Abstract
Expression of CD56 has recently been introduced as one of the adverse prognostic factors in acute promyelocytic leukemia (APL). However, the clinical significance of CD56 antigen in APL has not been well elucidated. We assessed the clinical significance of CD56 antigen in 239 APL patients prospectively treated with all-trans retinoic acid and chemotherapy according to the Japan Adult Leukemia Study Group APL97 protocol. All patients were prospectively treated by the Japan Adult Leukemia Study Group APL97 protocol. The median follow-up period was 8.5 years. Positive CD56 expression was found in 23 APL patients (9.6%). Expression of CD56 was significantly associated with lower platelet count (P = 0.04), severe disseminated intravascular coagulation (P = 0.04), and coexpression of CD2 (P = 0.03), CD7 (P = 0.04), CD34 (P < 0.01) and/or human leukocyte antigen-DR (P < 0.01). Complete remission rate and overall survival were not different between the two groups. However, cumulative incidence of relapse and event-free survival (EFS) showed an inferior trend in CD56+ APL (P = 0.08 and P = 0.08, respectively). Among patients with initial white blood cell counts of 3.0 × 109/L or more, EFS and cumulative incidence of relapse in CD56+ APL were significantly worse (30.8% vs 63.6%, P = 0.008, and 53.8% vs 28.9%, P = 0.03, respectively), and in multivariate analysis, CD56 expression was an unfavorable prognostic factor for EFS (P = 0.04). In conclusion, for APL with higher initial white blood cell counts, CD56 expression should be regarded as an unfavorable prognostic factor.
Keywords: Acute promyelocytic leukemia, all-trans retinoic acid, CD56 expression, chemotherapy, prognostic factor
The clinical introduction of ATRA has dramatically improved the outcome of APL.1–6 However, 13–33% of patients with APL still relapse after the first remission.6 Therefore, various prognostic factors predicting outcome are being continuously analyzed, and initial high WBC count, low platelet count, and older age have been recognized as significant factors.3,5–8 Recently, several investigators have suggested that the expression of CD56 antigen, a neural adhesion factor, is associated with higher incidence of relapse and poorer outcome in APL.9–12 However, the number of reported cases and follow-up periods are still limited, and there has been no recommendation so far to modify standard treatment of APL on the basis of CD56 expression.13,14 We analyzed the long-term outcome of 239 APL patients who were prospectively treated with ATRA combined with chemotherapies, including anthracycline and Ara-C, in the JALSG APL97 study, and assessed the clinical significance of CD56 expression in APL.
Materials and Methods
Patients
Adult patients with previously untreated APL were consecutively registered to the JALSG APL97 study between May 1997 and June 2002.15 Eligibility criteria were: (i) diagnosis of APL with t(15,17) and/or the PML-RARA fusion gene amplified by RT-PCR; (ii) age between 15 and 70 years; (iii) ECOG PS 0 to 3; and (iv) sufficient functioning of the heart, lung, liver, and kidney. This study was approved by the institutional review boards of each participating institution, and registered with the UMIN Clinical Trials Registry (http://www.umin.ac.jp/ctrj/) under trial number C000000206. Informed consent was obtained from each patient before registration to the study in accordance with the Declaration of Helsinki.
Study design and treatments
The detail of treatment schedule was as described previously.15 Remission induction therapy consisted of ATRA and chemotherapy with idarubicin and Ara-C, with dose and duration determined by initial WBC counts. After obtaining CR and receiving three courses of intensive consolidation chemotherapy including anthracyclines, Ara-C, and etoposide, patients negative for the PML-RARA fusion transcript were randomly allocated either to receive six courses of intensified maintenance chemotherapy or to observation. Patients who were positive for the PML-RARA fusion transcript received late ATRA therapy followed by maintenance therapy, and were scheduled to receive allogeneic hematopoietic stem cell transplantation, if they had a human leukocyte antigen-identical donor. Risk stratification according to initial WBC counts (<3.0 × 109/L; 3.0 × 109/L to less than 10.0 × 109/L; ≥10.0 × 109/L) used in the current JALSG APL study are based on the results of the JALSG APL92 study.3 In consideration of this background and the number of cases in each group, we adopted the value and divided the patients into two groups (i.e., initial WBC counts <3.0 × 109 and ≥3.0 × 109) to analyze the prognostic impact of CD56 expression.
Immunophenotypic analysis
Immunophenotypic analysis was carried out using bone marrow samples taken at diagnosis and analyzed in the reference laboratory by standard immunofluorescence methods. Cells were stained with anti-CD45 (mAb), gated by CD45 expression and analyzed by flow cytometer. Cells were additionally stained with fluorescein-conjugated mAb against CD2, CD5, CD7, CD4, CD8, CD19, CD20, CD11b, CD13, CD14, CD15, CD33, CD34, CD56, and HLA-DR surface antigens. According to the criteria defined by the European Group for the Immunological Characterization of Leukemias,16 surface markers were defined as positive if more than 20% of APL cells expressed a specific antigen.
Definition and evaluation of patients
Hematological response was evaluated by standard criteria.17 Molecular relapse detected by RT-PCR analysis of PML-RARA was also considered as a relapse. Overall survival was calculated from the first day of therapy to death or last visit. Event-free survival was determined from the first day of therapy to relapse, death from any cause, or last visit. Cumulative incidence of relapse (extramedullary relapse) was measured from the date of CR to the first relapse, whereas non-relapse mortality was censored as a competing risk event.
Statistical analysis
Categorical data were compared using the χ2-test or Fisher's exact test. Continuous data were compared using Wilcoxon's rank-sum test. The OS and EFS were estimated by Kaplan–Meier methods and compared by the log–rank test. The CIR was analyzed according to Kalbfleisch and Prentice, and differences were compared using Gray statistics. Cox's proportional hazards model was used for multivariate analysis of EFS. Factors significant at the 0.2 level in the univariate analysis were included in the multivariate analysis model. Statistical analyses were carried out using spss version 11.0 (SPSS Inc., Chicago, IL, USA) and R 2.12.1 (R Foundation for Statistical Computing, Vienna, Austria; available at http://www.r-project.org/). All hypothesis testing was two-tailed with a significance level of 0.05.
Results
Patient characteristics
Among 283 evaluable patients of 302 registered to the JALSG APL97 study,15 239 (85%) (median age, 48 years; range, 15–70 years) had satisfactory data for CD56 surface antigen expression, and were evaluated in this study. The median follow-up period was 8.5 years (0–12.2 years).
Of 239 patients, 23 (9.6%) were positive for CD56. The clinical and biological characteristics according to CD56 expression are shown in Tables1 and 2. Expression of CD56 was significantly associated with lower platelet count (<10 × 109/L) and severe DIC (P = 0.04 and P = 0.04, respectively); CD56+ APL significantly coexpressed CD2, CD7, CD34, and/or HLA-DR antigen. (P = 0.03, P = 0.04, P < 0.001, and P < 0.001, respectively).
Table 1.
Clinical features of acute promyelocytic leukemia (APL) patients according to CD56 expression (n = 239)
| Clinical features | CD56-positive |
CD56-negtive |
P-value | ||
|---|---|---|---|---|---|
| No. of patients (%) | Median (range) | No. of patients (%) | Median (range) | ||
| All patients | |||||
| No. of patients | 23 | 216 | |||
| Age, years | 48 (16–66) | 47 (15–70) | 0.84 | ||
| 15–59 | 20 (87) | 181 (84) | 0.69 | ||
| 60–65 | 3 (13) | 35 (16) | |||
| Sex | |||||
| Male | 9 (39) | 127 (59) | 0.07 | ||
| Female | 14 (61) | 89 (41) | |||
| Initial WBC counts, ×109/L | 2.1 (0.04–98) | 1.7 (0.01–257) | 0.47 | ||
| <3.0 | 12 (52) | 129 (60) | 0.78 | ||
| 3.0 to <10.0 | 6 (26) | 46 (21) | |||
| ≥10.0 | 5 (22) | 41 (19) | |||
| Initial APL cell counts, ×109/L | 1.8 (0–92) | 0.6 (0–253) | 0.53 | ||
| Initial platelet counts, ×109/L | 15 (6–120) | 30 (2–238) | 0.04 | ||
| <10 | 5 (22) | 28 (13) | 0.30 | ||
| 10 to <40 | 13 (56) | 111 (51) | |||
| ≥40 | 5 (22) | 77 (36) | |||
| ECOG performance status score | |||||
| 0–2 | 19 (83) | 202 (94) | 0.05 | ||
| 3 | 4 (17) | 13 (6) | |||
| Albumin level, g/dL | 4.2 (3.3–6.1) | 4.2 (2.3–6.0) | 0.51 | ||
| <3.5 | 2 (9) | 18 (9) | 0.96 | ||
| ≥3.5 | 20 (91) | 188 (91) | |||
| Fibrinogen level, mg/dL | 105 (55–389) | 139 (20–513) | 0.46 | ||
| FDP ratio† | 16.1 (4.0–322.4) | 11.6 (0.3–524) | 0.09 | ||
| DIC score‡ | |||||
| 0–2 | 0 (0) | 18 (9) | 0.04 | ||
| 3–9 | 17 (77) | 166 (82) | |||
| ≥10 | 5 (23) | 18 (9) | |||
| FAB subtype | |||||
| Typical | 23 (100) | 201 (93) | 0.32 | ||
| Variant | 0 (0) | 15 (7) | |||
| ACAs | 8 (42) | 64 (35) | 0.56 | ||
| Patients with intial WBC counts ≥3.0 × 109/L | |||||
| No. of patients | 11 | 87 | |||
| Age, years | 41 (21–66) | 45 (19–58) | 0.87 | ||
| 15–59 | 10 (91) | 73 (84) | 0.54 | ||
| 60–65 | 1 (9) | 14 (16) | |||
| Sex | |||||
| Male | 7 (64) | 52 (60) | 0.81 | ||
| Female | 4 (36) | 35 (40) | |||
| Initial WBC counts, ×109/L | 6.3 (3.2–98) | 8.9 (3.0–257) | 0.62 | ||
| ≥10.0 | 5 (45) | 41 (47) | 0.92 | ||
| Initial APL cell counts, ×109/L | 4.8 (0–92) | 7.0 (0.2–253) | 0.37 | ||
| Initial platelet counts, ×109/L | 14 (6–54) | 23 (2–92) | 0.38 | ||
| <10 | 3 (30) | 16 (18) | 0.78 | ||
| 10 to <40 | 5 (40) | 46 (53) | |||
| ≥40 | 3 (30) | 25 (29) | |||
| ECOG performance status score | |||||
| 0–2 | 0 (0) | 77 (90) | 0.45 | ||
| 3 | 11 (100) | 9 (10) | |||
| Albumin level, g/dL | 4.3 (3.5–4.7) | 4.2 (2.6–5.8) | 0.86 | ||
| <3.5 | 2 (9) | 8 (10) | 0.29 | ||
| ≥3.5 | 20 (91) | 76 (90) | |||
| Fibrinogen level, mg/dL | 104 (56–389) | 104 (21–438) | 0.84 | ||
| FDP ratio† | 26.7 (4.4–280) | 14.1 (0.3–303) | 0.24 | ||
| DIC score‡ | |||||
| 0–2 | 0 (0) | 3 (4) | 0.02 | ||
| 3–9 | 7 (64) | 75 (88) | |||
| ≥10 | 4 (36) | 7 (8) | |||
| FAB subtype | |||||
| Typical | 11 (100) | 74 (85) | 0.17 | ||
| Variant | 0 (0) | 13 (15) | |||
| ACAs | 2 (25) | 22 (30) | 0.76 | ||
Fibrinogen degradation product (FDP) ratio calculated by dividing the FDP value by its upper normal limit.
Disseminated intravascular coagulation (DIC) score:(18) 0–2 indicates improbable DIC; score 3, suspected DIC; score 4–9, definitive DIC; ≥10, severe DIC. ACAs, additional chromosomal abnormalities; APL, Acute promyelocytic leukemia; ECOG, Eastern Cooperative Oncology Group; FAB, French–American–British; FDP, fibrin degradation product; WBC, white blood cell.
Table 2.
Immunophenotypic features of acute promyelocytic leukemia patients (n = 239) according to CD56 expression
| Parameters | CD56-positive | CD56-negative | P-value |
|---|---|---|---|
| No. of patients (%) | No. of patients (%) | ||
| CD2 | |||
| Positive | 5 (22) | 16 (8) | 0.03 |
| Negative | 18 (78) | 191 (92) | |
| CD5 | |||
| Positive | 1 (5) | 3 (2) | 0.25 |
| Negative | 18 (95) | 195 (98) | |
| CD7 | |||
| Positive | 2 (9) | 4 (2) | 0.04 |
| Negative | 20 (91) | 208 (98) | |
| CD19 | |||
| Positive | 1 (4) | 5 (2) | 0.56 |
| Negative | 22 (96) | 210 (98) | |
| CD20 | |||
| Positive | 0 (0) | 1 (0.5) | 0.75 |
| Negative | 19 (100) | 191 (99.5) | |
| CD11b | |||
| Positive | 3 (19) | 11 (7) | 0.08 |
| Negative | 13 (81) | 157 (93) | |
| CD15 | |||
| Positive | 7 (54) | 50 (33) | 0.12 |
| Negative | 6 (46) | 103 (67) | |
| CD41a | |||
| Positive | 1 (5) | 19 (10) | 0.46 |
| Negative | 20 (95) | 177 (90) | |
| CD34 | |||
| Positive | 9 (41) | 27 (13) | P < 0.01 |
| Negative | 13 (59) | 185 (87) | |
| HLA-DR | |||
| Positive | 7 (30) | 16 (8) | P < 0.01 |
| Negative | 16 (70) | 197 (92) | |
HLA, human leukocyte antigen.
Treatment outcome
The CR rate and incidence of early death during induction therapy were not different between CD56+ and CD56− APL (91% vs 95%, P = 0.4, and 9% vs 5%, P = 0.54, respectively; Table3). Primary resistance to induction therapy was not observed in either group. The incidence of differentiation syndrome was not different between the two groups (22% vs 21%, P = 0.9; Table3).
Table 3.
Clinical outcomes of acute promyelocytic leukemia patients according to CD56 expression (n = 239)
| Clinical features | CD56-positive |
CD56-negative |
P-value | ||
|---|---|---|---|---|---|
| No. of patients (%) | No. of patients (%) | ||||
| No. of patients | 23 | 216 | |||
| Induction outcome | |||||
| CR rate | 21 (91) | 206 (95) | 0.40 | ||
| Differentiation syndrome | 5 (22) | 44 (21) | 0.90 | ||
| Induction death | 2 (9) | 10 (5) | 0.54 | ||
| Hemorrhage | 2 (100) | 6 (60) | 0.13 | ||
| Infection | 0 (0) | 1 (10) | 0.74 | ||
| Differentiation syndrome | 0 (0) | 2 (20) | 0.64 | ||
| Others | 0 (0) | 1 (10) | 0.74 | ||
| Postremission outcome | |||||
| No. of patients | 21 | 206 | |||
| Relapse | |||||
| All patients | 9 (43) | 49 (24) | 0.06 | ||
| Intial WBC counts <3.0 | 3 (14) | 27 (13) | 0.88 | ||
| Initial WBC counts ≥3.0 | 6 (29) | 22 (11) | 0.02 | ||
| Extramedullary relapse | |||||
| All patients | 1 (5) | 3 (1.5) | 0.27 | ||
| Intial WBC counts <3.0 | 0 (0) | 2 (1.0) | 0.65 | ||
| Initial WBC counts ≥3.0 | 1 (5) | 1 (0.5) | 0.05 | ||
| CIR (%) | |||||
| All patients | 39.1 | 24.3 | 0.08 | ||
| Intial WBC counts <3.0 | 20.0 | 20.1 | 0.98 | ||
| Initial WBC counts ≥3.0 | 53.8 | 28.9 | 0.03 | ||
| CIR (extramedullary relapse) (%) | |||||
| All patients | 5.0 | 1.5 | 0.27 | ||
| Intial WBC counts <3.0 | 0.0 | 1.8 | 0.69 | ||
| Initial WBC counts ≥3.0 | 9.3 | 1.1 | 0.07 | ||
CIR, cumulative incidence of relapse; CR, complete remission; WBC, white blood cell.
Overall survival was not different between the two groups (73.9% vs 79.2%, P = 0.52, at 9 years; Fig.1a), whereas EFS and CIR tended to be inferior in CD56+ APL (47.8% vs 64.8%, P = 0.08, and 39.1% vs 24.3%, P = 0.08, at 9 years, respectively; Figs2a,3a). In patients with initial WBC counts ≥3.0 × 109/L, EFS and CIR for 11 CD56+APL patients were significantly inferior to those for 87 CD56− APL patients (30.8% vs 63.6%, P = 0.008, and 53.8% vs 28.9%, P = 0.03, at 9 years, respectively; Figs2b,3b). In patients with initial WBC counts <3.0 × 109/L, EFS and CIR were not different between the two groups (P = 0.99 and P = 0.98, at 9 years, respectively). The OS in patients with initial WBC counts ≥3.0 × 109/L was similar between the two groups (61.5% vs 78.8%, P = 0.13, at 9 years; Fig.1b). Although the number was small, EFS and CIR for five CD56+ APL patients among those with initial WBC counts of ≥10 × 109/L were inferior to those for 41 CD56− APL patients (20.0% vs 60.9%, P = 0.03, and 60.0% vs 30.7%, P = 0.09, at 9 years, respectively). Cumulative incidence of extramedullary relapse tended to be more frequent in patients with CD56+ APL whose initial WBC counts were ≥3.0 × 109/L (9.3% vs 1.1%, at 9 years, P = 0.07). We also analyzed the influence of CD56 expression on clinical outcomes according to Sanz's relapse risk score.7 Both CIR and EFS in patients with CD56+ APL were inferior in the high risk group (60.0% vs 31.4%, P = 0.09 and 20.0% vs 62.5%, P = 0.02, respectively), but not in low and intermediate risk groups (P = 0.17 and P = 0.55, respectively).
Figure 1.
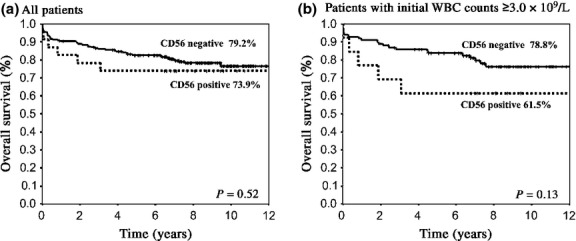
Overall survival (OS) of patients with acute promylocytic leukemia according to CD56 expression. (a) OS was not different between the two groups for all patients (73.9% vs 79.2% at 9 years, P = 0.52). (b) In patients whose white blood cell (WBC) count was ≥3.0 × 109/L, OS did not differ between the two groups (61.5% vs 78.8%, P = 0.13).
Figure 2.
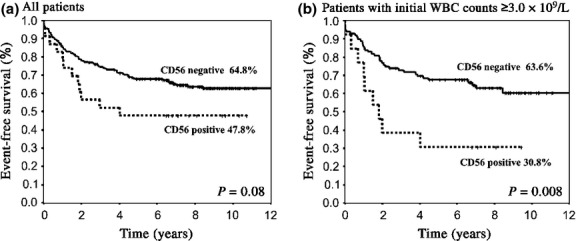
Event-free survival (EFS) of patients with acute promylocytic leukemia (APL) according to CD56 expression. (a) EFS for all patients showed an inferior trend in CD56+ APL (47.8% vs 64.8% at 9 years, P = 0.08). (b) In patients whose white blood cell (WBC) count was ≥3.0 × 109/L, EFS for CD56+ APL was significantly inferior to that for CD56− APL (30.8% vs 63.8%, P = 0.008).
Figure 3.
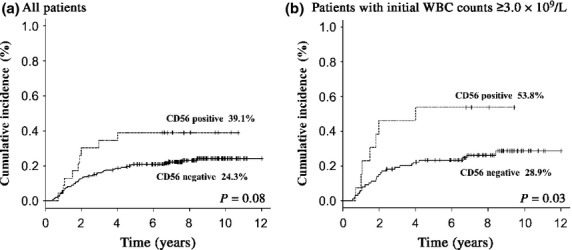
Cumulative incidence of relapse (CIR) of patients with acute promylocytic leukemia (APL) according to CD56 expression. (a) CIR for all patients showed an inferior trend in the CD56+ APL group (39.1% vs 24.3% at 9 years, P = 0.08). (b) In patients whose white blood cell (WBC) count was ≥3.0 × 109/L, CIR for the CD56+ group was significantly higher compared to that for the CD56− APL group (53.8% vs 28.9%, P = 0.03).
In the multivariate analysis, CD56 expression was an independent adverse prognostic factor for EFS in patients whose initial WBC counts were ≥3.0 × 109/L (hazard ratio = 2.54; 95% confidence interval, 1.07–6.06, P = 0.04) (Table4).
Table 4.
Prognostic factors affecting event-free survival of acute promyelocytic leukemia patients (initial white blood cell counts ≥3.0 × 109/L) (n = 239)
| Factors for event-free survival | Univariate analysis |
Multivariate analysis |
||
|---|---|---|---|---|
| P-value | Hazard ratio | 95% CI | P-value | |
| DIC score† >10 (vs DIC score ≤10) | 0.17 | 1.06 | 0.90–1.24 | 0.48 |
| Age >60 years (vs age ≤60 years) | 0.04 | 2.00 | 0.86–4.65 | 0.11 |
| HLA-DR antigen positive (vs negative) | 0.02 | 1.46 | 0.49–4.33 | 0.49 |
| CD56 antigen positive (vs negative) | 0.008 | 2.54 | 1.07–6.06 | 0.04 |
Disseminated intravascular coagulation (DIC) score:(18) 0–2 indicates improbable DIC; score 3, suspected DIC; score 4–9, definitive DIC; ≥10, severe DIC. Factors with P-value <0.20 in univariate analysis were included in the multivariate analysis. CI, confidence interval; HLA, human leukocyte antigen; HR, hazard ratio.
Discussion
Expression of CD56 has been reported as one of the adverse prognostic factors in AML with t(8;21), associated with a short remission duration and survival as well as higher incidence of extramedullary relapse.19,20 Recently, several investigators have suggested that CD56 expression is also associated with short remission duration in APL, higher CIR, and extramedullary relapse (Table5).9–12 However, large-scale studies with long-term follow-up are limited,12 and the prognostic significance of CD56 expression has not been fully elucidated.
Table 5.
Clinical features and outcomes in acute promyelocytic leukemia (APL) patients with CD56 expression, as reported in published works
| Authors | No. of patients | Treatment | CD56+ APL (%) | Clinical features in patients with CD56+ APL* | CR rate |
CIR |
CIR (extramedullary) |
DFS† |
OS |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CD56+ | CD56− | CD56+ | CD56− | CD56+ | CD56− | CD56+ | CD56− | CD56+ | CD56− | |||||
| Murray et al.9 | 50 | CT alone / ATRA alone / ATRA + CT | 24% | S-isoform↑, Fibrinogen↓ | 50%* | 84% | NA | NA | NA | NA | NA | NA | 5 weeks* | 232 weeks |
| Ferrara et al.10 | 100 | ATRA + CT | 15% | No effect | 87% | 94% | NA | NA | 13% | 8% | 22 months | NR | 62%* | 86% |
| Ito et al.11 | 28 | ATRA + CT | 14% | Coexpression of CD34 | 100% | 87% | NA | NA | 75%* | 0% | 4 months* | NR | 26 months | NR |
| Montesinos et al.12 | 651 | CT alone / ATRA + CT | 11% | Initial WBC counts↑, Albumin↓, S-isoform↑, Coexpression of CD2, CD7, CD15, CD34, CD117, and HLA-DR | 85% | 92% | 22%* | 10% | 7%* | 1% | 73%* | 85% | 78% | 84% |
| Present study (all patients) | 225 | ATRA + CT | 10% | Initial platelet counts↓, Severe DIC↑, Coexpression of CD2, CD7, CD34, and HLA-DR | 91% | 95% | 39% | 24% | 5% | 1.5% | 48% | 65% | 74% | 79% |
| Present study (initial WBC counts ≥3.0 × 109/L) | 112 | ATRA + CT | 12% | 92% | 94% | 54%* | 29% | 9.3% | 1.1% | 31%* | 64% | 62% | 79% | |
Significant difference.
Event-free survival in present study. APL, acute promyelocytic leukemia; ATRA, all-trans retinoic acid; CIR, cumulative incidence of relapse; CR, complete remission; CT, chemotherapy; DFS, disease-free survival; DIC, disseminated intravascular coagulation; HLA, human leukocyte antigen; NA, not available; NR, not reached; OS, overall survival; WBC, white blood cell.
Our study, analyzing 239 APL patients, showed a significant correlation between CD56 expression with lower platelet counts and severe DIC. In contrast to previous reports,9,10,12 CD56 expression was not associated with higher WBC counts, lower albumin levels, or higher frequency of M3 variant. Severity of DIC was related to platelet counts in CD56+ APL, although fibrinogen levels and fibrinogen degradation product ratios (fibrinogen degradation product value/its upper limit of normal value) were not different (Table1). The relationship between CD56 expression and DIC in AML, including APL, has not been elucidated. As statistically significant findings associated with CD56+ APL in previous reports were not the same as our present study, further studies with sufficient numbers of patients will be needed to clarify the characteristic features of CD56+ APL.
Consistent with the report from the PETHEMA/HOVON group,12 CD56+ APL cells frequently coexpressed CD2, CD7, CD34, and/or HLA-DR antigen in our study. Although the mechanism leading to aberrant expression of lymphoid markers, such as CD2 and CD7 in CD56+ APL cells, remains unclear, the expression of these antigens, as well as CD34 and HLA-DR, may indicate that CD56+ APL cells arise in more immature, undifferentiated, and progenitor cells, as previously suggested in acute leukemia.21
The PETHEMA/HOVON group have reported lower CR rates in their patients with CD56+ APL.12 However, our study showed no difference in CR and induction mortality rates. Their patients with CD56+ APL showed poorer ECOG PS scores and lower albumin levels compared with our patients. Higher ECOG PS scores and lower albumin levels were reportedly associated with induction mortality.22 Therefore, the difference may be explained by the characteristics of patients enrolled in both studies.
Our study indicated that CD56 expression was correlated with higher CIR and inferior EFS, and was an independent adverse prognostic factor for EFS by multivariate analysis among APL patients whose initial WBC counts were ≥3.0 × 109/L. These results verified that CD56 expression was one of the adverse prognostic factors in APL patients. However, the direct molecular mechanism why CD56 expression in APL is associated with poor prognosis still remains unclear. CD56 expression is reportedly associated with higher expression of P-glycoprotein in AML,23,24 but their adverse prognostic roles seem independent.24 Unfortunately, neither ours nor other studies focusing on CD56+ APL have tested the association between CD56 and P-glycoprotein. However, APL expressing CD34 was reportedly less sensitive to ATRA therapy.25,26 Therefore, coexpression of CD34 antigen might explain the higher CIR in CD56+ APL, although the RT-PCR negativity after the consolidation chemotherapy was not different between CD56+ and CD56− APL.
In this study, CD56 expression was not determined as one of the prognostic factors in APL patients whose initial WBC counts were <3.0 × 109/L. One explanation might be that it has become difficult to determine significant risk factors in patients with APL, whose prognosis has considerably improved.1–5 In particular, in patients with lower initial WBC counts, the outcome has been dramatically improved in the ATRA era.3,27 Another considerable reason is that there might be synergistic action between CD56 expression and some undetermined proliferation molecular factors. Additionally, extramedullary relapse, observed frequently in patients with CD56+ APL whose initial WBC counts are ≥3.0 × 109/L, might also be a reason. The molecular mechanism behind why CD56+ APL patients with higher initial WBC counts show poor prognosis should be clarified in a future study.
Recently, arsenic trioxide, gemtuzumab ozogamicin, and tamibarotene have been shown to be effective for APL,28–33 and, in fact, most of our relapsed patients received these drugs as well as stem cell transplantation. This may be a plausible reason why EFS and CIR tended to be worse in CD56+ APL, but not OS, because these drugs and transplantation salvaged the relapsed patients.
Although our study confirmed CD56 expression as an independent adverse prognostic factor in APL patients with higher initial WBC counts who were treated with ATRA and chemotherapy (Table4), the clinical significance of CD56 expression might change with the introduction of more potent agents as front-line therapy. Expression of CD56 has not been included so far in standard treatments recommended by the European LeukemiaNet.14 However, some recent published research, including ours (summarized in Table5), will promote the modification of treatment for CD56+ APL. In fact, it is proposed in some recently published studies. We should not only continue to monitor CD56 expression in APL patients, but use more effective therapeutic strategies for patients with CD56+ APL, especially those with higher initial WBC counts.
Acknowledgments
We thank the participating patients for consenting to enter this study and the participating physicians from 92 institutions who registered their patients and provided necessary data. We are deeply grateful to Drs. Miki Nishimura, Tohru Kobayashi, and Motohiro Tsuzuki for their assistance in designing the protocol. Dr. Hiroyuki Fujita updated collected data in March 2010. Dr. Ryuzo Ohno reviewed the manuscript. This work was supported in part by the National Cancer Center Research and Development Fund (23-A-23), Grants-in-Aid from the Cancer Research from the Japanese Ministry of Health, Labor and Welfare (Clinical Cancer Research 23-004 and 25100501), and Grants-in-Aid from the Project for Development of Innovative on Cancer Therapeutics (P-Direct).
Glossary
- AML
acute myeloid leukemia
- APL
acute promyelocytic leukemia
- Ara-C
cytosine arabinoside
- ATRA
all-trans retinoic acid
- CIR
cumulative incidence of relapse
- CR
complete remission
- DIC
disseminated intravascular coagulation
- ECOG
Eastern Cooperative Oncology Group
- EFS
event-free survival
- HLA
human leukocyte antigen
- HOVON
Hemato-Oncologie voor Volwassenen Nederland
- JALSG
Japan Adult Leukemia Study Group
- OS
overall survival
- PETHEMA
Programa de Estudio y Tratamiento de las Hemopatı′as Malignas
- PS
performance status
- WBC
white blood cell
Disclosure Statement
The authors have no conflict of interest.
Funding Information
National Cancer Center Research and Development Fund (23-A-23). Japanese Ministry of Health, Labor and Welfare (Clinical Cancer Research 23-004 and 25100501). Project for Development of Innovative on Cancer Therapeutics (P-Direct).
References
- 1.Fenaux P, Le Deley MC, Castaigne S, et al. Effect of all transretinoic acid in newly diagnosed acute promyelocytic leukemia. Results of a multicenter randomized trial. European APL 91 Group. Blood. 1993;82:3241–9. [PubMed] [Google Scholar]
- 2.Mandelli F, Diverio D, Avvisati G, et al. Molecular remission in PML/RAR alpha-positive acute promyelocytic leukemia by combined all-trans retinoic acid and idarubicin (AIDA) therapy. Gruppo Italiano-Malattie Ematologiche Maligne dell'Adulto and Associazione Italiana di Ematologia ed Oncologia Pediatrica Cooperative Groups. Blood. 1997;90:1014–21. [PubMed] [Google Scholar]
- 3.Asou N, Adachi K, Tamura J, et al. Analysis of prognostic factors in newly diagnosed acute promyelocytic leukemia treated with all-trans retinoic acid and chemotherapy. Japan Adult Leukemia Study Group. J Clin Oncol. 1998;16:78–85. doi: 10.1200/JCO.1998.16.1.78. [DOI] [PubMed] [Google Scholar]
- 4.Sanz MA, Martin G, Rayon C, et al. A modified AIDA protocol with anthracycline-based consolidation results in high antileukemic efficacy and reduced toxicity in newly diagnosed PML/RARalpha-positive acute promyelocytic leukemia. PETHEMA group. Blood. 1999;94:3015–21. [PubMed] [Google Scholar]
- 5.Lo-Coco F, Avvisati G, Vignetti M, et al. Front-line treatment of acute promyelocytic leukemia with AIDA induction followed by risk-adapted consolidation for adults younger than 61 years: results of the AIDA-2000 trial of the GIMEMA Group. Blood. 2010;116:3171–9. doi: 10.1182/blood-2010-03-276196. [DOI] [PubMed] [Google Scholar]
- 6.Ades L, Guerci A, Raffoux E, et al. Very long-term outcome of acute promyelocytic leukemia after treatment with all-trans retinoic acid and chemotherapy: the European APL Group experience. Blood. 2010;115:1690–6. doi: 10.1182/blood-2009-07-233387. [DOI] [PubMed] [Google Scholar]
- 7.Sanz MA, Lo Coco F, Martin G, et al. Definition of relapse risk and role of nonanthracycline drugs for consolidation in patients with acute promyelocytic leukemia: a joint study of the PETHEMA and GIMEMA cooperative groups. Blood. 2000;96:1247–53. [PubMed] [Google Scholar]
- 8.Ono T, Takeshita A, Kishimoto Y, et al. Long-term outcome and prognostic factors of elderly patients with acute promyelocytic leukemia. Cancer Sci. 2012;103:1974–8. doi: 10.1111/j.1349-7006.2012.02390.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murray CK, Estey E, Paietta E, et al. CD56 expression in acute promyelocytic leukemia: a possible indicator of poor treatment outcome? J Clin Oncol. 1999;17:293–7. doi: 10.1200/JCO.1999.17.1.293. [DOI] [PubMed] [Google Scholar]
- 10.Ferrara F, Morabito F, Martino B, et al. CD56 expression is an indicator of poor clinical outcome in patients with acute promyelocytic leukemia treated with simultaneous all-trans-retinoic acid and chemotherapy. J Clin Oncol. 2000;18:1295–300. doi: 10.1200/JCO.2000.18.6.1295. [DOI] [PubMed] [Google Scholar]
- 11.Ito S, Ishida Y, Oyake T, et al. Clinical and biological significance of CD56 antigen expression in acute promyelocytic leukemia. Leuk Lymphoma. 2004;45:1783–9. doi: 10.1080/10428190410001683624. [DOI] [PubMed] [Google Scholar]
- 12.Montesinos P, Rayon C, Vellenga E, et al. Clinical significance of CD56 expression in patients with acute promyelocytic leukemia treated with all-trans retinoic acid and anthracycline-based regimens. Blood. 2011;117:1799–805. doi: 10.1182/blood-2010-04-277434. [DOI] [PubMed] [Google Scholar]
- 13.Tallman MS, Altman JK. Curative strategies in acute promyelocytic leukemia. Hematology Am Soc Hematol Educ Program. 2008:391–9. doi: 10.1182/asheducation-2008.1.391. [DOI] [PubMed] [Google Scholar]
- 14.Sanz MA, Grimwade D, Tallman MS, et al. Management of acute promyelocytic leukemia: recommendations from an expert panel on behalf of the European LeukemiaNet. Blood. 2009;113:1875–91. doi: 10.1182/blood-2008-04-150250. [DOI] [PubMed] [Google Scholar]
- 15.Asou N, Kishimoto Y, Kiyoi H, et al. A randomized study with or without intensified maintenance chemotherapy in patients with acute promyelocytic leukemia who have become negative for PML-RARalpha transcript after consolidation therapy: the Japan Adult Leukemia Study Group (JALSG) APL97 study. Blood. 2007;110:59–66. doi: 10.1182/blood-2006-08-043992. [DOI] [PubMed] [Google Scholar]
- 16.Bene MC, Castoldi G, Knapp W, et al. Proposals for the immunological classification of acute leukemias. European Group for the Immunological Characterization of Leukemias (EGIL) Leukemia. 1995;9:1783–6. [PubMed] [Google Scholar]
- 17.Cheson BD, Bennett JM, Kopecky KJ, et al. Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. J Clin Oncol. 2003;21:4642–9. doi: 10.1200/JCO.2003.04.036. [DOI] [PubMed] [Google Scholar]
- 18.Kobayashi N, Maekawa T, Takada M, Tanaka H, Gonmori H. Criteria for diagnosis of DIC based on the analysis of clinical and laboratory findings in 345 DIC patients collected by the Research Committee on DIC in Japan. Bibl Haematol. 1983;49:265–75. doi: 10.1159/000408467. [DOI] [PubMed] [Google Scholar]
- 19.Baer MR, Stewart CC, Lawrence D, et al. Expression of the neural cell adhesion molecule CD56 is associated with short remission duration and survival in acute myeloid leukemia with t(8;21)(q22;q22) Blood. 1997;90:1643–8. [PubMed] [Google Scholar]
- 20.Byrd JC, Weiss RB, Arthur DC, et al. Extramedullary leukemia adversely affects hematologic complete remission rate and overall survival in patients with t(8;21)(q22;q22): results from Cancer and Leukemia Group B 8461. J Clin Oncol. 1997;15:466–75. doi: 10.1200/JCO.1997.15.2.466. [DOI] [PubMed] [Google Scholar]
- 21.Greaves MF, Chan LC, Furley AJ, Watt SM, Molgaard HV. Lineage promiscuity in hemopoietic differentiation and leukemia. Blood. 1986;67:1–11. [PubMed] [Google Scholar]
- 22.de la Serna J, Montesinos P, Vellenga E, et al. Causes and prognostic factors of remission induction failure in patients with acute promyelocytic leukemia treated with all-trans retinoic acid and idarubicin. Blood. 2008;111:3395–402. doi: 10.1182/blood-2007-07-100669. [DOI] [PubMed] [Google Scholar]
- 23.Raspadori D, Damiani D, Lenoci M, et al. CD56 antigenic expression in acute myeloid leukemia identifies patients with poor clinical prognosis. Leukemia. 2001;15:1161–4. doi: 10.1038/sj.leu.2402174. [DOI] [PubMed] [Google Scholar]
- 24.Raspadori D, Damiani D, Michieli M, et al. CD56 and PGP expression in acute myeloid leukemia: impact on clinical outcome. Haematologica. 2002;87:1135–40. [PubMed] [Google Scholar]
- 25.Warrell RP., Jr Retinoid resistance in acute promyelocytic leukemia: new mechanisms, strategies, and implications. Blood. 1993;82:1949–53. [PubMed] [Google Scholar]
- 26.Purton LE, Bernstein ID, Collins SJ. All-trans retinoic acid delays the differentiation of primitive hematopoietic precursors (lin-c-kit+Sca-1(+)) while enhancing the terminal maturation of committed granulocyte/monocyte progenitors. Blood. 1999;94:483–95. [PubMed] [Google Scholar]
- 27.Ono T, Takeshita A, Kishimoto Y, et al. Long-Term Outcome Of Acute Promyelocytic Leukemia (APL) With Lower Initial Leukocyte Counts By Using All-Trans Retinoic Acid (ATRA) Alone For Remission Induction Therapy: Japan Adult Leukemia Study Group (JALSG) APL97 Study. Blood. 2013;122 : Abstract #3950. [Google Scholar]
- 28.Estey E, Garcia-Manero G, Ferrajoli A, et al. Use of all-trans retinoic acid plus arsenic trioxide as an alternative to chemotherapy in untreated acute promyelocytic leukemia. Blood. 2006;107:3469–73. doi: 10.1182/blood-2005-10-4006. [DOI] [PubMed] [Google Scholar]
- 29.Ravandi F, Estey E, Jones D, et al. Effective treatment of acute promyelocytic leukemia with all-trans-retinoic acid, arsenic trioxide, and gemtuzumab ozogamicin. J Clin Oncol. 2009;27:504–10. doi: 10.1200/JCO.2008.18.6130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Takeshita A, Shinjo K, Naito K, et al. Efficacy of gemtuzumab ozogamicin on ATRA- and arsenic-resistant acute promyelocytic leukemia (APL) cells. Leukemia. 2005;19:1306–11. doi: 10.1038/sj.leu.2403807. [DOI] [PubMed] [Google Scholar]
- 31.Powell BL, Moser B, Stock W, et al. Arsenic trioxide improves event-free and overall survival for adults with acute promyelocytic leukemia: North American Leukemia Intergroup Study C9710. Blood. 2010;116:3751–7. doi: 10.1182/blood-2010-02-269621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Iland HJ, Bradstock K, Supple SG, et al. All-trans-retinoic acid, idarubicin, and IV arsenic trioxide as initial therapy in acute promyelocytic leukemia (APML4) Blood. 2012;120:1570–80. doi: 10.1182/blood-2012-02-410746. [DOI] [PubMed] [Google Scholar]
- 33.Shinagawa K, Ohtake S, Sakura T, et al. A Phase III Study of New Synthetic Retinoid Tamibarotene(Am80) Compared with ATRA in Maintenance Therapy for Newly Diagnosed Acute Promyelocytic Leukemia (APL): Japan Adult Leukemia Study Group (JALSG) APL204 Study. Blood. 2012;120 Abstract #410. (Abstract). [Google Scholar]


