. Ex vivo analysis of pancreatic cancer-infiltrating T lymphocytes reveals that ENO-specific Tregs accumulate in tumor tissue and inhibit Th1/Th17 effector cell functions. Cancer Immunol Immunother. 2013;62:1249–60. doi: 10.1007/s00262-013-1429-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ye ZJ, Zhou Q, Gu YY, et al. Generation and differentiation of IL-17-producing CD4+ T cells in malignant pleural effusion. J Immunol. 2010;185:6348–54. doi: 10.4049/jimmunol.1001728. [DOI] [PubMed] [Google Scholar]
- 14.Jain P, Javdan M, Feger FK, et al. Th17 and non-Th17 interleukin-17-expressing cells in chronic lymphocytic leukemia: delineation, distribution, and clinical relevance. Haematologica. 2012;97:599–607. doi: 10.3324/haematol.2011.047316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weaver CT, Hatton RD, Mangan PR, Harrington LE. IL-17 family cytokines and the expanding diversity of effector T cell lineages. Annu Rev Immunol. 2007;25:821–52. doi: 10.1146/annurev.immunol.25.022106.141557. [DOI] [PubMed] [Google Scholar]
- 16.Lin H, Tong ZH, Xu QQ, et al. Interplay of Th1 and Th17 Cells in Murine Models of Malignant Pleural Effusion. Am J Respir Crit Care Med. 2014;189:697–706. doi: 10.1164/rccm.201310-1776OC. [DOI] [PubMed] [Google Scholar]
- 17.Kryczek I, Banerjee M, Cheng P, et al. Phenotype, distribution, generation, and functional and clinical relevance of Th17 cells in the human tumor environments. Blood. 2009;114:1141–9. doi: 10.1182/blood-2009-03-208249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Beelen AJ, Zelinkova Z, Taanman-Kueter EW, et al. Stimulation of the intracellular bacterial sensor NOD2 programs dendritic cells to promote interleukin-17 production in human memory T cells. Immunity. 2007;27:660–9. doi: 10.1016/j.immuni.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 19.Wu C, Wang S, Wang F, et al. Increased frequencies of T helper type 17 cells in the peripheral blood of patients with acute myeloid leukaemia. Clin Exp Immunol. 2009;158:199–204. doi: 10.1111/j.1365-2249.2009.04011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ersvaer E, Liseth K, Skavland J, Gjertsen BT, Bruserud O. Intensive chemotherapy for acute myeloid leukemia differentially affects circulating TC1, TH1, TH17 and TREG cells. BMC Immunol. 2010;11:38. doi: 10.1186/1471-2172-11-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tian T, Yu S, Wang M, et al. Aberrant T helper 17 cells and related cytokines in bone marrow microenvironment of patients with acute myeloid leukemia. Clin Dev Immunol. 2013;2013:915873. doi: 10.1155/2013/915873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abousamra NK. Salah El-Din M, Helal R. Prognostic value of Th17 cells in acute leukemia. Med Oncol. 2013;30:732. doi: 10.1007/s12032-013-0732-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang S, Zhang Y, Zhuang Y, et al. Matrine induces apoptosis in human acute myeloid leukemia cells via the mitochondrial pathway and Akt inactivation. PLoS ONE. 2012;7:e46853. doi: 10.1371/journal.pone.0046853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kesselring R, Thiel A, Pries R, Trenkle T, Wollenberg B. Human Th17 cells can be induced through head and neck cancer and have a functional impact on HNSCC development. Br J Cancer. 2010;103:1245–54. doi: 10.1038/sj.bjc.6605891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yao Z, Fanslow WC, Seldin MF, et al. Herpesvirus Saimiri encodes a new cytokine, IL-17, which binds to a novel cytokine receptor. Immunity. 1995;3:811–21. doi: 10.1016/1074-7613(95)90070-5. [DOI] [PubMed] [Google Scholar]
- 26.Konopleva MY, Jordan CT. Leukemia stem cells and microenvironment: biology and therapeutic targeting. J Clin Oncol. 2011;29:591–9. doi: 10.1200/JCO.2010.31.0904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Toh ML, Kawashima M, Hot A, Miossec P. Role of IL-17 in the Th1 systemic defects in rheumatoid arthritis through selective IL-12Rbeta2 inhibition. Ann Rheum Dis. 2010;69:1562–7. doi: 10.1136/ard.2009.111757. [DOI] [PubMed] [Google Scholar]
- 28.Zheng Y, Danilenko DM, Valdez P, et al. Interleukin-22, a T(H)17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature. 2007;445:648–51. doi: 10.1038/nature05505. [DOI] [PubMed] [Google Scholar]
- 29.Shenghui Z, Yixiang H, Jianbo W, et al. Elevated frequencies of CD4(+) CD25(+) CD127lo regulatory T cells is associated to poor prognosis in patients with acute myeloid leukemia. Int J Cancer. 2011;129:1373–81. doi: 10.1002/ijc.25791. [DOI] [PubMed] [Google Scholar]
- 30.Mussai F, De Santo C, Abu-Dayyeh I, et al. Acute myeloid leukemia creates an arginase-dependent immunosuppressive microenvironment. Blood. 2013;122:749–58. doi: 10.1182/blood-2013-01-480129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang L, Anderson DE, Baecher-Allan C, et al. IL-21 and TGF-beta are required for differentiation of human T(H)17 cells. Nature. 2008;454:350–2. doi: 10.1038/nature07021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Manel N, Unutmaz D, Littman DR. The differentiation of human T(H)-17 cells requires transforming growth factor-beta and induction of the nuclear receptor RORgammat. Nat Immunol. 2008;9:641–9. doi: 10.1038/ni.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bettelli E, Carrier Y, Gao W, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–8. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 34.Acosta-Rodriguez EV, Napolitani G, Lanzavecchia A, Sallusto F. Interleukins 1beta and 6 but not transforming growth factor-beta are essential for the differentiation of interleukin 17-producing human T helper cells. Nat Immunol. 2007;8:942–9. doi: 10.1038/ni1496. [DOI] [PubMed] [Google Scholar]
- 35.Huang H, Kim HJ, Chang EJ, et al. IL-17 stimulates the proliferation and differentiation of human mesenchymal stem cells: implications for bone remodeling. Cell Death Differ. 2009;16:1332–43. doi: 10.1038/cdd.2009.74. [DOI] [PubMed] [Google Scholar]
- 36.Dunn GP, Koebel CM, Schreiber RD. Interferons, immunity and cancer immunoediting. Nat Rev Immunol. 2006;6:836–48. doi: 10.1038/nri1961. [DOI] [PubMed] [Google Scholar]
- 37.Sansone P, Bromberg J. Targeting the interleukin-6/Jak/stat pathway in human malignancies. J Clin Oncol. 2012;30:1005–14. doi: 10.1200/JCO.2010.31.8907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Subramaniam SV, Cooper RS, Adunyah SE. Evidence for the involvement of JAK/STAT pathway in the signaling mechanism of interleukin-17. Biochem Biophys Res Commun. 1999;262:14–9. doi: 10.1006/bbrc.1999.1156. [DOI] [PubMed] [Google Scholar]
- 39.Zhang JP, Yan J, Xu J, et al. Increased intratumoral IL-17-producing cells correlate with poor survival in hepatocellular carcinoma patients. J Hepatol. 2009;50:980–9. doi: 10.1016/j.jhep.2008.12.033. [DOI] [PubMed] [Google Scholar]
- 40.Sanchez-Correa B, Bergua JM, Campos C, et al. Cytokine profiles in acute myeloid leukemia patients at diagnosis: survival is inversely correlated with IL-6 and directly correlated with IL-10 levels. Cytokine. 2013;61:885–91. doi: 10.1016/j.cyto.2012.12.023. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1. The effects of IL-17A on cell cycle of AML cells.
Fig. S2. IL-17A treatment does not induce apoptosis in AML cells.
Fig. S3. The relative expression of IL-17R mRNA of AML cells.
Table S1. The sequences of the primers used for real-time qPCR.