Abstract
Aromatase inhibitors have played a central role in endocrine therapy for estrogen receptor (ER)-positive breast cancer in postmenopausal women. However, factors predictive of the efficacy of aromatase inhibitors, and prognostic factors, both for early and late recurrence in women treated with adjuvant aromatase inhibitors have not been identified. Whole genome analysis identified that a TP53 gene mutation exists in ER-positive breast cancers, although the frequency of TP53 gene mutation in luminal tumors is lower compared with basal-like or human epidermal growth factor receptor type 2 (HER2)-positive breast cancers. We examined expression of p53, as well as ER, progesterone receptor, HER2 and Ki-67 using immunohistochemistry in postmenopausal ER-positive breast cancer patients who were treated with aromatase inhibitors as adjuvant endocrine therapy. There were 53 (21%) tumors that contained 10% or more p53-positive cells. High p53 expression was positively correlated with tumor grade, HER2 score and Ki-67 expression. Significant association was observed between disease-free survival and high p53 expression in multivariate analysis (P < 0.0001). Compared with women without recurrence, women with early recurrence had significantly higher p53 expression (P < 0.0001), as did women with late recurrence (P = 0.037). The present study demonstrates that p53 accumulation is a strong predictor of both early and late recurrence in ER-positive breast cancer patients treated with aromatase inhibitors as adjuvant endocrine therapy. TP53 gene alteration might be a key biological characteristic of ER-positive breast cancer.
Keywords: Aromatase inhibitor, breast cancer, endocrine therapy, p53, prognosis
Endocrine therapy has become the most important systemic treatment for women with estrogen receptor (ER)-positive breast cancer. Local aromatization of androgens to estrogens is the primary source of estradiol in the breasts of postmenopausal women. Results of recent clinical trials have shown that third-generation aromatase inhibitors, such as anastrozole, letrozole and exemestane, are superior to tamoxifen in the treatment of postmenopausal women with either early stage or metastatic breast cancer.1 Thus, aromatase inhibitors are now considered to be the gold standard endocrine therapy in postmenopausal women in both adjuvant and metastatic settings.2,3 Characterization of the risk of recurrence for patients who receive aromatase inhibitors is important for the selection of treatment. Although recent analyses found multiple mechanisms of aromatase inhibitor resistance,4 factors predictive of the efficacy of aromatase inhibitors have not been identified.
Most studies analyzing prognostic factors in postmenopausal ER-positive breast cancer have been performed in patients treated with tamoxifen as adjuvant endocrine therapy or in patients treated with either tamoxifen or aromatase inhibitors. Therefore, biomarkers predicting outcome after adjuvant aromatase inhibitors, but not tamoxifen, are not completely understood. Furthermore, most prognostic markers and multigene scores, such as Ki-67 and recurrence score, are factors for predicting early recurrence.5,6 However, approximately half of all disease recurrences occur after 5 years of adjuvant endocrine therapy7,8 and prognostic factors might differ between early and late relapse in patients treated with adjuvant aromatase inhibitors.
Recent whole genome analysis identified the presence of a TP53 gene mutation in 12% of luminal A and 32% of luminal B breast cancers,9 although the frequency of TP53 gene mutation in luminal tumors is lower compared with basal-like (84%) or human epidermal growth factor receptor type 2 (HER2)-positive (75%) breast cancers. Functional p53 plays an important role in maintaining genomic stability, regulating the cell cycle and inducing apoptosis.10 As mutated p53 accumulates in the nucleus of tumor cells, immunohistochemical (IHC) staining for p53 is frequently used as a surrogate marker for p53 mutational status. We previously reported that 20% of ER-positive breast cancer patients showed p53 accumulation by IHC11 and that p53 accumulation predicted resistance to endocrine therapy and decreased post-relapse survival in metastatic breast cancer.12 We also investigated p53 expression in pretreatment biopsy tissues and post-treatment surgical specimens in postmenopausal patients with ER-positive breast cancer who were treated with exemestane as neoadjuvant endocrine therapy.13 Although p53 expression was low in most pretreatment tumors, expression levels of p53 were decreased in post-treatment specimens compared with the values in the pretreatment biopsies. Many studies have been performed on the prognostic and predictive value of p53 in breast cancer; however, the role of TP53 mutation and p53 accumulation has not yet been identified.10 It is suggested that the role of p53 alteration might differ according to breast cancer subtypes and treatments.
In the present study, we examined expression of p53, as well as ER, progesterone receptor (PR), HER2 and Ki-67 using IHC in ER-positive breast cancer patients who were treated with aromatase inhibitors as adjuvant endocrine therapy. Correlations between p53 accumulation and expression levels of these biological markers and clinicopatholoical factors and prognosis were analyzed.
Materials and Methods
Patients and samples
A total of 287 postmenopausal women with stage I–III breast cancer treated with adjuvant aromatase inhibitors between 2001 and 2010 at Hokkaido University Hospital were recruited in the present study (Table1). The study protocol was approved by the institutional review board and conformed to the guidelines of the 1996 Declaration of Helsinki. Written informed consent for the use of the surgically resected tumor tissues was provided by all patients prior to treatments. The samples were chosen from a continuous series of ER-positive breast cancer. All patients had undergone mastectomy or lumpectomy. Patients who were positive for axillary lymph nodes received neoadjuvant or adjuvant chemotherapy. Pretreatment specimens obtained using core needle biopsies were used for immunohistochemical analysis in patients treated with neoadjuvant chemotherapy. Of the remaining patients, tumor samples were obtained during surgery. All patients received aromatase inhibitors (anastrozole, letrozole or exemestane) as adjuvant endocrine therapy. The median follow-up period was 71.8 months (range, 1–114 months).
Table 1.
Clinicopathological characteristics of patients and tumors
| No. patients | 287 |
| Age, mean ± SD (range) (years) | 62.9 ± 8.0 (47–89) |
| Body mass index, mean ± SD (range) | 23.9 ± 4.0 (13.5–38.5) |
| Tumor category | |
| 1 (≤2 cm) | 198 (69%) |
| 2 (2.1–5.0 cm) | 74 (26%) |
| 3 (>5.0 cm) | 11 (4%) |
| 4 | 4 (1%) |
| No. positive lymph nodes | |
| 0 | 208 (73%) |
| 1–3 | 55 (19%) |
| 4–9 | 15 (5%) |
| ≥10 | 6 (2%) |
| Unknown | 3 |
| Tumor grade | |
| 1 | 62 (22%) |
| 2 | 185 (64%) |
| 3 | 40 (14%) |
| Histological type | |
| Invasive ductal carcinoma | 248 (86%) |
| Invasive lobular carcinoma | 24 (8%) |
| Other | 15 (5%) |
| ER (Allred score) | |
| 3–5 | 12 (4%) |
| 6–8 | 275 (96%) |
| PR (Allred score) | |
| 0, 2 | 93 (32%) |
| 3–5 | 70 (24%) |
| 6–8 | 124 (43%) |
| HER2 score | |
| 0 | 124 (43%) |
| 1+ | 87 (30%) |
| 2+ | 58 (20%) |
| 3+ | 18 (6%) |
| Ki-67 LI | |
| <14% | 168 (66%) |
| ≥14% | 86 (34%) |
| Unknown | 33 |
| p53 | |
| 0 | 125 (50%) |
| <1% | 38 (15%) |
| 1–9% | 35 (14%) |
| ≥10% | 53 (21%) |
| Unknown | 36 |
| Dosing period of aromatase inhibitors, mean ± SD (range) (months) | 47.6 ± 20.1 (1–114) |
| Follow-up time, mean ± SD (range) (months) | 71.8 ± 34.3 (1–143) |
ER, estrogen receptor; HER2, human epidermal growth factor receptor type 2; LI, labeling index; PR, progesterone receptor.
Immunohistochemical analysis
One 4-μm section of each submitted paraffin block was stained first with hematoxylin–eosin to verify that an adequate number of carcinoma cells were present and that the fixation quality was adequate for IHC analysis. Serial sections (4 μm) were prepared from selected blocks and float-mounted on adhesive-coated glass slides for IHC13. The IHC status of ER, PR and HER2 was determined using the PATHWAY rabbit monoclonal antibodies (clone SP1, 1E2 and 4B5, respectively) and iView DAB Detection Kit (Ventana Medical Systems, Inc., Tucson, AZ, USA). Expression of ER and PR were scored by assigning proportion and intensity scores, according to Allred's procedure.14 In brief, a proportion score represented the estimated proportion of tumor cells staining positive as follows: 0, none; 1, <1/100; 2, 1/100–1/10; 3, 1/10–1/3; 4, 1/3–2/3; and 5, >2/3. Any brown nuclear staining in breast epithelium counted towards the proportion score. An intensity score represented the average intensity of the positive cells as follows: 0, none; 1, weak; 2, intermediate; and 3, strong. The proportion and intensity scores were then added to obtain a total score that could range from 0 to 8. Tumors with ≥1% positive cells (proportion score ≥2) were evaluated as positive. To determine the level of HER2 expression, the membrane staining pattern was estimated and scored on a scale of 0 to 3+. For Ki-67 and p53 staining, antigens were retrieved in Dako EnVision FLEX Target Retrieval Solution, high pH (pH 9.0), using Dako PT Link for 20 min at 97°C according to the manufacturer's instructions (Dako, Glostrup, Denmark). The IHC for Ki-67 was performed using a mouse monoclonal anti-human Ki-67 antibody (MIB-1, Dako) at 1:200 dilution for 30 min at room temperature and the Dako Envision FLEX system was used for visualization. The labeling index (LI) was assessed as the percentage of tumor cells showing definite nuclear staining among >1000 invasive tumor cells15 analyzed using NanoZoomer 2.0-HT (Hamamatsu photonics, Hamamatsu, Japan) for slide scanning and Tissue Studio (Definiens, Munich, Germany) for automated scoring. If there were clear hot spots, data from these areas were assessed. The IHC for p53 was performed using a mouse monoclonal anti-human p53 antibody (DO-7, Dako) at 1:200 dilution for 30 min at room temperature with the Dako Envision FLEX system for visualization. p53 protein expression was measured as the percentage of cells showing definite nuclear staining. p53 was considered positive if there was ≥10% positive nuclear staining regardless of the intensity.12,16–18 Representative images of staining for Ki-67 and p53 are shown in Fig.1.
Figure 1.
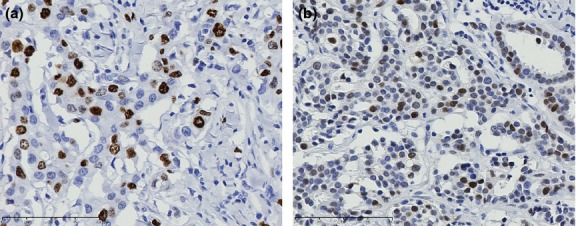
Immunohistochemical examination of Ki-67 (a) and p53 (b) in invasive carcinoma. Representative results of positive staining are shown. Nuclear staining of Ki-67 (a) and p53 (b) can be observed in breast cancer cells.
Preparation of formalin-fixed, paraffin-embedded cell blocks
Human cancer cell lines (Table2) were cultured in Dulbecco's modified Eagle medium with 10% fetal bovine serum (H1299, Hela, MCF-7, SK-BR-3, MDA-MB-231 and MDA-MB-468 cells) or RPMI 1640 with 10% fetal bovine serum (H2228, Lovo, T47D and BT-474 cells). All cell lines except MCF-7 and Hela cells were purchased from ATCC (Manassas, VA, USA). MCF-7 and Hela cells were purchased from RIKEN Cell Bank (Tsukuba, Japan). Cells were allowed to reach semiconfluence, then collected and fixed in 10% neutral formalin for 15 min. Cell blocks were prepared by embedding cells in 1% sodium alginate (Wako Pure Chemical Industries, Osaka, Japan) in a 1 M calcium chloride solution and processing as a piece of tissue. The IHC staining for p53 was performed using the same method as that used for breast cancer tissues.
Table 2.
p53 mutations in cancer cell lines
| Cell line | Primary site | Exon | Codon | Type | Nucleotide change | Residue change |
|---|---|---|---|---|---|---|
| H1299 | Lung | Null | ||||
| H2228 | Lung | Wild-type | ||||
| Lovo | Colon | Wild-type | ||||
| Hela | Cervix | Wild-type | ||||
| MCF-7 | Breast | Wild-type | ||||
| T47D | Breast | 6 | 194 | Missense | CTT → TTT | Leu (L) → Phe (F) |
| SK-BR-3 | Breast | 5 | 175 | Missense | CGC → CAC | Arg (R) → His (H) |
| BT-474 | Breast | 8 | 285 | Missense | GAG → AAG | Glu (E) → Lys (K) |
| MDA-MB-231 | Breast | 8 | 280 | Missense | AGA → AAA | Arg (R) → Lys (K) |
| MDA-MB-468 | Breast | 8 | 273 | Missense | CGT → CAT | Arg (R) → His (H) |
Statistical analysis
The Chi-squared test and Mann–Whitney U-test were used to study the relationships between p53 expression and clinicopathological characteristics or molecular markers and to compare characteristics among patients with early and late recurrence and without recurrence. Cox's proportional hazards model was used for univariate and multivariate analyses of prognostic values. Estimation of survival was performed using the Kaplan–Meier method and differences between survival curves were assessed uisng the log-rank test.
Results
p53 accumulation in breast cancer cells with missense mutation of TP53 gene
We first analyzed p53 expression by immunohistochemistry in a selection of cancer cell lines using formalin-fixed, paraffin-embedded cell block materials (Table2, Fig.2). Negative or week nuclear staining in only a few cells was observed in H1299 p53 null cells and in H2228, Lovo, Hela and MCF-7 cells without the TP53 gene mutation (Fig.2, upper panel). In contrast, strong nuclear staining in all cells was seen in T47D, SK-BR-3, BT-474, MDA-MB-231 and MDA-MB-468 cells with missense mutations of the TP53 gene (Fig.2, lower panel).
Figure 2.
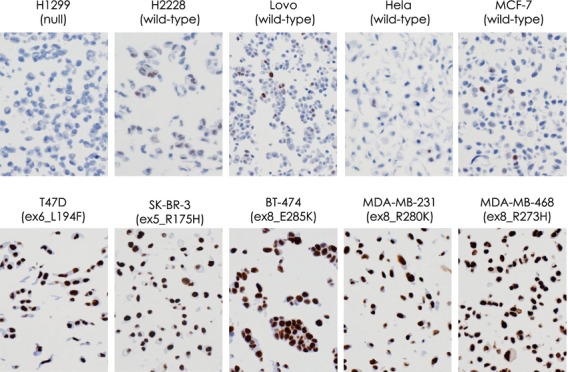
Immunohistochemical examination of p53 in various cancer cell lines using formalin-fixed, paraffin-embedded cell block materials. Representative results are shown. Negative or only weak staining in a few cells is observed in H1299 p53 null cells and in H2228, Lovo, Hela and MCF-7 cells that do not have the TP53 gene mutation (upper panel), whereas nuclear staining can be observed in all cells in T47D, SK-BR-3, BT-474, MDA-MB-231 and MDA-MB-468 cells that have missense mutations of the TP53 gene (lower panel).
p53 protein accumulation in postmenopausal ER-positive breast cancer
In 251 tumors that were analyzed for p53 expression, no expression was detected in 125 (50%) tumors (Table1). Of the remainder, 53 (21%) tumors contained 10% or more p53-positive cells. The cut-off points for p53 expression were set at 10% in previous studies that reported on the prognostic value of p53 accumulation in ER-positive breast cancer.12,16,17 Therefore, we compared clinicopathological factors and expression of ER, PR, HER2 and Ki-67 between tumors with high p53 expression (10% or more positive cells) and those with low p53 expression (<10% positive cells). High p53 expression was positively correlated with tumor grade (P = 0.001), HER2 score (P = 0.0004) and Ki-67 expression (P < 0.0001) and negatively correlated with age (P = 0.03; Table3).
Table 3.
Correlation between p53 expression and clinicopathological factors
| p53 < 10% | p53 ≥ 10% | P-value | ||
|---|---|---|---|---|
| No. of patients | 198 | 53 | ||
| Age, years | Mean ± SD (range) | 63.4 ± 8.2 (47–89) | 60.9 ± 6.9 (48–77) | 0.03* |
| Body mass index | Mean ± SD (range) | 23.7 ± 3.9 (13.5–37.7) | 23.9 ± 3.9 (14.5–33.3) | 0.66 |
| Tumor category | 1 (≤2 cm) | 137 (69%) | 39 (74%) | 0.52 |
| 2 (2.1–5.0 cm) | 51 (26%) | 12 (23%) | ||
| 3 (>5.0 cm) | 6 (3%) | 2 (4%) | ||
| 4 | 4 (2%) | 0 | ||
| No. of positive lymph nodes | 0 | 147 (75%) | 33 (62%) | 0.053 |
| 1–3 | 35 (18%) | 14 (26%) | ||
| 4–9 | 11 (6%) | 4 (8%) | ||
| ≥10 | 2 (1%) | 2 (4%) | ||
| Unknown | 3 | 0 | ||
| Tumor grade | 1 | 47 (24%) | 6 (11%) | 0.001* |
| 2 | 133 (67%) | 33 (62%) | ||
| 3 | 18 (9%) | 14 (26%) | ||
| Histological type | Invasive ductal carcinoma | 169 (85%) | 50 (94%) | 0.15 |
| Invasive lobular carcinoma | 20 (10%) | 1 (2%) | ||
| Others | 9 (5%) | 2 (4%) | ||
| ER (Allred score) | 3–5 | 6 (3%) | 2 (4%) | 0.15 |
| 6–8 | 192 (97%) | 51 (96%) | ||
| PgR (Allred score) | 0–2 | 56 (28%) | 23 (43%) | 0.12 |
| 3–5 | 52 (26%) | 9 (17%) | ||
| 6–8 | 90 (45%) | 21 (40%) | ||
| HER2 score | 0 | 96 (48%) | 16 (30%) | 0.0004* |
| 1+ | 62 (31%) | 13 (25%) | ||
| 2+ | 34 (17%) | 18 (34%) | ||
| 3+ | 6 (3%) | 6 (11%) | ||
| Ki-67 LI | <14% | 140 (75%) | 20 (38%) | <0.0001* |
| ≥14% | 47 (25%) | 32 (62%) | ||
| Unknown | 11 | 1 | ||
| Dosing period of aromatase inhibitors (months) | Mean ± SD (range) | 50.9 ± 18.9 (1–114) | 40.3 ± 21.1 (2–60) | |
| Follow-up time (months) | Mean ± SD (range) | 72.9 ± 31.2 (1–143) | 69.3 ± 39.0 (5–140) |
P < 0.05 is considered significant.
p53 accumulation is a strong prognostic factor in ER-positive breast cancer patients treated with adjuvant aromatase inhibitors
Univariate analysis demonstrated significant associations between disease-free survival and tumor size, number of positive lymph nodes and expression of Ki-67 and p53 (P < 0.0001, P < 0.0001, P = 0.001, P < 0.0001, respectively; Table4). In the multivariate analysis, significant associations were observed between disease-free survival and high p53 expression (P = 0.001), as well as tumor size (P = 0.001), suggesting that p53 accumulation might be a strong prognostic factor in postmenopausal ER-positive breast cancer patients treated with aromatase inhibitors as adjuvant endocrine therapy. Kaplan–Meier analysis showed that high expression of Ki-67 (≥14%) was significantly associated with decreased disease-free survival (P = 0.0007; Fig.3a). Furthermore, high expression of p53 (≥10%) was strongly and significantly associated with decreased disease-free survival (P < 0.0001; Fig.3b). Combination analysis of expression status for Ki-67 and p53 revealed that patients whose tumors showed both high Ki-67 and high p53 expression had significantly shorter disease-free survival compared with patients whose tumors showed both low Ki-67 and low p53 expression (P < 0.0001; Fig.3c).
Table 4.
Univariate and multivariate analysis of factors predicting disease-free survival
| Univariate |
Multivariate |
|||||
|---|---|---|---|---|---|---|
| RR | 95% CI | P-value | RR | 95% CI | P-value | |
| Age | 0.99 | 0.95–1.04 | 0.71 | |||
| Body mass index | 0.94 | 0.87–1.04 | 0.26 | |||
| Tumor category | 2.53 | 1.71–3.74 | <0.0001* | 2.3 | 1.39–3.78 | 0.001* |
| Lymph nodes status | 2.28 | 1.60–3.25 | <0.0001* | 1.46 | 0.95–2.26 | 0.09 |
| Tumor grade | 1.63 | 0.91–2.93 | 0.10 | |||
| ER | 1.02 | 0.70–1.48 | 0.93 | |||
| PgR | 0.76 | 0.51–1.14 | 0.18 | |||
| HER2 score | 1.07 | 0.75–1.53 | 0.71 | |||
| Ki-67 LI < 14% | 3.39 | 1.60–7.19 | 0.001* | 1.71 | 0.72–4.04 | 0.22 |
| p53 < 10% | 5.66 | 2.69–11.89 | <0.0001* | 4.82 | 2.01–11.52 | 0.001* |
| Dosing period of aromatase inhibitors (months) | 0.95 | 0.93–0.96 | <0.0001* | |||
| Chemotherapy | 2.06 | 1.00–4.24 | 0.051 | |||
P < 0.05 is considered significant. CI, Confidence interval; ER, estrogen receptor; HER2, human epidermal growth factor receptor type 2; LI, labeling index; PR, progesterone receptor; RR, relative risk.
Figure 3.
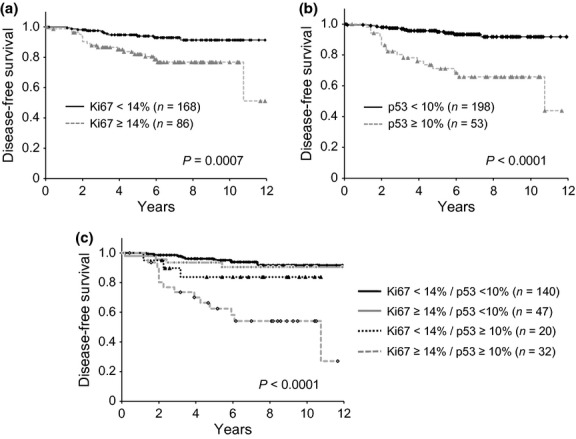
Disease-free survival according to expression of Ki-67 (a), p53 (b) and combination subgroups of Ki-67 and p53 (c) in estrogen-positive breast cancer patients treated with aromatase inhibitors as adjuvant endocrine therapy.
p53 accumulation is associated with both early and late recurrence in ER-positive breast cancer patients treated with adjuvant aromatase inhibitors
Next we analyzed whether p53 expression, as well as clinicopathological and biological factors predicts early recurrence (within 5 years) or late recurrence (after 5 years). Clinicopathological factors and expression of biological markers were compared between patients with no recurrence, early recurrence and late recurrence who were treated between 2001 and 2008 (Table5). Compared with women without recurrence, women with early recurrence had significantly larger tumor size (P = 0.0071), a higher number of positive lymph nodes (P = 0.0039) and higher expression levels of Ki-67 (P = 0.049) and p53 (P < 0.0001) compared with women without recurrence. In contrast, women with late recurrence had significantly larger tumor size (P = 0.045) and higher expression levels of p53 (P = 0.037) compared with those without recurrence. Only tumor size (P = 0.04) was significantly associated with disease-free survival using univariate analysis in patients with late recurrence and in those without recurrence. Lymph node status and Ki-67 expression did not differ between women with late recurrence and women without recurrence. Moreover, tumor grade and expression levels of ER, PR and HER2 were not different among women with early and late recurrence and women without recurrence.
Table 5.
Clinicopathological characteristics of patients and tumors according to recurrence pattern
| Early recurrence (within 5 years) | Late recurrence (after 5 years) | No recurrence | Early vs No | Late vs No | Early vs Late | |
|---|---|---|---|---|---|---|
| No. patients | 22 | 6 | 200 | |||
| Age, mean ± SD (years) | 61.5 ± 7.9 | 63.7 ± 4.5 | 62.6 ± 8.1 | 0.48 | 0.52 | 0.31 |
| Body mass index, mean ± SD | 23.7 ± 4.4 | 21.5 ± 1.9 | 24.0 ± 4.1 | 0.73 | 0.063 | 0.26 |
| Tumor category | ||||||
| 1 (≤2 cm) | 12 (55%) | 2 (33%) | 146 (73%) | 0.0071* | 0.045* | 0.95 |
| 2 (2.1–5.0 cm) | 6 (27%) | 4 (67%) | 48 (24%) | |||
| 3 (>5.0 cm) | 3 (14%) | 0 (0%) | 5 (3%) | |||
| 4 | 2 (9%) | 0 (0%) | 1 (1%) | |||
| No. positive lymph nodes | ||||||
| 0 | 11 (52%) | 3 (50%) | 152 (76%) | 0.0039* | 0.17 | 0.63 |
| 1–3 | 4 (19%) | 3 (50%) | 36 (18%) | |||
| 4–9 | 3 (14%) | 0 (0%) | 9 (5%) | |||
| ≥10 | 3 (14%) | 0 (0%) | 1 (1%) | |||
| Unknown | 1 | 0 | 2 | |||
| Tumor grade | ||||||
| 1 | 3 (14%) | 0 (0%) | 37 (19%) | 0.30 | 0.11 | 0.39 |
| 2 | 14 (64%) | 4 (67%) | 135 (68%) | |||
| 3 | 5 (23%) | 2 (33%) | 28 (14%) | |||
| Histological type | ||||||
| Invasive ductal carcinoma | 19 (86%) | 6 (100%) | 173 (87%) | 0.93 | 0.63 | 0.63 |
| Invasive lobular carcinoma | 2 (9%) | 0 (0%) | 15 (8%) | |||
| Other | 1 (5%) | 0 (0%) | 12 (6%) | |||
| ER (Allred score) | ||||||
| 3–5 | 1 (5%) | 0 (0%) | 7 (4%) | 0.66 | 0.50 | 0.70 |
| 6–8 | 21 (95%) | 6 (100%) | 193 (97%) | |||
| PR (Allred score) | ||||||
| 0, 2 | 10 (45%) | 3 (50%) | 60 (30%) | 0.32 | 0.57 | 0.98 |
| 3–5 | 4 (18%) | 1 (17%) | 53 (27%) | |||
| 6–8 | 8 (36%) | 2 (33%) | 87 (44%) | |||
| HER2 score | ||||||
| 0 | 11 (50%) | 2 (33%) | 85 (43%) | 0.86 | 0.38 | 0.46 |
| 1+ | 4 (18%) | 1 (17%) | 55 (28%) | |||
| 2+ | 4 (18%) | 2 (33%) | 47 (24%) | |||
| 3+ | 3 (14%) | 1 (17%) | 13 | |||
| Ki-67 LI | ||||||
| <14% | 9 (47%) | 2 (33%) | 124 (70%) | 0.049* | 0.060 | 0.55 |
| ≥14% | 10 (53%) | 4 (67%) | 54 (30%) | |||
| Unknown | 3 | 0 | 22 | |||
| p53 | ||||||
| 0 | 5 (26%) | 1 (17%) | 91 (53%) | <0.0001* | 0.037* | 0.73 |
| <1% | 2 (11%) | 1 (17%) | 27 (16%) | |||
| 1–9% | 1 (5%) | 1 (17%) | 26 (15%) | |||
| ≥10% | 11 (58%) | 3 (50%) | 29 (17%) | |||
| Unknown | 3 | 0 | 27 | |||
| Chemotherapy | ||||||
| None | 13 (59%) | 6 (100%) | 163 (82%) | 0.014* | 0.24 | 0.057 |
| Done | 9 (41%) | 0 (0%) | 37 (19%) | |||
| Dosing period of aromatase inhibitors, mean ± SD (months) | 24.7 ± 13.7 | 52.0 ± 19.6 | 52.6 ± 20.0 | <0.0001* | 0.85 | 0.0064* |
| Follow-up time, mean ± SD (months) | 29.7 ± 15.7 | 82.8 ± 23.9 | 80.6 ± 32.8 | |||
P < 0.05 is considered significant. ER, estrogen receptor; HER2, human epidermal growth factor receptor type 2; LI, labeling index; PR, progesterone receptor.
Discussion
We investigated expression of p53, as well as expression of ER, PR, HER2 and Ki-67 using IHC in postmenopausal ER-positive breast cancer patients who were treated with aromatase inhibitors as adjuvant endocrine therapy. The present study demonstrates that p53 accumulation is a strong predictor of both early and late recurrence.
Recent whole genome analysis demonstrated that TP53 gene mutation is one of the most frequent somatic mutations in breast cancer.9 Because frequencies of TP53 gene mutation differ in luminal A (12%) and luminal B (32%) tumors, p53 gene alteration should have some role in characterization, such as the response to treatments and prognosis in ER-positive breast cancer. The correlation between p53 accumulation measured using IHC and p53 mutation detected using sequencing has been estimated to be <75% in breast cancer.19 Not all mutations yield a stable protein and some mutations lead to a truncated protein not detected using IHC. Done and colleagues demonstrated strong p53 nuclear staining in all tumors known to have missense mutations, but in none of the tumors with truncation mutations.20 Our results using a variety of cancer cell lines also showed strong nuclear accumulation of p53 in cells with missense mutations, but not in cells without mutations. In contrast, wild-type p53 might accumulate in some tumors as a result of a response to DNA damage or by binding to other cellular proteins, giving a positive IHC result.10 It has been reported that p53 protein accumulation detected using IHC is present in non-invasive lesions surrounding p53 mutation positive breast cancers, suggesting that p53 mutation occurs prior to invasion in breast carcinogenesis.20
The clinical role of p53 alteration, both mutation and protein accumulation, in breast cancer might differ according to subtype and treatment. Because IHC analysis is more practical for application in the clinic than mutational analysis, the role of p53 accumulation should be analyzed in ER-positive breast cancer by treatment. In the present study, we examined p53 accumulation in postmenopausal ER-positive early breast cancer patients who were treated with aromatase inhibitors as adjuvant endocrine therapy. Our results demonstrate that p53 accumulation is correlated with an aggressive phenotype, such as high tumor grade and high Ki-67 expression. Moreover, patients with high p53 expression had significantly shorter disease-free survival. Importantly, the prognostic value of p53 accumulation was much higher than that of Ki-67 expression (P < 0.0001 vs P = 0.0007, by the log-rank test). Moreover, p53 accumulation, but not Ki-67 expression, was significantly associated with disease-free survival in multivariate analysis.
In the present study, we demonstrate that high expression of both Ki-67 and p53 is predictive of early recurrence in women treated with aromatase inhibitors as adjuvant endocrine therapy. It was recently reported that Mammostrat, an immunohistochemical multigene assay that can analyze positivity for p53, HTF9C, CEACAM5, NDRG1 and SLC7A5, predicted early relapse risk for patients treated with exemestane and patients treated with tamoxifen followed by exemestane.21 Recent studies also indicated that Ki-67 expression, IHC4 and multigene assays, such as OncotypeDx (recurrence score) and PAM50, were useful for predicting risk of recurrence after endocrine therapy.22 Moreover, Ki-67 expression and a recurrence score predict the benefit of chemotherapy.23–25 In contrast, it has been indicated that the predictive significance of Ki-67 expression and a recurrence score is mostly limited to the first 5 years (early recurrence)5,6 and these factors might not be useful to predict late recurrence. Viale and colleagues demonstrated that the greatest benefit of letrozole relative to tamoxifen with higher levels of Ki-67 LI was observed according to 4-year disease-free survival and that the risk of a disease-free survival event was reduced by approximately half in favor of letrozole for higher levels of Ki-67, which was a treatment effect of greater magnitude compared with patients with tumors having low levels of Ki-67 LI.26 Since the median follow-up time of the BIG 1-98 study by Viale et al. was 51 months, any predictive role of Ki-67 for late recurrence (beyond 5 years) in women treated with adjuvant letrozole could not be evaluated. In contrast, most studies analyzing biomarkers for late recurrence were performed in patients treated mainly with tamoxifen as adjuvant endocrine therapy.27–29 In the present study, we analyzed prognostic factors for patients treated with aromatase inhibitors only as adjuvant endocrine therapy. Our results indicate that high p53 expression, but not Ki-67 expression, is a predictor of late recurrence.
Ellis and colleagues studied pretreatment tumor biopsies from patients treated with neoadjuvant aromatase inhibitor therapy using whole-genome analysis.30 They identified that 38% of the aromatase inhibitor-resistant group had mutations in the TP53 pathway and that TP53 mutations were significantly enriched in luminal B tumors and correlated with higher Ki-67 levels and higher tumor grade. In addition to biological alteration in the tumors, it has been indicated that host factors, such as ineffective inhibition of aromatase and alternative sources of estrogen/estrogenic hormones, cause resistance to aromatase inhibitors.4 Recent analysis demonstrated that obese women treated with anastrozole rather than tamoxifen as adjuvant therapy had a poorer prognosis, suggesting that body mass index might affect risk of recurrence after adjuvant endocrine therapy.31
In conclusion, we demonstrated that p53 accumulation is a strong predictor of both early and late recurrence in ER-positive breast cancer patients treated with aromatase inhibitors as adjuvant endocrine therapy. TP53 gene alteration might therefore be a key biological characteristic of ER-positive breast cancer.
Acknowledgments
The authors thank Mr Jun Moriya and Mrs Mikiko Sato for their technical assistance.
Disclosure Statement
The authors have no conflict of interest.
Funding information
None declared.
References
- 1.Iwase H. Current topics and perspectives on the use of aromatase inhibitors in the treatment of breast cancer. Breast Cancer. 2008;15:278–90. doi: 10.1007/s12282-008-0071-y. [DOI] [PubMed] [Google Scholar]
- 2.Burstein HJ, Prestrud AA, Seidenfeld J, et al. American Society of Clinical Oncology clinical practice guideline: update on adjuvant endocrine therapy for women with hormone receptor-positive breast cancer. J Clin Oncol. 2010;28:3784–96. doi: 10.1200/JCO.2009.26.3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cardoso F, Senkus-Konefka E, Fallowfield L, et al. Locally recurrent or metastatic breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;21(Suppl 5):v15–9. doi: 10.1093/annonc/mdq160. [DOI] [PubMed] [Google Scholar]
- 4.Miller WR, Larionov AA. Understanding the mechanisms of aromatase inhibitor resistance. Breast Cancer Res. 2012;14:201. doi: 10.1186/bcr2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dowsett M, Cuzick J, Wale C, et al. Prediction of risk of distant recurrence using the 21-gene recurrence score in node-negative and node-positive postmenopausal patients with breast cancer treated with anastrozole or tamoxifen: a TransATAC study. J Clin Oncol. 2010;28:1829–34. doi: 10.1200/JCO.2009.24.4798. [DOI] [PubMed] [Google Scholar]
- 6.Cuzick J, Dowsett M, Pineda S, et al. Prognostic value of a combined estrogen receptor, progesterone receptor, Ki-67, and human epidermal growth factor receptor 2 immunohistochemical score and comparison with the Genomic Health recurrence score in early breast cancer. J Clin Oncol. 2011;29:4273–8. doi: 10.1200/JCO.2010.31.2835. [DOI] [PubMed] [Google Scholar]
- 7.Cuzick J, Sestak I, Baum M, et al. Effect of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: 10-year analysis of the ATAC trial. Lancet Oncol. 2010;11:1135–41. doi: 10.1016/S1470-2045(10)70257-6. [DOI] [PubMed] [Google Scholar]
- 8.Davies C, Godwin J, Gray R, et al. Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet. 2011;378:771–84. doi: 10.1016/S0140-6736(11)60993-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.The Cancer Genome Atlas Network. Comprehensive molecular portraits of human breast tumours. Nature. 2012;490:61–70. doi: 10.1038/nature11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lacroix M, Toillon RA, Leclercq G. p53 and breast cancer, an update. Endocr Relat Cancer. 2006;13:293–325. doi: 10.1677/erc.1.01172. [DOI] [PubMed] [Google Scholar]
- 11.Yamashita H, Nishio M, Toyama T, et al. Coexistence of HER2 over-expression and p53 protein accumulation is a strong prognostic molecular marker in breast cancer. Breast Cancer Res. 2004;6:R24–30. doi: 10.1186/bcr738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yamashita H, Toyama T, Nishio M, et al. p53 protein accumulation predicts resistance to endocrine therapy and decreased post-relapse survival in metastatic breast cancer. Breast Cancer Res. 2006;8:R48. doi: 10.1186/bcr1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yamashita H, Takahashi S, Ito Y, et al. Predictors of response to exemestane as primary endocrine therapy in estrogen receptor-positive breast cancer. Cancer Sci. 2009;100:2028–33. doi: 10.1111/j.1349-7006.2009.01274.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Allred DC, Harvey JM, Berardo M, et al. Prognostic and predictive factors in breast cancer by immunohistochemical analysis. Mod Pathol. 1998;11:155–68. [PubMed] [Google Scholar]
- 15.Dowsett M, Nielsen TO, A'Hern R, et al. Assessment of Ki67 in breast cancer: recommendations from the International Ki67 in Breast Cancer working group. J Natl Cancer Inst. 2011;103:1656–64. doi: 10.1093/jnci/djr393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kobayashi T, Iwaya K, Moriya T, et al. A simple immunohistochemical panel comprising 2 conventional markers, Ki67 and p53, is a powerful tool for predicting patient outcome in luminal-type breast cancer. BMC Clin Pathol. 2013;13:5. doi: 10.1186/1472-6890-13-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Millar EK, Graham PH, McNeil CM, et al. Prediction of outcome of early ER+ breast cancer is improved using a biomarker panel, which includes Ki-67 and p53. Br J Cancer. 2011;105:272–80. doi: 10.1038/bjc.2011.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jung SY, Jeong J, Shin SH, et al. Accumulation of p53 determined by immunohistochemistry as a prognostic marker in node negative breast cancer; analysis according to St Gallen consensus and intrinsic subtypes. J Surg Oncol. 2011;103:207–11. doi: 10.1002/jso.21819. [DOI] [PubMed] [Google Scholar]
- 19.Norberg T, Lennerstrand J, Inganas M, et al. Comparison between p53 protein measurements using the luminometric immunoassay and immunohistochemistry with detection of p53 gene mutations using cDNA sequencing in human breast tumors. Int J Cancer. 1998;79:376–83. doi: 10.1002/(sici)1097-0215(19980821)79:4<376::aid-ijc12>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 20.Done SJ, Arneson CR, Ozcelik H, et al. P53 protein accumulation in non-invasive lesions surrounding p53 mutation positive invasive breast cancers. Breast Cancer Res Treat. 2001;65:111–8. doi: 10.1023/a:1006425809069. [DOI] [PubMed] [Google Scholar]
- 21.Bartlett JM, Bloom KJ, Piper T, et al. Mammostrat as an immunohistochemical multigene assay for prediction of early relapse risk in the tamoxifen versus exemestane adjuvant multicenter trial pathology study. J Clin Oncol. 2012;30:4477–84. doi: 10.1200/JCO.2012.42.8896. [DOI] [PubMed] [Google Scholar]
- 22.Dowsett M, Sestak I, Lopez-Knowles E, et al. Comparison of PAM50 risk of recurrence score with oncotype DX and IHC4 for predicting risk of distant recurrence after endocrine therapy. J Clin Oncol. 2013;31:2783–90. doi: 10.1200/JCO.2012.46.1558. [DOI] [PubMed] [Google Scholar]
- 23.Hugh J, Hanson J, Cheang MC, et al. Breast cancer subtypes and response to docetaxel in node-positive breast cancer: use of an immunohistochemical definition in the BCIRG 001 trial. J Clin Oncol. 2009;27:1168–76. doi: 10.1200/JCO.2008.18.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Penault-Llorca F, Andre F, Sagan C, et al. Ki67 expression and docetaxel efficacy in patients with estrogen receptor-positive breast cancer. J Clin Oncol. 2009;27:2809–15. doi: 10.1200/JCO.2008.18.2808. [DOI] [PubMed] [Google Scholar]
- 25.Paik S, Tang G, Shak S, et al. Gene expression and benefit of chemotherapy in women with node-negative, estrogen receptor-positive breast cancer. J Clin Oncol. 2006;24:3726–34. doi: 10.1200/JCO.2005.04.7985. [DOI] [PubMed] [Google Scholar]
- 26.Viale G, Giobbie-Hurder A, Regan MM, et al. Prognostic and predictive value of centrally reviewed Ki-67 labeling index in postmenopausal women with endocrine-responsive breast cancer: results from Breast International Group Trial 1-98 comparing adjuvant tamoxifen with letrozole. J Clin Oncol. 2008;26:5569–75. doi: 10.1200/JCO.2008.17.0829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang Y, Schnabel CA, Schroeder BE, et al. Breast cancer index identifies early-stage estrogen receptor-positive breast cancer patients at risk for early- and late-distant recurrence. Clin Cancer Res. 2013;19:4196–205. doi: 10.1158/1078-0432.CCR-13-0804. [DOI] [PubMed] [Google Scholar]
- 28.Dubsky P, Filipits M, Jakesz R, et al. EndoPredict improves the prognostic classification derived from common clinical guidelines in ER-positive, HER2-negative early breast cancer. Ann Oncol. 2013;24:640–7. doi: 10.1093/annonc/mds334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ahn SG, Lee HM, Cho SH, et al. The difference in prognostic factors between early recurrence and late recurrence in estrogen receptor-positive breast cancer: nodal stage differently impacts early and late recurrence. PLoS ONE. 2013;8:e63510. doi: 10.1371/journal.pone.0063510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ellis MJ, Ding L, Shen D, et al. Whole-genome analysis informs breast cancer response to aromatase inhibition. Nature. 2012;486:353–60. doi: 10.1038/nature11143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sestak I, Distler W, Forbes JF, et al. Effect of body mass index on recurrences in tamoxifen and anastrozole treated women: an exploratory analysis from the ATAC trial. J Clin Oncol. 2010;28:3411–5. doi: 10.1200/JCO.2009.27.2021. [DOI] [PubMed] [Google Scholar]


