Abstract
The antitumor activity of pladienolide B, a novel splicing inhibitor, against gastric cancer is totally unknown and no predictive biomarker of pladienolide B efficacy has been reported. We investigated the antitumor activity of pladienolide B and its derivative on gastric cancer cell lines and primary cultured cancer cells from carcinomatous ascites of gastric cancer patients. The effect of pladienolide B and its derivative on six gastric cancer cell lines was investigated using a MTT assay and the mean IC50 values determined to be 1.6 ± 1.2 (range, 0.6–4.0) and 1.2 ± 1.1 (range, 0.4–3.4) nM, respectively, suggesting strong antitumor activity against gastric cancer. The mean IC50 value of pladienolide B derivative against primary cultured cells from 12 gastric cancer patients was 4.9 ± 4.7 nM, indicative of high antitumor activity. When 18 SCID mice xenografted with primary cultured cells from three patients were administered the pladienolide B derivative intraperitoneally, all tumors completely disappeared within 2 weeks after treatment. Histological examination revealed a pathological complete response for all tumors. In the xenograft tumors after treatment with pladienolide B derivative, immature mRNA were detected and apoptotic cells were observed. When the expressions of cell-cycle proteins p16 and cyclin E in biopsied gastric cancer specimens were examined using immunohisctochemistry, positivities for p16 and cyclin E were significantly and marginally higher, respectively, in the low-IC50 group compared with the high-IC50 group, suggesting the possibility that they might be useful as predictive biomarkers for pladienolide B. In conclusion, pladienolide B was very active against gastric cancer via a mechanism involving splicing impairment and apoptosis induction.
Keywords: Apoptosis, ascites, gastric cancer, pladienolide B, RNA splicing
Gastric cancer is associated with a high worldwide mortality rate and is ranked as the third and fifth most common form of cancer in men and women, respectively.1 Gastric cancer is associated with a particularly high mortality rate in Asia and is the second leading cause of mortality due to malignant tumors in Japan.2,3
Currently, combination chemotherapy with anticancer drugs such as 5-fluorouracil with cisplatin, taxanes and irinotecans has been used for the treatment of metastatic gastric cancer.4–6 However, standard treatment has not yet been established and the average survival period is only approximately 1 year. Diffuse-type gastric cancer is particularly resistant to a variety of anticancer drugs and often causes carcinomatous peritonitis at a relatively early stage with a very poor prognosis. Therefore, the development of new effective drugs for gastric cancer is an urgent priority.
Recently, considerable attention has been drawn to a new class of anticancer agents targeting the spliceosome. In particular, spliceostatin A, GEX1 and pladienolides were reported to show strong antitumor activity and were expected to emerge as new anticancer drug candidates. These substances directly bind splicing factor 3b (SF3b) in the spliceosome and inhibit the splicing process in tumor cells.7–11 One of these agents, pladienolide, is a novel 12-membered macrolide produced by Streptomyces platensis Mer-11107.12,13 Among several related macrolides, pladienolide B was found to be the most active form with an IC50 value in the low nanomolar range against human cancer cell lines.14–16 Moreover, a new pladienolide B derivative, which has strong in vitro antitumor activity and preferable physicochemical properties, was synthesized.9,17 It was shown to have very strong antitumor activity in a xenograft model using lung cancer and breast cancer cell lines. However, no data are available regarding the antitumor efficacy of pladienolides against gastric cancer cells, and their efficacy has not been determined in primary cultured cancer cells of any type. Moreover, no predictive biomarkers for evaluating the efficacy of pladienolides have been reported. Therefore, in the present study, we first investigated the antitumor activity of pladienolide B and its derivative on various gastric cancer cell lines. We then investigated its antitumor activity against primary cultured gastric cancer cells from carcinomatous ascites obtained from patients with gastric cancer. We also assessed the correlation between the antitumor activity of pladienolides and expression of the cell cycle regulatory proteins cyclin E and p16 in biopsied gastric cancer cells.
Materials and Methods
Anticancer agents
Pladienolide B was purified as described previously.9 The pladienolide B derivative, (3R,6R,7S,8E,10 S,11S,12E,14E,16R,18R,19R,20R,21S)-7-((4-cyclo-heptylpiperazin-1-yl)carbonyl)oxy-3,6,16,21-tetrahydroxy-6,10,12,16,20-penta-methyl-18,19-epoxytrichosa-8,12,14-trien-11-olide (compound 7), was also synthesized as reported previously (Fig. 1).9
Figure 1.
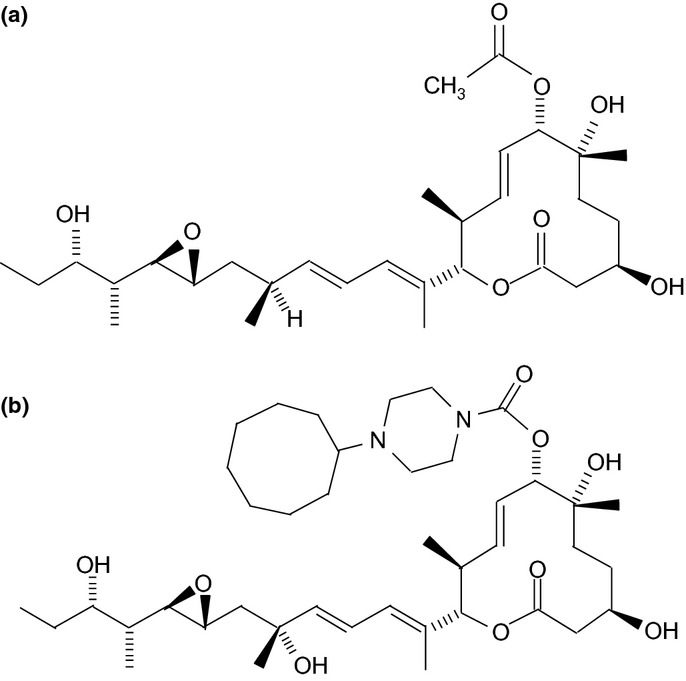
Structures of pladienolide B (a) and its derivative (b). To increase the stability and antitumor activity of pladienolide B, the acetyl group at the C7 position was substituted with 4-cyclo-heptylpiperazin-1-yl and a hydroxyl group was added to the C16 position.
Cell lines and cell culture
Six human gastric cancer cell lines composed of various grades of histological differentiation were used; MKN74 and IM95 were derived from moderately differentiated adenocarcinoma, MKN45 from poorly differentiated adenocarcinoma, HGC27 from undifferentiated carcinoma, NUGC-4 from signet ring cell carcinoma and MKN1 from adenosquamous carcinoma. Lung cancer cell lines SBC-3, Lu99 and T3M-11 and breast cancer cell line MDA-MB-453 were used as controls. MKN74, IM95, MKN45, MKN1 and SBC-3 were obtained from Health Science Research Resources Bank (HSRRB, Tokyo, Japan). HGC27, NUGC-4, Lu99, T3M-11 and MDA-MB-453 were obtained from RIKEN BioResource Center (RIKEN BRC, Ibaraki, Japan). Each cell line was cultured in the recommended medium containing 10% fetal calf serum (FCS) at 37°C with 5% CO2.
Patients
Twelve patients with gastric cancer who were confirmed to have carcinomatous peritonitis by fine needle aspiration cytology of ascites were involved in the present study. Baseline characterisctics of the patients are shown in Table 1. The present study was approved by the Institutional Review Board (IRB) of Tokushima University Hospital and written informed consent was obtained from all patients.
Table 1.
Baseline characteristics of patients and cytotoxic activity of pladienolide B derivative for primary cultured gastric cancer cells
| Patient no. | Sex | Age (years) | PS | Diffuse or intestinal† | Aspirated ascites volume (mL)‡ | Other metastatic sites | Survival time (months)§ | IC50 (nM) (95% CI) |
|---|---|---|---|---|---|---|---|---|
| 1 | F | 80 | 0 | Intestinal | 1360 | Ovary | 7.3 | 2.5 (0.28–4.8) |
| 2 | F | 86 | 1 | Intestinal | 780 | LN | 10.8 | 5.5 (0.11–11) |
| 3 | M | 75 | 1 | Diffuse | 1240 | PD | 12.6 | 16 (7.8–23) |
| 4 | M | 75 | 0 | Intestinal | 2530 | Liver | 9.7 | 12 (0.75–23) |
| 5 | M | 47 | 0 | Diffuse | 970 | LN | 13.4 | 2.5 (0.94–3.9) |
| 6 | F | 83 | 2 | Diffuse | 1650 | Bone, LN | 6.8 | 2.0 (0.79–3.1) |
| 7 | F | 52 | 0 | Diffuse | 1820 | Bone | 10.3 | 6.0 (5.1–7.1) |
| 8 | F | 70 | 0 | Intestinal | 950 | – | 11.2 | 5.8 (2.4–9.1) |
| 9 | F | 53 | 0 | Intestinal | 1290 | Bone | 11.4 | 0.4 (0.25–0.74) |
| 10 | F | 65 | 1 | Diffuse | 2320 | PD | 15.7 | 4.0 (0.051–8.1) |
| 11 | F | 73 | 1 | Intestinal | 880 | PD, ovary | 9.6 | 2.4 (0.12–4.7) |
| 12 | M | 59 | 0 | Intestinal | 2250 | Lung | 18.6 | 0.3 (0.28–0.37) |
Diagnosed by biopsy from primary lesions.
Calculated using multi-detector computed tomography.
Survival time after diagnosis of gastric cancer. CI, confidence interval; IC50, half maximal (50%) inhibitory concentration; LN, lymph node; PD, pleural dissemination; PS, performance status.
Primary culture of gastric cancer cells
Ascites were collected aseptically from cancer patients with carcinomatous peritonitis by aspiration and centrifuged at 300 g for 5 min. Ten millilitres of supernatant from the ascites and 10 mL of DMEM with 10% FCS were added to the pelleted cells. The mixture was cultured in a 25-mL tissue culture flask at 37°C with 5% CO2. The first passage was performed approximately 1 week later and a total of 15 passages were performed. Prior to the experiments, the cells were immunostained with anti-CEA antibody (Cell Signaling Technology Inc., Danvers, MA, USA) or anti-CA19-9 antibody (Dako, Tokyo, Japan) to confirm that more than 95% of the cells were cancerous.
In vitro cytotoxicity assay
The sensitivities of each cancer cell to pladienolide B and its derivative were determined using MTT assay, as described previously.9 Each assay was performed three times and the mean value and 95% confidence intervals were calculated.
Xenotransplantation
Gastric cancer cells (2 × 106) were inoculated into the flank of 6-week-old SCID mice (CLEA Japan Inc., Tokyo, Japan) and the mice were then randomly assigned to receive either the pladienolide derivative or vehicle. The primary cultured cells from cases 6, 8 and 9 were used for xenotransplantation. These samples were selected because an appreciable number of cells were obtained from ascites and grew well continuously up to a number of more than 2 × 107 cells. Tumor volume (V) was calculated according to the formula: V = length × (width)2 × 0.5, as described previously.18 When the tumor volume reached 100–300 mm3, the pladienolide B derivative (10 mg/kg) or vehicle was administered intraperitoneally to each mouse on days 0, 2, 4 and 6. Chronological changes in tumor volume were expressed as the relative tumor volume (RTV) in comparison to the tumor volume at the start of drug administration.19 All animal experiments were performed according to the Guideline for Animal Experiments at Tokushima University.
Reverse transcription–polymerase chain reaction (RT-PCR)
For the detection of unspliced and spliced RNA of RIOK3 and DNAJB1 genes in MKN74 cells (in vitro), RT-PCR was performed as previously reported.9 For in vivo experiments, MKN 74 cells were inoculated into the flank of SCID mice and the pladienolide B derivative (10 mg/kg) or vehicle was administered intraperitoneally four times. The tumor was then excised, the RNA was extracted and RT-PCR was performed.
TUNEL assay
The MKN74 cells were plated on chamber slides and treated with pladienolide B derivative (1, 10, 100 or 10 000 nM) for 48 h, followed by fixation with 10% formalin. The TUNEL assay was then performed using an Apotosis in situ Detection Kit (Takara, Shiga, Japan) according to the manufacturer's instructions. Apoptotic cells were calculated as the mean percentage of darkly stained nuclei at six randomly selected 400 × 400 μm fields.
For the in vivo experiments, formalin-fixed paraffin-embedded sections of excised xenograft tumors were placed on glass slides and the TUNEL assay was performed.
Immunohistochemistry
Immunohistochemical staining was performed using the streptavidin-biotin peroxidase method with labeled streptavidin-biotin (Dako), as described previously.20 A mouse anti-human p16 monoclonal antibody (Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA) or rabbit anti-human cyclin E polyclonal antibody (Santa Cruz Biotechnology Inc.) was used as the primary antibody.
Positive immunostaining was evaulated according to the percentage of positive cells and staining intensity and was classfied as negative, weak positive or strong positive using a previously published method,21 with a minor modification. In brief, scores for the percentage of positive cells were assigned as follows: ≤10% of cells positive, 0; 11–25% of cells positive, 1; 26–50% of cells positive, 2; 51–75% of cells positive, 3; and >75% of cells positive, 4. Scores for staining intensity were assigned as follows: no staining, 0; light brown, 1; brown, 2; and dark brown, 3. Overall scores were obtained by multiplying the percentage score by the intensity score. Overall scores ≤2 were defined as negative, overall scores >2 but ≤7 were defined as weak positive and overall scores >7 were defined as strong positive. Two independent pathologists examined five random fields (300 μm2) per sample and assigned scores without knowledge of the patient outcome. The average value of the two scores was calculated.
Statistical analysis
The tumor sizes of the xenografts were compared between the treatment and control groups using the Student's t-test. The percentage of TUNEL-positive cells was compared between the pladienolide B derivative and vehicle groups using the Student's t test. Positivity for p16 or cyclin E was compared between the high- and low-IC50 groups using the two-tailed Chi-squared test. P < 0.05 was defined as statistically significant. spss software version 11.05 (SPSS Inc., Chicago, IL, USA) was used for the analysis.
Results
Antitumor activity of pladienolide B and its derivative on gastric cancer cell lines
First we investigated the antitumor activity of pladienolide B against a variety of gastric cancer cell lines using the MTT assay. The mean IC50 value was 1.6 ± 1.2 nM (range, 0.6–4.0) (Table 2). This value was equivalent to the values for lung cancer and breast cancer cell lines, for which a high antitumor effect of pladienolide B has already been shown,14 suggesting that pladienolide B also has high antitumor activity against gastric cancer. Next we examined the antitumor activity of the pladienolide B derivative, which has greater stability and greater antitumor activity than pladienolide B.9,17 Its mean IC50 value against the six gastric cancer cell lines was 1.2 ± 1.1 nM (range, 0.4–3.4 nM). Again, these values were similar to those for lung cancer and breast cancer cell lines, indicating that the pladienolide B derivative is also highly active against gastric cancer cell lines. The IC50 value for the derivative was significantly correlated with that of pladienolide B in each cell line (P < 0.01 using Pearson's test). Moreover, the IC50 value for the derivative was lower than that for pladienolide B in all cell lines, indicating that the former has greater antitumor activity than pladienolide B. In this context, subsequent studies were carried out using the pladienolide B derivative.
Table 2.
Cytotoxic activity of pladienolide B and its derivative on cancer cell lines
| Cell lines | Origin | Pladienolide B IC50 (nM) | 95% CI (nM) | Pladienolide B derivative IC50 (nM) | 95% CI (nM) |
|---|---|---|---|---|---|
| MKN1 | Stomach | 4.0 | 2.9–5.1 | 3.4 | 2.1–4.7 |
| MKN45 | Stomach | 1.6 | 1.3–1.9 | 0.4 | 0.34–0.52 |
| MKN74 | Stomach | 1.4 | 1.3–1.5 | 1.1 | 0.96–1.30 |
| IM95 | Stomach | 0.9 | 0.65–1.20 | 0.6 | 0.57–0.66 |
| HGC27 | Stomach | 1.2 | 1.11–1.31 | 1.4 | 1.3–1.5 |
| NUGC-4 | Stomach | 0.6 | 0.47–0.76 | 0.4 | 0.29–0.51 |
| SBC-3 | Lung | 0.9 | 0.72–1.12 | 0.6 | 0.54–0.69 |
| Lu99 | Lung | 1.1 | 1.0–1.2 | 1.0 | 0.9–1.1 |
| T3M-11 | Lung | 2.5 | 2.3–2.8 | 1.2 | 1.2–1.3 |
| MDA-MB-453 | Breast | 2.9 | 1.2–4.1 | 1.0 | 0.31–1.50 |
CI, confidence interval; IC50, half maximal (50%) inhibitory concentration.
Antitumor activity of the pladienolide B derivative on primary cultured gastric cancer cells
Since the pladienolide B derivative showed high antitumor activity against gastric cancer cell lines, we next examined its efficacy on primary cultured gastric cancer cells. Its mean IC50 value against primary cultured cells from the 12 cases was 4.9 ± 4.7 nM (range, 0.3–16 nM) (Table 1). Appreciably high antitumor activity (IC50 ≤ 6 nM), similar to that for gastric cancer cell lines, was observed in 10 cases, although the activity seemed to be insufficient in the remaining two cases (cases 3 and 4). These results indicate that the pladienolide B derivative has high antitumor activity not only against cultured gastric cancer cells but also against actual gastric cancer cells from patients. No significant correlations were observed between the IC50 values and sex, age, performance status, histological type, ascites volume or survival time (data not shown).
Inhibitory activity of the pladienolide B derivative on xenografts of primary cultured gastric cancer cells in SCID mice
Because the pladienolide B derivative showed in vitro antitumor activity against primary cultured gastric cancer cells, we investigated its effect in vivo using a xenograft model with primary cultured cells. Figure 2 shows a representative result of chronological changes in xenograft volume from primary cultured cells of case 8. The tumor volume of the vehicle group continued to increase over time and was approximately 3.3 times larger at 50 days. In contrast, the tumor volume of the pladienolide B derivative group significantly decreased and the tumors disappeared completely within 2 weeks in all mice. Moreover, no tumor regrowth was seen in any of these mice, indicating a long-lasting complete response (CR). When the mice were killed at 50 days, no tumors were observed either macroscopically or microscopically. The same results were obtained in cases 6 and 9 (Table 3). Thus, in all three cases, the xenograft tumors completely disappeared within 14 days and did not recur until 50 days in the treatment group, while all tumors increased over time in the vehicle group (P < 0.01). These results suggest that the pladienolide B derivative cured xenograft tumors in all mice with remarkable efficacy.
Figure 2.
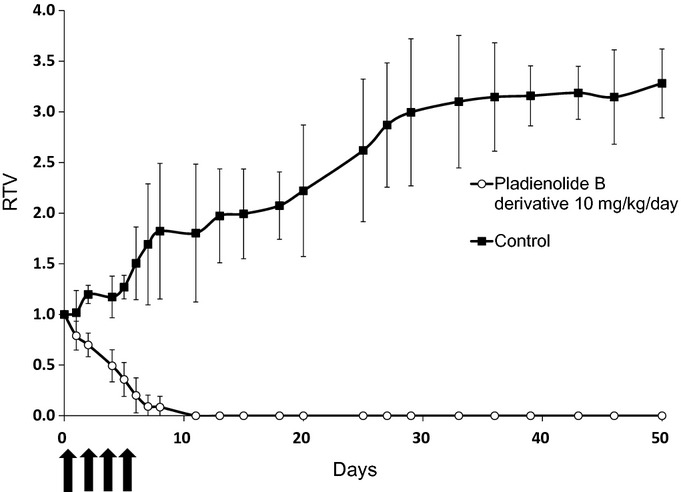
Inhibitory effect of pladienolide B derivative on xenograft tumors from primary cultured gastric cancer cells in SCID mice. Representative data from xenograft experiments for case 8 are shown. The primary cultured cancer cells (2 × 106) were inoculated subcutaneously into the flank of SCID mice. When the tumor volume reached 100–300 mm3, the pladienolide B derivative (10 mg/kg) or vehicle was administered by intraperitoneal injection every other day (four injections in total). Relative tumor volume (RTV) was calculated using the following formula: RTV = (Vx/V1), where Vx is the tumor volume on day X and V1 is the tumor volume at the start of drug administration.
Table 3.
Summary of xenograft experiments using primary cultured gastric cancer cells treated with pladienolide B derivative
| Vehicle group |
Pladienolide B derivative group |
||||||
|---|---|---|---|---|---|---|---|
| No. mice examined | RTV | CR rate (%) | No. mice examined | RTV | CR rate (%) | Recurrence rate† (%) | |
| Case 6 | 5 | 5.5 ± 1.3 | 0/5 (0) | 5 | 0.0 ± 0.0 | 5/5 (100) | 0/5 (0) |
| Case 8 | 5 | 3.3 ± 0.3 | 0/5 (0) | 5 | 0.0 ± 0.0 | 5/5 (100) | 0/5 (0) |
| Case 9 | 8 | 5.2 ± 2.2 | 0/8 (0) | 8 | 0.0 ± 0.0 | 8/8 (100) | 0/8 (0) |
50-day recurrence rate. CR, complete response; RTV, relative tumor volume.
With regard to toxicity, only temporary weight loss of <10% of total bodyweight was observed approximately 1 week after the start of drug administration.
Inhibition of splicing in gastric cancer cells in vitro and in vivo by the pladienolide B derivative
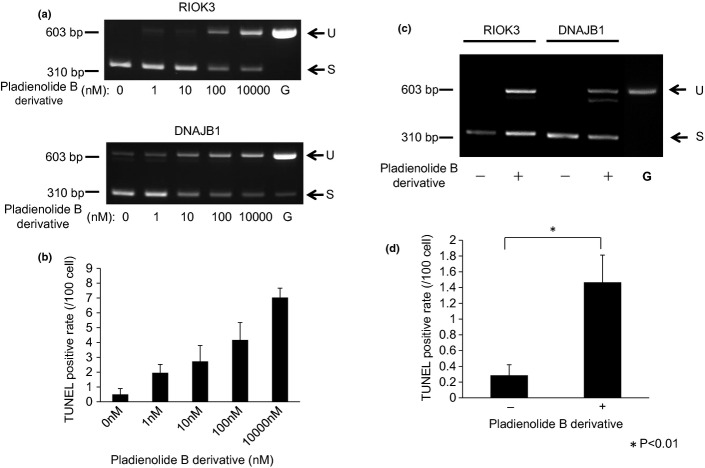
To determine whether the pladienolide B derivative inhibits splicing of pre-mRNA in gastric cancer cells, we first treated MKN74 cells in vitro with various doses of the drug and evaluated the amount of unspliced mRNA of the RIOK3 or DNAJB1 genes, as described previously.9,13 In untreated cells, only mature mRNA (spliced mRNA) were identified and unspliced mRNA was not observed or was observed very faintly. However, in the treatment group, unspliced mRNA of RIOK3 and DNAJB1 genes were clearly observed and the amounts of unspliced mRNA increased in a dose-dependent manner (Fig. 3a).
Figure 3.
Splicing inhibition and apoptosis induction in gastric cancer cells in vitro and in vivo by the pladienolide B derivative. (a) MKN74 cells were treated with pladienolide B derivative for 4 h and unspliced mRNA of RIOK3 and DNAJB1 genes were evaluated using RT-PCR. G, Genomic DNA as a control; S, spliced mRNA; U, unspliced mRNA. (b) MKN74 cells were treated with the pladienolide B derivative and apoptotic cells were detected using TUNEL staining. (c) Mice with xenografts from MKN74 cells were treated with pladienolide B derivative (10 mg/kg) or vehicle four times and unspliced mRNA of excised xenografts were evaluated using RT-PCR. (d) Apoptotic cells in the xenograft tumors were detected using TUNEL staining. *P < 0.01.
We also examined in vivo splicing impairment in xenograft tumors from MKN 74 cells. In vehicle-treated mice, only mature mRNA of the RIOK3 and DNAJB1 genes were observed. However, unspliced mRNA of the RIOK3 or DNAJB1 genes were observed in tumors treated with the pladienolide B derivative. These data indicate that the pladienolide B derivative actually caused splicing impairment in the tumor cells in vivo (Fig. 3c).
Increase in apoptosis by pladienolide B derivative
Apoptotic cells in cultured MKN74 cells and in xenografted tumors were detected using a TUNEL assay. The percentages of TUNEL-positive cells in cultured cells treated with 1 μM pladienolide B derivative was significantly higher than in the untreated cells (P < 0.05) and increased in a dose-dependent manner (Fig. 3b). The percentage of TUNEL-positive cells in xenograft tumors treated with the pladienolide B derivative was significantly higher than in the vehicle-treated tumors (P < 0.01; Fig. 3d). These results indicate that pladienolide B derivative induces apoptosis in colorectal cancer cells.
Expression of p16 and cyclin E in primary cultured gastric cancer cells
We previously found preliminary data showing a positive correlation between pladienolide B derivative sensitivity and expression of p16 or cyclin E in lung cancer and breast cancer cell lines.22 Therefore, in the present study, we examined p16 and cyclin E expression in biopsied human gastric cancer tissue using immunohistochemical staining. Figure 4(A) shows three representative strong positive (panels a,d), weak positive (panels b,e) and negative (panels c,f) staining patterns for p16. In Figure 4(A-D), the nuclei of the majority of cancer cells (>75%) were stained dark brown and were categorized as strong positive according to the evaluation scheme described in the Materials and Methods. In Figure 4(A-E), the nuclei of 25–50% of cancer cells were stained brown (weak positive). In Figure 4(A-F), <25% of cells were stained light brown (negative). Out of a total of 12 cases, three cases were strong positive, three cases were weak positive and six cases were negative. When the primary cultured cells were classified according to whether the IC50 was equal to or greater than the median value of 3.25 nM (high IC50 group) or lower than 3.25 nM (low IC50 group), p16 positivity in the low IC50 group was significantly higher than in the high IC50 group (P = 0.047; Fig. 4C). These results indicate that cancer cells with high expression of p16 had a low IC50 and were more sensitive to the pladienolide B derivative.
Figure 4.
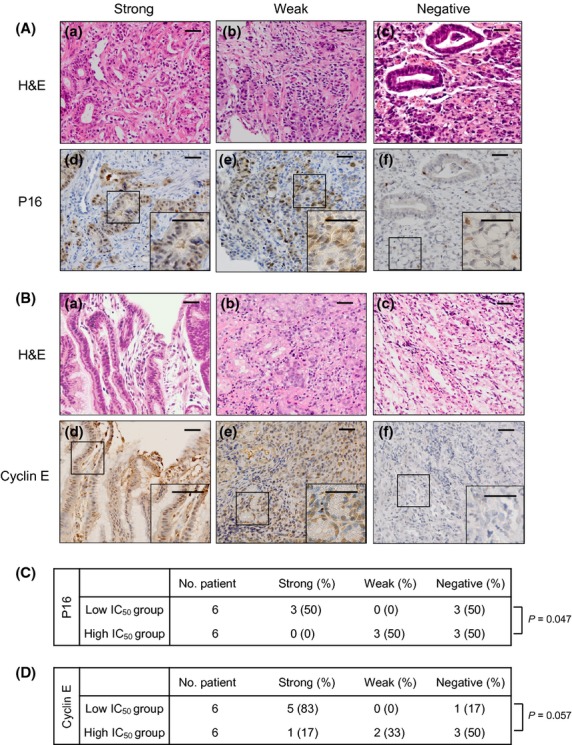
Immunohistochemical analysis for p16 or cyclin E expression in gastric cancer tissue. (A, B) Representative microphotographs of strong, weak and negative staining for p16 or cyclin E (original magnification, ×200). Panels a–c represent H&E staining. Panels d–f represent the corresponding immunohistochemical staining for p16 or cyclin E. A magnified view (×400) of the area in the square is shown in the inset at the lower right. (C, D) Summary of immunohistochemical staining for p16 or cyclin E. The low IC50 group was defined as cases with IC50 values lower than the median IC50 value of 3.25 nM. The high IC50 group was defined as cases with IC50 values equal to or greater than 3.25 nM. Bar, 100 μm.
Figure 4(B) shows the representative strong positive, weak positive and negative staining patterns for cyclin E. In a total of 12 cases, three cases were strong positive, three cases were weak positive and six cases were negative. In the low IC50 group, cyclin E positivity was marginally higher compared with the high IC50 group (Fig. 4D) (P = 0.057). These results indicate that cancer cells with high expression of cyclin E had a low IC50 and tend to be more sensitive to the pladienolide B derivative.
Discussion
In the present study, we showed for the first time that pladienolide B and its derivative have very high antitumor activity against gastric cancer. We also showed that these compounds were not only very active against cultured cell lines, but also against primary cultured cells from gastric cancer in vitro and in vivo. In particular, it is notable that all xenograft tumors of the 18 mice completely disappeared within 2 weeks and a pathological CR was confirmed with no relapse, suggesting that this pladienolide B derivative might be active enough to cure gastric cancer. The in vitro IC50 values of these primary cultured cells from cases 6, 8 and 9 were 2.0, 5.8 and 0.4 nM, respectively. The finding that xenograft tumors disappeared completely from case 8 with relatively high IC50 values (5.8 nM), as well from cases 6 and 9 with low IC50 values, suggests that pladienolide B derivative has very strong antitumor activity. Because the IC50 values in the majority of cases were equal to or <6.0 nM, similar results would be expected when xenograft experiments were performed using these primary cultured cells.
All primary cultured cells in the present study were cultivated from ascites of patients with carcinomatous peritonitis. In general, cancer cells in ascites fluid grow as single free cells that have lost homophilic cell-to-cell contact, a characteristic of diffuse-type gastric cancer. In this context, our data may suggest that pladienolide B and its derivative are very active against diffuse-type gastric cancer.
In preliminary experiments, the pladienolide B derivative was administered to mice at a dose of 2.5, 5, 10 or 20 mg/kg (n = 5 each) for 5 days. The mean bodyweight loss of each dosage was 7, 5, 10 and 19%, respectively. Therefore, in the present study, we set the pladienolide B derivative dosage at 10 mg/kg. As expected, no significant side-effects were observed. In future experiments, it will be necessary to evaluate and optimize the dose and dosing schedule of this agent as well as any drug-related side-effects.
It has been reported that pladienolide B inhibits splicing of pre-mRNA by binding to SF3b (SAP130) and that the expression of immature RNA of genes such as RIOK3 and DNAJB1 is increased by treatment with the pladienolide B derivative.9 In the present study, immature mRNA were apparently observed in the cultured cells and xenograft tumors following treatment with the pladienolide B derivative. Moreover, apoptosis was induced in the cultured cells by treatment with pladienolide B derivative in a dose-dependent manner. Apoptosis was also induced in the xenograft tumors of mice treated with this agent. Thus, it was confirmed that the mechanism of the pladienolide B derivative against gastric cancer involves inhibition of pre-mRNA splicing and apoptosis induction. It has been reported that spliceostatin A, another splicing inhibitor that binds SF3b, strongly inhibited splicing of MDM2 and produced several alternative splicing variants in a rhabdomyosarcoma cell line.23 Because MDM2 is closely associated with apoptosis inhibition,24 it is surmised that one mechanism by which pladienolide B derivative induces apoptosis is through impairment of MDM2.
Because several cell cycle regulatory factors are included in the splisceosome,9,22,25 we previously examined the association between pladienolide sensitivity and expression of these factors including cyclin E, p16, pRB and p53 in cancer cells. Our preliminary results showed that sensitivity to the pladienolide B derivative correlates with the expression of p16 and cyclin E in lung and breast cancer cell lines.22 In the present study, p16 positivity in gastric cancer tissues was significantly higher in the low IC50 group than in the high IC50 group (P = 0.047). Similarly, cyclin E positivity in the low IC50 group was marginally higher (P = 0.057) than in the high IC50 group. These results suggest the possibility that p16 and cyclin E might serve as predictive markers for sensitivity to pladienolide B derivative. However, the number of cases examined in the present study was not sufficient to be definitive and it is also known that gastric cancer cells sometimes exhibit heterogeneity within the cancer tissue. Therefore, the usefulness of p16 and cyclin E as biomarkers remains to be confirmed in a large-scale study.
In conclusion, the pladienolide B derivative showed very high antitumor activity against gastric cancer cell lines and primary cultured gastric cancer cells derived from ascites of patients with carcinomatous peritonitis. The mechanism of the antitumor activity of pladienolide B involves splicing impairment and apoptosis induction.
Acknowledgments
The authors are grateful to Eriko Aoyagi and Hiroko Nakanishi for their expert technical assistance.
Disclosure Statement
The authors have no conflict of interest.
Funding information
None declared.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics, 2011. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Jing JJ, Liu HY, Hao JK, et al. Gastric cancer incidence and mortality in Zhuanghe, China, between 2005 and 2010. World J Gastroenterol. 2012;18:1262–9. doi: 10.3748/wjg.v18.i11.1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cancer Statistics zrin Japan-2012. [Cited 29 Jul 2013.] Available from URL: http://ganjoho.jp/professional/statistics/backnumber/2012_jp.html.
- 4.Webb A, Cunningham D, Scarffe JH, et al. Randomized trial comparing epirubicin, cisplatin, and fluorouracil versus fluorouracil, doxorubicin, and methotrexate in advanced esophagogastric cancer. J Clin Oncol. 1997;15:261–7. doi: 10.1200/JCO.1997.15.1.261. [DOI] [PubMed] [Google Scholar]
- 5.Van Cutsem E, Moiseyenko VM, Tjulandin S, et al. Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: a report of the V325 Study Group. J Clin Oncol. 2006;24:4991–7. doi: 10.1200/JCO.2006.06.8429. [DOI] [PubMed] [Google Scholar]
- 6.Koizumi W, Narahara H, Hara T, et al. S-1 plus cisplatin versus S-1 alone for first-line treatment of advanced gastric cancer (SPIRITS trial): a phase III trial. Lancet Oncol. 2008;9:215–21. doi: 10.1016/S1470-2045(08)70035-4. [DOI] [PubMed] [Google Scholar]
- 7.Kaida D, Motoyoshi H, Tashiro E, et al. Spliceostatin A targets SF3b and inhibits both splicing and nuclear retention of pre-mRNA. Nat Chem Biol. 2007;3:576–83. doi: 10.1038/nchembio.2007.18. [DOI] [PubMed] [Google Scholar]
- 8.Hasegawa M, Miura T, Kuzuya K, et al. Identification of SAP155 as the target of GEX1A (Herboxidiene), an antitumor natural product. ACS Chem Biol. 2011;6:229–33. doi: 10.1021/cb100248e. [DOI] [PubMed] [Google Scholar]
- 9.Kotake Y, Sagane K, Owa T, et al. Splicing factor SF3b as a target of the antitumor natural product pladienolide. Nat Chem Biol. 2007;3:570–5. doi: 10.1038/nchembio.2007.16. [DOI] [PubMed] [Google Scholar]
- 10.Bonnal S, Vigevani L, Valcárcel J. The spliceosome as a target of novel antitumour drugs. Nat Rev Drug Discov. 2012;11:847–59. doi: 10.1038/nrd3823. [DOI] [PubMed] [Google Scholar]
- 11.Kaida D, Schneider-Poetsch T, Yoshida M. Splicing in oncogenesis and tumor suppression. Cancer Sci. 2012;103:1611–6. doi: 10.1111/j.1349-7006.2012.02356.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sakai T, Sameshima T, Matsufuji M, Kawamura N, Dobashi K, Mizui Y. Pladienolides, new substances from culture of Streptomyces platensis Mer-11107. I. Taxonomy, fermentation, isolation and screening. J Antibiot (Tokyo) 2004;57:173–9. doi: 10.7164/antibiotics.57.173. [DOI] [PubMed] [Google Scholar]
- 13.Sakai T, Asai N, Okuda A, Kawamura N, Mizui Y. Pladienolides, new substances from culture of Streptomyces platensis Mer-11107. II. Physico-chemical properties and structure elucidation. J Antibiot (Tokyo) 2004;57:180–7. doi: 10.7164/antibiotics.57.180. [DOI] [PubMed] [Google Scholar]
- 14.Mizui Y, Sakai T, Iwata M, et al. Pladienolides, new substances from culture of Streptomyces platensis Mer-11107. III. In vitro and in vivo antitumor activities. J Antibiot (Tokyo) 2004;57:188–96. doi: 10.7164/antibiotics.57.188. [DOI] [PubMed] [Google Scholar]
- 15.Asai N, Kotake Y, Niijima J, Fukuda Y, Uehara T, Sakai T. Stereochemistry of 6pladienolide B. J Antibiot (Tokyo) 2007;60:364–9. doi: 10.1038/ja.2007.49. [DOI] [PubMed] [Google Scholar]
- 16.Yokoi A, Kotake Y, Takahashi K, et al. Biological validation that SF3b is a target of the antitumor macrolide pladienolide. FEBS J. 2011;278:4870–80. doi: 10.1111/j.1742-4658.2011.08387.x. [DOI] [PubMed] [Google Scholar]
- 17.Iwata M, Ozawa Y, Uenaka T, et al. E7107, a new 7-urethan derivative of pladienplide D, displays curative effect against several human tumor xenografts. Proc Am Assoc Cancer Res. 2004;45:691-a. [Google Scholar]
- 18.Geran RI, Greenberg NH, Macdonald MM, Schumacher AM, Abbott BJ. Protocols for screening chemical agents and natural products against animal tumors and other biological systems. Cancer Chemother Rept. 1972;3:1–103. [Google Scholar]
- 19.Arrouss I, Nemati F, Roncal F, et al. Specific targeting of caspase-9/PP2A interaction as potential new anti-cancer therapy. PLoS ONE. 2013;8:e60816. doi: 10.1371/journal.pone.0060816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takeuchi H, Kimura T, Okamoto K, et al. A mechanism for abnormal angiogenesis in human radiation proctitis: analysis of expression profile for angiogenic factors. J Gastroenterol. 2012;47:56–64. doi: 10.1007/s00535-011-0470-2. [DOI] [PubMed] [Google Scholar]
- 21.Takayama T, Sato Y, Sagawa T, et al. Phase I study of S-1, docetaxel and cisplatin combination chemotherapy in patients with unresectable metastatic gastric cancer. Br J Cancer. 2007;97:851–6. doi: 10.1038/sj.bjc.6603957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iwata M, Uenaka T, Ozawa Y, Kotake Y, Mizui Y, Asada M. E7107: Antitumor activity on human SCLC and cervical cancer xenografts in relation to its potential predictive markers of response . [Cited 29 Jun 2013.] Available from URL: http://www.aacrmeetingabstracts.org/cgi/content/meeting_abstract/2007/1_Annual_Meeting/5608?maxtoshow=&hits=10&RESULTFORMAT=&fulltext=E7107&andorexactfulltext=and&searchid=1&FIRSTINDEX=0&sortspec=relevance&resourcetype=HWCIT.
- 23.Fan L, Lagisetti C, Edwards CC, Webb TR, Potter PM. Sudemycins, novel small molecule analogues of FR901464, induce alternative gene splicing. ACS Chem Biol. 2011;6:582–9. doi: 10.1021/cb100356k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oliner JD, Pietenpol JA, Thiagalingam S, Gyuris J, Kinzler KW, Vogelstein B. Oncoprotein MDM2 conceals the activation domain of tumour suppressor p53. Nature. 1993;362:857–60. doi: 10.1038/362857a0. [DOI] [PubMed] [Google Scholar]
- 25.Seghezzi W, Chua K, Shanahan F, Gozani O, Reed R, Lees E. Cyclin E associates with components of the pre-mRNA splicing machinery in mammalian cells. Mol Cell Biol. 1998;18:4526–36. doi: 10.1128/mcb.18.8.4526. [DOI] [PMC free article] [PubMed] [Google Scholar]