Abstract
Patients with extrahepatic cholangiocarcinoma (EHCC) have a poor prognosis; postoperative survival depends on cancer progression and therapeutic resistance. The mechanism of EHCC progression needs to be clarified to identify ways to improve disease prognosis. Stathmin1 (STMN1) is a major cytosolic phosphoprotein that regulates microtubule dynamics and is associated with malignant phenotypes and chemoresistance in various cancers. Recently, STMN1 was reported to interact with p27, an inhibitor of cyclin-dependent kinase complexes. Eighty EHCC cases were studied using immunohistochemistry and clinical pathology to determine the correlation between STMN1 and p27 expression; RNA interference to analyze the function of STMN1 in an EHCC cell line was also used. Cytoplasmic STMN1 expression correlated with venous invasion (P = 0.0021) and nuclear p27 underexpression (P = 0.0011). Patients in the high-STMN1-expression group were associated with shorter recurrence-free survival and overall survival than those in the low-expression group. An in vitro protein-binding assay revealed that cytoplasmic STMN1 bound to p27 in the cytoplasm, but not in the nucleus of EHCC cells. Moreover, p27 accumulated in EHCC cells after STMN1 suppression. STMN1 knockdown inhibited proliferation and increased the sensitivity of EHCC cells to paclitaxel. STMN1 contributes to a poor prognosis and cancer progression in EHCC patients. Understanding the regulation of p27 by STMN1 could provide new insights for overcoming therapeutic resistance in EHCC.
Keywords: Cancer progression, drug resistance, extrahepatic cholangiocarcinoma, p27, stathmin1
Cholangiocarcinoma is associated with poor prognosis and its incidence and mortality are increasing worldwide.1 The 5-year survival rate for cholangiocarcinoma is 10–40%.2 Cholangiocarcinoma is defined as intrahepatic or extrahepatic (EHCC), the latter of which consists of hilar or bile duct tumors. Surgical therapy is the only effective curative treatment for EHCC; postoperative survival is dependent on the existence of invasion and metastasis.3,4 Therefore, to improve patient prognoses, we must understand the mechanism of cancer progression in EHCC.
Stathmin1 (STMN1) is a major cytosolic phosphoprotein that regulates microtubule dynamics by preventing tubulin polymerization and promoting microtubule destabilization. STMN1 plays an important role in a variety of biological processes, including carcinogenesis. STMN1 is highly expressed in various types of human malignancies and is therefore also known as oncoprotein 18 (OP18). Moreover, STMN1 expression correlates with tumor progression and poor prognosis in the following cancers: breast cancer,5–7 prostate cancer,8 gastric cancer,9,10 hepatocellular carcinoma,11,12 oral squamous cell carcinoma,13 colorectal cancer,14,15 malignant mesothelioma16 and urothelial carcinoma.17 Thus, STMN1 is a fundamental cancer-associated gene and a potential target for diagnosis and treatment. To our knowledge, STMN1 expression in EHCC has not been reported; therefore, we explored the role of STMN1 in EHCC.
STMN1 regulates microtubule metabolism and contributes to tumor progression. Baldassarre et al.18 reported that STMN1 bound to p27 suppressed the function of p27 and enhanced the proliferation of tumor cells. p27 was discovered as an inhibitor of cyclin-dependent kinase (CDK) complexes in TGF (transforming growth factor) β-arrested cells and was classified as a member of the Cip/Kip family of cyclin-dependent kinase inhibitors (CKI).19 The CKI associate with a broad spectrum of cyclin–CDK complexes to negatively regulate progression through the G1 phase of the cell cycle. More recently, cytoplasmic p27 was shown to play a role in the regulation of cell migration.20 However, there are still few reports that discuss the relationship of STMN1 and p27 in malignancy, including EHCC. Therefore, we examined the relationship between STMN1 and p27 in EHCC.
The purpose of the present study was to clarify the function of STMN1 in EHCC cell lines in vitro, determine the clinical significance of STMN1 in primary EHCC and evaluate the relationship between STMN1 and p27. To this end, we performed immunohistochemistry to evaluate the relationships between STMN1, p27 and clinicopathological factors in clinical EHCC samples. We also examined the in vitro effects of siRNA-mediated STMN1 suppression on the proliferation, chemotherapeutic sensitivity and p27 expression in human EHCC cell lines.
Material and Methods
Patients and samples
Imunohistochemistry was performed on 80 EHCC patients who had undergone curative surgery in our department between 1995 and 2011. Patients ranged in age from 43 to 94 years. Tumor stage was classified according to the seventh tumor–node–metastasis (TNM) classification of the Union for International Cancer Control (UICC).21 Forty-four (53%) patients received adjuvant therapy following chemotherapy: 11 received UFT (tegafur-uracil; Taiho Pharmaceutical, Tokyo, Japan); nine received Gemcitabine (Eli Lilly and Company, Indianapolis, IN, USA); 21 received S-1 (TS-1; Taiho Pharmaceutical); and three received Gemcitabine+S-1. All patients provided written informed consent, as per institutional guidelines.
Immunohistochemical staining
A 4-μm section was cut from paraffin blocks of EHCC samples. Each section was mounted on a silane-coated glass slide, deparaffinized and soaked for 30 min at room temperature in 0.3% H2O2/methanol to block endogenous peroxidases. The sections were then heated in boiled water and Immunosaver (Nishin EM, Tokyo, Japan) at 98°C for 45 min. Non-specific binding sites were blocked by incubating with Protein Block Serum-Free (DAKO, Carpinteria, CA, USA) for 30 min. A mouse monoclonal anti-STMN1 (OP18) antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA) and a mouse monoclonal anti-p27 antibody (Santa Cruz Biotechnology) were applied at a dilution of 1:100 for 24 h at 4°C. The primary antibody was visualized using the Histofine Simple Stain PO (M) Kit (Nichirei, Tokyo, Japan) according to the instruction manual. The chromogen 3,3¢-diaminobenzidine tetrahydrochloride was applied as a 0.02% solution containing 0.005% H2O2 in 50 mM ammonium acetate-citrate acid buffer (pH 6.0). The sections were lightly counterstained with Mayer's hematoxylin and mounted. Negative controls were established by omitting the primary antibody and no detectable staining was evident. We previously confirmed that esophageal carcinomas express STMN1 and p27; therefore, esophageal carcinomas were used as a positive control (Figs S1–S3).
Immunohistostaining results were evaluated as described by Altan et al.22 The intensity of cytoplasmic STMN1, nuclear p27 and cytoplasmic p27 staining was scored as follows: 0, no staining; 1+, weak staining; 2+, moderate staining; and 3+, strong staining relative to the positive control (Figs S1–S3). The percentage of nuclear-stained cells was calculated by examining at least 1000 cancer cells in five representative areas. Cytoplasmic STMN1, nuclear p27 and cytoplasmic p27 were scored as follows: 0, no staining; 1+, 1–10%; 2+, 11–50%; and 3+, 51–100%. The score was defined as the percentage score multiplied by the intensity score according to the criteria presented in Table S1 (0, 1+, 2+, 3+, 4+, 6+ and 9+). The optimal cut-off point was defined as follows: grades 0, 1, 2, 3 and 4 were considered low expression, while grades 6 and 9 were designated as high expression. All samples were evaluated by two observers (AW, TY). The Ki-67 labeling index was used to calculate the percentage of cells with high nuclear expression cells in approximately 1000 cells per sample.23
Cell culture
The human EHCC cell line HuCCT-1 was used in the present study. All cells were obtained from RIKEN BRC through the National Bio-Resource Project of MEXT, Tokyo, Japan. The cells were cultured in RPMI 1640 medium (Wako, Osaka, Japan) supplemented with 10% FBS and 1% penicillin–streptomycin (Invitrogen, Carlsbad, CA, USA).
siRNA transfection
STMN1-specific siRNA (Silencer Pre-designed siRNA) was purchased from Bonac Corporation (Fukuoka, Japan). HuCCT-1 cells were seeded in six-well, flat-bottom microtiter plates at a density of 1 × 105 cells per well in a volume of 2 mL and incubated in a humidified atmosphere (37°C and 5% CO2). After incubation, 500 μL of Opti-MEM I Reduced Serum Medium (Invitrogen), 5 μL Lipofectamine RNAi MAX (Invitrogen) and 5 μL STMN1-specific siRNA (50 nM final concentration in each well) were mixed and incubated for 20 min to form chelate bonds. The siRNA reagents were then added to the cells. The experiments were performed after 24–96 h of incubation.
Proximity ligation assay (PLA)
HuCCT1 cells were seeded and incubated on Chamber Slides (Lab-Tek II, Thermo Scientific, Waltham, MA, USA) for 24 h. The cells were fixed with 4% paraformaldehyde for 30 min and 100% methanol for 10 min. The slides were then blocked in 4% bovine serum albumin (Millipore, Biillerica, MA, USA) for 30 min and incubated for 48 h at 4°C with the appropriate combinations of mouse, rabbit and goat antibodies diluted 1:100 (STMN1 rabbit antibody; Cell Signaling Technology, Danvers, MA, USA; and p27 mouse antibody; Santa Cruz Biotechnology) in antibody dilution solution (Olink Bioscience, Uppsala, Sweden). After washing, the slides were incubated with Duolink PLA Rabbit MINUS and PLA Mouse PLUS proximity probes (Olink Bioscience) and a proximity ligation was performed using the Duolink Detection Reagent Kit (Olink Bioscience) according to the manufacturer's protocol. Nuclei were stained with Duolink In Situ Mounting Medium with DAPI (Olink Bioscience). Images were acquired with an All-in-one Fluorescence Microscope (Keyence Corporation, Osaka, Japan).
Protein extraction and western blot analysis
Transfected cells were incubated for 96 h. Total protein was extracted using the PRO-PREP Protein Extraction Solution Kit (iNtRON Biotechnology, Sungnam, Kyungki-Do, Korea) and nuclear protein was extracted with the NE-PER Nuclear and Cytoplasmic Extraction Kit (Thermo Scientific, Kanagawa, Japan). The proteins were separated on 4–12% Bis-Tris Mini Gels (Life Technologies Corporation, Carlsbad, CA, USA) and transferred to membranes using an iBlot Dry Blotting System (Life Technologies Corporation). The membranes were incubated overnight at 4°C with mouse monoclonal antibodies against STMN1 (1:1000; Santa Cruz Biotechnology), p27 (1:1000; Santa Cruz Biotechnology), β-actin (1:1000; Sigma, St Louis, MO, USA) and Histone H1 (1:1000; Santa Cruz Biotechnology). The membranes were then treated with horseradish peroxidase-conjugated anti-mouse secondary antibodies and the proteins were detected with the ECL Prime Western Blotting Detection System (GE Healthcare, Tokyo, Japan).
Proliferation assay
Cell proliferation was measured with the Cell Counting Kit-8 (Dojindo Laboratories, Kumamoto, Japan). At 48 h after transfection, the HuCCT1 cells were plated (approximately 5000 cells per well) in 96-well plates in 100 μL of medium containing 10% FBS. Evaluations were performed at the following time points: 0, 24, 48, 72 and 96 h. To determine cell viability, 10 μL of cell counting solution was added to each well and incubated at 37°C for 2 h. Next, the absorbance of each well was detected at 450 nm using a xMark Microplate Absorbance Spectrophotometer (Bio Rad, Hercules, CA, USA).
Paclitaxel assay
A water-soluble tetrazolium (WST)-8 test and the Cell Counting Kit-8 (Dojindo Laboratories) were used to evaluate paclitaxel sensitivity. At 48 h after transfection, HuCCT1 cells were seeded (10 000 cells/well) into 96-well plates in 100 μL medium containing 10% FBS prior to drug exposure. After 24 h pre-incubation the cells were treated with various concentrations of paclitaxel for 48 h. Then, 10 μL WST-8 reagent was added and the cells were incubated for an additional 2 h at 37°C. Viability was determined using colorimetry by absorbance at 450 nm (xMark Microplate Absorbance Spectrophotometer).
Statistical analysis
Data for the continuous variables are expressed as the mean ± SEM. Significance was determined using Student's t-tests and anova. Statistical analysis of the immunohistochemical staining data was performed using the chi-squared test. Survival curves were calculated using the Kaplan–Meier method and analyzed with the log-rank test. Prognostic factors were examined by univariate and multivariate analyses using a Cox proportional hazards model. All differences were deemed significant at P < 0.05 and all statistical analyses were performed with jmp software, version 5.01 (SAS Institute Inc., Cary, NC, USA).
Results
Immunohistochemical staining of STMN1 and p27 in EHCC tissues
STMN1 expression was evaluated using immunohistochemistry in 80 EHCC samples. Thirty-three samples (41.2%) were negative for STMN1 expression (Fig.1a) and 47 samples (58.8%) were positive for cytoplasmic STMN1 expression (Fig.1b). Nuclear p27 expression was also evaluated in these samples. Twenty-five (31.2%) samples were positive for p27 expression (Fig.1c) and 55 samples (68.8%) were negative (Fig.1d). High STMN1 expression was associated with low p27 nuclear expression (P = 0.0011; Table1). Cytoplasmic p27 expression was evaluated in the same EHCC samples; 43 (54.4%) samples demonstrated high cytoplasmic staining for p27 expression (Fig.1d), whereas 36 (45.6%) samples exhibited low p27 expression (Fig.1c). High STMN1 expression was associated with high p27 cytoplasmic expression (P = 0.0063; Table1).
Figure 1.
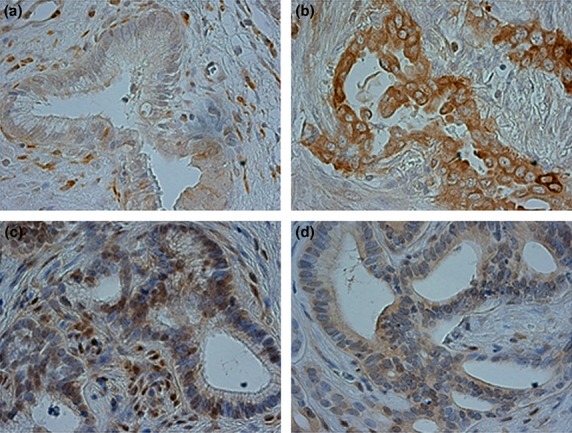
Immunohistochemical staining of stathmin1 (STMN1) and p27 in primary extrahepatic cholangiocarcinoma (EHCC) samples. (a) Low STMN1 expression in a primary EHCC specimen (original magnification, ×400). (b) High STMN1 expression in a primary EHCC specimen (original magnification, ×400). (c) High nuclear p27 expression and low cytoplasmic p27 expression in a primary EHCC specimen (original magnification, ×400). (d) Low nuclear p27 expression and high cytoplasmic p27 expression in a primary EHCC specimen (original magnification, ×400). Figures a, c and b, d show images from the same cases.
Table 1.
Clinicopathological characteristics of extrahepatic cholangiocarcinoma patients according to stathmin expression
| Factor | STMN1 low expression (n = 33) | STMN1 high expression (n = 47) | P-value |
|---|---|---|---|
| Age (years) | |||
| ≤65 | 13 | 18 | 0.9211 |
| >65 | 20 | 29 | |
| Sex | |||
| Male | 23 | 35 | 0.6389 |
| Female | 10 | 12 | |
| Differentiation | |||
| Well | 9 | 9 | 0.2563 |
| Moderate | 15 | 26 | |
| Poor | 8 | 14 | |
| Tumor stage | |||
| T1–2 | 20 | 19 | 0.0745 |
| T3–4 | 13 | 28 | |
| Lymph node metastasis | |||
| − | 19 | 27 | 0.9908 |
| + | 14 | 20 | |
| Lymphatic invasion | |||
| − | 7 | 4 | 0.1070 |
| + | 26 | 43 | |
| Venous invasion | |||
| − | 12 | 4 | 0.002* |
| + | 21 | 43 | |
| Distant metastasis | |||
| − | 32 | 45 | 0.7740 |
| + | 1 | 2 | |
| Infiltration | |||
| α | 0 | 2 | 0.1179 |
| β | 17 | 31 | |
| γ | 15 | 14 | |
| TNM stage (UICC) | |||
| 0, I | 11 | 11 | 0.3297 |
| II, III, IV | 22 | 36 | |
| Nuclear p27 | |||
| High expression | 17 | 8 | 0.001 |
| Low expression | 16 | 39 | |
| Cytoplasmic p27 | |||
| High expression | 12 | 31 | 0.006 |
| Low expression | 21 | 15 | |
| Ki-67 labeling index (mean ± SD) | 9.28 ± 13.5 | 62.1 ± 31.9 | <0.0001 |
P < 0.05. STMN1, stathmin1; TNM, tumor–node–metastasis; UICC, Union for International Cancer Control.
STMN1 expression correlates with venous invasion and Ki-67 labeling index in EHCC tissues
The correlations between STMN1 expression and clinicopathological findings are shown in Table1. We considered the following factors: patient age, patient gender, tumor stage, lymph node metastasis, lymphatic invasion, venous invasion, nerve invasion, infiltrating type and TNM stage (UICC 7th).21 The results showed a correlation between high STMN1 expression and venous invasion (P = 0.0021). We also examined the association between STMN1 expression and the Ki-67 labeling index to evaluate proliferation. High-STMN1-expressing patients had a significantly higher Ki-67 labeling index in comparison to low-STMN1-expressing patients (P < 0.0001).
Prognostic significance of STMN1 expression in EHCC
The prognostic significance of STMN1 expression on postoperative recurrence-free survival (RFS) and cancer-specific survival (CSS) is shown in Figure2. The STMN1-positive group had significantly poorer prognoses than the STMN1-negative group, regarding both RFS (P = 0.0222) and CSS (P = 0.0061). For CSS, STMN1 expression was prognostic for poor survival in the univariate analysis (Table2; P = 0.0044). Multivariate analysis also showed STMN1 expression is prognostic for poor survival (Table2; P = 0.0165). Interestingly, other existing clinicopathological factors were not significantly and independently associated with shorter CSS, whereas STMN1 expression in EHCC remained more significant than the presence of lymph node metastasis (Table2; hazard ratio [HR], 1.696; 95% confidence interval [CI], 1.10–2.76).
Figure 2.
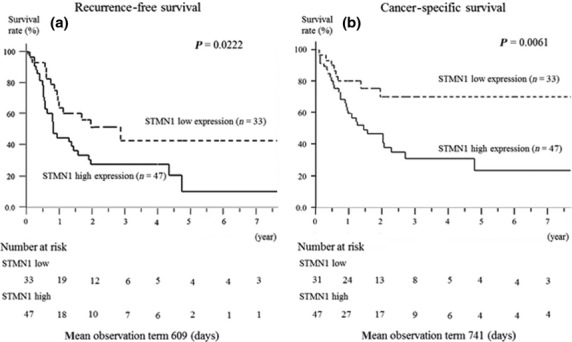
Relationship between postoperative survival and stathmin1 (STMN1) expression. Kaplan–Meier curves of the low-STMN1-expression and high-STMN1-expression groups are shown. (a) High STMN1 expression indicated a poor prognosis for recurrence-free survival (P = 0.0222). (b) High STMN1 expression also indicated a poor prognosis for cancer-specific survival (P = 0.0061).
Table 2.
Univariate and multivariate analysis of prognostic factors using the Cox proportional hazards model
| Factor | Univariate analysis |
Multivariate analysis |
||||
|---|---|---|---|---|---|---|
| RR | 95% CI | P-value | RR | 95% CI | P-value | |
| Age (≤65/>66) | 0.994 | 0.70–1.38 | 0.9720 | – | – | – |
| Sex (M/F) | 0.753 | 0.49–1.09 | 0.1392 | – | – | – |
| Tumor stage (T1–2/3–4) | 2.314 | 1.18–4.71 | 0.0131* | 2.031 | 0.97–4.39 | 0.0577 |
| Lymph node metastasis (−/+) | 1.742 | 1.23–2.50 | 0.0013 | 1.48 | 1.02–2.20 | 0.0379 |
| Lymphatic invasion (−/+) | 1.631 | 0.97–3.33 | 0.0649 | – | – | – |
| Venous invasion (−/+) | 1.619 | 1.02–2.97 | 0.0410 | 1.039 | 0.59–1.99 | 0.898 |
| Distant metastasis (−/+) | 1.803 | 0.29–5.99 | 0.4582 | – | – | – |
| Adjuvant therapy (−/+) | 0.467 | 0.25–0.90 | 0.0242 | 0.346 | 0.17–0.70 | 0.0035 |
| Nuclear p27 expression (−/+) | 0.596 | 0.36–0.89 | 0.0011 | 0.811 | 0.47–1.30 | 0.401 |
| STMN1 expression (−/+) | 1.688 | 1.16–2.58 | 0.0044 | 1.696 | 1.10–2.76 | 0.0165 |
P < 0.05. CI, confidence interval; RR, relative risk; STMN1, stathmin1.
STMN1 and p27 cross-interact in cultured EHCC cells
To examine the protein complexes of STMN1 and p27 in HuCCT1 cells, we performed PLA, which revealed the complexes as red spots in the cytoplasm (Fig.3a). Thus, STMN1 interacts with p27 in the cytoplasm, but not in the nucleus, of EHCC cells.
Figure 3.
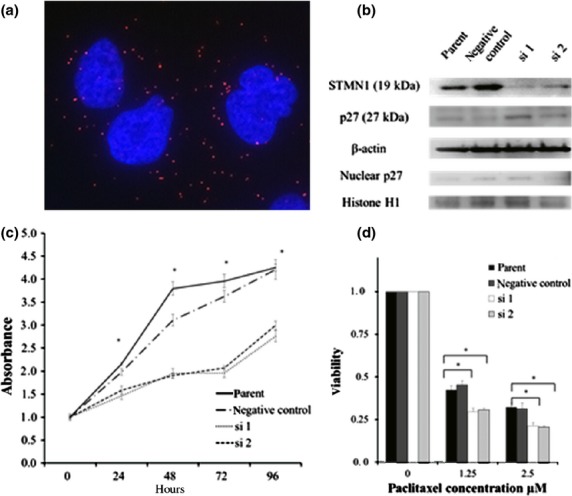
(a) Stathmin1 (STMN1) bound to p27 in the cytoplasm of HuCCT1 cells. Primary STMN1 rabbit and p27 mouse antibodies bound to STMN1 and p27 complexes were combined with secondary proximity ligation assay probes. The interaction events are visible as red dots (nuclear staining with DAPI). (b) siRNA-mediated STMN1 suppression. STMN1 protein levels were measured using western blotting after transfection with STMN1 siRNA. p27 expression in STMN1 siRNA-transfected and untreated HuCCT-1 cells was assessed using western blotting of total and nuclear protein. β-Actin was used as a loading control for total protein and Histone H1 was used as a loading control for nuclear protein. (c) HuCCT-1 cells were transfected with STMN1 siRNA and WST-8 proliferative assays were performed and compared with untransfected cells. (d) Paclitaxel sensitivity was determined using a WST-8 assay. STMN1 siRNA transfection significantly increased paclitaxel sensitivity compared with untransfected cells. Each time point represents the mean ± SD of triplicate determinations in two independent experiments. *P < 0.05.
siRNA-mediated STMN1 suppression and p27 expression in HuCCT-1 cells
Two siRNA complexes were used to knock down STMN1 expression in HuCCT-1 cells. Suppression of STMN1 by siRNA1 and siRNA2 was demonstrated using western blotting 96 h after transfection (Fig.3b). Next, we examined the effect of STMN1 knockdown on p27 expression. STMN1 depletion induced total p27 protein expression and also had a tendency to increase nuclear p27 expression (Fig.3b).
Suppression of STMN1 reduces proliferation and sensitizes EHCC cells to paclitaxel
We assessed the relationship between EHCC proliferation and STMN1 expression. WST assays revealed that the proliferation of STMN1-knockdown cells was significantly lower than in the parent and negative-control cells (P < 0.01; Fig.3c). STMN1-knockdown cells were also significantly more sensitive to paclitaxel than the control cells (P < 0.01; Fig.3d).
Immunohistochemical staining of STMN1 and p27 in EHCC tissues
STMN1 expression was evaluated using immunohistochemistry in 80 EHCC samples. Overall, 33 (41.2%) EHCC samples were negative for STMN1 expression (Fig.1a), whereas 47 (58.8%) samples exhibited positive cytoplasmic staining for STMN1 expression (Fig.1b). Nuclear p27 expression was also evaluated in the 80 EHCC samples. Twenty-five (31.2%) samples demonstrated positive nucleic staining for p27 expression (Fig.1c), whereas 55 (68.8%) samples were negative for p27 expression (Fig.1d). Moreover, the samples exhibiting high STMN1 expression were associated with low p27 nuclear expression (P = 0.0011; Table1). In addition, p27 expression was also evaluated on each EHCC samples, 43 (54.4%) samples demonstrated high cytoplasmic staining for p27 expression (Fig.1d), whereas 36 (45.6%) samples were low staining for p27 expression (Fig.1c). The samples exhibiting high STMN1 expression were also associated with high p27 cytoplasmic expression (P = 0.0063; Table1).
STMN1 expression correlates with venous invasion and Ki-67 labeling index in EHCC tissues
The correlations between STMN1 expression and clinicopathological findings are displayed in Table1. Regarding the clinicopathological findings, we considered the following factors: patient age, patient gender, tumor stage, lymph node metastasis, lymphatic invasion, venous invasion, nerve invasion, infiltrating type and TNM stage (UICC 7th). The results revealed that STMN1-high expression was correlated with venous invasion (P = 0.0021). In addition, we examined the association between STMN1 expression and the Ki-67 labeling index to evaluate proliferation ability. The STMN1-high-expression patients had a significantly higher Ki-67 labeling index than the low-expression patients (P < 0.0001).
Prognostic significance of STMN1 expression in EHCC
The prognostic significance of STMN1 expression on postoperative RFS and CSS rates are displayed in Figure2. The STMN1-positive group had significantly poorer prognoses than the STMN1-negative group, regarding both RFS (P = 0.0222) and CSS (P = 0.0061). For CSS, STMN1 expression was a prognostic factor for poor survival in the univariate analysis (Table2; P = 0.0044). In the multivariate analysis, STMN1 expression was also a prognostic factor for poor survival (Table2; P = 0.0165). Interestingly, other existing clinicopathological factors were not significantly and independently associated with shorter CSS, whereas the detection of STMN1 expression in EHCC remained prognostically more significant than the presence of lymph node metastasis with regard to CSS (Table2; HR, 1.696; 95% CI, 1.10–2.76).
STMN1 and p27 cross-interactinn EHCC cells in vitro
To examine the protein complexes formed between STMN1 and p27 in HuCCT1 cells, we performed PLA. As a result, the formations of STMN1 and p27 complexes were detected in the cytoplasm as red spots (Fig.3a). This result demonstrates that STMN1 interacts with p27 in the cytoplasm, but not in the nucleus, of EHCC cells.
Effect of siRNA-mediated STMN1 suppression on p27 expression in HuCCT-1 cells
Two siRNA complexes were used to knockdown STMN1 expression in the HuCCT-1 cells. The suppression of STMN1 by siRNA1 and siRNA2 was demonstrated using western blotting 96 h after transfection (Fig.3b). Next, we examined the effect of STMN1 knockdown on p27 expression. The depletion of STMN1 increased p27 expression on total protein and also had a tendency to increase nuclear p27 expression (Fig.3b).
Suppression of STMN1 reduces proliferation and sensitizes EHCC cells to paclitaxel
We assessed the relationship between the proliferative ability of EHCC cells and STMN1 expression. The cellular proliferation ability was evaluated using the WST assay, which revealed that the proliferation of STMN1-knockdown cells significantly diminished compared with the parent and negative-control cells (P < 0.01; Fig.3c). Moreover, STMN1-knockdown cells exhibited significantly higher sensitivity to paclitaxel than the control cells (P < 0.01; Fig.3d).
Discussion
In the present study, we demonstrated that high STMN1 expression is associated with poor prognosis in primary EHCC samples. Moreover, high STMN1 expression is related to low nuclear p27 expression and high cytoplasmic p27 expression. In the in vitro STMN1 knockdown analysis, proliferative ability was reduced and paclitaxel sensitivity was increased in transfected cells compared with the control cells. In addition, STMN1 knockdown increased p27 expression.
In the immunohistochemical analysis, high STMN1 expression correlated to poor RFS and CSS prognosis. Moreover, based on the multivariate analysis of CSS, high STMN1 expression translated into an independent prognostic factor. Some reports have also revealed that high STMN1 expression is related to poor prognosis in the following cancers: oral squamous cell carcinoma;13 diffuse type gastric cancer;9 colon cancer;14 hepatocellular cancer;12 and urothelial carcinoma.17 The results of the present study are in agreement with these previous reports. Therefore, STMN1 is expected to serve as a prognostic-predictive marker of EHCC.
In the present study, high STMN1 expression was associated with venous invasion in EHCC. Jeon et al.9 reported this association in diffuse type gastric carcinoma and showed that in vitro STMN1 suppression inhibited migration and invasion in gastric cancer cells. Baldassare et al.18 reported that STMN1 promotes invasion in sarcoma and regulates microtubule stability following adhesion to the extracellular matrix. STMN1 also regulated invasion in hepatocellular carcinoma.12 Our results were consistent with these previous reports and indicate that STMN1 is associated with invasion in EHCC.
Previous studies have examined the relationship between STMN1 and p27.24,25 Baldassarre et al.18 reported that STMN1 bound to p27 in the cytoplasm of sarcoma cells and that high-STMN1-expressing and low-p27-expressing cells demonstrate increased proliferation and invasion ability. However, the authors did not describe in detail whether cytoplasmic p27 regulates STMN1 function or whether cytoplasmic STMN1 inhibits the import of p27 into the nucleus. In our immunohistochemical analysis of EHCC, high-STMN1-expressing samples exhibited a significant tendency for low nuclear p27 expression and high cytoplasmic p27 expression. Using a PLA assay, STMN1 was shown to interact with p27 directly in the cytoplasm of HuCCT1 cells in vitro. Moreover, siRNA-mediated STMN1 knockdown triggered increased p27 expression and the proliferative ability of STMN1-suppressed cells significantly diminished. Interestingly, p27-degradation promotion has been reported in the cytoplasm but not in the nucleus during the G0–G1 transition. From these results it is suggested that STMN1 interacts with p27 in the cytoplasm, inhibits the function of nuclear p27 via the degradation of cytoplasmic p27 and leads to the progression of cancer. S phase kinase-associated protein 2 (SKP2) promotes p27 degradation via the ubiquitin–proteasome system26 and p27 upregulation facilitated by a SKP2 inhibitor significantly suppresses the cell cycle and results in lower proliferation potency.27 Therefore, p27 regulation by STMN1 targeting might provide a promising therapeutic tool for several cancers including EHCC.
Some studies have examined the relationship between STMN1 expression and taxane anticancer drugs. STMN1 overexpression levels are associated with paclitaxel sensitivity in ovarian cancer and breast cancer cells.6,28 Iancu et al.29 reported that taxol and anti-STMN1 therapy produced a synergistic anticancer effect on a leukemic cell line. Moreover, Alli et al.7 also reported that STMN1 overexpression increases the rate of cell death, decreases microtubule polymerization, which markedly decreases taxane binding, and prevents cells from entering mitosis. In the present study, STMN1 knockdown increased paclitaxel sensitivity, which is consistent with previous reports. Although few anti-microtubule cancer drugs have produced a positive effect on EHCC in current clinical practice, the combination of taxane and anti-STMN1 drugs may provide a prospective therapy against various cancers in the future.
In conclusion, STMN1 expression contributed to a shorter duration of RFS and reduced CSS in patients with EHCC. Therefore, the evaluation of STMN1 expression in EHCC might be a useful predictor of recurrence and poor prognosis. Moreover, high STMN1 expression was associated with low p27 expression in clinical EHCC samples and STMN1 suppression regulated proliferation and paclitaxel sensitivity in an EHCC cell line. Our results suggest that STMN1 in EHCC may be a promising molecular target for controlling cancer progression and taxane resistance via p27 regulation.
Acknowledgments
The authors thank Ms Yukie Saito, Ms Tomoko Yano, Ms Tomoko Ubukata, Ms Yuka Matsui, Ms Ayaka Ishida and Ayaka Ishikubo for their excellent assistance.
Disclosure Statement
The authors have no conflicts of interest.
Funding information
None declared.
Supporting Information
Additional supporting information may be found in the online version of this article:
Immunohistochemical staining of stathmin1 (STMN1) in primary esophageal cancer served as a positive control.
Immunohistochemical staining of nuclear p27 in primary esophageal cancer served as a positive control.
Immunohistochemical staining of cytoplasmic p27 in primary esophageal cancer served as a positive control.
Criteria of Immunohisochemistry evaluation.
References
- 1.Vasilieva LE, Papadhimitriou SI, Dourakis SP. Modern diagnostic approaches to cholangiocarcinoma. Hepatobiliary Pancreat Dis Int. 2012;11:349–59. doi: 10.1016/s1499-3872(12)60192-1. [DOI] [PubMed] [Google Scholar]
- 2.Skipworth JR, Olde Damink SW, Imber C, Bridgewater J, Pereira SP, Malago M. Review article: surgical, neo-adjuvant and adjuvant management strategies in biliary tract cancer. Aliment Pharmacol Ther. 2011;34:1063–78. doi: 10.1111/j.1365-2036.2011.04851.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pichlmayr R, Weimann A, Klempnauer J, et al. Surgical treatment in proximal bile duct cancer. A single-center experience. Ann Surg. 1996;224:628–38. doi: 10.1097/00000658-199611000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klempnauer J, Ridder GJ, Werner M, Weimann A, Pichlmayr R. What constitutes long-term survival after surgery for hilar cholangiocarcinoma? Cancer. 1997;79:26–34. [PubMed] [Google Scholar]
- 5.Alli E, Yang JM, Hait WN. Silencing of stathmin induces tumor-suppressor function in breast cancer cell lines harboring mutant p53. Oncogene. 2007;26:1003–12. doi: 10.1038/sj.onc.1209864. [DOI] [PubMed] [Google Scholar]
- 6.Alli E, Yang JM, Ford JM, Hait WN. Reversal of stathmin-mediated resistance to paclitaxel and vinblastine in human breast carcinoma cells. Mol Pharmacol. 2007;71:1233–40. doi: 10.1124/mol.106.029702. [DOI] [PubMed] [Google Scholar]
- 7.Alli E, Bash-Babula J, Yang JM, Hait WN. Effect of stathmin on the sensitivity to antimicrotubule drugs in human breast cancer. Cancer Res. 2002;62:6864–9. [PubMed] [Google Scholar]
- 8.Ghosh R, Gu G, Tillman E, et al. Increased expression and differential phosphorylation of stathmin may promote prostate cancer progression. Prostate. 2007;67:1038–52. doi: 10.1002/pros.20601. [DOI] [PubMed] [Google Scholar]
- 9.Jeon TY, Han ME, Lee YW, et al. Overexpression of stathmin1 in the diffuse type of gastric cancer and its roles in proliferation and migration of gastric cancer cells. Br J Cancer. 2010;102:710–8. doi: 10.1038/sj.bjc.6605537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kang W, Tong JH, Chan AW, et al. Stathmin1 plays oncogenic role and is a target of microRNA-223 in gastric cancer. PLoS ONE. 2012;7:e33919. doi: 10.1371/journal.pone.0033919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singer S, Ehemann V, Brauckhoff A, et al. Protumorigenic overexpression of stathmin/Op18 by gain-of-function mutation in p53 in human hepatocarcinogenesis. Hepatology. 2007;46:759–68. doi: 10.1002/hep.21736. [DOI] [PubMed] [Google Scholar]
- 12.Hsieh SY, Huang SF, Yu MC, et al. Stathmin1 overexpression associated with polyploidy, tumor-cell invasion, early recurrence, and poor prognosis in human hepatoma. Mol Carcinog. 2010;49:476–87. doi: 10.1002/mc.20627. [DOI] [PubMed] [Google Scholar]
- 13.Kouzu Y, Uzawa K, Koike H, et al. Overexpression of stathmin in oral squamous-cell carcinoma: correlation with tumour progression and poor prognosis. Br J Cancer. 2006;94:717–23. doi: 10.1038/sj.bjc.6602991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zheng P, Liu YX, Chen L, et al. Stathmin, a new target of PRL-3 identified by proteomic methods, plays a key role in progression and metastasis of colorectal cancer. J Proteome Res. 2010;9:4897–905. doi: 10.1021/pr100712t. [DOI] [PubMed] [Google Scholar]
- 15.Ogino S, Nosho K, Baba Y, et al. A cohort study of STMN1 expression in colorectal cancer: body mass index and prognosis. Am J Gastroenterol. 2009;104:2047–56. doi: 10.1038/ajg.2009.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim JY, Harvard C, You L, et al. Stathmin is overexpressed in malignant mesothelioma. Anticancer Res. 2007;27:39–44. [PubMed] [Google Scholar]
- 17.Lin WC, Chen SC, Hu FC, et al. Expression of stathmin in localized upper urinary tract urothelial carcinoma: correlations with prognosis. Urology. 2009;74:1264–9. doi: 10.1016/j.urology.2009.04.088. [DOI] [PubMed] [Google Scholar]
- 18.Baldassarre G, Belletti B, Nicoloso MS, et al. p27(Kip1)-stathmin interaction influences sarcoma cell migration and invasion. Cancer Cell. 2005;7:51–63. doi: 10.1016/j.ccr.2004.11.025. [DOI] [PubMed] [Google Scholar]
- 19.Nakayama K, Ishida N, Shirane M, et al. Mice lacking p27(Kip1) display increased body size, multiple organ hyperplasia, retinal dysplasia, and pituitary tumors. Cell. 1996;85:707–20. doi: 10.1016/s0092-8674(00)81237-4. [DOI] [PubMed] [Google Scholar]
- 20.Denicourt C, Dowdy SF. Cip/Kip proteins: more than just CDKs inhibitors. Genes Dev. 2004;18:851–5. doi: 10.1101/gad.1205304. [DOI] [PubMed] [Google Scholar]
- 21.Sobin LH, Gospodarowicz MK, Wittekind C. TMN Classification of Malignant Tumors. Wiley-Blackwell: 2009. eds., 7th edn. Washington, DC. [Google Scholar]
- 22.Altan B, Yokobori T, Mochiki E, et al. Nuclear karyopherin-alpha2 expression in primary lesions and metastatic lymph nodes was associated with poor prognosis and progression in gastric cancer. Carcinogenesis. 2013;34:2314–21. doi: 10.1093/carcin/bgt214. [DOI] [PubMed] [Google Scholar]
- 23.Suzuki S, Miyazaki T, Tanaka N, et al. Prognostic significance of CD151 expression in esophageal squamous cell carcinoma with aggressive cell proliferation and invasiveness. Ann Surg Oncol. 2011;18:888–93. doi: 10.1245/s10434-010-1387-3. [DOI] [PubMed] [Google Scholar]
- 24.Iancu-Rubin C, Atweh GF. p27(Kip1) and stathmin share the stage for the first time. Trends Cell Biol. 2005;15:346–8. doi: 10.1016/j.tcb.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 25.Karst AM, Levanon K, Duraisamy S, et al. Stathmin 1, a marker of PI3K pathway activation and regulator of microtubule dynamics, is expressed in early pelvic serous carcinomas. Gynecol Oncol. 2011;123:5–12. doi: 10.1016/j.ygyno.2011.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hara T, Kamura T, Nakayama K, Oshikawa K, Hatakeyama S. Degradation of p27(Kip1) at the G(0)–G(1) transition mediated by a Skp2-independent ubiquitination pathway. J Biol Chem. 2001;276:48937–43. doi: 10.1074/jbc.M107274200. [DOI] [PubMed] [Google Scholar]
- 27.Wu L, Grigoryan AV, Li Y, Hao B, Pagano M, Cardozo TJ. Specific small molecule inhibitors of Skp2-mediated p27 degradation. Chem Biol. 2012;19:1515–24. doi: 10.1016/j.chembiol.2012.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Balachandran R, Welsh MJ, Day BW. Altered levels and regulation of stathmin in paclitaxel-resistant ovarian cancer cells. Oncogene. 2003;22:8924–30. doi: 10.1038/sj.onc.1207060. [DOI] [PubMed] [Google Scholar]
- 29.Iancu C, Mistry SJ, Arkin S, Atweh GF. Taxol and anti-stathmin therapy: a synergistic combination that targets the mitotic spindle. Cancer Res. 2000;60:3537–41. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Immunohistochemical staining of stathmin1 (STMN1) in primary esophageal cancer served as a positive control.
Immunohistochemical staining of nuclear p27 in primary esophageal cancer served as a positive control.
Immunohistochemical staining of cytoplasmic p27 in primary esophageal cancer served as a positive control.
Criteria of Immunohisochemistry evaluation.


