Abstract
Hepatocyte growth factor activator inhibitor type 1 (HAI-1) is a membrane-bound serine protease inhibitor that is expressed on the surface of epithelial and carcinoma cells. On the cell surface, HAI-1 regulates membrane-anchored serine proteases, with matriptase being the most critical target. Matriptase is involved in pericellular processing of biologically active molecules, including protease-activated receptor-2 (PAR-2). Previously we reported that S2-CP8 cells, a metastatic variant of the SUIT-2 human pancreatic adenocarcinoma cell line, showed markedly decreased HAI-1 expression. To assess the significance of HAI-1 loss in invasion and spontaneous metastasis of S2-CP8 cells, we established stable S2-CP8 sublines that expressed HAI-1 under the control of a tetracycline-regulated promoter. In vitro migration and invasion assays revealed inhibitory effects of HAI-1 on S2-CP8 cell migration and invasion. Matriptase activity was suppressed by the expression of HAI-1. As the enhanced invasiveness in the absence of HAI-1 was alleviated by knockdown of matriptase by 81% and of PAR-2 completely, and PAR-2 antagonist also suppressed the invasion, matriptase-mediated PAR-2 activation is involved in HAI-1 loss-induced invasion of S2-CP8 cells. We then analyzed the effect of HAI-1 expression on metastasis of S2-CP8 cells in vivo using a nude mouse orthotopic xenograft model. Although approximately 50% of the control mice developed distant metastasis, mice treated with doxycycline to induce HAI-1 expression did not develop metastasis. These data indicate that HAI-1 loss contributes to invasion and dissemination of a highly metastatic subline of SUIT-2, suggesting crucial roles for the balance of pericellular serine proteases/inhibitors in pancreatic cancer progression.
Keywords: HAI-1, invasion, metastasis, pancreatic cancer, PAR-2
Pancreatic ductal adenocarcinoma (typical pancreatic cancer) remains a challenging disease with a 5-year survival rate of <5%. The lethal nature of this disease is attributed mainly to the propensity for significant invasiveness and rapid metastatic spreading by mechanisms that are not well understood.1 The tumor cell microenvironment plays a critical role in the malignant progression of tumors and consists of a complex mixture of tumor and stromal cells, modification to the extracellular matrix and proteins expressed on and secreted from tumor and stromal cells.2 Pericellular proteolysis significantly influences biological activities of proteins around the constituent cells and thereby determines how the microenvironment participates in tumor progression. Hepatocyte growth factor activator inhibitor type 1 (HAI-1) is a membrane-bound serine protease inhibitor expressed on the surface of epithelial cells.3 HAI-1 inhibits hepatocyte growth factor (HGF) activator4–6 as well as kallikrein 1-related peptidase,7 and also interacts with and inhibits several membrane-anchored serine proteases, such as matriptase, prostasin, hepsin, TMPRSS13, TMPRSS4 and human airway trypsin-like protease.8–11 Among these proteins, matriptase, the most important cognate protease of HAI-1,8 mediates activation of growth factors such as HGF, macrophage stimulating protein (MSP), platelet-derived growth factors (PDGF), protease-activated receptor-2 (PAR-2), and other proteases such as prostasin and urokinase-type plasminogen activator (uPA) in the pericellular microenvironment.12–17 Therefore, insufficient HAI-1 function on the epithelial cell surface may result in deregulated pericellular proteolysis followed by abnormal activation of bioactive molecules and subsequent downstream signaling, which eventually accelerates malignancy progression.18 The amount of cell surface-associated HAI-1 can be significantly decreased via enhanced shedding of the extracellular domain18–20 or decreased mRNA levels,21–23 and a loss of cell surface HAI-1 protein, indeed, occurs in carcinoma cells in vivo.18,20,24,25 Previously we reported that S2-CP8 cells, a metastatic subline of SUIT-2 pancreatic adenocarcinoma cells,26 showed markedly decreased HAI-1 expression compared to SUIT-2 and acquired an epithelial to mesenchymal transition (EMT) phenotype.10 Consequently, HAI-1 knockdown (KD) in SUIT-2 cells resulted in enhanced invasion and MMP-9 expression in vitro,10 and increased pulmonary colonization after injection of the cells into mouse tail veins (i.e. experimental metastasis assay).27 Although these effects of HAI-1 KD were supposed to be mediated by its cognate cell surface proteases,10 the molecular mechanism underlying this HAI-1 KD-induced enhanced invasion and the role of HAI-1 in spontaneous metastasis of cancer cells remains unclear.
For studying metastatic behavior of cancer cells in mouse models, orthotopic transplantation of tumor cells provides a marked improvement over simple subcutaneous implantation and experimental metastasis after tail vein injection.28,29 To assess the function of HAI-1 in invasion and metastasis of S2-CP8 cells more rigorously, we established stable S2-CP8 sublines that expressed HAI-1 under the control of a tetracycline-regulated promoter. Using this system, we analyzed the roles of HAI-1 and its possible downstream effector molecule, PAR-2, in cellular invasion in vitro and examined the effect of HAI-1 expression on metastatic spreading in vivo using a nude mouse orthotopic (i.e. intra-pancreas) xenograft model.
Materials and Methods
Cell culture
The human pancreatic adenocarcinoma cell line SUIT-2 and its metastatic subline S2-CP8 were kindly provided by Dr Takeshi Iwamura (Junwakai Memorial Hospital, Miyazaki, Japan). S2-CP8 was established by cutis–pulmonary metastasis-culture (eight times), via subcutaneous injection of SUIT-2 cells into nude mice.26 The human pancreatic adenocarcinoma cell line AsPC1 was obtained from the American Type Culture Collection (Manassas, VA, USA) through Summit Pharmaceuticals International (Tokyo, Japan). S2-CP8 and AsPC1 cells were cultured in DMEM and RPMI1640, respectively, containing 10% FBS.
RT-PCR and matriptase activity assay
RT-PCR reactions and primer sets for HAI-1, HAI-2, matriptase, TMPRSS13, TMPRSS4, prostasin and GAPDH are described previously.30 Primer sets for HGF, c-MET, PAR-2 and uPA are indicated in Supplementary Table S1. Total RNA was prepared with TRIzol (Life Technologies, Carlsbad, CA, USA). Matriptase activity in concentrated (10 × ) serum-free culture supernatant was measured using the fluorogenic substrate t-butyloxycarbonyl-[(2S0-2-amino-4-(benzyloxycarnony)butanoyl]-L-alanyl-L-arginine4-methyl-coumaryl-7-amide (Boc-E(OBzl)AR-MCA [Peptide Institute, Osaka, Japan]) at a final concentration of 10 μM as described previously.25
Immunoblot analysis and immunohistochemistry
The primary Abs used in this study are anti-human HAI-1 polyclonal Ab (R&D Systems, Minneapolis, MN, USA) and mAb (1N7),3 anti-human matriptase mAb M24,30 anti-human PAR-2 (Santa Cruz Biotechnology, Santa Cruz, CA, USA) and anti-human β-actin (Sigma, St. Louis, MI, USA) mAbs. To detect cellular HAI-1 protein, cultured cells were washed in PBS on ice and extracted with 1% Triton X-100 in PBS. Immunoblot analysis and immunohistochemistry of HAI-1 in formalin-fixed paraffin-embedded tissue sections were performed as described previously.3 For detection of PAR-2 in immunoblotting, non-reducing condition was applied.
Engineered gene expression and knockdown
Human HAI-1 cDNA was subcloned into the lentiviral vector pLenti6.3/TO/V5 and co-infected with pLenti3.3/TR to S2-CP8 cells to establish blasticidin-resistant sublines (S2-CP8_HAI-1tet) according to the manufacturer instructions (Life Technologies). To induce HAI-1 expression in vitro, 1 μg/mL of tetracycline (Tet) (Life Technologies) was added to the culture medium. For KD of HAI-1 or PAR-2, transient transfection of a siRNA pool (ON-TARGETplus SMARTpool siRNA, Thermo Scientific, Yokohama, Japan) was used with the siGENOME Non-Targeting siRNA pool serving as a control. For KD of matriptase, transient transfection of matriptase siRNA (Stealth RNAi; Life Technologies) was used with Stealth RNAi negative control duplex (Life Technologies) as a control. Sequences of siRNA are indicated in Table S1. Lipofectamine 2000 reagent (Life Technologies) was used for transfection.
Matrigel invasion and cellular proliferation assays
In vitro migration and invasion assays were performed using chemotaxis chambers (ThinCert, pore size 8 μm [Greiner Bio-One, Tokyo, Japan]) coated with type-IV collagen (3.6 μg per filter) and Matrigel-coated invasion chambers (BD BioCoat invasion chamber [BD Biosciences, Bedford, MA, USA]), respectively. Cells (1 × 105) in DMEM, 0.1% BSA were placed in the upper compartment with 5% FBS added to the lower compartment as a chemoattractant. For S2-CP8_HAI-1tet sublines, cells were incubated with or without 1 μg/mL Tet. To examine the role of PAR-2 in Matrigel invasion, cells were incubated in the presence or absence of 100 μM of the PAR-2 activating peptide SLIGR-NH2 and/or 100 μM of the PAR-2 selective antagonist, FSLLRY-NH2 (Peptides International, Louisville, KY, USA). After the indicated incubation period at 37°C, the chambers were rubbed with a cotton swab to remove non-invading cells, and the invading cells were fixed and stained with hematoxylin before counting at 200 × fields under a light microscope. Ten randomly selected fields were counted for each membrane. In vitro cellular proliferation was assessed using the Tetra-Color One assay (Seikagaku, Tokyo, Japan).
Animal experiments
All animal work was carried out using protocols approved by the University of Miyazaki Animal Research Committee, in accordance with international guiding principles for biomedical research involving animals. Six-week-old male nude mice (Balb/CAJcl-nu/nu) were obtained from Kyudo (Saga, Japan). The mice were anesthetized by isoflurane (DS Pharma Animal Health, Osaka, Japan) inhalation and a small transverse incision was made in the left lateral flank. The tail of the pancreas was exposed and S2-CP8 cells (5 × 105/50 μL PBS) were injected. The pancreas was returned to the abdomen, and the peritoneum and skin were closed with a continuous suture. The mice were given water with or without 1 mg/mL doxycycline (Dox) (Sigma). The mice were killed by a lethal dose of isoflurane and autopsied 26 days after the implantation. Tissue samples were fixed in 4% formaldehyde in PBS and processed for H&E staining and immunohistochemical evaluation.
Statistical analysis
A comparison between two unpaired groups was done with a repeated measure anova or Mann–Whitney U-tests using spss 15.0 software (SPSS JAPAN, Tokyo, Japan). The threshold for statistical significance was P < 0.05.
Results
Establishment of S2-CP8 sublines that express hepatocyte growth factor activator inhibitor type 1 under the control of a Tet-inducible promoter
Previously we reported that S2-CP8 cells, a highly metastatic subline from lungs and selected from SUIT-2, showed markedly decreased HAI-1 expression compared to the parent line. To determine whether the loss of HAI-1 is directly involved in S2-CP8 cell invasion and metastasis or is simply an epiphenomenon that occurred after the selection of the metastatic variant, we generated S2-CP8 cells that expressed HAI-1 under the control of a Tet-inducible promoter using the pLenti6.3 expression vector system (S2-CP8_HAI-1tet). Two sublines (S2-CP8_HAI-1tet#1 and #2) were isolated in which significant HAI-1 protein expression was induced by Tet treatment (Fig.1a). In accordance with the previous report, HAI-1 expression was barely detectable in parent S2-CP8 cells (not shown) and mock-transfected S2-CP8 cells (S2-CP8_mock) (Fig.1a). It should be noted that even in the absence of Tet, very low HAI-1 levels were detectable in S2-CP8_HAI-1tet cells by immunoblotting and RT-PCR (Fig.1a,b). The parent S2-CP8 cells expressed membrane-anchored serine proteases that can be regulated by HAI-1, such as matriptase, TMPRSS13, TMPRSS4 and prostasin. The mRNA levels of these proteases were not altered by the forced overexpression of HAI-1 (Fig.1b). Among presumed matriptase substrates, PAR-2 and uPA were also expressed by S2-CP8 cells. Although HGF, another important matriptase substrate, was not expressed by these cells, its receptor c-MET was expressed (Fig.1b). The Tet-induced overexpression of HAI-1 modestly enhanced the growth rate of S2-CP8 cells (Fig.1c) and resulted in a more epithelial morphology in vitro (Fig.1d).
Figure 1.
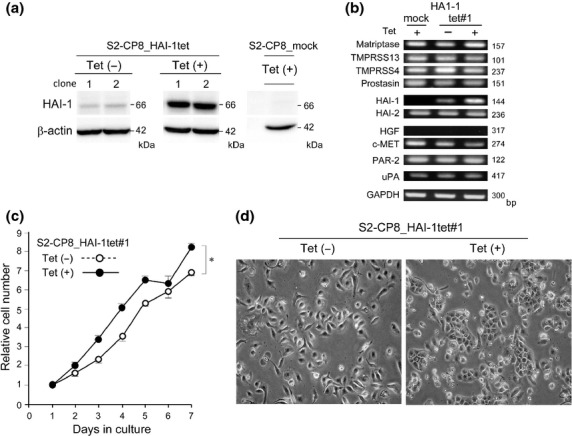
Establishment of S2-CP8 subclones with Tet-inducible HAI-1 expression. (a) Immunoblot analysis of cellular proteins from two subclones with Tet-inducible HAI-1 expression (S2-CP8_HAI-1tet#1, S2-CP8_HAI-1tet#2) and a mock-transfected subclone (S2-CP8/mock). The same blot was reprobed with an anti-β-actin antibody. (b) RT-PCR for cell surface proteases, HAIs, HGF, c-MET, PAR-2, uPA and GAPDH. (c) Growth curves of S2-CP8_HAI-1tet#1 cells with or without Tet treatment. P = 0.02 (one-way anova). (d) Morphology of cultured S2-CP8_HAI-1tet#1 cells without or with Tet treatment.
Suppressive effect of hepatocyte growth factor activator inhibitor type 1 on Matrigel invasion of pancreatic cancer cell lines
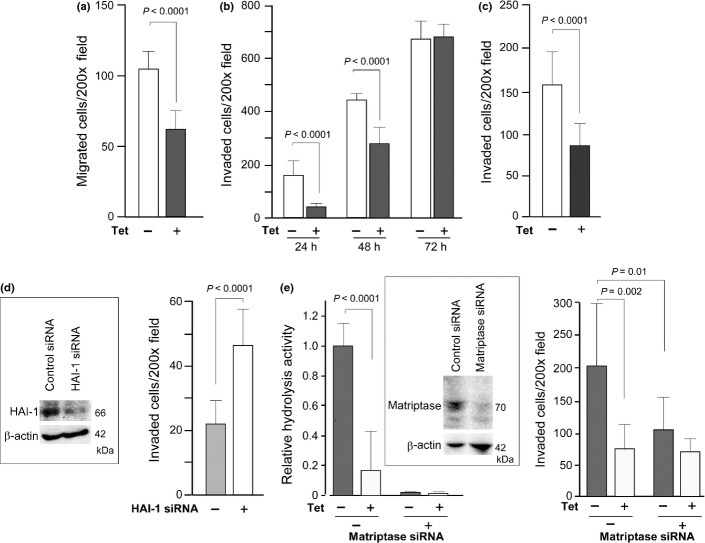
As S2-CP8 is a metastatic subline, we examined the effect of Tet-induced HAI-1 overexpression on cellular migration and invasion in vitro. As shown in Figure2a, migratory activity was suppressed by the HAI-1 expression. Then, a Matrigel invasion assay was performed. Judging by the number of invaded cells after 24, 48 and 72 h of incubation, S2-CP8 cell invasion was clearly delayed by Tet-induced HAI-1 expression (Fig.2b). Twenty-four hours after plating of S2-CP8_HAI-1tet#1 cells, the number of cells that had migrated was approximately four times higher in the absence of Tet compared to Tet-treated cells. The number of invaded cells was similar after 72 h of incubation, which may be due to the higher growth rate of Tet-treated cells compared to untreated cells (Fig.1c) or the saturation of cells on the lower filter area. Another clone, S2-CP8_HAI-1tet#2, also showed decreased invasiveness in response to Tet treatment (Fig.2c). To further confirm the suppressive role of HAI-1 on invasion of pancreatic cancer cells, we examined the effect of HAI-1 KD on Matrigel invasion of another human pancreatic cancer cell line, AsPC1. While AsPC1 cells were less invasive compared to S2-CP8 cells, transient transfection of siRNA that knocked down HAI-1 expression resulted in enhanced Matrigel invasion (Fig.2d). These data indicate a potentially suppressive role for HAI-1 in the invasiveness of pancreatic cancer cells.
Figure 2.
Effect of HAI-1 on migration and invasion of pancreatic cancer cells in vitro. (a) Reduced migration by Tet-induced HAI-1 overexpression in S2-CP8_HAI-1tet#1 cells (6 h incubation). (b) Suppression of Matrigel invasion by Tet-induced expression of HAI-1 in S2-CP8_HAI-1tet#1 cells. The number of invading cells was counted after 24, 48 and 72 h of incubation. (c) Suppression of Matrigel invasion (48 h incubation) by Tet-induced expression of HAI-1 in S2-CP8_HAI-1tet#2 cells. (d) Enhanced Matrigel invasion following HAI-1 KD in AsPC1 cells. Forty-eight hours after siRNA transfection, the cells were applied to a Matrigel invasion assay (72 h incubation). (e) Decreased matriptase activity by HAI-1 expression and effect of matriptase KD on Matrigel invasion of S2-CP8_HAI-1tet#1 cells. Boc-E(OBzl)AR-MCA hydrolysis (relative velocity max) in 24-h culture supernatants (left graph) and Matrigel invasion of S2-CP8_HAI-1tet#1 cells with or without treatment with matriptase siRNA in the presence or absence of Tet (right graph) are shown. Immunoblot for cellular matriptase is also shown. *P < 0.0001; **P = 0.002; #P = 0.01 (Mann–Whitney U-tests). Data are the mean ± SD.
Involvement of matriptase/protease-activated receptor-2 axis in hepatocyte growth factor activator inhibitor type 1 loss-induced enhanced migration and invasion
As matriptase is a cognate protease of HAI-1 on the epithelial cell surface,8 we tested the effect of HAI-1 overexpression on pericellular matriptase activity. Hydrolysis of Boc-E(OBzl)AR-MCA was significantly suppressed in culture supernatant from Tet-treated S2-CP8_HAI-1tet#1 cells compared with that from non-treated S2-CP8_HAI-1tet#1 cells (Fig.2e). The activity was significantly suppressed by KD of matriptase expression. Moreover, matriptase KD alleviated HAI-1-loss induced enhanced invasiveness of S2-CP8_HAI-1tet#1 cells by 81% (Fig.2e).
Protease-activated receptor-2 is an epithelial cell surface protein that is activated by matriptase,16,17 and is suggested to be involved in the invasive growth of pancreatic cancer.31,32 As S2-CP8 cells express PAR-2, we hypothesized that excess PAR-2 activation may be involved in HAI-1 loss-induced enhanced migration and invasion. To test this hypothesis, we examined the effects of PAR-2 antagonist on the migration and invasion of S2-CP8_HAI-1tet#1 cells with or without Tet treatment. A selective antagonist of PAR-2 (FSLLRY) inhibited migration of S2-CP8_HAI-1tet#1 cells in the absence but not presence of Tet, which cancelled out the advantage of cellular migration conferred by loss of HAI-1 (Fig.3a). In contrast, addition of a PAR-2-activating peptide (SLIGR) significantly enhanced the migration of both HAI-1-expressing and control S2-CP8 cells. In a Matrigel invasion assay, a PAR-2 antagonist also showed a suppressive effect on the invasion activity of control S2-CP8, but not on HAI-1-expressing S2-CP8 cells (Fig.3b). While the suppression effect was significant in S2-CP8_mock and S2-CP8_HAI-1tet#2, it was modest and not statistically significant in S2-CP8/HAI-1tet#1 after 48 h incubation. However, at an earlier time point (24 h incubation), the difference was apparent (P = 0.017) in S2-CP8_HAI-1#1, and PAR-2 KD, instead of the antagonist treatment, resulted in significant suppression of the invasion of S2-CP8/HAI-1tet#1 cells in the absence of Tet (Fig.4). Taken together, these results indicate that HAI-1 loss-induced enhanced invasion of S2-CP8 cells is mediated, at least partly, by matriptase-mediated activation of PAR-2.
Figure 3.
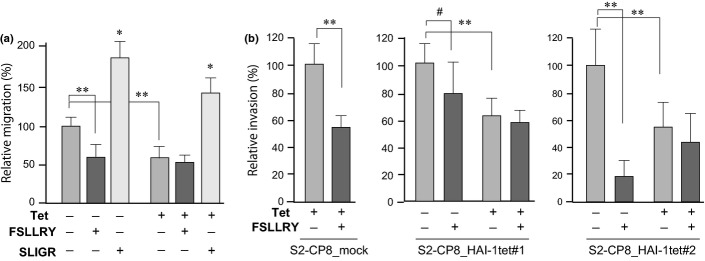
Effect of PAR-2 antagonist (FSLLRY) and agonist (SLIGR) on migration and invasion of S2-CP8 cells. (a) Effect on migration (6 h incubation) of S2-CP8_HAI-1tet#1 cells. The PAR-2 antagonist cancelled the enhanced migration caused by HAI-1 loss while the PAR-2 agonist significantly enhanced the migration. (b) Effects of PAR-2 antagonist on Matrigel invasion of S2-CP8_mock, S2-CP8_HAI-1tet#1 and #2 cells (48 h incubation). *P < 0.0001; **P < 0.0005; #P = 0.073 (Mann–Whitney U-tests). Data are mean ± SD.
Figure 4.
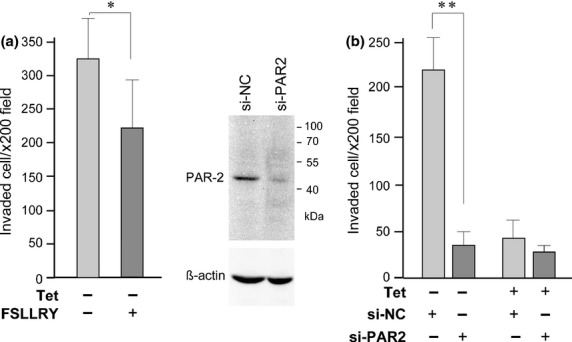
Effect of PAR-2 KD on Matrigel invasion of S2-CP8_HAI-1tet#1 cells. (a) Effect of PAR-2 antagonist on Matrigel invasion (24 h incubation). (b) Effect of PAR-2 KD on Matrigel invasion (24 h incubation). The KD effect was verified by immunoblot and shown. Data are the mean ± SD. *P = 0.017; **P < 0.0001 (Mann–Whitney U-tests).
Suppression of tumor growth and metastasis of S2-CP8 cells by forced expression of hepatocyte growth factor activator inhibitor type 1 in orthotopic transplantation model in vivo
Finally, we examined the role of HAI-1 expression in metastatic spreading of S2-CP8 cells using a nude mouse orthotopic transplantation model. Dox was used instead of Tet to induce HAI-1 expression in this experiment. After implantation of S2-CP8_HAI-1tet#1 cells into the pancreas, mice were given drinking water that either did or did not contain 1 mg/mL Dox. The mice were killed and autopsied 26 days after cell implantation (Fig.5a). Dox treatment indeed induced significant expression of cell surface HAI-1. While the cells formed poorly differentiated tumors regardless of Dox treatment, intratumoral or peritumoral fibroblastic cells were more pronounced in the untreated tumors compared with those from Dox-treated mice (Fig.5b). Notably, mice treated with Dox to induce HAI-1 overexpression did not show metastasis to distant organs (n = 10) (Table1). In contrast, 50% of mice without Dox treatment (n = 9) showed metastases to lungs (44%, 4/9) and/or liver (22%, 2/9) (Table1 and Fig.5c). Dox treatment itself did not alter the metastatic capability of the cells, as pulmonary metastasis was equally observed after orthotopic implantation of parent S2-CP8 cells in both non-treated (67%, 2/3) and treated (75%, 3/4) groups. Interestingly, although induced HAI-1 expression enhanced cellular growth of S2-CP8_HAI-1tet#1 cells in vitro, in vivo tumor growth was significantly reduced by HAI-1 (Fig.5a).
Figure 5.
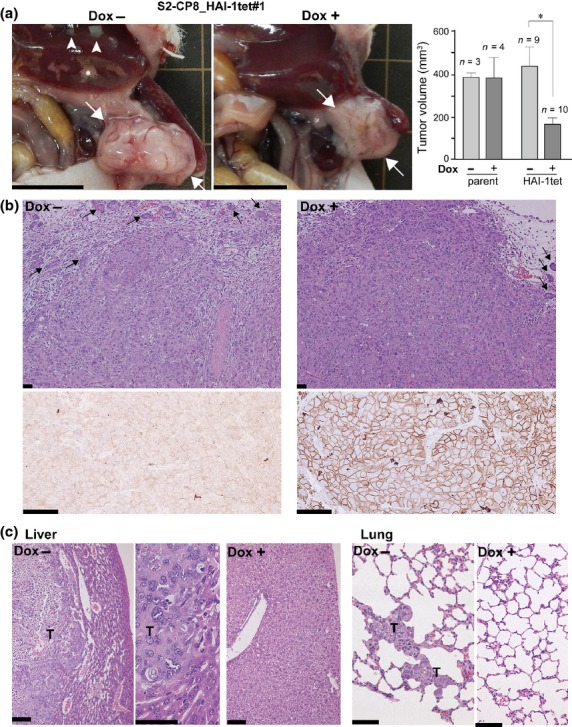
Intra-pancreatic transplantation of S2-CP8_HAI-1tet#1 cells in nude mice. The treated mice were maintained with or without Dox administration and killed 26 days after injection. (a) Macroscopic findings of pancreatic tumors (arrows). Metastases and infarction in the liver are indicated by arrow heads and asterisks, respectively. Bar, 1 cm. Primary tumor volumes of parent S2-CP8 and S2-CP8_HAI-1tet#1 cells without or with Dox treatment are also shown (mean ± SEM). *P < 0.001 (Mann–Whitney U-tests). (b) Histology of pancreatic tumors (upper panel, H&E) and immunohistochemistry of HAI-1 (lower panel). Arrows indicate pancreatic acinar tissue. Bars, 50 μm. (c) Histology of metastatic lesions (liver and lungs) formed in the absence of Dox treatment. T, metastatic tumor. Bars, 50 μm.
Table 1.
Effect of doxycycline-induced HAI-1 expression on spontaneous metastasis after orthotopic transplantation of S2-CP8_HAI-1tet#1 cells
| Dox treatment | Infarction of liver† | Metastasis |
|||
|---|---|---|---|---|---|
| Positive | Liver and lungs | Liver only | Lungs only | ||
| No (n = 9) | 44% (4/9) | 56% (5/9) | 1/9 | 1/9 | 3/9 |
| Yes (n = 10) | 10% (1/10) | 0% (0/10) | |||
Infarction due to portal vein thrombosis. HAI-1, hepatocyte growth factor activator inhibitor type 1.
Discussion
In this study, we analyzed the effect of HAI-1 expression on invasion and metastasis of a metastatic subline (S2-CP8) of the human pancreatic adenocarcinoma cell line SUIT-2 using a Tet ON/OFF system. Induced HAI-1 expression alleviated migration and Matrigel invasion in vitro, and, more importantly, suppressed metastatic spreading of S2-CP8 cells in vivo.
Cancer metastasis is a very complex process that involves migration, invasion, seeding, adhesion and cell growth. In order to analyze a role for specific molecules in metastasis, animal models that represent human processes are required. Tail vein injection to verify pulmonary colonization and subsequent growth is a simple method, but this model bypasses several steps in the metastatic process and, as such, is not a true representation of the metastasis process in human cancer. Subcutaneous implantation is a commonly-used assay of spontaneous metastasis, but the subcutaneous microenvironment of human visceral cancers is known to differ significantly from their original milieu.33 Therefore, orthotopic injection of cell suspensions is a marked improvement over simple subcutaneous implantation.28,29 We previously reported that HAI-1 KD significantly enhanced early pulmonary colonization of SUIT-2 cells after tail vein injection.27 However, in a subcutaneous implantation assay in nude mice, HAI-1 KD in SUIT-2 cells did not result in an increased incidence of pulmonary metastasis, although the number of metastatic lesions in each metastatic case was increased.10 Therefore, convincing evidence for the role of HAI-1 in human cancer cell metastasis in a mouse model has been lacking to date. In this study, using an orthotopic implanted model with a Tet ON/OFF system, we provide for the first time experimental evidence for the suppressive role of HAI-1 in spontaneous metastasis of pancreatic cancer cells.
Although forced HAI-1 expression did not suppress, but rather enhanced, cellular growth in vitro, the expression of HAI-1 suppressed the growth of S2-CP8 cells in the pancreas in vivo. This result may indicate that the biological functions of HAI-1 are dependent on the complex tissue microenvironment where interactions between tumor and host cells occur. These interactions may result in altered expression of extracellular bioactive proteins in the tissue milieu, which could be further modified by pericellular proteolysis that is regulated by HAI-1. For example, pro-HGF is secreted by stromal cells in response to stimulation by cancer cells and must be activated by extracellular serine proteases to transduce c-MET signaling in tumor cells.6 S2-CP8 cells express HGF-activating proteases such as matriptase and TMPRSS13,9,12 which are both inhibited by HAI-1. Matriptase also activates pericellular PDGF-C, PDGF-D and plasma-derived MSP, which, in turn, stimulate both stromal and tumor cells.13–15 Therefore, the roles of HAI-1 in cancer biology should be further analyzed in the context of the cancer tissue microenvironment.
Our in vitro analysis also suggested that the loss of HAI-1, indeed, enhances invasion of cancer cells themselves, and in S2-CP8 cells, upregulation of PAR-2 signaling may be involved in this effect. PAR is a family of seven transmembrane-spanning domain G protein-coupled receptors and consists of PAR-1, PAR-2, PAR-3 and PAR-4.34 After cleavage of the amino-terminal ectodomain, PAR are irreversibly activated upon binding of the newly generated amino-terminal peptide to the core receptor. Whereas PAR-1, PAR-3 and PAR-4 are activated by thrombin, PAR-2 is activated by multiple trypsin-like enzymes34 and is suggested to be involved in pancreatic cancer progression.31,32 Recent studies reveal that matriptase is a potent activator of PAR-216,17, and matriptase KD also alleviates HAI-1 loss-induced enhanced invasiveness. Hence, in the present study, deregulated activity of matriptase in the absence of HAI-1 resulted in PAR-2 activation and eventually enhanced cellular invasion in vitro. In contrast in vitro growth was somewhat enhanced by HAI-1 overexpression, a result that may conflict with the possible growth-promoting activity of PAR-2 signaling.34 However, in accordance with this observation, HAI-1 KD resulted in reduced in vitro cellular growth of human oral squamous and prostatic carcinoma cell lines.25,35 At present, the mechanism by which HAI-1 loss reduces cellular growth in vitro is not known, but acquisition of an EMT phenotype may be involved in this phenomenon.10,25,36
An important question that remains unanswered by this study is the clinical relevance of these findings. HAI-1 expression may be augmented in response to adverse cellular situations in tissue injury,3,37 which occasionally results in enhanced HAI-1 immunoreactivity in some cancer cells at the invasion front.20 However, at the same time, significantly reduced HAI-1 immunoreactivity can also be observed in invading cancer cells,25 which is likely mediated by ectodomain shedding by membrane type-1 MMP18,19 and/or decreased HAI-1 mRNA levels in cancer cells.21–23 In clinicopathological studies, possible correlation between decreased HAI-1 expression and disease progression has been suggested in gynecological, mammary, prostatic and gastrointestinal cancers.22,23,25,36,38–41 In contrast, normal hepatocytes do not express HAI-1 and increased HAI-1 expression in hepatocellular carcinomas predicts a worse prognosis.42 Clearly, detailed clinicopathological analyses of the relationship between cell surface HAI-1 levels and pancreatic ductal adenocarcinoma prognosis using surgically resected specimens will be required.
Acknowledgments
We are grateful to Ms Yumiko Nomura for technical assistance. This study was supported by Grant-in-Aid for Scientific Research No. 24390099 (H. K.) and No. 23790250 (M. K.) from the Ministry of Education, Science, Sports and Culture, Japan, and by the Japan−China Sasagawa Medical Scholarship (J. Y.).
Disclosure Statement
The authors have no conflict of interest.
Funding information
Ministry of Education, Science, Sports and Culture, Japan (24390099 [H. K.] and 23790250 [M. K.]). Japan-China Sasagawa Medical Scholarship (J. Y.).
Supporting Information
Additional supporting information may be found in the online version of this article:
Sequences of siRNA and RT-PCR primers.
References
- 1.Karamitopoulou E. Tumor budding cells, cancer stem cells and epithelial–mesenchymal transition-type cells inpancreatic cancer. Front Oncol. 2012;2:209. doi: 10.3389/fonc.2012.00209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hanahan D, Coussens LM. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell. 2012;21:309–22. doi: 10.1016/j.ccr.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 3.Kataoka H, Suganuma T, Shimomura T, et al. Distribution of hepatocyte growth factor activator inhibitor type 1 (HAI-1) in human tissues. Cellular surface localization of HAI-1 in simple columnar epithelium and its modulated expression in injured and regenerative tissues. J Histochem Cytochem. 1999;47:673–82. doi: 10.1177/002215549904700509. [DOI] [PubMed] [Google Scholar]
- 4.Shimomura T, Denda K, Kitamura A, et al. Hepatocyte growth factor activator inhibitor, a novel Kunitz-type serine protease inhibitor. J Biol Chem. 1997;272:6370–6. doi: 10.1074/jbc.272.10.6370. [DOI] [PubMed] [Google Scholar]
- 5.Kataoka H, Kawaguchi M. Hepatocyte growth factor activator (HGFA): pathophysiological functions in vivo. FEBS J. 2010;277:2230–7. doi: 10.1111/j.1742-4658.2010.07640.x. [DOI] [PubMed] [Google Scholar]
- 6.Kataoka H, Miyata S, Uchinokura S, Itoh H. Roles of hepatocyte growth factor (HGF) activator and HGF activator inhibitor in the pericellular activation of HGF/scatter factor. Cancer Metast Rev. 2003;22:223–36. doi: 10.1023/a:1023051500010. [DOI] [PubMed] [Google Scholar]
- 7.Mukai S, Fukushima T, Naka D, Tanaka H, Osada Y, Kataoka H. Activation of hepatocyte growth factor activator zymogen (pro-HGFA) by human kallikrein 1-related peptidases. FEBS J. 2008;275:1003–17. doi: 10.1111/j.1742-4658.2008.06265.x. [DOI] [PubMed] [Google Scholar]
- 8.Toni M, Anatalis TM, Buzza MS, Hodge KM, Hooper JD, Netzel-Arnett S. The cutting edge: membrane-anchored serine protease activities in the pericellular microenvironment. Biochem J. 2010;428:325–46. doi: 10.1042/BJ20100046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hashimoto T, Kato M, Shimomura T, Kitamura N. TMPRSS13, a type II transmembrane serine protease, is inhibited by hepatocyte growth factor activator inhibitor type 1 and activates pro-hepatocyte growth factor. FEBS J. 2010;277:4888–900. doi: 10.1111/j.1742-4658.2010.07894.x. [DOI] [PubMed] [Google Scholar]
- 10.Cheng H, Fukushima T, Takahashi N, Tanaka H, Kataoka H. Hepatocyte growth factor activator inhibitor type 1 regulates epithelial to mesenchymal transition through membrane-bound serine proteinases. Cancer Res. 2009;69:1828–35. doi: 10.1158/0008-5472.CAN-08-3728. [DOI] [PubMed] [Google Scholar]
- 11.Kato M, Hashimoto T, Shimomura T, Kataoka H, Ohi H, Kitamura N. Hepatocyte growth factor activator inhibitor type 1 inhibits protease activity and proteolytic activation of human airway trypsin-like protease. J Biochem. 2012;151:179–87. doi: 10.1093/jb/mvr131. [DOI] [PubMed] [Google Scholar]
- 12.Owen KA, Qiu D, Alves J, et al. Pericellular activation of hepatocyte growth factor by the transmembrane serine proteases matriptase and hepsin, but not by the membrane-associated protease uPA. Biochem J. 2010;426:219–28. doi: 10.1042/BJ20091448. [DOI] [PubMed] [Google Scholar]
- 13.Bhatt AS, Welm A, Farady CJ, Vásquez M, Wilson K, Craik CS. Coordinate expression and functional profiling identify an extracellular proteolytic signaling pathway. Proc Natl Acad Sci USA. 2007;104:5771–6. doi: 10.1073/pnas.0606514104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ustach CV, Huang W, Conley-LaComb MK, et al. A novel signaling axis of matriptase/PDGF-D/ß-PDGFR in human prostate cancer. Cancer Res. 2010;70:9631–40. doi: 10.1158/0008-5472.CAN-10-0511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hurst NJ, Najy AJ, Ustach CV, Movilla L, Kim HR. Platelet-derived growth factor-C (PDGF-C) activation by serine proteases: implications for breast cancer progression. Biochem J. 2012;441:909–18. doi: 10.1042/BJ20111020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bocheva G, Rattenholl A, Kempkes C, et al. Role of matriptase and proteinase-activated receptor-2 in nonmelanoma skin cancer. J Invest Dermatol. 2009;129:1816–23. doi: 10.1038/jid.2008.449. [DOI] [PubMed] [Google Scholar]
- 17.Camerer E, Barker A, Duong DN, et al. Local protease signaling contributes to neural tube closure in the mouse embryo. Dev Cell. 2010;18:25–38. doi: 10.1016/j.devcel.2009.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoshiko S, Kawaguchi M, Fukushima T, et al. Hepatocyte growth factor activator inhibitor type 1 is a suppressor of intestinal tumorigenesis. Cancer Res. 2013;73:2659–70. doi: 10.1158/0008-5472.CAN-12-3337. [DOI] [PubMed] [Google Scholar]
- 19.Domoto T, Takino T, Guo L, Sato H. Cleavage of hepatocyte growth factor activator inhibitor-1 by membrane-type MMP-1 activates matriptase. Cancer Sci. 2012;103:448–54. doi: 10.1111/j.1349-7006.2011.02162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nagaike K, Kohama K, Uchiyama S, et al. Paradoxically enhanced immunoreactivity of hepatocyte growth factor activator inhibitor type 1 (HAI-1) in cancer cells at the invasion front. Cancer Sci. 2004;95:728–35. doi: 10.1111/j.1349-7006.2004.tb03253.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kataoka H, Uchino H, Denda K, et al. Evaluation of hepatocyte growth factor activator inhibitor expression in normal and malignant colonic mucosa. Cancer Lett. 1998;128:219–27. doi: 10.1016/s0304-3835(98)00067-6. [DOI] [PubMed] [Google Scholar]
- 22.Zeng L, Cao J, Zhang X. Expression of serine protease SNC19/matriptase and its inhibitor hepatocyte growth factor activator inhibitor type 1 in normal and malignant tissues of gastrointestinal tract. World J Gastroenterol. 2005;11:6202–7. doi: 10.3748/wjg.v11.i39.6202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vogel LK, Saebo M, Skjelbred CF, et al. The ratio of matriptase/HAI-1 mRNA is higher in colorectal cancer adenomas and carcinomas than corresponding tissue from control individuals. BMC Cancer. 2006;6:176. doi: 10.1186/1471-2407-6-176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kataoka H, Hamasuna R, Itoh H, Kitamura N, Koono M. Activation of hepatocyte growth factor/scatter factor in colorectal carcinoma. Cancer Res. 2000;60:6148–59. [PubMed] [Google Scholar]
- 25.Baba T, Kawaguchi M, Fukushima T, et al. Loss of membrane-bound serine protease inhibitor HAI-1 induces oral squamous cell carcinoma cells' invasiveness. J Pathol. 2012;228:181–92. doi: 10.1002/path.3993. [DOI] [PubMed] [Google Scholar]
- 26.Kitamura N, Iwamura T, Taniguchi S, et al. High collagenolytic activity in spontaneously highly metastatic variants derived from a human pancreatic cancer cell line (SUIT-2) in nude mice. Clin Exp Metastasis. 2000;18:561–71. doi: 10.1023/a:1011900818419. [DOI] [PubMed] [Google Scholar]
- 27.Fukushima T, Kawaguchi M, Yamasaki M, Tanaka H, Yorita K, Kataoka H. Hepatocyte growth factor activator inhibitor type 1 suppresses metastatic pulmonary colonization of pancreatic carcinoma cells. Cancer Sci. 2011;102:407–13. doi: 10.1111/j.1349-7006.2010.01808.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hoffman RM. Orthotopic is orthodox: why are orthotopic-transplant metastatic models different from all other models? J Cell Biochem. 1994;56:1–3. doi: 10.1002/jcb.240560102. [DOI] [PubMed] [Google Scholar]
- 29.Loukopoulos P, Kanetaka K, Takamura M, Shibata T, Sakamoto M, Hirohashi S. Orthotopic transplantation models of pancreatic adenocarcinoma derived from cell lines and primary tumors and displaying varying metastatic activity. Pancreas. 2004;29:193–203. doi: 10.1097/00006676-200410000-00004. [DOI] [PubMed] [Google Scholar]
- 30.Kohama K, Kawaguchi M, Fukushima T, Lin CY, Kataoka H. Regulation of pericellular proteolysis by hepatocyte growth factor activator inhibitor type 1 (HAI-1) in trophoblast cells. Hum Cell. 2012;25:100–10. doi: 10.1007/s13577-012-0055-2. [DOI] [PubMed] [Google Scholar]
- 31.Ikeda O, Egami H, Ishiko T, et al. Expression of proteinase-activated receptor-2 in human pancreatic cancer: a possible relation to cancer invasion and induction of fibrosis. Int J Oncol. 2003;22:295–300. [PubMed] [Google Scholar]
- 32.Yada K, Shibata K, Matsumoto T, Ohta M, Yokoyama S, Kitano S. Protease-activated receptor-2 regulates cell proliferation and enhances cyclooxygenase-2 mRNA expression in human pancreatic cancer cells. J Surg Oncol. 2005;89:79–85. doi: 10.1002/jso.20197. [DOI] [PubMed] [Google Scholar]
- 33.Fidler IJ. Critical factors in the biology of human cancer metastases: twenty-eighth G.H.A. Clowes memorial award lecture. Cancer Res. 1990;50:6130–8. [PubMed] [Google Scholar]
- 34.Elste AP, Petersen I. Expression of proteinase-activated receptor 1–4 (PAR 1–4) in human cancer. J Mol Histol. 2010;41:89–99. doi: 10.1007/s10735-010-9274-6. [DOI] [PubMed] [Google Scholar]
- 35.Sanders AJ, Parr C, Mason MD, Jiang WG. Suppression of hepatocyte growth factor activator inhibitor-1 leads to a more aggressive phenotype of prostate cancer cells in vitro. Int J Mol Med. 2007;20:613–9. [PubMed] [Google Scholar]
- 36.Nakamura K, Hongo A, Kodama J, Hiramatsu Y. The role of hepatocyte growth factor activator inhibitor (HAI)-1 and HAI-2 in endometrial cancer. Int J Cancer. 2011;128:2613–24. doi: 10.1002/ijc.25606. [DOI] [PubMed] [Google Scholar]
- 37.Komaki W, Fukushima T, Tanaka H, Itoh H, Chosa E, Kataoka H. Expression of hepatocyte growth factor activator inhibitor type 1 on the epithelial cell surface is regulated by hypoxic and oxidative stresses. Virchow Arch. 2008;453:347–57. doi: 10.1007/s00428-008-0662-1. [DOI] [PubMed] [Google Scholar]
- 38.Nakamura K, Abarzua F, Kodama J, et al. Expression of hepatocyte growth factor activator inhibitors (HAI-1 and HAI-2) in ovarian cancer. Int J Oncol. 2009;34:345–53. [PubMed] [Google Scholar]
- 39.Nakamura K, Abarzua F, Hongo A, et al. The role of hepatocyte growth factor activator inhibitor-1 (HAI-1) as a prognostic indicator in cervical cancer. Int J Oncol. 2009;35:239–48. [PubMed] [Google Scholar]
- 40.Saleem M, Adhami VM, Zhong W, et al. A novel biomarker for staging human prostate adenocarcinoma: overexpression of matriptase with concomitant loss of its inhibitor, hepatocyte growth factor activator inhibitor-1. Cancer Epidemiol Biomarkers Prev. 2006;15:217–27. doi: 10.1158/1055-9965.EPI-05-0737. [DOI] [PubMed] [Google Scholar]
- 41.Kauppinen JM, Kosma VM, Soini Y, et al. ST14 gene variant and decreased matriptase protein expression predict poor breast cancer survival. Cancer Epidemiol Biomarkers Prev. 2010;19:2133–42. doi: 10.1158/1055-9965.EPI-10-0418. [DOI] [PubMed] [Google Scholar]
- 42.Funagayama M, Kondo K, Chijiiwa K, Kataoka H. Expression of hepatocyte growth factor activator inhibitor type 1 in human hepatocellular carcinoma and postoperative outcomes. World J Surg. 2010;34:1563–71. doi: 10.1007/s00268-010-0517-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Sequences of siRNA and RT-PCR primers.